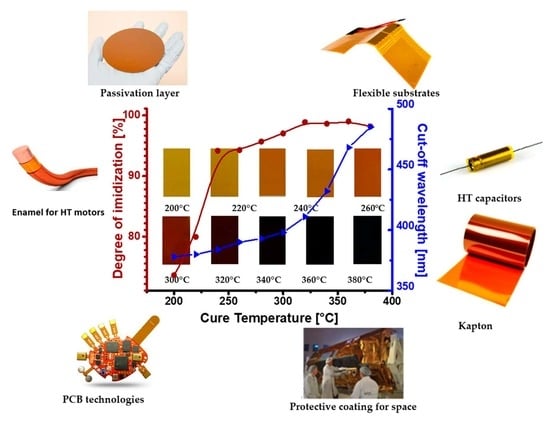
A Universal Study on the Effect Thermal Imidization Has on the Physico-Chemical, Mechanical, Thermal and Electrical Properties of Polyimide for Integrated Electronics Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Summary of the Thermal Imidization Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaham, S. (Ed.) Polyimide in Electronics: Applications and Processability Overview. In Polyimide for Electronic and Electrical Engineering Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Endrey, A.L. Process for Preparing Polyimides by Treating Polyamides-Acids with Lower Fatty Monocarboxylic Acid Anhydrides. U.S. Patent No. 3179630, 20 April 1965. [Google Scholar]
- Endrey, A.L. Ammonium Salts of Aromatic Polyamides-Acids and Process for Preparing Polyimides Therefrom. U.S. Patent No. 3242136, 22 March 1966. [Google Scholar]
- Ishii, J.; Shimizu, N.; Ishihara, N.; Ikeda, Y.; Sensui, N.; Matano, T.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (4): Casting- and melt-induced in-plane orientation. Eur. Polym. J. 2010, 46, 69–80. [Google Scholar] [CrossRef]
- Kotov, B.; Gordina, T.; Voishchev, V.; Kolninov, O.; Pravednikov, A. Aromatic polyimides as charge transfer complexes. Polym. Sci. USSR 1977, 19, 711–716. [Google Scholar] [CrossRef]
- Fowkes, F.M.; Mostafa, M.A. Acid-Base Interactions in Polymer Adsorption. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 3–7. [Google Scholar] [CrossRef]
- Bell, V.L.; Stump, B.L.; Gager, H. Polyimide structure–property relationships. II. Polymers from isomeric diamines. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 2275–2291. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoon, D.Y.; Parrish, W. Molecular order in condensed states of semiflexible poly(amic acid) and polyimide. Macromolecules 1984, 17, 2583–2588. [Google Scholar] [CrossRef]
- Lamb, R.N.; Baxter, J.; Grunze, M.; Kong, C.W.; Unertl, W.N. An XPS study of the composition of thin polyimide films formed by vapor deposition. Langmuir 1988, 4, 249–256. [Google Scholar] [CrossRef]
- Ito, Y.; Hikita, M.; Kimura, T.; Mizutani, T. Effect of Degree of Imidization in Polyimide Thin Films Prepared by Vapor Deposition Polymerization on the Electrical Conduction. Jpn. J. Appl. Phys. 1990, 29, 1128–1131. [Google Scholar] [CrossRef]
- Hsu, T.-C.J.; Liu, Z.-L. Solvent effect on the curing of polyimide resins. J. Appl. Polym. Sci. 1992, 46, 1821–1833. [Google Scholar] [CrossRef]
- Kan, L.; Kao, K.C. Ultraviolet absorption and photoconduction spectra of polyimide films fabricated at various curing temperatures. J. Chem. Phys. 1993, 98, 3445–3451. [Google Scholar] [CrossRef]
- Iida, K.; Imamura, Y.; Liao, C.; Nakamura, S.; Saw, A.G. Evaluation of Molecular Orientation in Aromatic Polyimide Films by FT-IR Reflection Absorption Spectroscopy. Polym. J. 1996, 28, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Karamancheva, I.; Stefov, V.; Šoptrajanov, B.; Danev, G.; Spasova, E.; Assa, J. FTIR spectroscopy and FTIR microscopy of vacuum-evaporated polyimide thin films. Vib. Spectrosc. 1999, 19, 369–374. [Google Scholar] [CrossRef]
- Li, W.S.; Shen, Z.X.; Zheng, J.Z.; Tang, S.H. FT-IR Study of the Imidization Process of Photosensitive Polyimide PMDA/ODA. Appl. Spectrosc. 1998, 52, 985–989. [Google Scholar] [CrossRef]
- Jou, J.H.; Huang, P.T. Effect of thermal curing on the structures and properties of aromatic polyimide films. ACS Macro 1991, 24, 3796–3803. [Google Scholar] [CrossRef] [Green Version]
- Kook, H.J.; Kim, D. In situ measurements and analysis of imidization extent, thickness, and stress during the curing of polyimide films. J. Mater. Sci. 2000, 35, 2949–2954. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Alfonso, E.L.; Harding, D.R.; Chen, S.H. Processing vapour-deposited polyimide. J. Phys. D Appl. Phys. 2001, 34, 3011. [Google Scholar] [CrossRef]
- Lee, B.-J.; Kim, H.-G.; Lee, D.-C. Electrical properties of polyimide thin films formed by the vapor deposition polymerization method. Surf. Coat. Technol. 2002, 150, 182–187. [Google Scholar] [CrossRef]
- Spassova, E. Vacuum deposited polyimide thin films. Vacuum 2003, 70, 551–561. [Google Scholar] [CrossRef]
- Anthamatten, M.; Letts, S.A.; Day, K.; Cook, R.C.; Gies, A.P.; Hamilton, T.P.; Nonidez, W.K. Solid-state amidization and imidization reactions in vapor-deposited poly(amic acid). J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5999–6010. [Google Scholar] [CrossRef]
- Shin, T.; Ree, M. Thermal Imidization and Structural Evolution of Thin Films of Poly(4,4’-oxydiphenylene p-pyromellitamic diethyl ester). J. Polym. Sci. 2007, 111, 13894–13900. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.; Zhang, B.; Yang, S.; Liu, C.-Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Chang, J.-H.; Park, K.M. Thermal cyclization of the poly(amic acid): Thermal, mechanical, and morphological properties. Eur. Polym. J. 2000, 36, 2185–2191. [Google Scholar] [CrossRef]
- Saeed, M.; Zhan, M.-S. Effects of monomer structure and imidization degree on mechanical properties and viscoelastic behavior of thermoplastic polyimide films. Eur. Polym. J. 2006, 42, 1844–1854. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, A.Y.; Yokota, R. Structure and Properties of Novel Asymmetric Biphenyl Type Polyimides. Homo- and Copolymers and Blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Chen, K.-M.; Wang, T.-H.; King, J.-S.; Hung, A. Effect of imidization temperature on properties of polymide films. J. Appl. Polym. Sci. 1993, 48, 291–297. [Google Scholar] [CrossRef]
- Pryde, C.A. FTIR studies of polyimides. II. Factors affecting quantitative measurement. J. Polym. Sci. Part A: Polym. Chem. 1993, 31, 1045–1052. [Google Scholar] [CrossRef]
- Bjellheim, P.; Helcee, B. Aromatic Polyimides: Synthesis, Characterization, and Evaluation of Electric strength. J. Appl. Polym. Sci. 1993, 48, 1587–1596. [Google Scholar] [CrossRef]
- Pramoda, K.; Liu, S.; Chung, T.-S. Thermal Imidization of the Precursor of a Liquid Crystalline Polyimide. Macromol. Mater. Eng. 2002, 287, 931–937. [Google Scholar] [CrossRef]
- Diaham, S.; Locatelli, M.; Lebey, T.; Malec, D. Thermal imidization optimization of polyimide thin films using Fourier transform infrared spectroscopy and electrical measurements. Thin Solid Films 2011, 519, 1851–1856. [Google Scholar] [CrossRef]
- Al-Ajaj, I.A.; Kareem, A.A. Synthesis and characterization of polyimide thin films obtained by thermal evaporation and solid state reaction. Mater. Sci. 2016, 34, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Krasovskii, A.; Antonov, N.; Koton, M.; Kalnin’Sh, K.; Kudryavtsev, V. Methods of investigation determining the degree of imidization of polyamidoacids. Polym. Sci. USSR 1979, 21, 1038–1043. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.M.; Wessling, M.; Strathmann, H. Plasticization-resistant glassy polyimide membranes for CO2/CH4 separations. Sep. Purif. Technol. 1998, 14, 27–39. [Google Scholar] [CrossRef]
- Wilson, D.; Stenzenberger, H.D.; Hergenrother, P.M. Polyimides, Blackie, Glasgow. Polym. Int. 1991, 25, 199. [Google Scholar] [CrossRef]
- Lan, Z.; Li, C.; Yu, Y.; Wei, J. Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility. Polymers 2019, 11, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of Aromatic Polyimides and Electronic Properties of Their Source Materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless Polyimides Derived from an Alicyclic Tetracarboxylic Dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.S.; Kim, I.; Ha, C.-S. Synthesis, characterization, and properties of fully aliphatic polyimides and their derivatives for microelectronics and optoelectronics applications. Macromol. Res. 2007, 15, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Abe, A.; Nakano, T.; Yamashita, W.; Fukukawa, K.; Okazaki, M.; Tamai, S. Theoretical and Experimental Studies on the Mechanism of Coloration of Polyimides. ChemPhysChem 2011, 12, 1367–1377. [Google Scholar] [CrossRef]
- Yang, Y.; Jung, Y.; Cho, M.D.; Lee, S.G.; Kwon, S. Transient color changes in oxidative-stable fluorinated polyimide film for flexible display substrates. RSC Adv. 2015, 5, 57339–57345. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Jia, Z.; Qi, S.; Wu, D. Structural Evolution of Macromolecular Chain During Pre-imidization Process and Its Effects on Polyimide Film Properties. J. Phys. Chem. B 2020, 124, 7969–7978. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Mikawa, M.; Nagaoka, S. Gas transport properties in thermally cured aromatic polyimide membranes. J. Membr. Sci. 1996, 118, 223–230. [Google Scholar] [CrossRef]
- Tang, H.; Feng, H.; Luo, H.; Dong, L.; Feng, Z. The aggregation state of polyimide. Eur. Polym. J. 1997, 33, 519–523. [Google Scholar] [CrossRef]
- Mikawa, M.; Nagaoka, S.; Kawakami, H. Gas transport properties and molecular motions of 6FDA copolyimides. J. Membr. Sci. 1999, 163, 167–176. [Google Scholar] [CrossRef]
- Luo, L.; Yao, J.; Wang, X.; Li, K.; Huang, J.; Li, B.; Wang, H.; Feng, Y.; Liu, X. The evolution of macromolecular packing and sudden crystallization in rigid-rod polyimide via effect of multiple H-bonding on charge transfer (CT) interactions. Polymers 2014, 55, 4258–4269. [Google Scholar] [CrossRef]
- Sabrina, M.P.; Geoffrey, D.S. Light Absorption by Charge Transfer Complexes in Brown Carbon Aerosols. Environ. Sci. Technol. Lett. 2014, 1, 382–386. [Google Scholar]
- García, M.G.; Marchese, J.; Ochoa, N.A. Aliphatic–aromatic polyimide blends for H2 separation. Int. J. Hydrogen Energy 2010, 35, 8983–8992. [Google Scholar] [CrossRef]
- Zhuang, Y.; Gu, Y. Probing structural evolution of the poly(amic acid) containing benzoxazole moieties in backbone during thermal imidization. J. Polym. Res. 2012, 19, 14. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Chang, F.-C. Novel Organosoluble Poly(pyridine−imide) with Pendent Pyrene Group: Synthesis, Thermal, Optical, Electrochemical, Electrochromic, and Protonation Characterization. Macromolecules 2007, 40, 3568–3574. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-Time Characterization of Physical Changes in Polyimide Film Formation: From Casting to Imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, H.; Mita, I.; Yokota, R. Isothermal Imidization of an Aromatic Polyimide Precursor Studied by Fluorescence Spectroscopy. Polym. J. 1990, 22, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Kochi, M.; Mita, I.; Yokota, R. Molecular aggregation and fluorescence spectra of aromatic polyimides. Eur. Polym. J. 1989, 25, 349–354. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Frank, C.W. Effect of cure history on the morphology of polyimide: Fluorescence spectroscopy as a method for determining the degree of cure. Polymer 1988, 29, 1191–1197. [Google Scholar] [CrossRef]
- Cho, D.; Yang, G.; Drzal, L.T. Effect of thermal imidization and curing on fluorescence behavior of a phenylethynyl-terminated poly(amic acid). Macromol. Res. 2003, 11, 297–302. [Google Scholar] [CrossRef]
- Jadhav, N.-R.; Gaikwad, V.-L.; Nair, K.-J.; Kadam, H.-M. Glass transition temperature: Basics and application in pharmaceutical sector. Asian J. Pharm. 2009, 3. [Google Scholar] [CrossRef]
- Billmeyer, F.W. Textbook of Polymer Science, 3rd ed.; AWiley-Interscience Publication: Singapore, 1994; pp. 320–326, 337–340. [Google Scholar]
- Huang, C.; Li, J.; Sun, D.; Xuan, R.; Sui, Y.; Li, T.; Shang, L.; Zhang, G.; Sun, R.; Wong, C.P. Comprehensive properties study of low-temperature imidized polyimide with curing accelerators. J. Mater. Chem. C 2020, 8, 14886–14894. [Google Scholar] [CrossRef]
- Sava, I.; Ştefan, C.; Brumă, M.; Lisa, G. Effect of thermal curing on the properties of thin films based on benzophenonetetracarboxylic dianhydride and 4,4′-diamino-3,3′-dimethyldiphenylmethane. J. Therm. Anal. 2011, 104, 1135–1143. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Cheng, J.-O. Thermal imidization behavior of aromatic polyimides by rigid-body pendulum rheometer. J. Appl. Polym. Sci. 2008, 108, 3973–3981. [Google Scholar] [CrossRef]
- Wallach, M.L. Structure–property relations of polyimide films. J. Polym. Sci. Part A 1968, 6, 953–960. [Google Scholar] [CrossRef]
- Khazaka, R.; Locatelli, M.L.; Diaham, S.; Bidan, P.; Dupuy, L.; Grosset, G. Broadband dielectric spectroscopy of BPDA/ODA polyimide films. J. Phys. D Appl. Phys. 2013, 46, 065501. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, J.; Gan, F.; Li, Z.; Zhang, Q. Structural evolution from poly(amic acid) to polyimide fibers during thermal imidization process. High Perform. Polym. 2019, 31, 600–610. [Google Scholar] [CrossRef]
- Diaham, S.; Bechara, M.; Locatelli, M.-L.; Tenailleau, C. Electrical Conductivity of Parylene F at High Temperature. J. Electron. Mater. 2010, 40, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Diaham, S.; Bechara, M.; Locatelli, M.-L.; Khazaka, R.; Tenailleau, C.; Kumar, R. Dielectric strength of parylene HT. J. Appl. Phys. 2014, 115, 054102. [Google Scholar] [CrossRef] [Green Version]






| Main Polyimide Properties | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physico-Chemical | Thermal | Mechanical | Electrical | |||||||||||||
| Polyimide Type | Imidization Curing (°C) | Process | FTIR | XRD | XPS | UV–Vis | TGA (Td) | DSC (Tg) | DMTA (E’, E”, tanδm) | Tensile Strength and Elongation | CTE | Thickness | BDRS (ε’, ε”, tanδd, σAC) | CC (σDC) | BD (EBR) | References |
| PMDA-ODA | 250–400 | Spin-coat | + | [8] | ||||||||||||
| PMDA-ODA | 97–447 | VDP | + | [9] | ||||||||||||
| PMDA-ODA | 150 vs. time | VDP | + | + | [10] | |||||||||||
| PMDA-ODA | 85–400 | Spin-coat | + | + | + | [11] | ||||||||||
| PMDA-ODA | 135–350 | Spin-coat | + | + | [12] | |||||||||||
| PMDA-ODA | 200–350 | VDP | + | + | + | [13] | ||||||||||
| PMDA-ODA | 170–350 | VDP | + | [14] | ||||||||||||
| PMDA-ODA | 100–500 | Spin-coat | + | + | [15] | |||||||||||
| PMDA-ODA PMDA-PDA | 100–400 | Casting | + | + | + | [16] | ||||||||||
| PMDA-ODA | 100–400 | Spin-coat | + | + | + | [17] | ||||||||||
| PMDA-ODA | 300 vs. time | VDP | + | + | + | [18] | ||||||||||
| PMDA-ODA | 150–350 | VDP | + | + | + | + | + | [19] | ||||||||
| PMDA-ODA | 170–350 | VDP | + | + | [20] | |||||||||||
| PMDA-ODA | 80–300 | VDP | + | + | [21] | |||||||||||
| PMDA-ODA | 30–380 | Spin-coat | + | + | + | [22] | ||||||||||
| PMDA-ODA | 70–400 | Casting | + | + | + | + | + | + | [23] | |||||||
| PMDA-BDA | 100–250 | Casting | + | + | + | + | + | [24] | ||||||||
| BPDA-ODA ODPA-ODA | 180–380 | Casting | + | + | + | [25] | ||||||||||
| BPDA-PDA BDPA-ODA | 200–400 | Dr.-blade | + | + | + | + | + | + | + | [26] | ||||||
| BPDA-ODA-PDA | 200–350 | Spin-coat | + | + | + | + | + | + | + | + | [27] | |||||
| BTDA-ODA-MPDA | 125–400 | Spin-coat | + | + | [28] | |||||||||||
| BTDA-type | 25–300 | N/A | + | + | + | + | [29] | |||||||||
| PMDA-BACB | 140–250 | N/A | + | + | + | + | [30] | |||||||||
| BPDA-PDA | 175–450 | Spin-coat | + | + | + | + | + | + | [31] | |||||||
| PMDA-ODA | 200–380 | Spin-coat | + | + | + | + | + | + | + | + | + | + | + | + | + | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benfridja, I.; Diaham, S.; Laffir, F.; Brennan, G.; Liu, N.; Kennedy, T. A Universal Study on the Effect Thermal Imidization Has on the Physico-Chemical, Mechanical, Thermal and Electrical Properties of Polyimide for Integrated Electronics Applications. Polymers 2022, 14, 1713. https://doi.org/10.3390/polym14091713
Benfridja I, Diaham S, Laffir F, Brennan G, Liu N, Kennedy T. A Universal Study on the Effect Thermal Imidization Has on the Physico-Chemical, Mechanical, Thermal and Electrical Properties of Polyimide for Integrated Electronics Applications. Polymers. 2022; 14(9):1713. https://doi.org/10.3390/polym14091713
Chicago/Turabian StyleBenfridja, Imadeddine, Sombel Diaham, Fathima Laffir, Grace Brennan, Ning Liu, and Tadhg Kennedy. 2022. "A Universal Study on the Effect Thermal Imidization Has on the Physico-Chemical, Mechanical, Thermal and Electrical Properties of Polyimide for Integrated Electronics Applications" Polymers 14, no. 9: 1713. https://doi.org/10.3390/polym14091713
APA StyleBenfridja, I., Diaham, S., Laffir, F., Brennan, G., Liu, N., & Kennedy, T. (2022). A Universal Study on the Effect Thermal Imidization Has on the Physico-Chemical, Mechanical, Thermal and Electrical Properties of Polyimide for Integrated Electronics Applications. Polymers, 14(9), 1713. https://doi.org/10.3390/polym14091713