Anticorrosion Properties of a Novel Hybrid Sol–Gel Coating on Aluminum 3003 Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
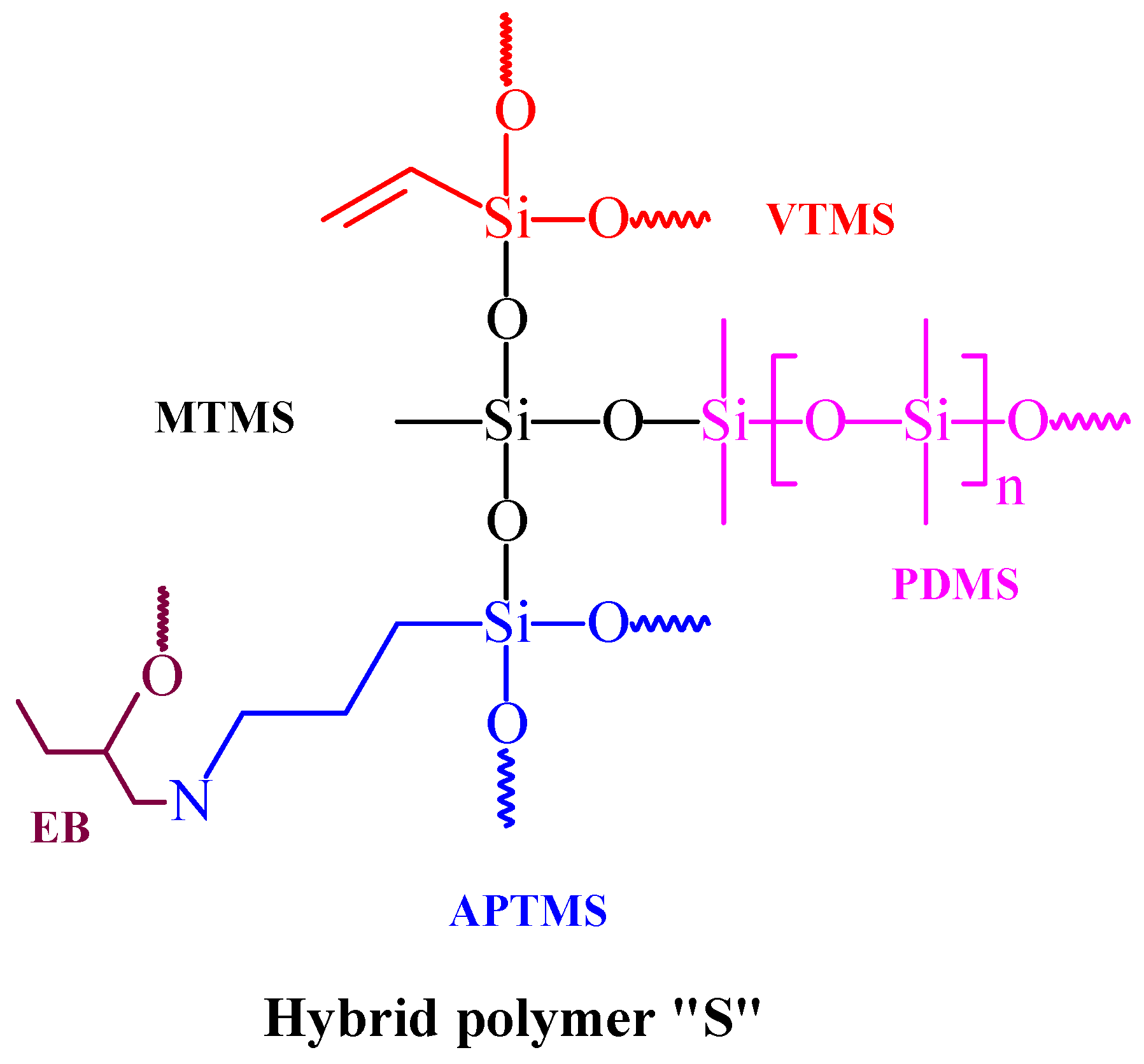
2.2. Synthesis of the Host Hybrid Coating
2.3. Formulation of the Waste-Additive-Embedded Coatings
2.4. Pretreatment of Aluminum Alloy Substrates
2.5. Deposition of Functional Coatings
2.6. Characterization of Coatings
3. Results and Discussion
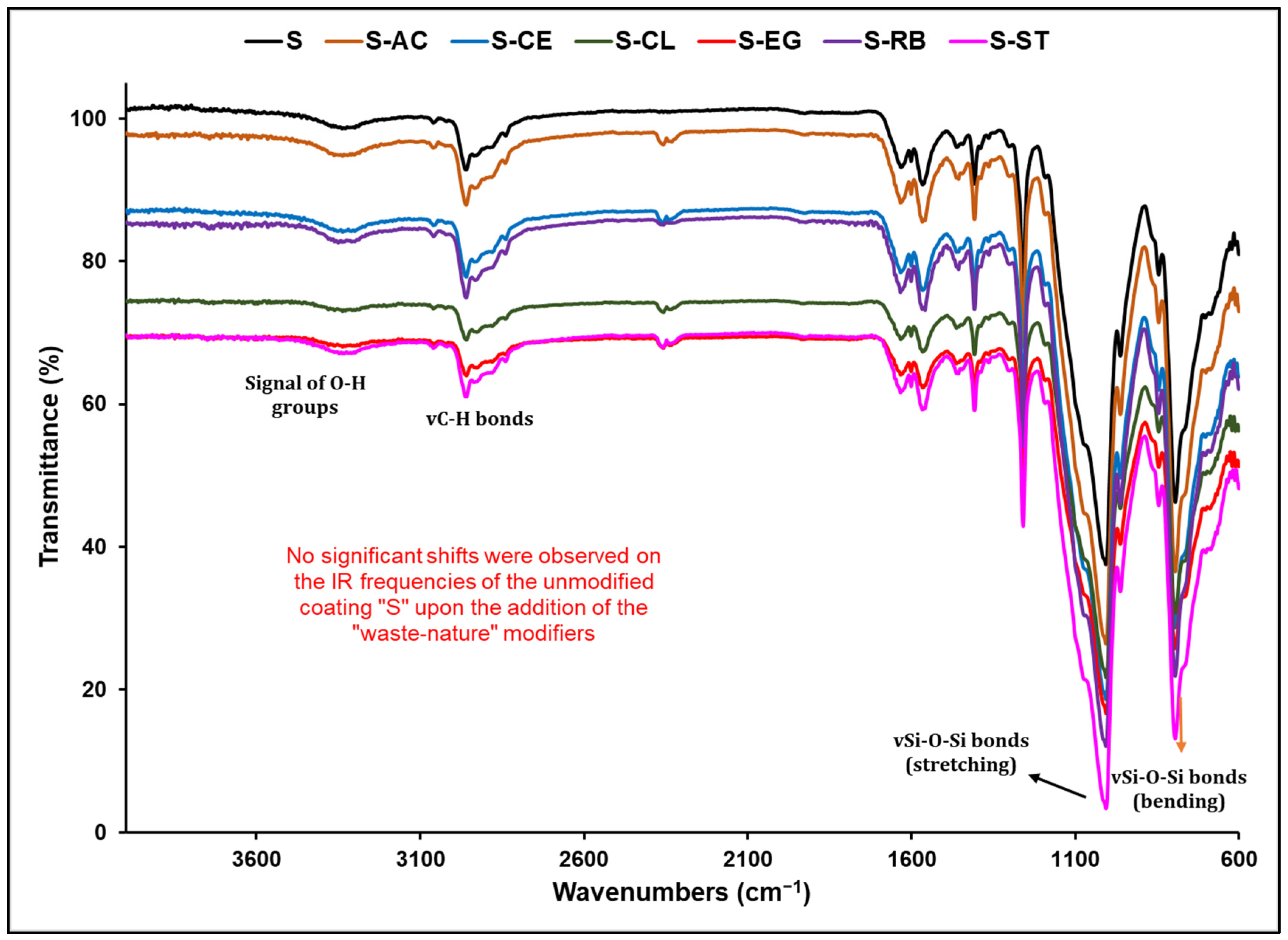
3.1. Structural Analysis of the Functional Coatings
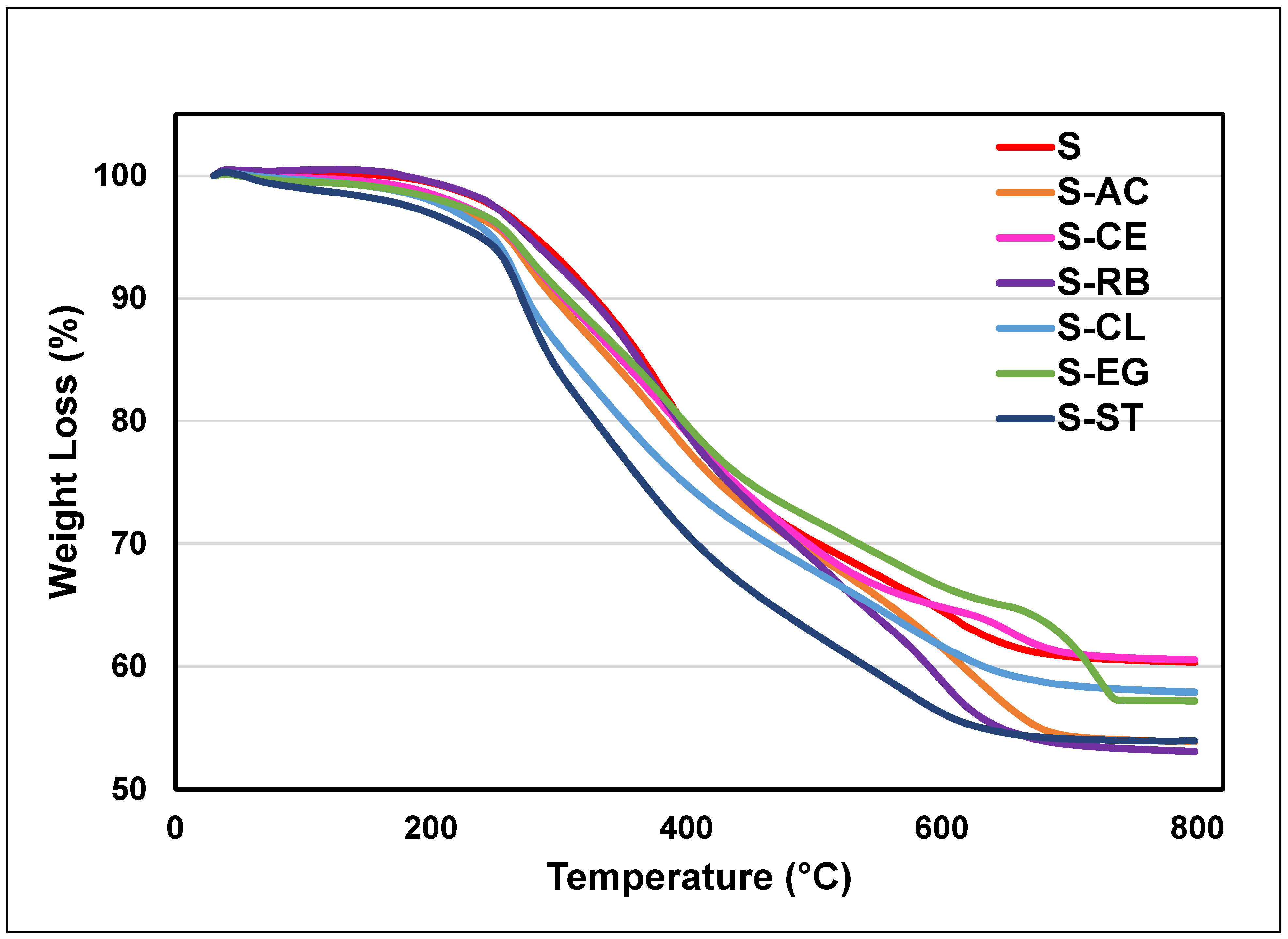
3.2. Gravimetric Thermal Analysis of the Cured Functional Coatings
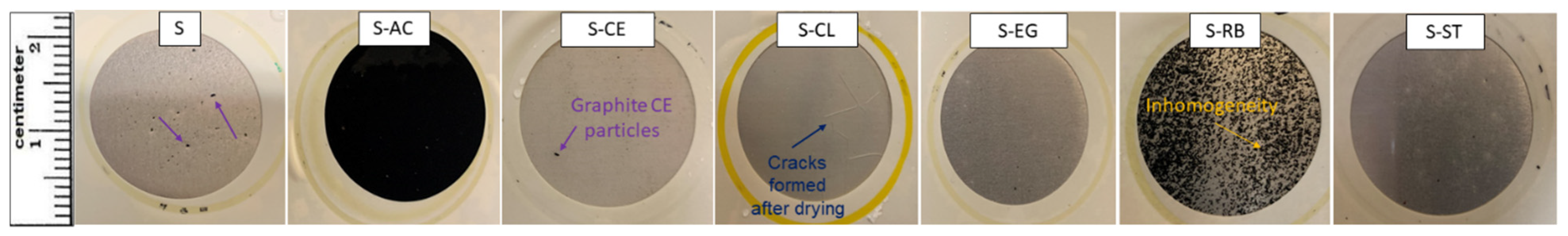
3.3. Surface Morphology of the Coating Formulations on Aluminum
3.4. Corrosion Behavior of the Hybrid Coating Formulations on Aluminum Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.M.; Kapun, B.; Tiringer, U.; Šekularac, G.; Milošev, I. Protection of Aluminum Alloy 3003 in Sodium Chloride and Simulated Acid Rain Solutions by Commercial Conversion Coatings Containing Zr and Cr. Coatings 2019, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Epstein, A.J.; Smallfield, J.A.O.; Guan, H.; Fahlman, M. Corrosion protection of aluminum and aluminum alloys by polyanilines: A potentiodynamic and photoelectron spectroscopy study. Synth. Met. 1999, 102, 1374–1376. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Abrahami, S.T.; de Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Hernández, M.; Nóvoa, X.R.; Pérez, C. Characterization of hybrid sol–gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys. Prog. Org. Coat. 2010, 68, 91–99. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Aruna, S.T. Corrosion protection behaviour of silica–titania hybrid coatings embedded with silica nanoparticles. Surf. Eng. 2017, 33, 467–473. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Santa Coloma, P.; Fernández-Carretero, F.J.; Brusciotti, F.; Brizuela, M. Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application. Coatings 2020, 10, 441. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Kumar, A.M.; Al-Aqeeli, N.; Ram, J.S.N. Influence of an anodized layer on the adhesion and surface protective performance of organic coatings on AA2024 aerospace Al alloy. Prog. Org. Coat. 2020, 138, 105396. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Kumar, A.M.; Adesina, A.Y.; Al-Badour, F.A.; Meliani, M.H.; Saleh, T.A. Hybrid Organosilicon-Metal oxide Composites and their Corrosion Protection Performance for Mild Steel in 3.5% NaCl Solution. Corros. Sci. 2020, 169, 108637. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Kumar, A.M.; Rahman, M.M.; Al-Badour, F.A.; Meliani, M.H.; Saleh, T.A. Effect of metal oxide additives on the structural and barrier properties of a hybrid organosilicon sol-gel coating in 3.5% NaCl medium. Prog. Org. Coat. 2020, 148, 105825. [Google Scholar] [CrossRef]
- Gobara, M. Effects of TiO2/SiO2 Reinforced Nanoparticles on the Mechanical Properties of Green Hybrid Coating. Int. Lett. Chem. Phys. Astron. 2015, 47, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Niknahad, M.; Mannari, V. Corrosion protection of aluminum alloy substrate with nano-silica reinforced organic–inorganic hybrid coatings. J. Coat. Technol. Res. 2016, 13, 1035–1046. [Google Scholar] [CrossRef]
- Kumar, N.; Jyothirmayi, A.; Raju, K.R.C.S.; Uma, V.; Subasri, R. One-Step Anodization/Sol-Gel Deposition of Ce3+-Doped Silica-Zirconia Self-Healing Coating on Aluminum. ISRN Corros. 2013, 2013, 424805. [Google Scholar] [CrossRef] [Green Version]
- Whelan, M.; Cassidy, J.; Duffy, B. Sol–gel sealing characteristics for corrosion resistance of anodised aluminium. Surf. Coat. Technol. 2013, 235, 86–96. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Suleiman, R.K. Improved mechanical and anticorrosion properties of metal oxide-loaded hybrid sol–gel coatings for mild steel in a saline medium. J. Adhes. Sci. Technol. 2020, 34, 1315–1330. [Google Scholar] [CrossRef]
- Savignac, P.; Menu, M.-J.; Gressier, M.; Denat, B.; Khadir, Y.; Manov, S.; Ansart, F. Improvement of Adhesion Properties and Corrosion Resistance of Sol-Gel Coating on Zinc. Molecules 2018, 23, 1079. [Google Scholar] [CrossRef] [Green Version]
- Sakai, R.T.; da Cruz, F.M.D.L.; de Melo, H.G.; Benedetti, A.V.; Santilli, C.V.; Suegama, P.H. Electrochemical study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA films on tin coated steel in 3.5% NaCl solution. Prog. Org. Coat. 2012, 74, 288–301. [Google Scholar] [CrossRef]
- Suleiman, R.; Estaitie, M.; Mizanurahman, M. Hybrid organosiloxane coatings containing epoxide precursors for protecting mild steel against corrosion in a saline medium. J. Appl. Polym. Sci. 2016, 133, 43947. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Martín-Ugarte, E.; Jorcin, J.B.; Imbuluzqueta, G.; Santa Coloma, P.; Izagirre-Etxeberria, U. Effect of organic precursor in hybrid sol–gel coatings for corrosion protection and the application on hot dip galvanised steel. J. Sol-Gel Sci. Technol. 2019, 89, 264–283. [Google Scholar] [CrossRef]
- Eduok, U.; Suleiman, R.; Gittens, J.; Khaled, M.; Smith, T.J.; Akid, R.; El Ali, B.; Khalil, A. Anticorrosion/antifouling properties of bacterial spore-loaded sol–gel type coating for mild steel in saline marine condition: A case of thermophilic strain of Bacillus licheniformis. RSC Adv. 2015, 5, 93818–93830. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, R.K.; Saleh, T.A.; Al Hamouz, O.C.S.; Ibrahim, M.B.; Sorour, A.A.; El Ali, B. Corrosion and fouling protection performance of biocide-embedded hybrid organosiloxane coatings on mild steel in a saline medium. Surf. Coat. Technol. 2017, 324, 526–535. [Google Scholar] [CrossRef]
- Zand, R.Z.; Verbeken, K.; Adriaens, A. Influence of the cerium concentration on the corrosion performance of ce-doped silica hybrid coatings on hot dip galvanized steel substrates. Int. J. Electrochem. Sci. 2013, 8, 548–563. [Google Scholar]
- Adesina, A.Y.; Zainelabdeen, I.H.; Dalhat, M.A.; Mohammed, A.S.; Sorour, A.A.; Al-Badour, F.A. Influence of micronized waste tire rubber on the mechanical and tribological properties of epoxy composite coatings. Tribol. Int. 2020, 146, 106244. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Shoja Razavi, R.; Loghman-Estarki, M.R.; Ramazani, M.; Barekat, M.; Mishra, A.K.; Fattahi, H. The effects of Cloisite 20A content on the adhesion strength and corrosion behavior of poly (amide-imide)/cloisite 20A nanocomposite coatings. Compos. Part B Eng. 2019, 175, 107154. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Najafisayar, P.; Hessam, R.; Zakerin, N. Characterization of Anodic Films Produced on Anodized AA1050 Aluminum Alloy: Effect of Bio-additive. Metall. Mater. Trans. A 2020, 51, 5475–5483. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Kumar, A.M.; Sorour, A.A.; Al-Badour, F.A.; El Ali, B. Hybrid organosiloxane material/metal oxide composite as efficient anticorrosive coatings for mild steel in a saline medium. J. Appl. Polym. Sci. 2018, 135, 46718. [Google Scholar] [CrossRef]
- Qu, H.; Bhattacharyya, S.; Ducheyne, P. 4.34 Sol–Gel Processed Oxide Controlled Release Materials ☆. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 617–643. [Google Scholar]
- Barberena-Fernández, A.M.; Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Eduok, U.; Suleiman, R.; Khaled, M.; Akid, R. Enhancing water repellency and anticorrosion properties of a hybrid silica coating on mild steel. Prog. Org. Coat. 2016, 93, 97–108. [Google Scholar] [CrossRef]
- Mohd Yusop, H.; Mohd Ismail, A.I.H.; Wan Ismail, W.N. Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent. Polymers 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Oliveira, P.C.; Giordani, D.S.; de Castro, H.F. Chitosan/siloxane hybrid polymer: Synthesis, characterization and performance as a support for immobilizing enzyme. J. Braz. Chem. Soc. 2011, 22, 1407–1417. [Google Scholar] [CrossRef]
- Farhadi, S.S.; Aliofkhazraei, M.; Barati Darband, G.; Abolhasani, A.; Sabour Rouhaghdam, A. Wettability and Corrosion Behavior of Chemically Modified Plasma Electrolytic Oxidation Nanocomposite Coating. J. Mater. Eng. Perform. 2017, 26, 4797–4806. [Google Scholar] [CrossRef]
- Purcar, V.; Rădițoiu, V.; Rădițoiu, A.; Manea, R.; Raduly, F.M.; Ispas, G.C.; Frone, A.N.; Nicolae, C.A.; Gabor, R.A.; Anastasescu, M.; et al. Preparation and characterization of some sol-gel modified silica coatings deposited on polyvinyl chloride (Pvc) substrates. Coatings 2021, 11, 11. [Google Scholar] [CrossRef]
- Su, H.-Y.; Chen, P.-L.; Lin, C.-S. Sol–gel coatings doped with organosilane and cerium to improve the properties of hot-dip galvanized steel. Corros. Sci. 2016, 102, 63–71. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Schreiner, W.; Pellice, S.; Ceré, S. Hybrid sol-gel coatings containing clay nanoparticles for corrosion protection of mild steel. Electrochim. Acta 2016, 203, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Yasakau, K.A.; Starykevich, M.; Ferreira, M.G.S.; Zheludkevich, M.L. A critical look at interpretation of electrochemical impedance spectra of sol-gel coated aluminium. Electrochim. Acta 2021, 378, 138091. [Google Scholar] [CrossRef]
- Kakaei, M.N.; Neshati, J.; Rezaierod, A.R. On the Extraction of the Effective Capacitance from Constant Phase Element Parameters. Prot. Met. Phys. Chem. Surf. 2018, 54, 548–556. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Wu, M.; Huang, R. Study of depassivation of carbon steel in simulated concrete pore solution using different equivalent circuits. Constr. Build. Mater. 2017, 157, 357–362. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Fan, H.; Hong, R.; Li, W. Construction of MOF-based superhydrophobic composite coating with excellent abrasion resistance and durability for self-cleaning, corrosion resistance, anti-icing, and loading-increasing research. Chem. Eng. J. 2021, 408, 127343. [Google Scholar] [CrossRef]
- McCafferty, E. Sequence of steps in the pitting of aluminum by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]


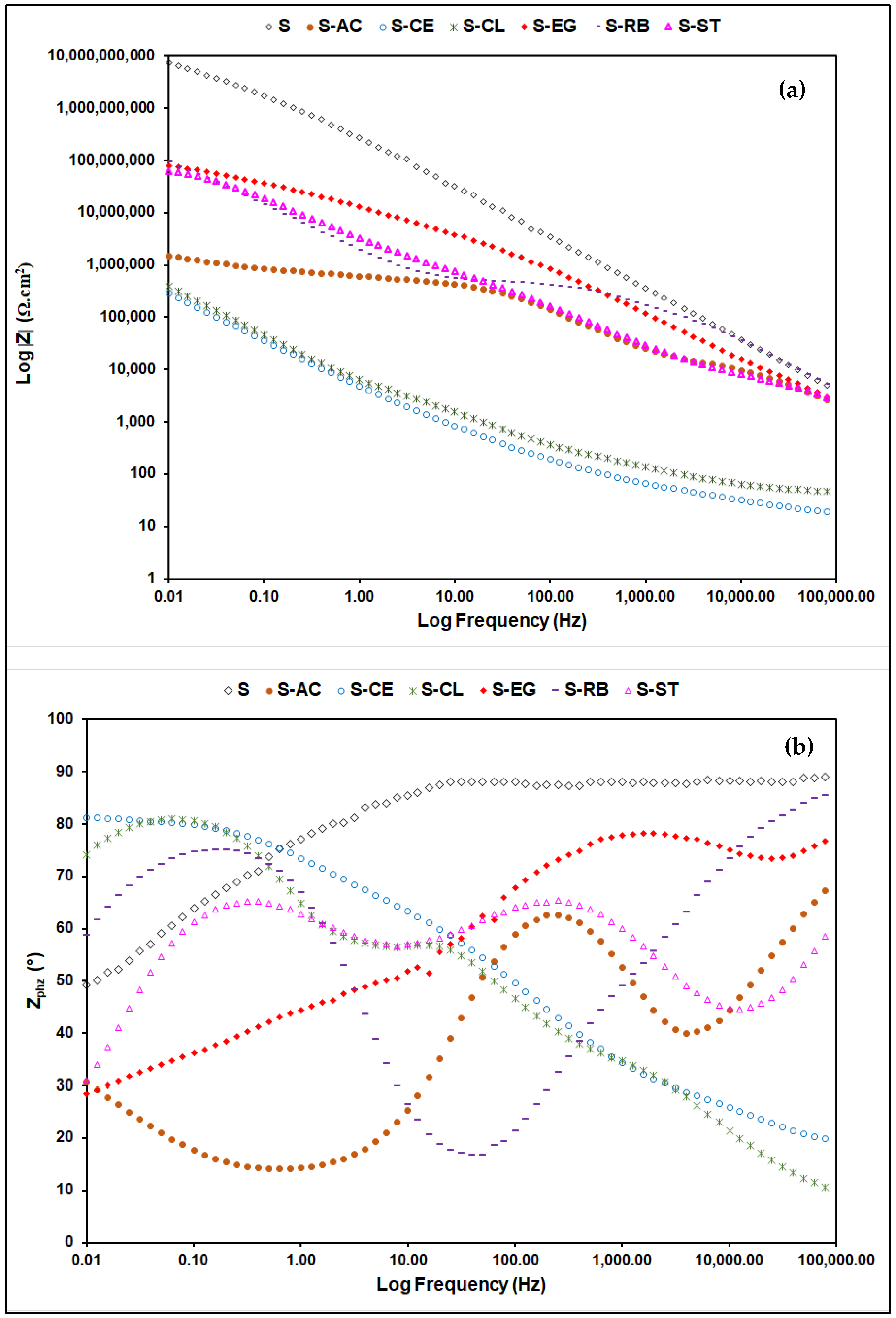
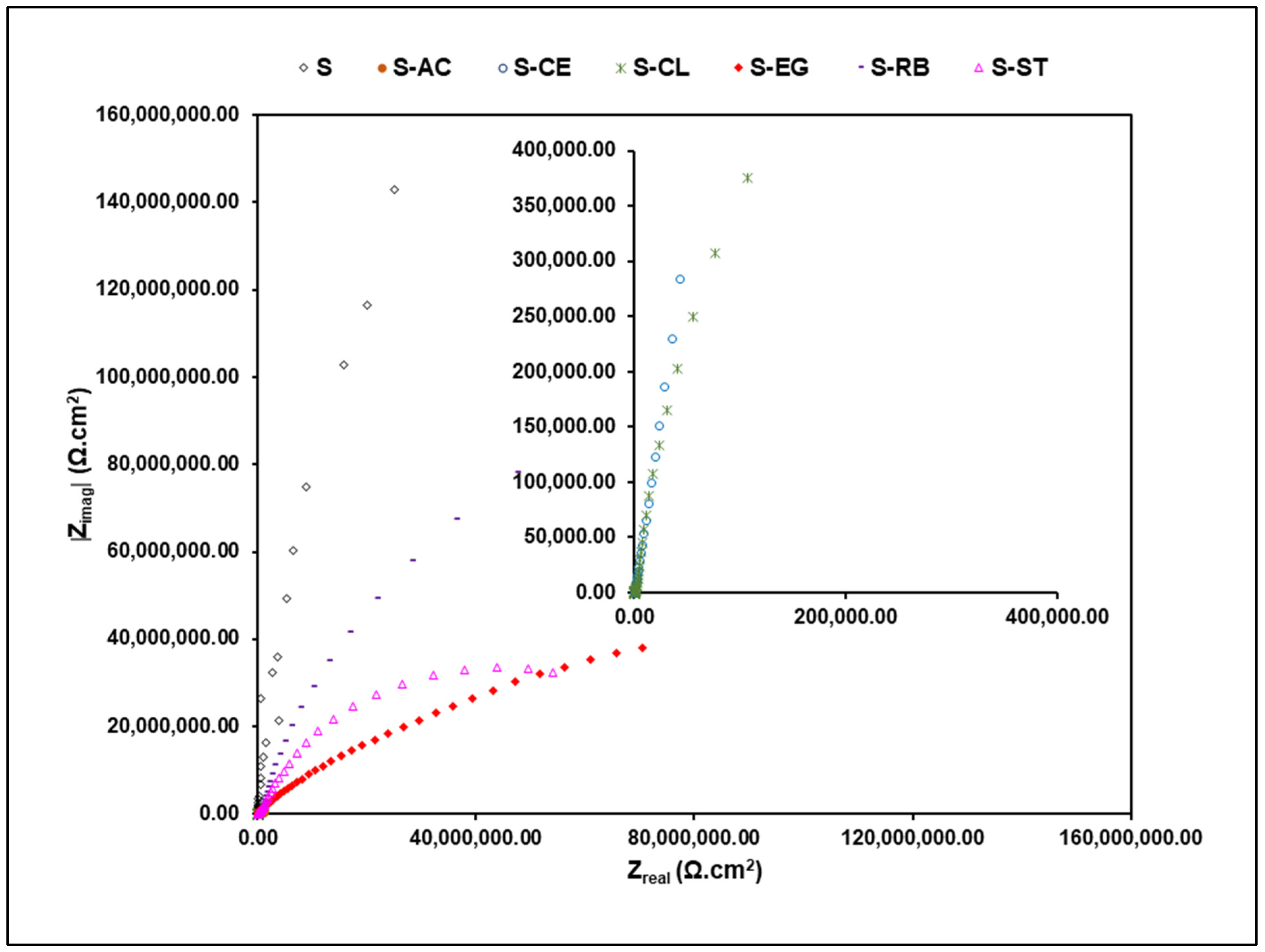
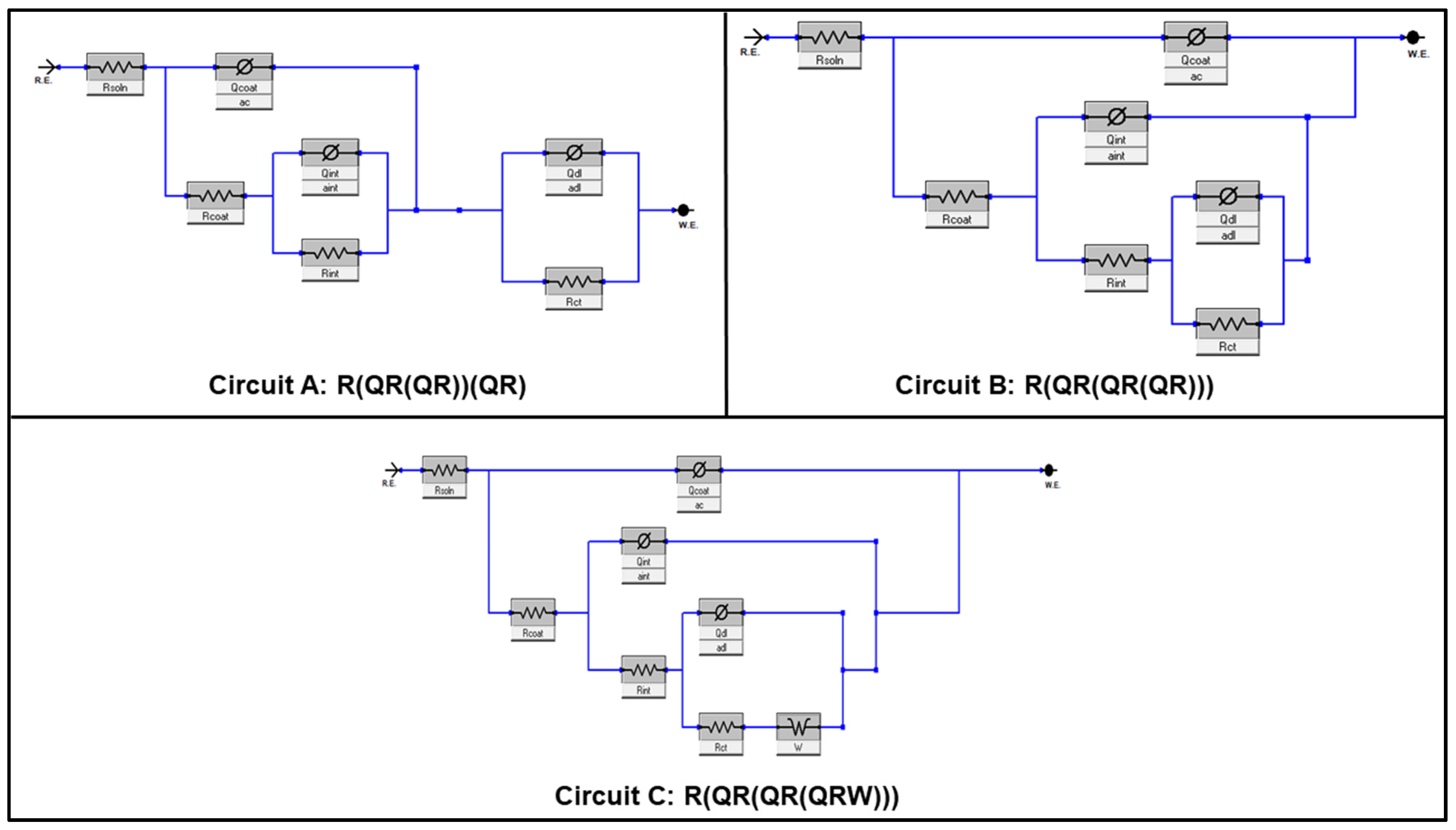
| Sample | CA (°, Mean ± SD *) |
|---|---|
| S | 94 ± 1 |
| S-AC | 91 ± 3 |
| S-CE | 81± 1 |
| S-CL | 95 ± 4 |
| S-EG | 85 ± 2 |
| S-RB | 93 ± 1 |
| S-ST | 87 ± 2 |
| Ra (nm) | Rms (nm) | Rpv (nm) | |
|---|---|---|---|
| S | 0.40 | 0.87 | 37.53 |
| S-AC | 0.81 | 1.16 | 29.34 |
| S-CE | 1.03 | 2.01 | 62.04 |
| S-CL | 3.32 | 5.34 | 59.64 |
| S-EG | 1.61 | 2.47 | 42.82 |
| S-RB | 0.93 | 1.43 | 30.14 |
| S-ST | 18.05 | 21.44 | 96.34 |
| Sample | Circuit A | Circuit B | Circuit C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qdl | RCT | χ2 | Qdl | RCT | χ2 | Qdl | RCT | χ2 | |
| S | 64.53 | 2.24 | 1.20 | 3.77 × 10−3 | 625.20 × 103 | 1.21 | NP | NP | NP |
| S-AC | NP | NP | NP | NP | NP | NP | 333.30 | 0.11 | 0.21 |
| S-CE | 45.09 × 103 | 23.78 | 2.23 | NF | NF | NF | NF | NF | NF |
| S-CL | 0.034 | 1.91 | 0.12 | 0.014 | 6.98 | 2.77 | NP | NP | NP |
| S-EG | 9.01 | 1.33 | 1.80 | 27.42 | 422.00 | 1.86 | NP | NP | NP |
| S-RB | 105.50 | 249.60 | 6.78 | 105.60 | 250.10 | 6.78 | NP | NP | NP |
| S-ST | 75.84 | 87.96 | 1.50 | 22.13 | 105.20 | 1.34 | NP | NP | NP |
| Parameter | Sample | ||||||
|---|---|---|---|---|---|---|---|
| S | S-AC | S-CE | S-CL | S-EG | S-RB | S-ST | |
| Rsoln (Ω) | 10.00 | 26.40 | 15.00 | 40.21 | 22.00 | 50.00 | 18.83 |
| Rcoat (kΩ) | 11.50 × 103 | 14.45 | 12.03 | 0.499 | 4.25 × 103 | 453.90 | 19.18 |
| Qcoat (nF cm−2 s−(1−αc)) | 507.20 × 10−3 | 3.46 | 0.39 | 0.049 | 4.02 | 2.64 | 44.93 |
| ac | 0.98 | 0.88 | 0.45 | 0.58 | 0.87 | 0.85 | 0.68 |
| Rint (MΩ) | 2.78 | 0.49 | 0.155 | 0.0030 | 0.0026 | 250.10 | 2.26 |
| Qint (nF cm−2 s−(1−αc)) | 3.83 × 10−3 | 23.38 | 3.42 × 103 | 7.53 × 103 | 5.91 | 105.60 | 7.22 |
| aint | 0.44 | 0.86 | 0.51 | 0.89 | 0.83 | 0.88 | 0.91 |
| Rct (MΩ) | 625.20 × 103 | 0.11 | 23.78 | 1.91 | 422.00 | 250.10 | 105.2 |
| Qdl (nF cm−2 s−(1−αc)) | 3.77 × 10−3 | 333.30 | 45.09 × 103 | 0.034 | 27.42 | 105.60 | 22.13 |
| adl | 0.83 | 1.00 | 0.92 | 0.95 | 0.36 | 0.19 | 0.90 |
| W (μF cm−2 s0.5) | - | 3.74 | - | - | - | - | |
| χ2 (× 103) | 1.21 | 0.21 | 2.23 | 0.12 | 1.86 | 6.78 | 1.34 |
| Circuit | B | C | A | A | B | B | B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleiman, R.K.; Adesina, A.Y.; Kumar, A.M.; Rahman, M.M.; Al-Badour, F.A.; El Ali, B. Anticorrosion Properties of a Novel Hybrid Sol–Gel Coating on Aluminum 3003 Alloy. Polymers 2022, 14, 1798. https://doi.org/10.3390/polym14091798
Suleiman RK, Adesina AY, Kumar AM, Rahman MM, Al-Badour FA, El Ali B. Anticorrosion Properties of a Novel Hybrid Sol–Gel Coating on Aluminum 3003 Alloy. Polymers. 2022; 14(9):1798. https://doi.org/10.3390/polym14091798
Chicago/Turabian StyleSuleiman, Rami K., Akeem Y. Adesina, Arumugam Madhan Kumar, Mohammad Mizanur Rahman, Fadi A. Al-Badour, and Bassam El Ali. 2022. "Anticorrosion Properties of a Novel Hybrid Sol–Gel Coating on Aluminum 3003 Alloy" Polymers 14, no. 9: 1798. https://doi.org/10.3390/polym14091798








