Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites
2.3. Characterization
2.3.1. Mechanical Properties
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Differential Scanning Calorimeter (DSC)
2.3.4. Scanning Electron Microscope (SEM)
2.3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.6. Contact Angle Measurements
2.4. Statistics
3. Results and Discussion
3.1. Mechanical Properties
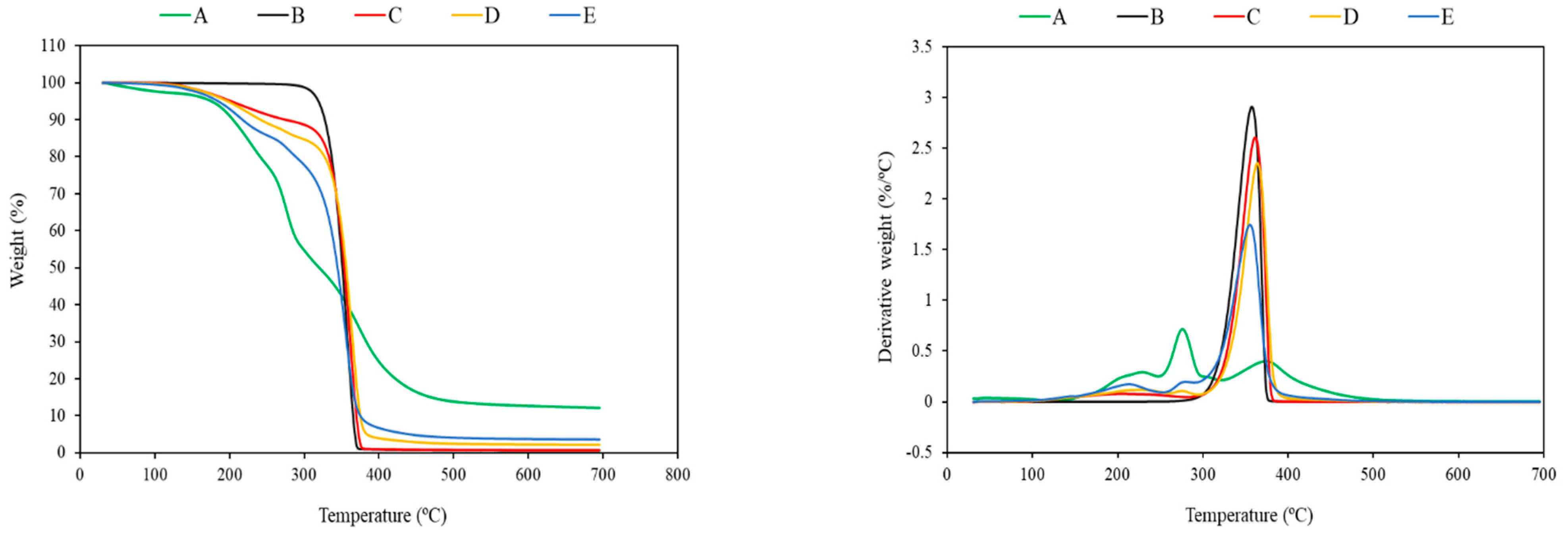
3.2. Thermogravimetric Analysis (TGA)
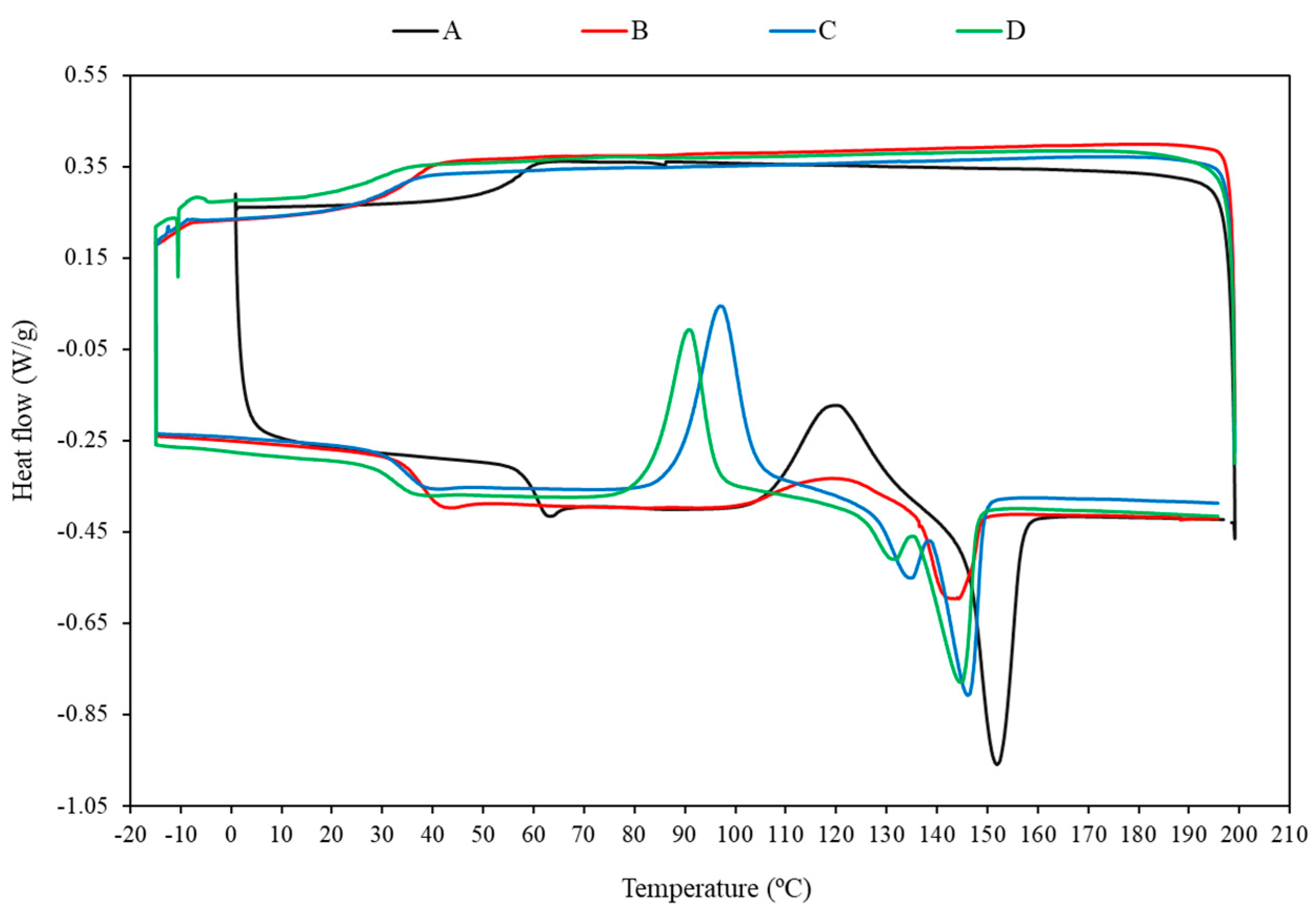
3.3. Differential Scanning Calorimeter (DSC)
3.4. Scanning Electron Microscope (SEM)
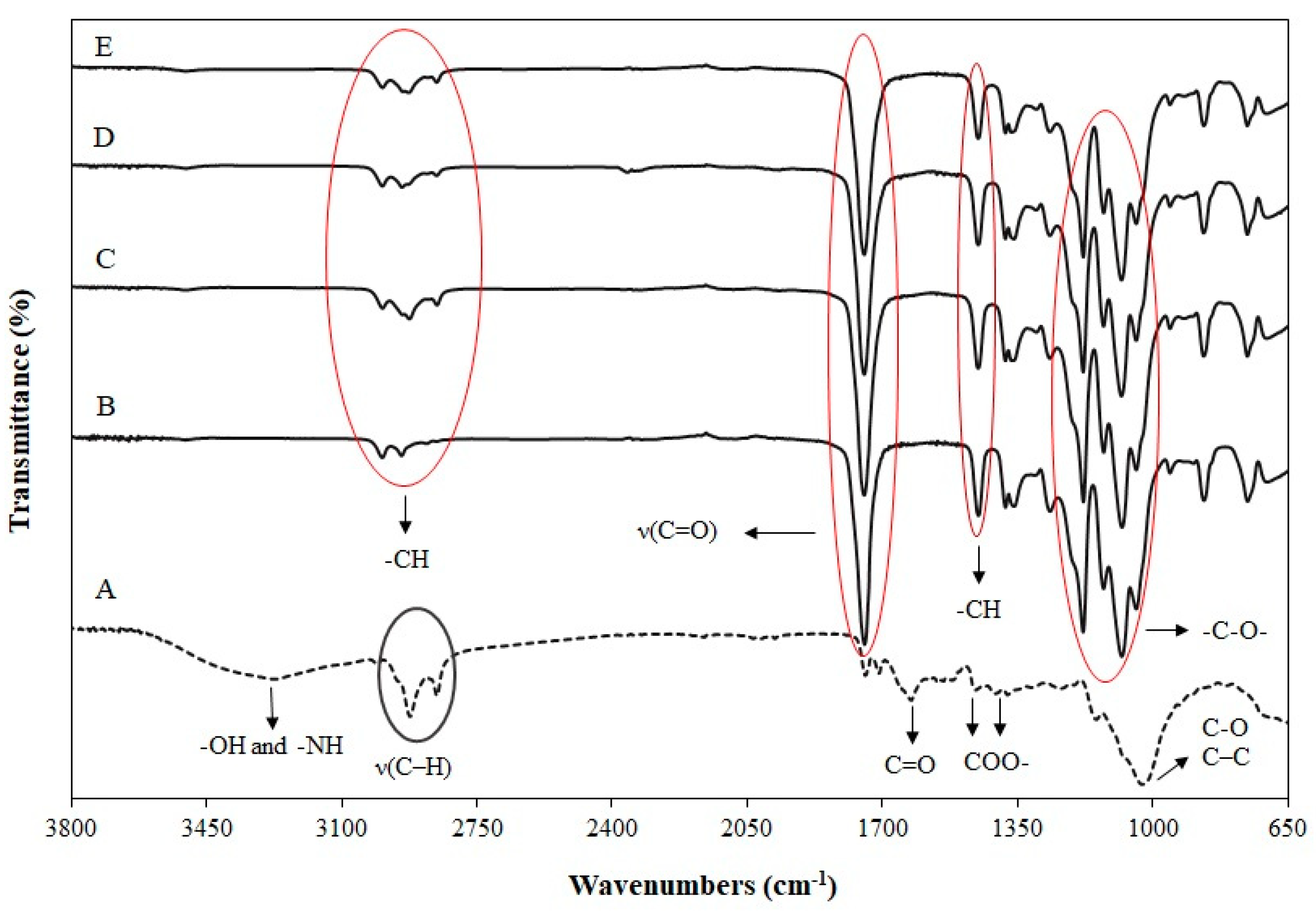
3.5. Fourier Transform Infrared (FT-IR)
3.6. Contact Angle Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [Green Version]
- Andrew, J.J.; Dhakal, H.N. Sustainable biobased composites for advanced applications: Recent trends and future opportunities—A critical review. Compos. Part C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, N.; Galiwango, E.; Haris, S.; Al-Marzouqi, A.H.; Abu-Jdayil, B.; Caires, Y.L. A New Green Composite Based on Plasticized Polylactic Acid Mixed with Date Palm Waste for Single-Use Plastics Applications. Polymers 2022, 14, 574. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.-J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Mechanical Stability of Polyhydroxyalkanoate (PHA)-Based Wood Plastic Composites (WPCs). J. Polym. Environ. 2020, 28, 1571–1577. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Z.; Zhao, X.; Dong, J.; Li, X.; Zhang, Q. Spent coffee Grounds/Poly(butylene succinate) biocomposites with Robust mechanical property and heat resistance via reactive compatibilization. Compos. Commun. 2022, 29, 101003. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials 2022, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Peidayesh, H.; Mosnáčková, K.; Špitalský, Z.; Heydari, A.; Šišková, A.O.; Chodák, I. Thermoplastic Starch-Based Composite Reinforced by Conductive Filler Networks: Physical Properties and Electrical Conductivity Changes during Cyclic Deformation. Polymers 2021, 13, 3819. [Google Scholar] [CrossRef]
- Hu, H.; Xu, A.; Zhang, D.; Zhou, W.; Peng, S.; Zhao, X. High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization. Molecules 2020, 25, 5951. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Righetti, M.C.; Cinelli, P.; Mallegni, N.; Massa, C.A.; Bronco, S.; Stäbler, A.; Lazzeri, A. Thermal, Mechanical, and Rheological Properties of Biocomposites Made of Poly(lactic acid) and Potato Pulp Powder. Int. J. Mol. Sci. 2019, 20, 675. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Maio, A.; Lopresti, F. Physical properties of green composites based on poly-lactic acid or Mater-Bi® filled with Posidonia Oceanica leaves. Compos. Part A Appl. Sci. Manuf. 2018, 112, 315–327. [Google Scholar] [CrossRef]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl Citrate (TEC) as a Dispersing Aid in Polylactic Acid/Chitin Nanocomposites Prepared via Liquid-Assisted Extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.A.; Genovese, M.E.; Mancini, G.; Marini, L.; Athanassiou, A. Green Processing Route for Polylactic Acid–Cellulose Fiber Biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4128–4136. [Google Scholar] [CrossRef]
- Ambone, T.; Joseph, S.; Deenadayalan, E.; Mishra, S.; Jaisankar, S.; Saravanan, P. Polylactic Acid (PLA) Biocomposites Filled with Waste Leather Buff (WLB). J. Polym. Environ. 2017, 25, 1099–1109. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, C.M.; Park, H.J. Development of biodegradable hot-melt adhesive based on poly-ε-caprolactone and soy protein isolate for food packaging system. LWT Food Sci. Technol. 2006, 39, 591–597. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Zhang, J. Compatibilizing Effects of Maleated Poly(lactic acid) (PLA) on Properties of PLA/Soy Protein Composites. Ind. Eng. Chem. Res. 2012, 51, 7786–7792. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid) (PLA)/thermoplastic starch (TPS) blends. Polym.-Plast. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Koh, J.J.; Zhang, X.; He, C. Fully biodegradable Poly(lactic acid)/Starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, B.; Jain, P. Green Natural Protein Isolate based composites and nanocomposites: A review. Polym. Test. 2021, 99, 106626. [Google Scholar] [CrossRef]
- Boontima, B.; Noomhorm, A.; Puttanlek, C.; Uttapap, D.; Rungsardthong, V. Mechanical Properties of Sugarcane Bagasse Fiber-Reinforced Soy Based Biocomposites. J. Polym. Environ. 2015, 23, 97–106. [Google Scholar] [CrossRef]
- Bartos, A.; Nagy, K.; Anggono, J.; Antoni; Purwaningsih, H.; Móczó, J.; Pukánszky, B. Biobased PLA/sugarcane bagasse fiber composites: Effect of fiber characteristics and interfacial adhesion on properties. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106273. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. The use of alkali treated walnut shells as filler in plasticized poly(lactic acid) matrix composites. Ind. Crops Prod. 2020, 145, 111993. [Google Scholar] [CrossRef]
- Guo, J.; Tsou, C.-H.; De Guzman, M.R.; Wu, C.-S.; Zhang, X.; Chen, Z.; Wen, Y.-H.; Yang, T.; Zhuang, Y.-J.; Ge, F.; et al. Preparation and characterization of bio-based green renewable composites from poly(lactic acid) reinforced with corn stover. J. Polym. Res. 2021, 28, 199. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Alaimo, G.; Morreale, M. Green Composites Based on PLA and Agricultural or Marine Waste Prepared by FDM. Polymers 2021, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Ozyhar, T.; Baradel, F.; Zoppe, J. Effect of functional mineral additive on processability and material properties of wood-fiber reinforced poly(lactic acid) (PLA) composites. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105827. [Google Scholar] [CrossRef]
- Madyan, O.A.; Wang, Y.; Corker, J.; Zhou, Y.; Du, G.; Fan, M. Classification of wood fibre geometry and its behaviour in wood poly(lactic acid) composites. Compos. Part A Appl. Sci. Manuf. 2020, 133, 105871. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Woźniak-Braszak, A.; Baranowski, M.; Sterzyński, T.; Di Lorenzo, M.L. Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites. Materials 2020, 13, 3776. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, M.; Han, W.; Li, P. Waste office paper filled polylactic acid composite filaments for 3D printing. Compos. Part B Eng. 2021, 221, 108998. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Chopped glass and recycled newspaper as reinforcement fibers in injection molded poly(lactic acid) (PLA) composites: A comparative study. Compos. Sci. Technol. 2006, 66, 1813–1824. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Misra, M.; Mohanty, A.K.; Williams, K.; Mielewski, D.F. A Study on Biocomposites from Recycled Newspaper Fiber and Poly(lactic acid). Ind. Eng. Chem. Res. 2005, 44, 5593–5601. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Jin, X.; Nie, S.; Yang, R. Study on Mechanical and Thermal Properties of Poly(Lactic acid)/Poly(Butylene adipate-co-terephthalate)/Office Wastepaper Fiber Biodegradable Composites. J. Polym. Environ. 2019, 27, 1273–1284. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Evtuguin, D.V. New poly(lactic acid) composites produced from coffee beverage wastes. J. Appl. Polym. Sci. 2022, 139, 51434. [Google Scholar] [CrossRef]
- Akderya, T.; Özmen, U.; Baba, B.O. Investigation of long-term ageing effect on the thermal properties of chicken feather fibre/poly(lactic acid) biocomposites. J. Polym. Res. 2020, 27, 162. [Google Scholar] [CrossRef]
- Molins, G.; Álvarez, M.D.; Garrido, N.; Macanás, J.; Carrillo, F. Environmental Impact Assessment of Polylactide(PLA)/Chicken Feathers Biocomposite Materials. J. Polym. Environ. 2018, 26, 873–884. [Google Scholar] [CrossRef]
- Baba, B.O.; Özmen, U. Preparation and mechanical characterization of chicken feather/PLA composites. Polym. Compos. 2017, 38, 837–845. [Google Scholar] [CrossRef]
- Cañavate, J.; Aymerich, J.; Garrido, N.; Colom, X.; Macanás, J.; Molins, G.; Álvarez, M.D.; Carrillo, F. Properties and optimal manufacturing conditions of chicken feathers/poly(lactic acid) biocomposites. J. Compos. Mater. 2015, 50, 1671–1683. [Google Scholar] [CrossRef] [Green Version]
- Özmen, U.; Baba, B.O. Thermal characterization of chicken feather/PLA biocomposites. J. Therm. Anal. Calorim. 2017, 129, 347–355. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and mechanics of fungal mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaza-Perez, F.; Ramirez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-Lopez, C. Potential of residual fungal biomass: A review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar] [CrossRef]
- Filipova, I.; Irbe, I.; Spade, M.; Skute, M.; Dāboliņa, I.; Baltiņa, I.; Vecbiskena, L. Mechanical and Air Permeability Performance of Novel Biobased Materials from Fungal Hyphae and Cellulose Fibers. Materials 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Barjas, G.; Gallardo, F.; Nesic, A.; Taboada, E.; Marican, A.; Mirabal-Gallardo, Y.; Avila-Salas, F.; Delgado, N.; de Armas-Ricard, M.; Valdes, O. Utilization of industrial by-product fungal biomass from Aspergillus niger and Fusarium culmorum to obtain biosorbents for removal of pesticide and metal ions from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 104355. [Google Scholar] [CrossRef]
- Núñez Decap, M.; Ballerini Arroyo, A.; Alarcón Énos, J. Evaluation of single cell protein from yeast for the development of wood adhesives. Eur. J. Wood Wood Prod. 2016, 74, 821–828. [Google Scholar] [CrossRef]
- Bustillos, J.; Loganathan, A.; Agrawal, R.; Gonzalez, B.A.; Perez, M.G.; Ramaswamy, S.; Boesl, B.; Agarwal, A. Uncovering the Mechanical, Thermal, and Chemical Characteristics of Biodegradable Mushroom Leather with Intrinsic Antifungal and Antibacterial Properties. ACS Appl. Bio Mater. 2020, 3, 3145–3156. [Google Scholar] [CrossRef]
- Wijayarathna, E.R.K.B.; Mohammadkhani, G.; Soufiani, A.M.; Adolfsson, K.H.; Ferreira, J.A.; Hakkarainen, M.; Berglund, L.; Heinmaa, I.; Root, A.; Zamani, A. Fungal textile alternatives from bread waste with leather-like properties. Resour. Conserv. Recycl. 2022, 179, 106041. [Google Scholar] [CrossRef]
- Bruscato, C.; Malvessi, E.; Brandalise, R.N.; Camassola, M. High performance of macrofungi in the production of mycelium-based biofoams using sawdust—Sustainable technology for waste reduction. J. Clean. Prod. 2019, 234, 225–232. [Google Scholar] [CrossRef]
- Svensson, S.E.; Ferreira, J.A.; Hakkarainen, M.; Adolfsson, K.H.; Zamani, A. Fungal textiles: Wet spinning of fungal microfibers to produce monofilament yarns. Sustain. Mater. Technol. 2021, 28, e00256. [Google Scholar] [CrossRef]
- Irbe, I.; Filipova, I.; Skute, M.; Zajakina, A.; Spunde, K.; Juhna, T. Characterization of Novel Biopolymer Blend Mycocel from Plant Cellulose and Fungal Fibers. Polymers 2021, 13, 1086. [Google Scholar] [CrossRef]
- Appels, F.V.W.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A.B. Fungal mycelium classified in different material families based on glycerol treatment. Commun. Biol. 2020, 3, 334. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.W.; Lee, K.-Y.; Kontturi, E.; Bismarck, A.; Mautner, A. Surface properties of chitin-glucan nanopapers from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 148, 677–687. [Google Scholar] [CrossRef]
- Yousefi, N.; Jones, M.; Bismarck, A.; Mautner, A. Fungal chitin-glucan nanopapers with heavy metal adsorption properties for ultrafiltration of organic solvents and water. Carbohydr. Polym. 2021, 253, 117273. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.W.; Jones, M.P.; Kontturi, E.; Mautner, A.; Bismarck, A. Plastic to elastic: Fungi-derived composite nanopapers with tunable tensile properties. Compos. Sci. Technol. 2020, 198, 108327. [Google Scholar] [CrossRef]
- Janesch, J.; Jones, M.; Bacher, M.; Kontturi, E.; Bismarck, A.; Mautner, A. Mushroom-derived chitosan-glucan nanopaper filters for the treatment of water. React. Funct. Polym. 2020, 146, 104428. [Google Scholar] [CrossRef]
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kählig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-Derived Low-Cost Mycelium Nanopapers with Tunable Mechanical and Surface Properties. Biomacromolecules 2019, 20, 3513–3523. [Google Scholar] [CrossRef] [Green Version]
- Fazli Wan Nawawi, W.M.; Lee, K.-Y.; Kontturi, E.; Murphy, R.J.; Bismarck, A. Chitin Nanopaper from Mushroom Extract: Natural Composite of Nanofibers and Glucan from a Single Biobased Source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496. [Google Scholar] [CrossRef] [Green Version]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Attias, N.; Reid, M.; Mijowska, S.C.; Dobryden, I.; Isaksson, M.; Pokroy, B.; Grobman, Y.J.; Abitbol, T. Biofabrication of Nanocellulose–Mycelium Hybrid Materials. Adv. Sustain. Syst. 2021, 5, 2000196. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully Bio-Based Hybrid Composites Made of Wood, Fungal Mycelium and Cellulose Nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef]
- Gurram, R.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. A Solvent-Free Approach for Production of Films from Pectin and Fungal Biomass. J. Polym. Environ. 2018, 26, 4282–4292. [Google Scholar] [CrossRef] [Green Version]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. Improving the ductility and heat deflection temperature of injection molded Poly(lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Zych, A.; Athanassiou, A. Effect of Green Plasticizer on the Performance of Microcrystalline Cellulose/Polylactic Acid Biocomposites. ACS Appl. Polym. Mater. 2021, 3, 3071–3081. [Google Scholar] [CrossRef]
- Gzyra-Jagieła, K.; Sulak, K.; Draczyński, Z.; Podzimek, S.; Gałecki, S.; Jagodzińska, S.; Borkowski, D. Modification of Poly(lactic acid) by the Plasticization for Application in the Packaging Industry. Polymers 2021, 13, 3651. [Google Scholar] [CrossRef]
- ISO 527-1:2012; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization of Standardization: Geneva, Switzerland, 2012.
- Gigante, V.; Cinelli, P.; Sandroni, M.; D’ambrosio, R.; Lazzeri, A.; Seggiani, M. On the Use of Paper Sludge as Filler in Biocomposites for Injection Moulding. Materials 2021, 14, 2688. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M. Utilization of linseed cake as a postagricultural functional filler for poly(lactic acid) green composites. J. Appl. Polym. Sci. 2019, 136, 47152. [Google Scholar] [CrossRef]
- Surya, I.; Olaiya, N.G.; Rizal, S.; Zein, I.; Sri Aprilia, N.A.; Hasan, M.; Yahya, E.B.; Sadasivuni, K.K.; Abdul Khalil, H.P.S. Plasticizer Enhancement on the Miscibility and Thermomechanical Properties of Polylactic Acid-Chitin-Starch Composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.-H. Thermal Degradation and Fire Properties of Fungal Mycelium and Mycelium—Biomass Composite Materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [Green Version]
- Gáplovská, K.; Šimonovičová, A.; Halko, R.; Okenicová, L.; Žemberyová, M.; Čerňanský, S.; Brandeburová, P.; Mackuľak, T. Study of the binding sites in the biomass of Aspergillus niger wild-type strains by FTIR spectroscopy. Chem. Pap. 2018, 72, 2283–2288. [Google Scholar] [CrossRef]



| Composition | Value (%) |
|---|---|
| Moisture | 3.5 ± 0.18 |
| Ash | 3.8 ± 0.13 |
| Total lipid | 21.3 ± 0.61 |
| Crude protein | 37.6 ± 1.20 |
| Other components 1 | 33.8 ± 1.53 |
| Sample Code | Poly(lactic acid) (PLA) %(w/w) | Triethyl Citrate (TEC) %(w/w) | Fungal Biomass (FB) %(w/w) |
|---|---|---|---|
| Neat PLA | 100 | - | - |
| PT5 | 95 | 5 | - |
| PT10 | 90 | 10 | - |
| PT15 | 85 | 15 | - |
| PT5F10 | 85 | 5 | 10 |
| PT10F10 | 80 | 10 | 10 |
| PT15F10 | 75 | 15 | 10 |
| PT5F20 | 75 | 5 | 20 |
| PT10F20 | 70 | 10 | 20 |
| PT15F20 | 65 | 15 | 20 |
| Sample Code | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (GPa) | Charpy Impact Strength (kJ/m2) |
|---|---|---|---|---|
| Neat PLA | 67.8 ± 1.0 | 3.8 ± 0.6 | 4.17 ± 0.60 | 15.31 ± 1.06 |
| PT5 | 52.1 ± 1.0 | 6.3 ± 0.9 | 3.50 ± 0.94 | 19.44 ± 3.05 |
| PT10 | 43.2 ± 1.7 | 10.2 ± 2.0 | 2.33 ± 0.78 | 24.52 ± 2.96 |
| PT15 | 18.4 ± 2.9 | 199.8 ± 11.7 | 0.43 ± 0.06 | 92.67 ± 3.60 |
| PT5F10 | 29.3 ± 1.5 | 13.0 ± 1.3 | 4.36 ± 0.85 | 10.85 ± 1.42 |
| PT10F10 | 24.1 ± 1.0 | 42.9 ± 7.0 | 2.85 ± 0.61 | 12.72 ± 0.79 |
| PT15F10 | 10.9 ± 1.1 | 224.0 ± 14.5 | 0.44 ± 0.06 | 65.92 ± 4.36 |
| PT5F20 | 25.5 ± 3.7 | 10.8 ± 1.6 | 3.19 ± 0.61 | 8.36 ± 1.56 |
| PT10F20 | 19.7 ± 4.0 | 25.1 ± 4.3 | 2.24 ± 0.49 | 12.12 ± 0.75 |
| PT15F20 | 9.3 ± 1.7 | 200.5 ± 10.5 | 0.07 ± 0.02 | 41.21 ± 4.75 |
| Sample Code | T5% (°C) | Tmax (°C) | T50% (°C) | Residue (%) |
|---|---|---|---|---|
| Fungal biomass | 175.7 | 291.6 | 320.1 | 12.1 |
| Neat PLA | 318.9 | 358.3 | 351.1 | 0.7 |
| PT5 | 285.6 | 368.9 | 362.4 | 0.8 |
| PT10 | 202.9 | 362.6 | 354.5 | 0.7 |
| PT15 | 196.0 | 349.2 | 340.8 | 0.7 |
| PT5F10 | 222.9 | 363.0 | 357.5 | 2.1 |
| PT10F10 | 196.8 | 361.5 | 355.5 | 2.2 |
| PT15F10 | 178.9 | 361.4 | 354.0 | 2.1 |
| PT5F20 | 212.5 | 361.4 | 355.0 | 3.5 |
| PT10F20 | 186.8 | 360.2 | 350.5 | 3.3 |
| PT15F20 | 175.8 | 361.0 | 350.2 | 3.2 |
| Sample Code | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J/g) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| Neat PLA | 55.9 | 120.3 | 151.9 | 24.5 | 25.3 | 0.9 |
| PT5 | 43.5 | 124.7 | 148.5 | 5.2 | 5.4 | 0.2 |
| PT10 | 30.5 | 119.4 | 144.1 | 9.3 | 9.4 | 0.1 |
| PT15 | 21.4 | 110.4 | 137.9 | 14.7 | 15.1 | 0.5 |
| PT5F10 | 36.7 | 106.9 | 149.0 | 30.9 | 31.2 | 0.4 |
| PT10F10 | 27.8 | 96.8 | 145.8 | 26.9 | 27.6 | 1.0 |
| PT15F10 | 20.9 | 85.6 | 141.9 | 21.8 | 22.0 | 0.2 |
| PT5F20 | 34.5 | 99.9 | 147.6 | 27.1 | 28.4 | 1.8 |
| PT10F20 | 25.5 | 90.3 | 144.5 | 24.0 | 24.2 | 0.3 |
| PT15F20 | 16.8 | 82.9 | 142.5 | 20.2 | 20.5 | 0.4 |
| Sample Code | WCA Mean [°] 0 SEC | WCA Mean [°] 10 SEC |
|---|---|---|
| Neat PLA | 73.68 ± 2.81 | 73.19 ± 2.54 |
| PT10 | 73.75 ± 2.64 | 72.18 ± 2.52 |
| PT10F10 | 72.76 ± 3.85 | 72.46 ± 3.97 |
| PT10F20 | 64.35 ± 3.47 | 60.63 ± 4.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadollahzadeh, M.; Mahboubi, A.; Taherzadeh, M.J.; Åkesson, D.; Lennartsson, P.R. Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites. Polymers 2022, 14, 1738. https://doi.org/10.3390/polym14091738
Asadollahzadeh M, Mahboubi A, Taherzadeh MJ, Åkesson D, Lennartsson PR. Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites. Polymers. 2022; 14(9):1738. https://doi.org/10.3390/polym14091738
Chicago/Turabian StyleAsadollahzadeh, Mohammadtaghi, Amir Mahboubi, Mohammad J. Taherzadeh, Dan Åkesson, and Patrik R. Lennartsson. 2022. "Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites" Polymers 14, no. 9: 1738. https://doi.org/10.3390/polym14091738








