Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Palladium Catalyst with Star-Shaped Structure
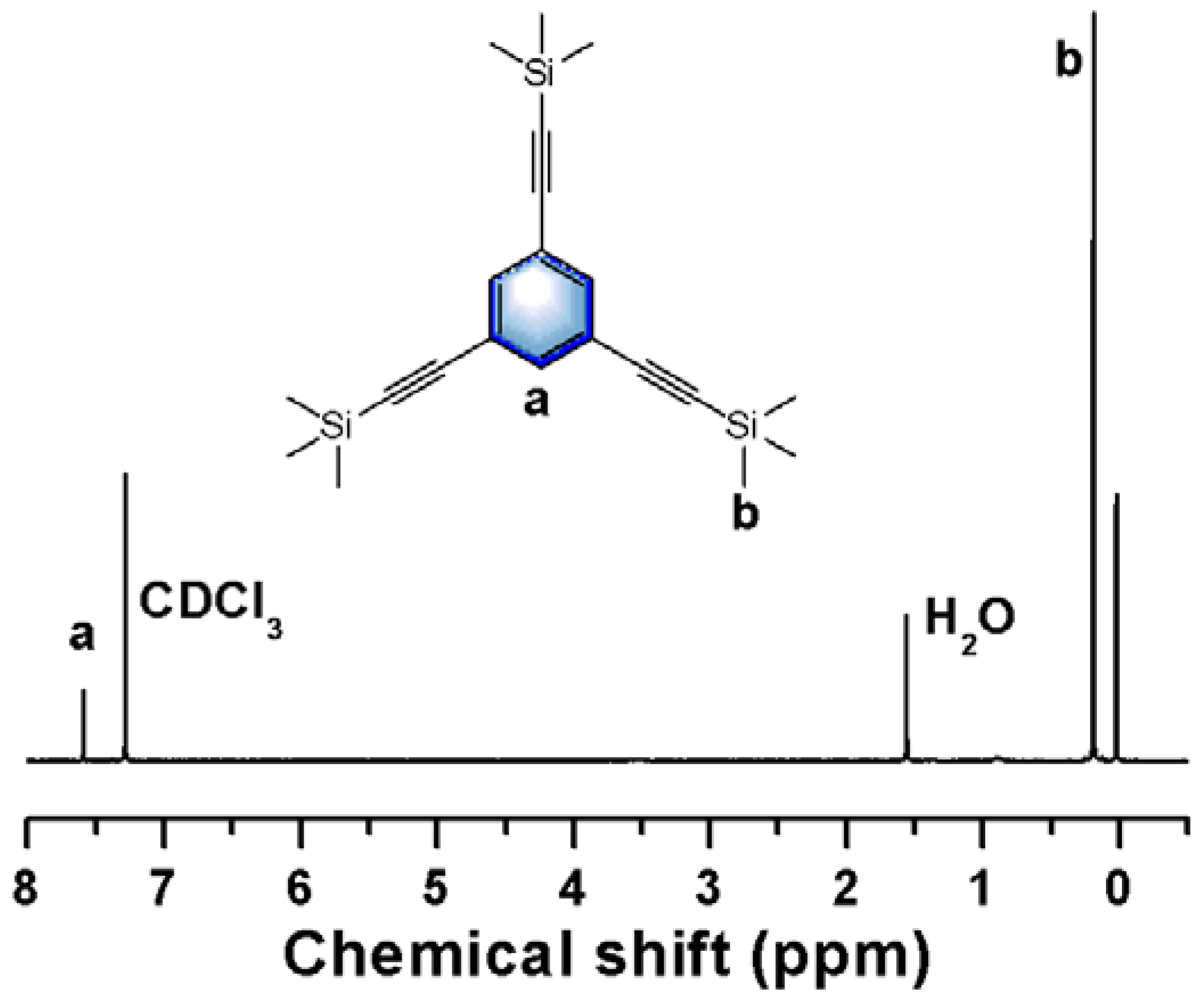
2.2.1. Synthesis of Intermediate a
2.2.2. Synthesis of Intermediate b
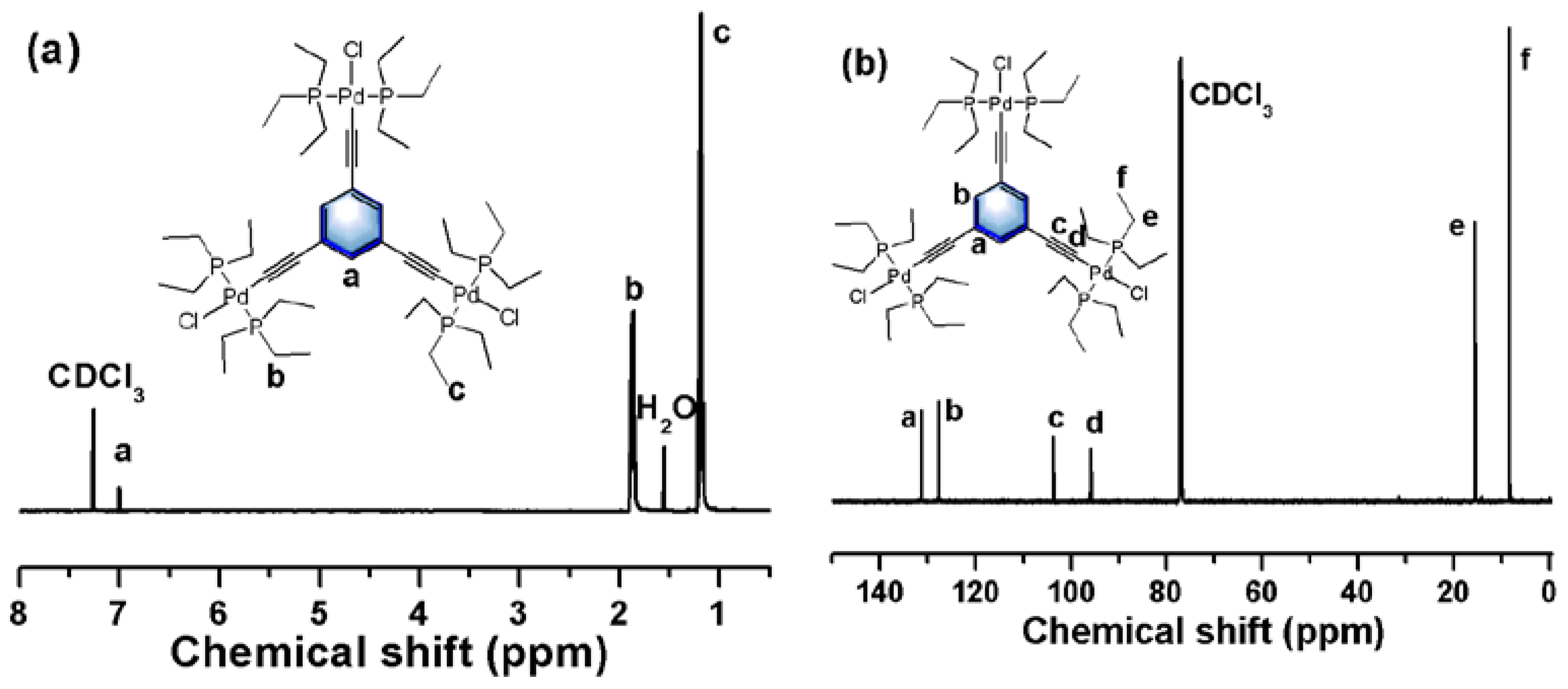
2.2.3. Synthesis of Palladium Catalyst
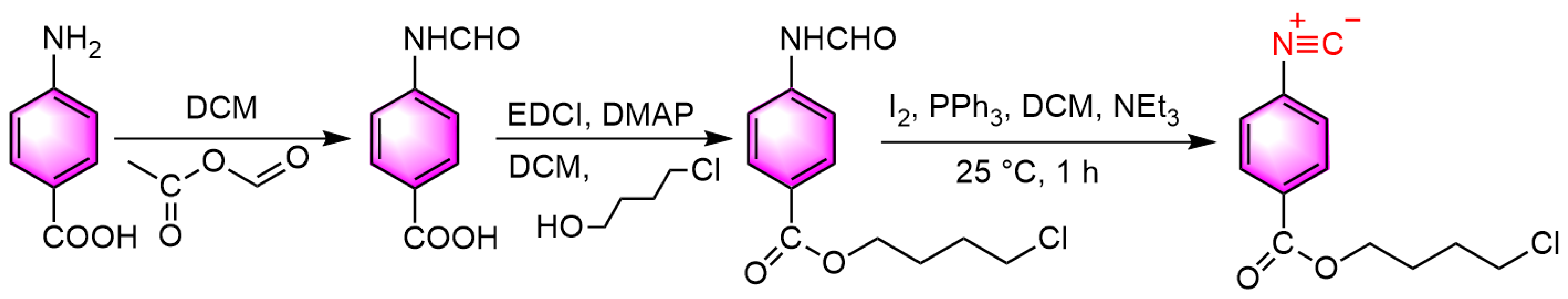
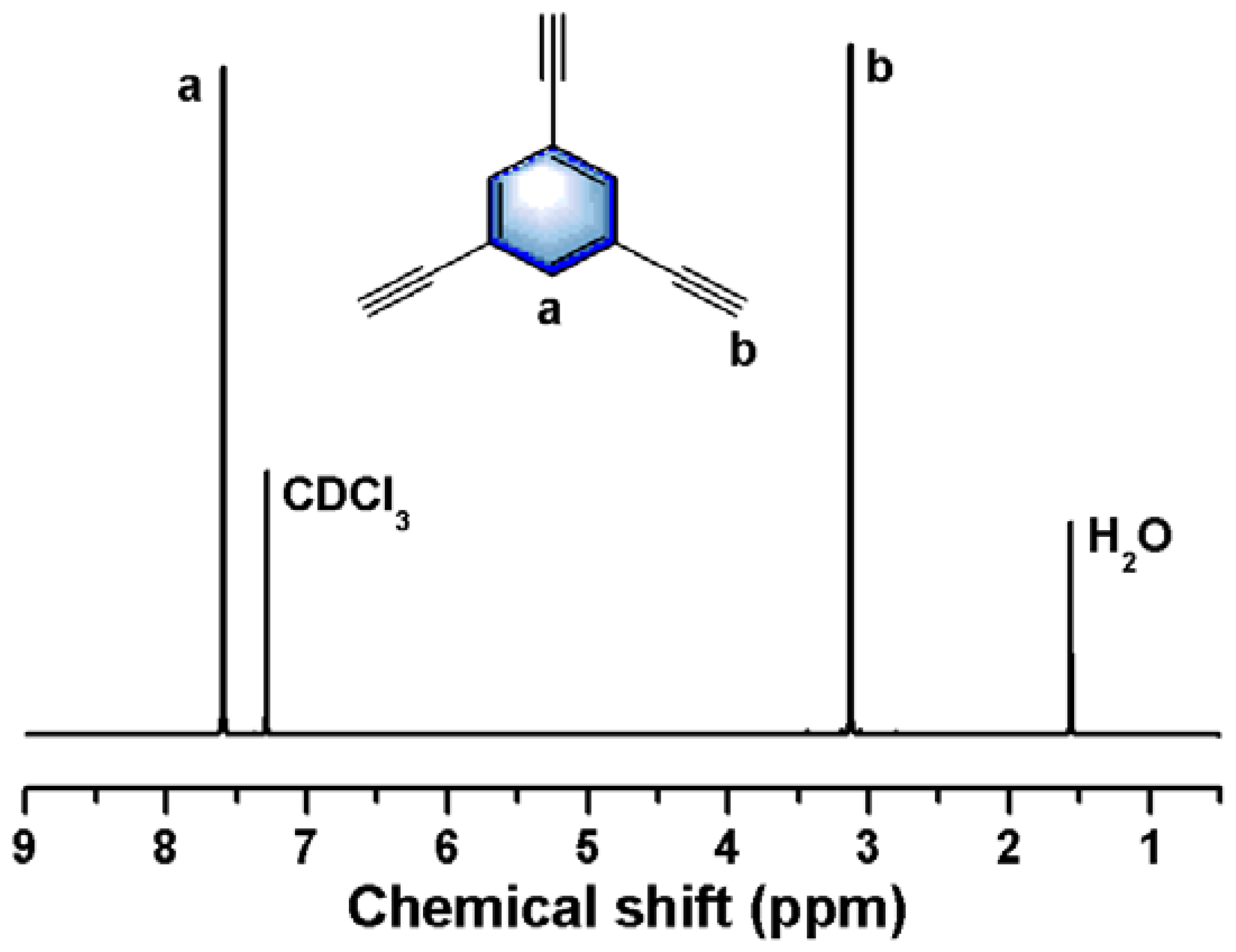
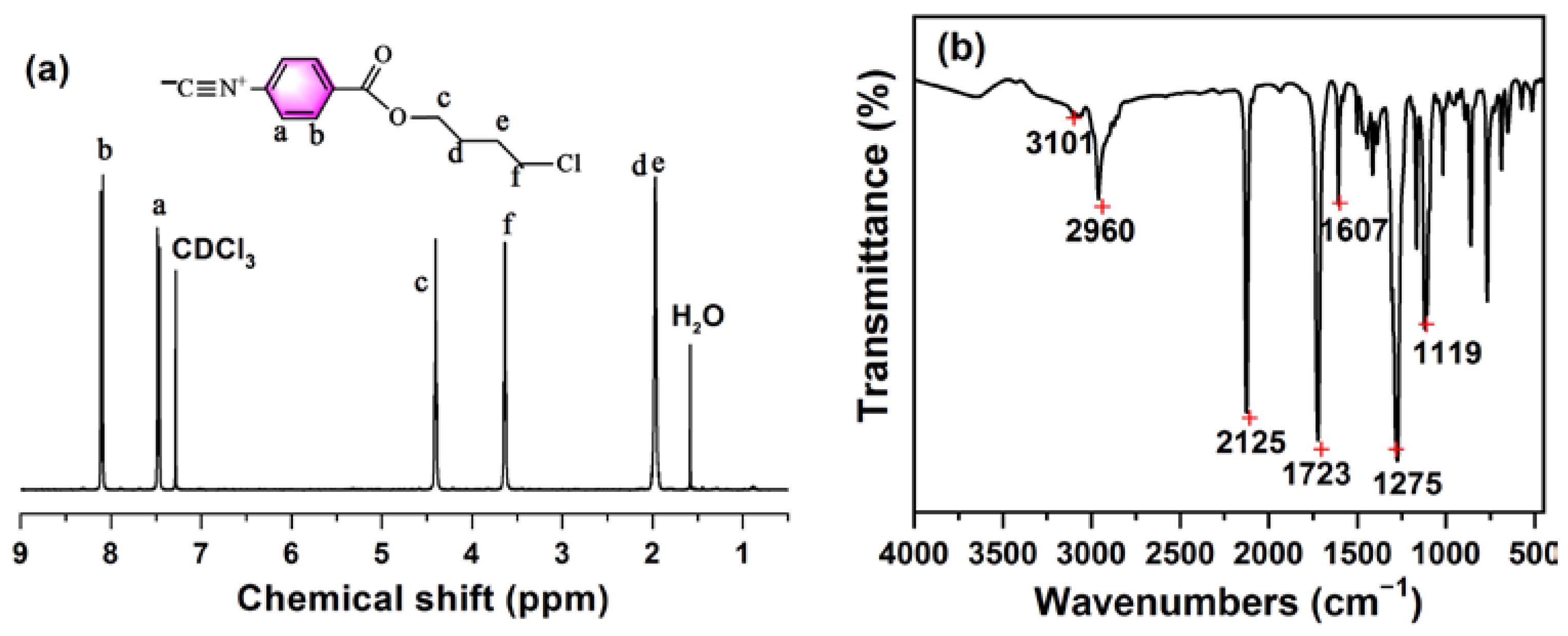
2.3. Synthesis of Isonitrile Monomer
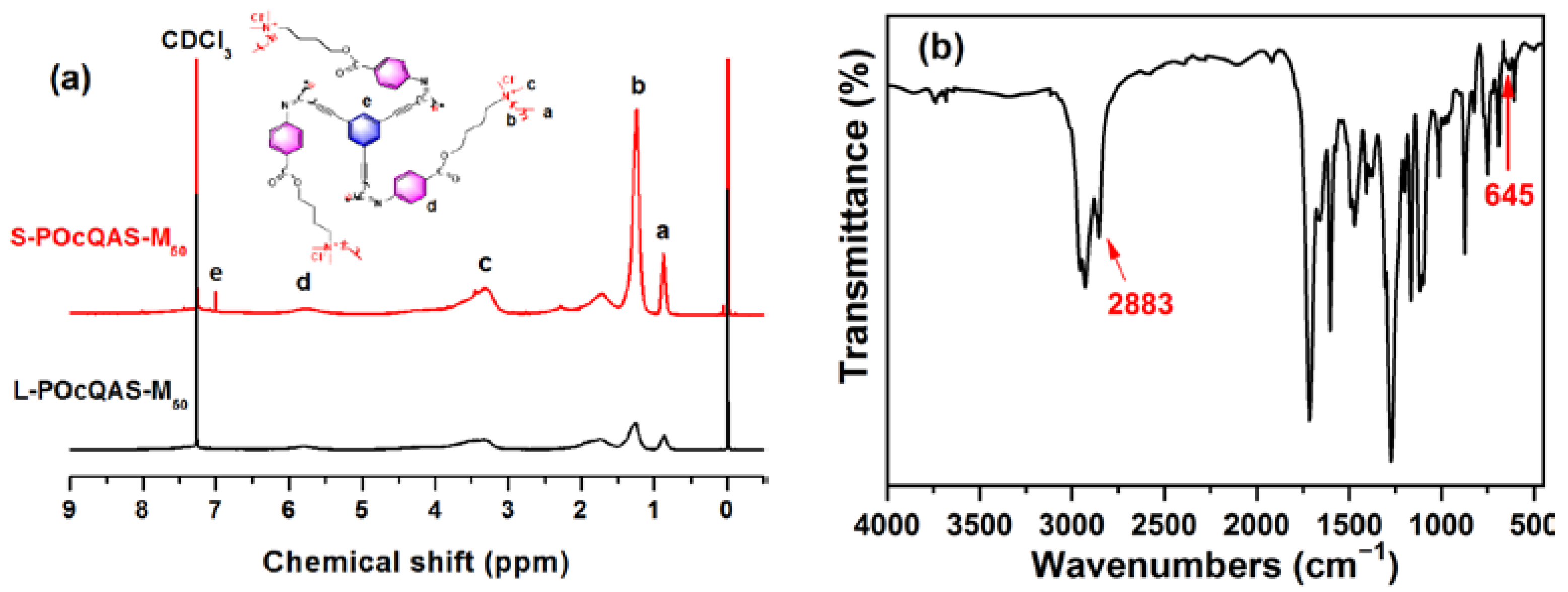
2.4. Synthesis of S-POcQASs
2.5. Antimicrobial Activity Test of S-POcQASs
2.6. Characterizations
3. Results
3.1. Characterization of Polymers and Compounds
3.2. Solubility and Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Li, L.; Duan, L.; Yang, M.; Zhou, X.; Zheng, Q.; Ou, Y.; Li, Z.; Lai, F.Y. Exploring antibiotic consumption between urban and sub-urban catchments using both parent drugs and related metabolites in wastewater-based epidemiology. Sci. Total Environ. 2022, 827, 154171. [Google Scholar] [CrossRef] [PubMed]
- Durand-Reville, T.F.; Miller, A.A.; O’Donnell, J.P.; Wu, X.; Sylvester, M.A.; Guler, S.; Iyer, R.; Shapiro, A.B.; Carter, N.M.; Velez-Vega, C.; et al. Rational design of a new antibiotic class for drug-resistant infections. Nature 2021, 597, 698–702. [Google Scholar] [CrossRef]
- Wu, S.; Hulme, J.P. Recent advances in the detection of antibiotic and multi-drug resistant salmonella: An update. Int. J. Mol. Sci. 2021, 22, 3499. [Google Scholar] [CrossRef] [PubMed]
- Haitao, Y.; Yifan, C.; Mingchao, S.; Shuaijuan, H. A novel polymeric nanohybrid antimicrobial engineered by antimicrobial peptide MccJ25 and chitosan nanoparticles exerts strong antibacterial and anti-inflammatory activities. Front. Immunol. 2021, 12, 811381. [Google Scholar] [CrossRef]
- Bueno, J.; Virto, L.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Sanz, M.; Herrera, D. Antibacterial effect of functionalized polymeric nanoparticles on titanium surfaces using an in vitro subgingival biofilm model. Polymers 2022, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Lv, S.; Zhang, L.; Ye, R.; Ge, C.; Huang, D.; Zhang, S.; Cai, Z. New type of borneol-based fluorine-free superhydrophobic antibacterial polymeric coating. Des. Monomers. Polym. 2021, 24, 145–155. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Olmos, D.; Gonzalez-Benito, J. Polymeric materials with antibacterial activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.Q.; Tan, P.F.; Gu, Z.P.; Liu, Z.M.; Tan, L. Polymeric antibacterial materials: Design, platforms and applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef]
- Durak, S.; Arasoglu, T.; Ates, S.C.; Derman, S. Enhanced antibacterial and antiparasitic activity of multifunctional polymeric nanoparticles. Nanotechnology 2020, 31, 175705. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.L.; Terenteva, O.S.; Akhmedov, A.A.; Iksanova, A.G.; Shtyrlin, N.V.; Nikitina, E.V.; Krylova, E.S.; Shtyrlin, Y.G.; Stoikov, I.I. Thiacalixarene based quaternary ammonium salts as promising antibacterial agents. Bioorg. Med. Chem. 2021, 29, 115905. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chen, Y.T.; Lee, B.S.; Chang, C.C. Suppressing antibacterial resistance: Chemical binding of monolayer quaternary ammonium salts to polymethyl methacrylate in an aqueous solution and its clinical efficacy. Int. J. Mol. Sci. 2019, 20, 4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; He, X.; Ding, M.; He, W.; Li, J.; Li, J.; Tan, H. Antibacterial and biocompatible cross-linked waterborne polyurethanes containing gemini quaternary ammonium salts. Biomacromolecules 2018, 19, 279–287. [Google Scholar] [CrossRef]
- Ermolaev, V.V.; Arkhipova, D.M.; Miluykov, V.A.; Lyubina, A.P.; Amerhanova, S.K.; Kulik, N.V.; Voloshina, A.D.; Ananikov, V.P. Sterically hindered quaternary phosphonium salts (QPSs): Antimicrobial activity and hemolytic and cytotoxic properties. Int. J. Mol. Sci. 2021, 23, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Erisen, D.E.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2022. [Google Scholar] [CrossRef]
- Yuan, H.; Li, W.; Song, C.; Huang, R. An injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties as potential carriers for drug delivery. Int. J. Biol. Macromol. 2022, 205, 563–573. [Google Scholar] [CrossRef]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The microstructure, antibacterial and antitumor activities of chitosan oligosaccharides and derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Shagdarova, B.; Sinitsyna, O.; Sinitsyn, A.; Varlamov, V. Evaluation of antibacterial and antifungal properties of low molecular weight chitosan extracted from hermetia illucens relative to crab chitosan. Molecules 2022, 27, 577. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Pei, P.; Hao, J.; Lu, Y.; Wan, P.; Peng, X.; Lv, W.; Xiong, W.; Zeng, Z. In vitro antibacterial activity of isopropoxy benzene guanidine against multidrug-resistant enterococci. Infect. Drug Resist. 2019, 12, 3943–3953. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, S.; Wicha, S.G.; Broker, A.; Naundorf, T.; Catmadim, T.; Oellingrath, E.K.; Rohnke, M.; Streit, W.R.; Vollstedt, C.; Kipphardt, H.; et al. Contact-active antibacterial polyethylene foils via atmospheric air plasma induced polymerisation of quaternary ammonium salts. Colloids Surf. B Biointerfaces 2020, 186, 110679. [Google Scholar] [CrossRef]
- Liu, W.S.; Wang, C.H.; Sun, J.F.; Hou, G.G.; Wang, Y.P.; Qu, R.J. Synthesis, characterization and antibacterial properties of dihydroxy quaternary ammonium salts with long chain alkyl bromides. Chem. Biol. Drug Des. 2015, 85, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Xie, X.R.; Liu, W.S.; Hou, G.G.; Sun, J.F.; Zhao, F.; Cong, W.; Li, H.J.; Xin, W.Y. Quaternary ammonium salts substituted by 5-phenyl-1,3,4-oxadiazole-2-thiol as novel antibacterial agents with low cytotoxicity. Chem. Biol. Drug Des. 2017, 90, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.Z.; Xiao, Z.Q.; Fan, G.R.; Chen, S.X.; Liao, S.L.; Luo, H.; Wang, Z.D. Design, synthesis, antibacterial, antifungal and anticancer evaluations of novel beta-pinene quaternary ammonium salts. Int. J. Mol. Sci. 2021, 22, 11299. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, L.; Sun, J.; Peng, W.; Dong, X.; Gu, Y.; Ma, Z.; Gan, D.; Liu, P. Phosphonate/quaternary ammonium copolymers as high-efficiency antibacterial coating for metallic substrates. J. Mater. Chem. B 2021, 9, 8321–8329. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing quaternary ammonium-modified poly(amidoamine) dendrimers as dual action antibacterial agents. Bioconjugate Chem. 2014, 25, 918–927. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Hou, P.; Liu, J.; Fu, S. Design, synthesis, antibacterial, and antitumor activity of linear polyisocyanide quaternary ammonium salts with different structures and chain lengths. Molecules 2021, 26, 5686. [Google Scholar] [CrossRef]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, H.; Agarwal, S. Hyperbranched polyesters as biodegradable and antibacterial additives. J. Mater. Chem. B 2017, 5, 6827–6834. [Google Scholar] [CrossRef]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A shape-adaptive, antibacterial-coating of immobilized quaternary-ammonium compounds tethered on hyperbranched polyurea and its mechanism of action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Li, H.; Liu, L.; Zhao, F.; Wang, G.; Wang, Q.; Yu, Q. Antibacterial and drug-release dual-function membranes of cross-linked hyperbranched cationic polymers. React. Funct. Polym. 2020, 157, 104749. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure− activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Cuneo, T.; Gao, H. Recent advances on synthesis and biomaterials applications of hyperbranched polymers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1640. [Google Scholar] [CrossRef]
- Xue, Y.X.; Zhu, Y.Y.; Gao, L.M.; He, X.Y.; Liu, N.; Zhang, W.Y.; Yin, J.; Ding, Y.; Zhou, H.; Wu, Z.Q. Air-stable (phenylbuta-1,3-diynyl)palladium(II) complexes: Highly active initiators for living polymerization of isocyanides. J. Am. Chem. Soc. 2014, 136, 4706–4713. [Google Scholar] [CrossRef]



| Entry | M/C a | Solvent | Polymer | Mn (g/mol) b | Mw/Mnc | Yield |
|---|---|---|---|---|---|---|
| 1 | 50 | THF | L-P-M50 | 10,125 | 1.12 | 88.2% |
| 2 | 100 | THF | L-P-M100 | 21,310 | 1.09 | 92.1% |
| 3 | 150 | THF | L-P-M150 | 30,051 | 1.13 | 79.4% |
| 4 | 50 | THF | S-P-M50 | 29,775 | 1.16 | 88.4% |
| 5 | 100 | THF | S-P-M100 | 59,611 | 1.11 | 94.6% |
| Entry | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Compound | L-POcQAS-M50 | L-POcQAS-M100 | S-POcQAS-M50 | S-POcQAS-M100 |
| Solubility | 14 mg/mL | 11 mg/mL | 13 mg/mL | 9 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, L.; Hou, P.; Pan, H.; Fu, S. Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties. Polymers 2022, 14, 1737. https://doi.org/10.3390/polym14091737
Zhang H, Liu L, Hou P, Pan H, Fu S. Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties. Polymers. 2022; 14(9):1737. https://doi.org/10.3390/polym14091737
Chicago/Turabian StyleZhang, Hongguang, Lijia Liu, Peng Hou, Hong Pan, and Shuang Fu. 2022. "Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties" Polymers 14, no. 9: 1737. https://doi.org/10.3390/polym14091737
APA StyleZhang, H., Liu, L., Hou, P., Pan, H., & Fu, S. (2022). Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties. Polymers, 14(9), 1737. https://doi.org/10.3390/polym14091737






