Synthesis and Characterization of Crosslinked Castor Oil-Based Polyurethane Nanocomposites Based on Novel Silane-Modified Isocyanate and Their Potential Application in Heat Insulating Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
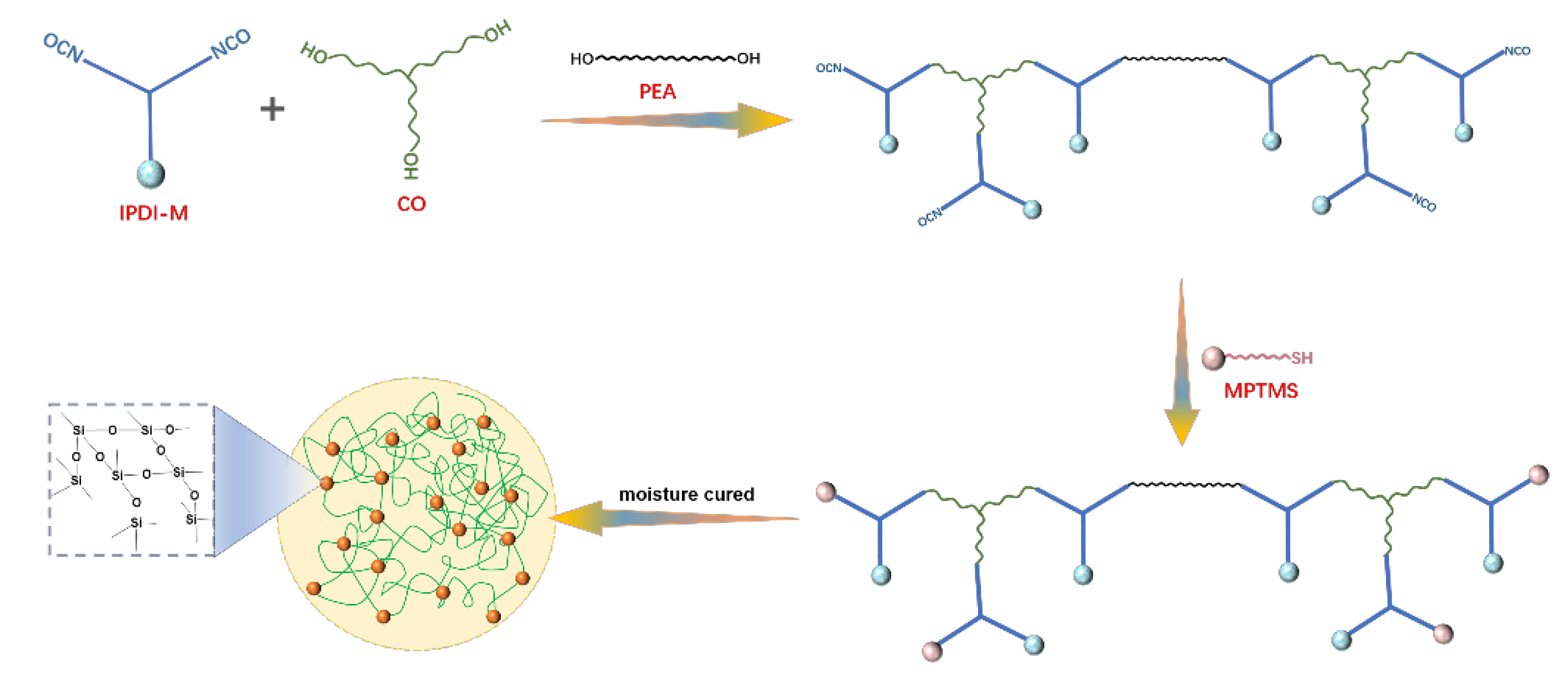
2.2. Preparation of MPTMS Grafted IPDI-Tri (IPDI-M)
2.3. Preparation of Silicone/Castor-Based Polyurethane (CPUSi) Hybrids Coating
2.4. Preparation of CPUSi/ATO Hybrids Coating
2.5. Characterization
3. Results
3.1. The Structural Characterization of CPUSi Coatings
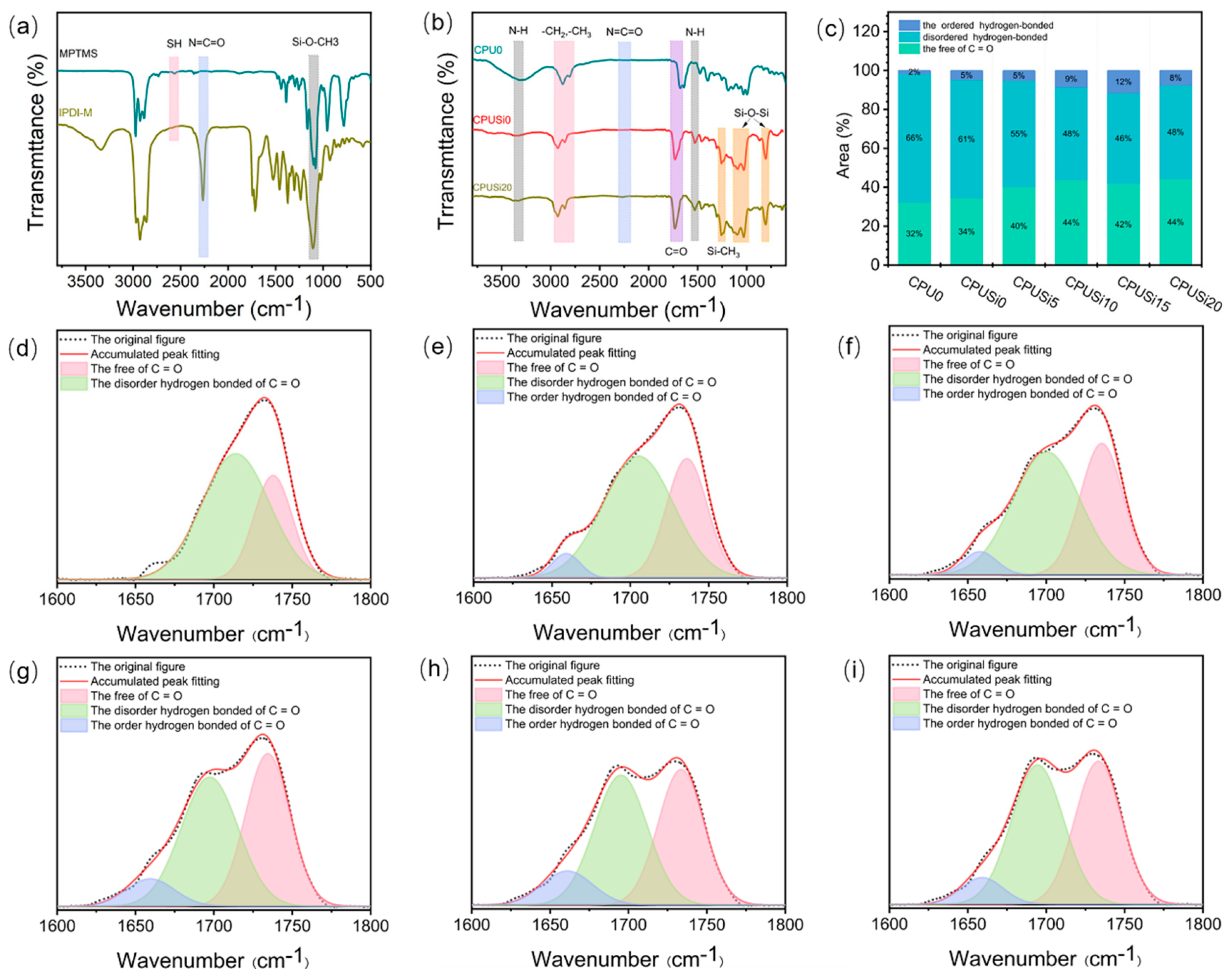
3.1.1. Infrared Spectroscopy Analysis
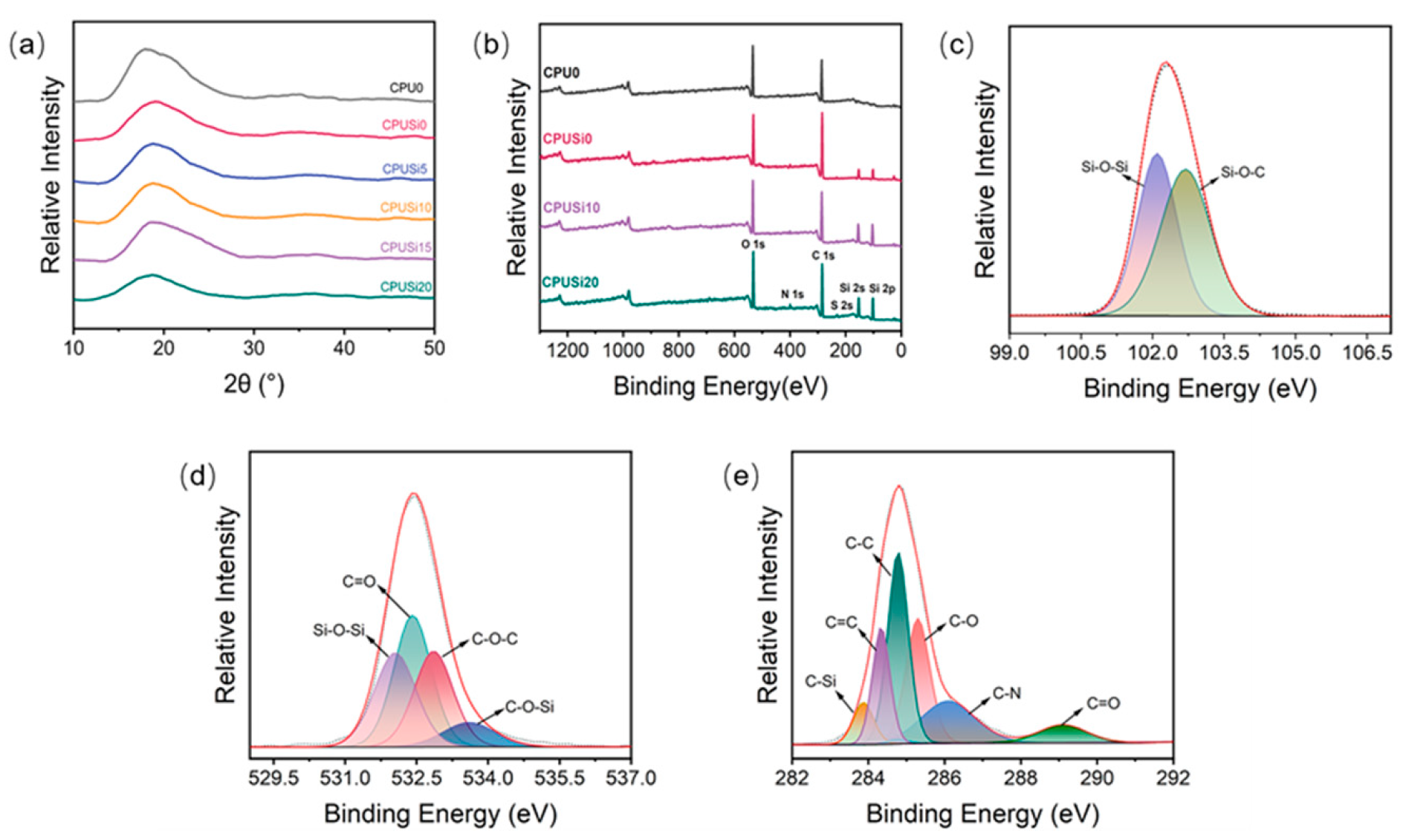
3.1.2. X-ray Diffraction Analysis
3.1.3. XPS Analysis
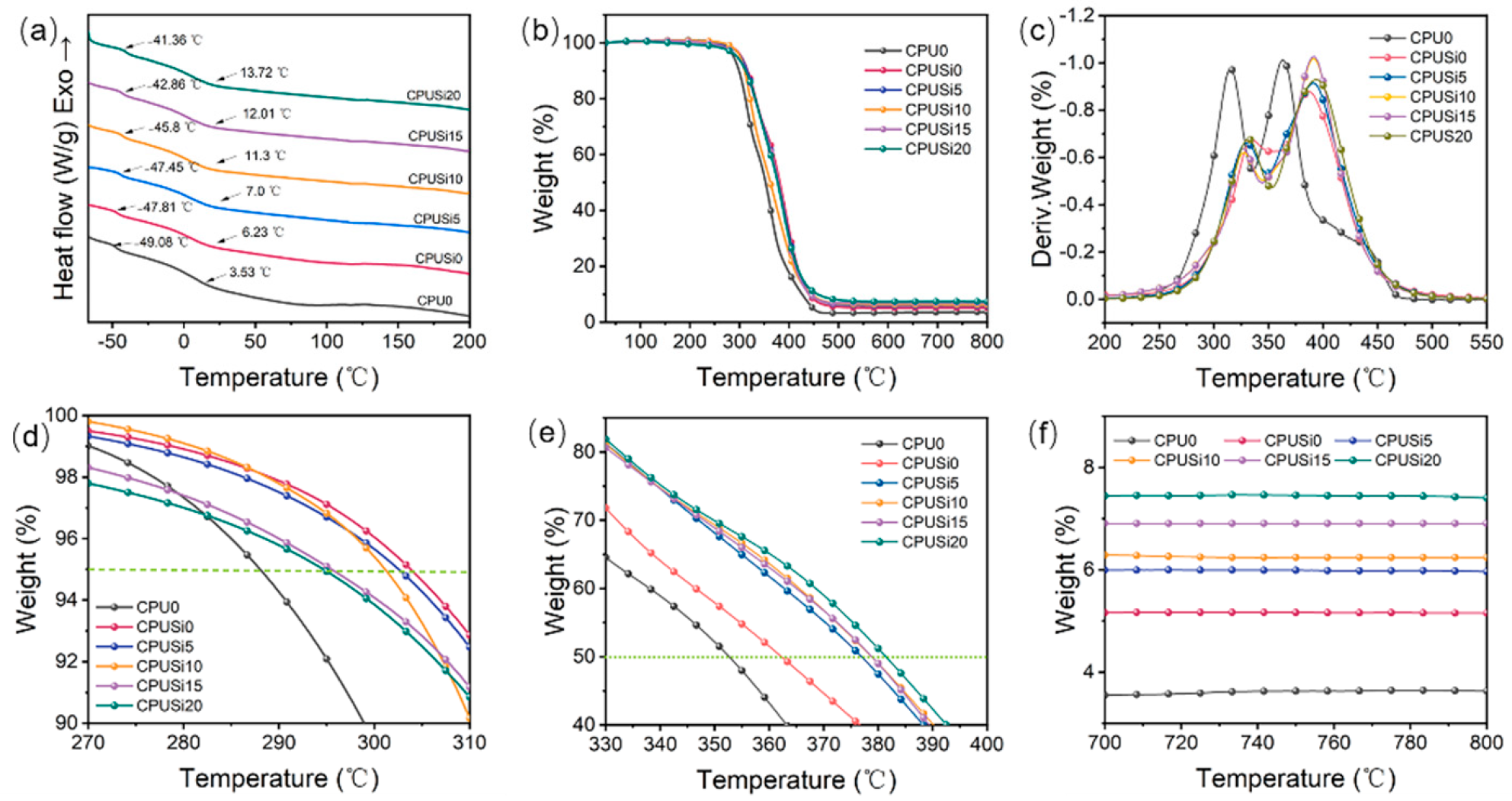
3.1.4. DSC Analysis
3.1.5. TGA Analysis
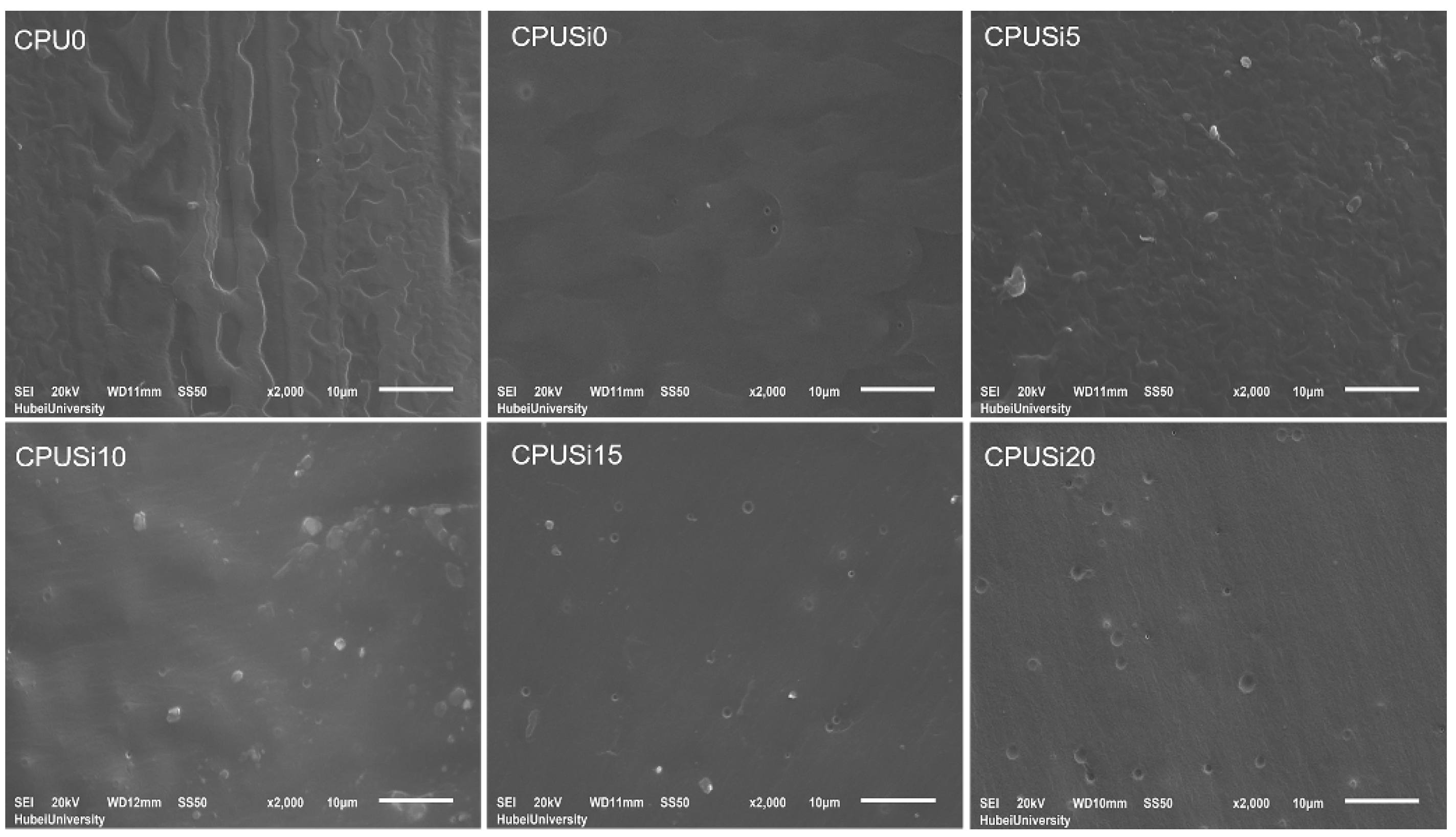
3.1.6. SEM Analysis
3.1.7. AFM Analysis
3.2. The Performance of CPUSi Coatings
3.2.1. Optical Performance
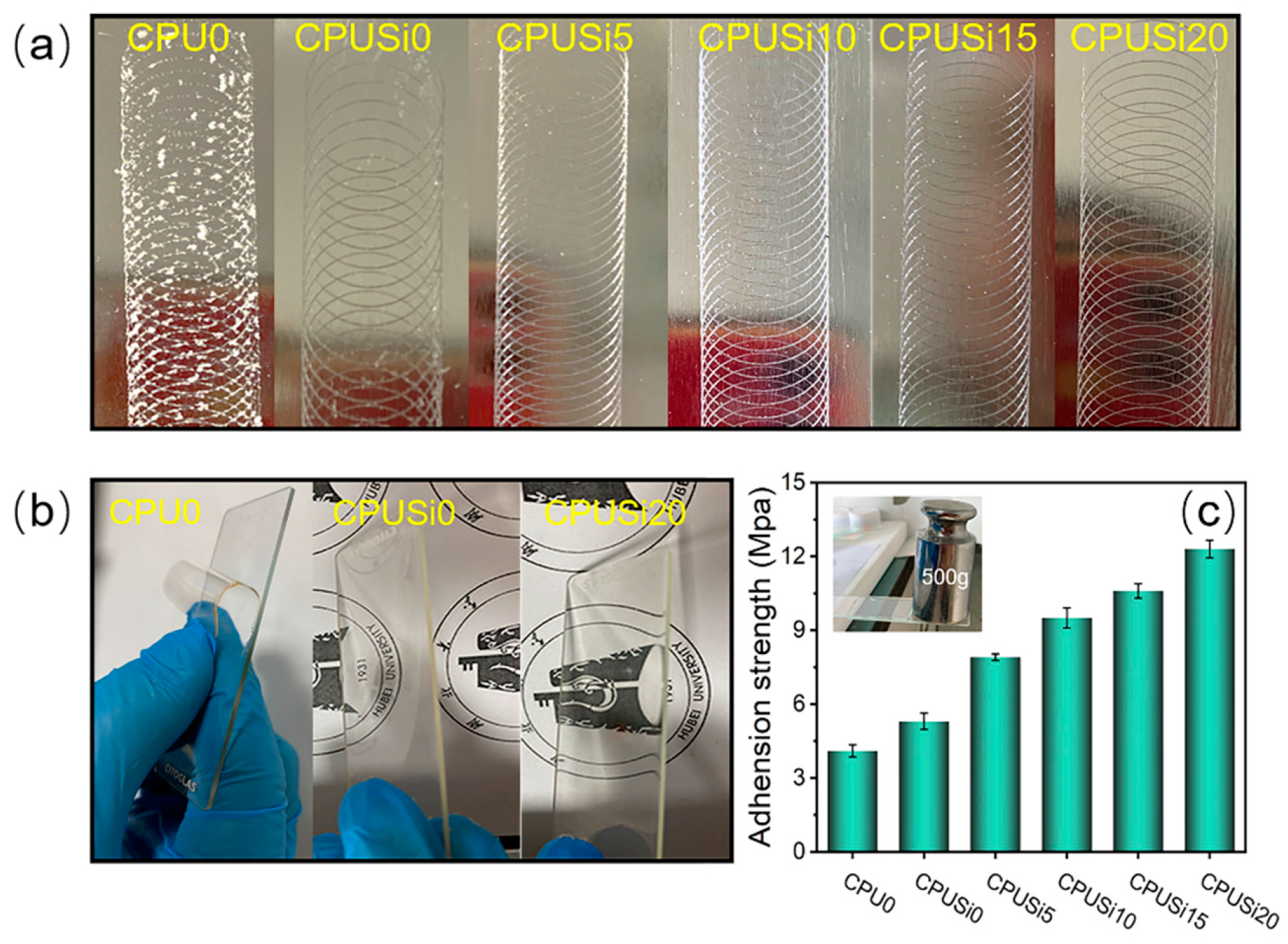
3.2.2. Surface Property
3.2.3. Chemical Resistance Property
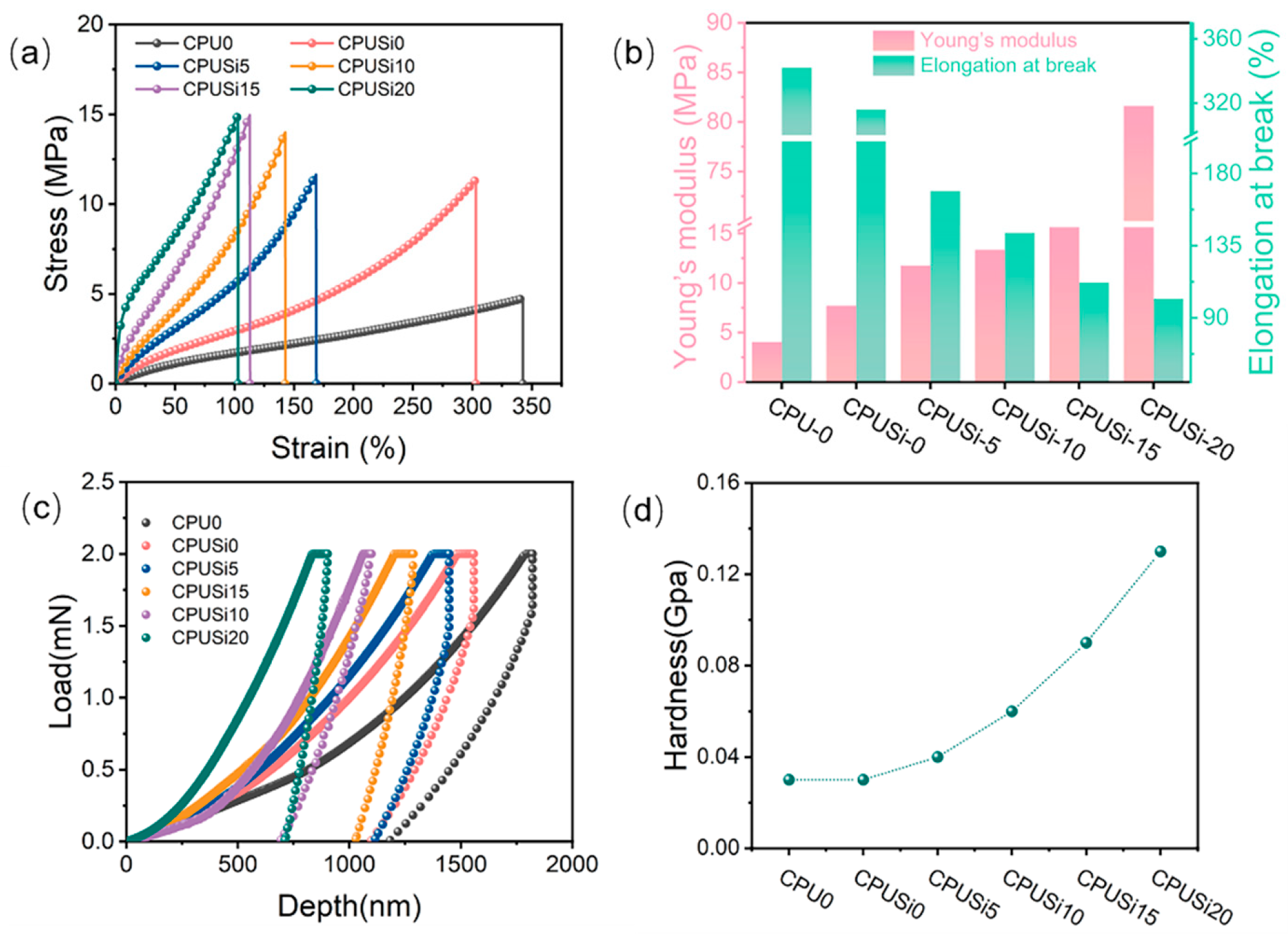
3.2.4. Mechanical Performance
3.2.5. Adhesive Property
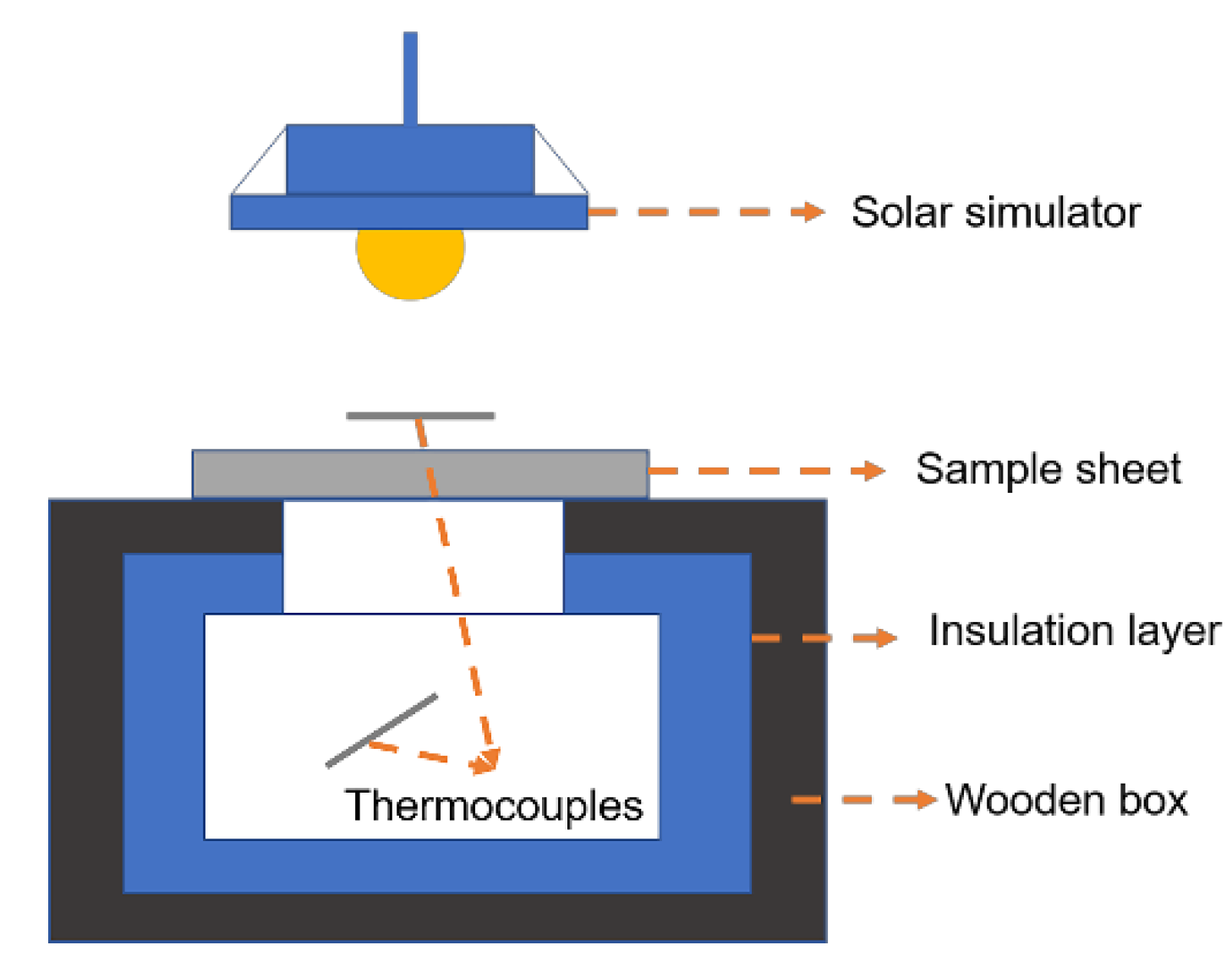
3.2.6. Thermal Insulation Performance of CPUSi/ATO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsui, H.; Tabata, H. Sn-Doped In2O3Nanoparticles as Thermal Insulating Materials for Solar–Thermal Shielding in the Infrared Range. ACS Appl. Nano Mater. 2021, 4, 6269–6279. [Google Scholar] [CrossRef]
- Wang, M.; Bu, J.; Xu, Y.; Liu, Y.; Yang, Y. Sb-doped SnO2 (ATO) hollow submicron spheres for solar heat insulation coating. Ceram. Int. 2021, 47, 547–555. [Google Scholar] [CrossRef]
- Wu, K.; Xiang, S.; Zhi, W.; Bian, R.; Wang, C.; Cai, D. Preparation and characterization of UV curable waterborne poly(urethane-acrylate)/antimony doped tin oxide thermal insulation coatings by sol-gel process. Prog. Org. Coat. 2017, 113, 39–46. [Google Scholar] [CrossRef]
- Fang, D.; Yu, H.; Dirican, M.; Tian, Y.; Xie, J.; Jia, D.; Yan, C.; Liu, Y.; Li, C.; Liu, H.; et al. Disintegrable, transparent and mechanically robust high-performance antimony tin oxide/nanocellulose/polyvinyl alcohol thermal insulation films. Carbohydr. Polym. 2021, 266, 118175. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Y.; Yang, Z.; Zhang, W.; Wang, S. Construction of ultraviolet protection, thermal insulation, superhydrophobic and aromatic textile with Al-doped ZnO–embedded lemon microcapsule coatings. Text. Res. J. 2019, 89, 3860–3870. [Google Scholar] [CrossRef]
- Farshchi, N.; Gedan-Smolka, M. Polyurethane Powder Coatings: A Review of Composition and Characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Gao, W.C.; Wu, W.; Chen, C.Z.; Zhao, H.; Liu, Y.; Li, Q.; Huang, C.X.; Hu, G.H.; Wang, S.F.; Shi, D.; et al. Design of a Superhydrophobic Strain Sensor with a Multilayer Structure for Human Motion Monitoring. ACS Appl Mater Interfaces 2022, 14, 1874–1884. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, W.C.; Li, Q.; Khan, M.R.; Hu, G.H.; Liu, Y.; Wu, W.; Huang, C.X.; Li, R.K.Y. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B. Advancements in the Development of Biostable Polyurethanes. Polym. Rev. 2018, 59, 391–417. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable Polyurethanes from Sustainable Biological Precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Seoane-Rivero, R.; Navarro, R.; Marcos-Fernandez, A. Coumarins into Polyurethanes for Smart and Functional Materials. Polymers 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Li, Y.; Huang, S.; Huang, K.; Zeng, X.; Dong, Q.; Liu, C.; Feng, P.; Zhang, C. Tailoring the Performance of Vegetable Oil-Based Waterborne Polyurethanes through Incorporation of Rigid Cyclic Rings into Soft Polymer Networks. ACS Sustain. Chem. Eng. 2019, 8, 914–925. [Google Scholar] [CrossRef]
- Li, C.; Sung, J.; Sun, X.S. Network from Dihydrocoumarin via Solvent-Free Metal-Mediated Pathway: A Potential Structure for Substantial Toughness Improvement of Epoxidized Plant Oil Materials. ACS Sustain. Chem. Eng. 2015, 4, 1231–1239. [Google Scholar] [CrossRef]
- An, X.-P.; Chen, J.-H.; Li, Y.-D.; Zhu, J.; Zeng, J.-B. Rational design of sustainable polyurethanes from castor oil: Towards simultaneous reinforcement and toughening. Sci. China Mater. 2018, 61, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Jian, X.-Y.; He, Y.; Li, Y.-D.; Wang, M.; Zeng, J.-B. Curing of epoxidized soybean oil with crystalline oligomeric poly(butylene succinate) towards high performance and sustainable epoxy resins. Chem. Eng. J. 2017, 326, 875–885. [Google Scholar] [CrossRef]
- Zhang, C.; Kessler, M.R. Bio-based Polyurethane Foam Made from Compatible Blends of Vegetable-Oil-based Polyol and Petroleum-based Polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Meticulous study on curing kinetics of green polyurethane-clay nanocomposite adhesive derived from plant oil: Evaluation of decomposition activation energy using TGA analysis. J. Macromol. Sci. Phys. Part A 2017, 54, 819–826. [Google Scholar] [CrossRef]
- Heidarian, M.; Shishesaz, M.R.; Kassiriha, S.M.; Nematollahi, M. Characterization of structure and corrosion resistivity of polyurethane/organoclay nanocomposite coatings prepared through an ultrasonication assisted process. Prog. Org. Coat. 2010, 68, 180–188. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Daga, B.; Aranguren, M.I.; Mucci, V. Bio-based waterborne polyurethanes reinforced with cellulose nanocrystals as coating films. Prog. Org. Coat. 2020, 144, 105649. [Google Scholar] [CrossRef]
- Pinto, E.R.P.; Barud, H.S.; Silva, R.R.; Palmieri, M.; Polito, W.L.; Calil, V.L.; Cremona, M.; Ribeiro, S.J.L.; Messaddeq, Y. Transparent composites prepared from bacterial cellulose and castor oil based polyurethane as substrates for flexible OLEDs. J. Mater. Chem. C 2015, 3, 11581–11588. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino–Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Akram, D.; Hakami, O.; Sharmin, E.; Ahmad, S. Castor and Linseed oil polyurethane/TEOS hybrids as protective coatings: A synergistic approach utilising plant oil polyols, a sustainable resource. Prog. Org. Coat. 2017, 108, 1–14. [Google Scholar] [CrossRef]
- Acuña, P.; Zhang, J.; Yin, G.-Z.; Liu, X.-Q.; Wang, D.-Y. Bio-based rigid polyurethane foam from castor oil with excellent flame retardancy and high insulation capacity via cooperation with carbon-based materials. J. Mater. Sci. 2020, 56, 2684–2701. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H. Fabrication of silane-grafted graphene oxide and its effect on the structural, thermal, mechanical, and hysteretic behavior of polyurethane. Sci. Rep. 2020, 10, 19152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Song, F.; Shang, Q.; Hu, Y.; Jia, P.; Liu, C.; Hu, L.; Zhu, G.; Huang, J.; et al. Castor-oil-based, robust, self-healing, shape memory, and reprocessable polymers enabled by dynamic hindered urea bonds and hydrogen bonds. Chem. Eng. J. 2022, 429, 131848. [Google Scholar] [CrossRef]
- Huo, L.; Wang, D.; Liu, H.; Jia, P.; Gao, J. Cytoxicity, dynamic and thermal properties of bio-based rosin-epoxy resin/castor oil polyurethane/carbon nanotubes bio-nanocomposites. J. Biomater. Sci. Polym. Ed. 2016, 27, 1100–1114. [Google Scholar] [CrossRef]
- Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Synthesis and Properties of Alkoxysilane Castor Oil and Their Polyurethane/Urea–Silica Hybrid Coating Films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Seeni Meera, K.M.; Murali Sankar, R.; Jaisankar, S.N.; Mandal, A.B. Physicochemical studies on polyurethane/siloxane cross-linked films for hydrophobic surfaces by the sol-gel process. J. Phys. Chem. B 2013, 117, 2682–2694. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Lai, X.; Li, Z.; Liu, M. Synthesis and properties of crosslinked waterborne polyurethane. J. Polym. Res. 2010, 18, 469–476. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, J.; Li, F.; Wu, Z.; Liu, Y.; Xiong, D. Synthesis and properties of self-crosslinking waterborne polyurethane with side chain for water-based varnish. Prog. Org. Coat. 2021, 150, 105972. [Google Scholar] [CrossRef]
- Zhao, H.; Hao, T.H.; Hu, G.H.; Shi, D.; Huang, D.; Jiang, T.; Zhang, Q.C. Preparation and Characterization of Polyurethanes with Cross-Linked Siloxane in the Side Chain by Sol-Gel Reactions. Materials 2017, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.; Yang, Z.; Zheng, Z.; Shen, L. Properties of alkoxysilane castor oil synthesized via thiol-ene and its polyurethane/siloxane hybrid coating films. Prog. Org. Coat. 2014, 77, 1241–1248. [Google Scholar] [CrossRef]
- Guo, S.; Xu, P.; Yu, H.; Li, X.; Cheng, Z. Hyper-branch sensing polymer batch self-assembled on resonant micro-cantilevers with a coupling-reaction route. Sens. Actuators B Chem. 2015, 209, 943–950. [Google Scholar] [CrossRef]
- Shaik, A.; Narayan, R.; Raju, K.V.S.N. Synthesis and properties of siloxane-crosslinked polyurethane-urea/silica hybrid films from castor oil. J. Coat. Technol. Res. 2014, 11, 397–407. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Qiu, T.; Xiao, W.; Du, D.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films. Appl. Surf. Sci. 2016, 377, 66–74. [Google Scholar] [CrossRef]
- Li, J.W.; Lee, H.T.; Tsai, H.A.; Suen, M.C.; Chiu, C.W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, T.; Chung, J.S. Synthesis of aminosilane crosslinked cationomeric waterborne polyurethane nanocomposites and its physicochemical properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 124–132. [Google Scholar] [CrossRef]
- Jeon, H.T.; Jang, M.K.; Kim, B.K.; Kim, K.H. Synthesis and characterizations of waterborne polyurethane–silica hybrids using sol–gel process. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 559–567. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, D.; Hao, T.-H.; Hu, G.-H.; Ye, G.-B.; Jiang, T.; Zhang, Q.-C. Synthesis and investigation of well-defined silane terminated and segmented waterborne hybrid polyurethanes. New J. Chem. 2017, 41, 9268–9275. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Yu, B.; Meng, Y.; Cao, X.; Zhang, Q.; Wu, W.; Shi, D.; Jiang, T.; Li, R.K.Y. Facile preparation of phosphorus containing hyperbranched polysiloxane grafted graphene oxide hybrid toward simultaneously enhanced flame retardancy and smoke suppression of thermoplastic polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106614. [Google Scholar] [CrossRef]
- Alagi, P.; Choi, Y.J.; Seog, J.; Hong, S.C. Efficient and quantitative chemical transformation of vegetable oils to polyols through a thiol-ene reaction for thermoplastic polyurethanes. Ind. Crop. Prod. 2016, 87, 78–88. [Google Scholar] [CrossRef]
- Dai, M.; Song, P.; Zhang, Y. Preparation and characterization of modified castor oil via photo-click chemistry for UV-curable waterborne polyurethane with enhanced water resistance and low conductive percolation threshold. J. Appl. Polym. Sci. 2020, 138, 49913. [Google Scholar] [CrossRef]
- Ahn, B.U.; Lee, S.K.; Lee, S.K.; Park, J.H.; Kim, B.K. UV curable polyurethane dispersions from polyisocyanate and organosilane. Prog. Org. Coat. 2008, 62, 258–264. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, M.; Chen, J.; Jiang, Z.; Ma, Y. Synthesis and properties of polyurethane elastomers based on renewable castor oil polyols. J. Appl. Polym. Sci. 2019, 136, 47309. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.k. Effect of nanosilica on the physicochemical, morphological and curing characteristics of transesterified castor oil based polyurethane coatings. Prog. Org. Coat. 2016, 97, 233–243. [Google Scholar] [CrossRef]
- Bockel, S.; Harling, S.; Grönquist, P.; Niemz, P.; Pichelin, F.; Weiland, G.; Konnerth, J. Characterization of wood-adhesive bonds in wet conditions by means of nanoindentation and tensile shear strength. Eur. J. Wood Wood Prod. 2020, 78, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.K.; Singh, B.P.; Dhakate, S.R.; Singh, V.N.; Mathur, R.B. Improved nanoindentation and microwave shielding properties of modified MWCNT reinforced polyurethane composites. J. Mater. Chem. A 2013, 1, 9138–9149. [Google Scholar] [CrossRef]
- Lin, C.; Ying, P.; Huang, M.; Zhang, P.; Yang, T.; Liu, G.; Wang, T.; Wu, J.; Levchenko, V. Synthesis of robust and self-healing polyurethane/halloysite coating via in-situ polymerization. J. Polym. Res. 2021, 28, 375. [Google Scholar] [CrossRef]
- Cai, D.; Jin, J.; Yusoh, K.; Rafiq, R.; Song, M. High performance polyurethane/functionalized graphene nanocomposites with improved mechanical and thermal properties. Compos. Sci. Technol. 2012, 72, 702–707. [Google Scholar] [CrossRef]
- Jindal, P.; Kumar, N.; Kumar, D. Elastic Modulus Behavior of Multi-Walled Carbon Nano-Tubes/Polyurethane Composites using Nano-Indentation Techniques. Indian J. Sci. Technol. 2017, 10, 17. [Google Scholar] [CrossRef]
- Shen, R.; Long, M.; Lei, C.; Dong, L.; Yu, G.; Tang, J. Anticorrosive waterborne polyurethane coatings derived from castor oil and renewable diols. Chem. Eng. J. 2022, 433, 134470. [Google Scholar] [CrossRef]
- Cassales, A.; Ramos, L.A.; Frollini, E. Synthesis of bio-based polyurethanes from Kraft lignin and castor oil with simultaneous film formation. Int. J. Biol. Macromol. 2020, 145, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Feng, Y.; Hu, Y.; Yuan, T.; Yang, Z. A renewable and multifunctional eco-friendly coating from novel tung oil-based cationic waterborne polyurethane dispersions. J. Clean. Prod. 2019, 241, 118341. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile synthesis and characterization of novel multi-functional bio-based acrylate prepolymers derived from tung oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Li, C.; Xiao, H.; Wang, X.; Zhao, T. Development of green waterborne UV-curable vegetable oil-based urethane acrylate pigment prints adhesive: Preparation and application. J. Clean. Prod. 2018, 180, 272–279. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Q.; Kong, F.; Liu, T.; Qian, H. Synthesis and surface properties of novel fluorinated polyurethane base on F-containing chain extender. Polym. Adv. Technol. 2019, 31, 616–629. [Google Scholar] [CrossRef]
- Li, K.; Qi, Y.; Zhou, Y.; Sun, X.; Zhang, Z. Microstructure and Properties of Poly(ethylene glycol)-Segmented Polyurethane Antifouling Coatings after Immersion in Seawater. Polymers 2021, 13, 573. [Google Scholar] [CrossRef]
- Lyu, J.; Xu, K.; Zhang, N.; Lu, C.; Zhang, Q.; Yu, L.; Feng, F.; Li, X. In Situ Incorporation of Diamino Silane Group into Waterborne Polyurethane for Enhancing Surface Hydrophobicity of Coating. Molecules 2019, 24, 1667. [Google Scholar] [CrossRef] [Green Version]





| Sample | IPDI-M/g(mol) | IPDI /g(mol) | CO/g (mol) | PEA /g(mol) | MPTMS /g(mol) | |
|---|---|---|---|---|---|---|
| IPDI-T | MPTMS | |||||
| CPU0 | 0 | 0 | 8.0 (0.072) | 13.8 (0.015) | 6 (0.02) | 0 |
| CPUSi0 | 0 | 0 | 8.0 (0.072) | 13.8 (0.015) | 6 (0.02) | 2.36 (0.012) |
| CPUSi5 | 1.7 (0.0075) | 0.5 (0.0025) | 7.4 (0.067) | 13.8 (0.015) | 6 (0.02) | 2.36 (0.012) |
| CPUSi10 | 3.3 (0.0150) | 1.0 (0.0050) | 6.9 (0.062) | 13.8 (0.015) | 6 (0.02) | 2.36 (0.012) |
| CPUSi15 | 5.0 (0.0225) | 1.5 (0.0075) | 6.3 (0.057) | 13.8 (0.015) | 6 (0.02) | 2.36 (0.012) |
| CPUSi20 | 6.7 (0.0300) | 2.0 (0.0100) | 5.8 (0.052) | 13.8 (0.015) | 6 (0.02) | 2.36 (0.012) |
| Sample | Atomic Concentration/% | Si Atomic Concentration/% | ||||
|---|---|---|---|---|---|---|
| C | N | O | S | Theoretical Bulk | Experimental on the Surface | |
| CPU0 | 57.63 | 0.21 | 42.16 | 0 | 0 | 0 |
| CPUSi0 | 49.05 | 0.38 | 43.73 | 0.41 | 1.12 | 6.43 |
| CPUSi10 | 37.34 | 0.43 | 48.13 | 0.6 | 1.44 | 13.5 |
| CPUSi20 | 34.14 | 0.68 | 49.01 | 0.92 | 1.7 | 15.25 |
| Samples | Td5/°C | Td50/°C | Tmax1/°C | Tmax2/°C | Residual Rate/% |
|---|---|---|---|---|---|
| CPU0 | 288.2 | 352.7 | 314.5 | 362.5 | 3.63 |
| CPUSi0 | 304.1 | 362.3 | 333.8 | 387.0 | 5.15 |
| CPUSi5 | 302.0 | 377 | 329.3 | 390.7 | 5.96 |
| CPUSi10 | 301.2 | 378.8 | 327.3 | 391.5 | 6.24 |
| CPUSi15 | 296.3 | 378.8 | 326.2 | 392.8 | 6.9 |
| CPUSi20 | 294.8 | 381.5 | 330.5 | 393.3 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Chen, K.; Yang, Y.; Jiang, T.; Hao, T.; Lu, X.; Zhang, Q. Synthesis and Characterization of Crosslinked Castor Oil-Based Polyurethane Nanocomposites Based on Novel Silane-Modified Isocyanate and Their Potential Application in Heat Insulating Coating. Polymers 2022, 14, 1880. https://doi.org/10.3390/polym14091880
Meng Y, Chen K, Yang Y, Jiang T, Hao T, Lu X, Zhang Q. Synthesis and Characterization of Crosslinked Castor Oil-Based Polyurethane Nanocomposites Based on Novel Silane-Modified Isocyanate and Their Potential Application in Heat Insulating Coating. Polymers. 2022; 14(9):1880. https://doi.org/10.3390/polym14091880
Chicago/Turabian StyleMeng, Yuan, Ken Chen, Yuyin Yang, Tao Jiang, Tonghui Hao, Xiaoju Lu, and Qunchao Zhang. 2022. "Synthesis and Characterization of Crosslinked Castor Oil-Based Polyurethane Nanocomposites Based on Novel Silane-Modified Isocyanate and Their Potential Application in Heat Insulating Coating" Polymers 14, no. 9: 1880. https://doi.org/10.3390/polym14091880
APA StyleMeng, Y., Chen, K., Yang, Y., Jiang, T., Hao, T., Lu, X., & Zhang, Q. (2022). Synthesis and Characterization of Crosslinked Castor Oil-Based Polyurethane Nanocomposites Based on Novel Silane-Modified Isocyanate and Their Potential Application in Heat Insulating Coating. Polymers, 14(9), 1880. https://doi.org/10.3390/polym14091880





