Porosity Tunable Poly(Lactic Acid)-Based Composite Gel Polymer Electrolyte with High Electrolyte Uptake for Quasi-Solid-State Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
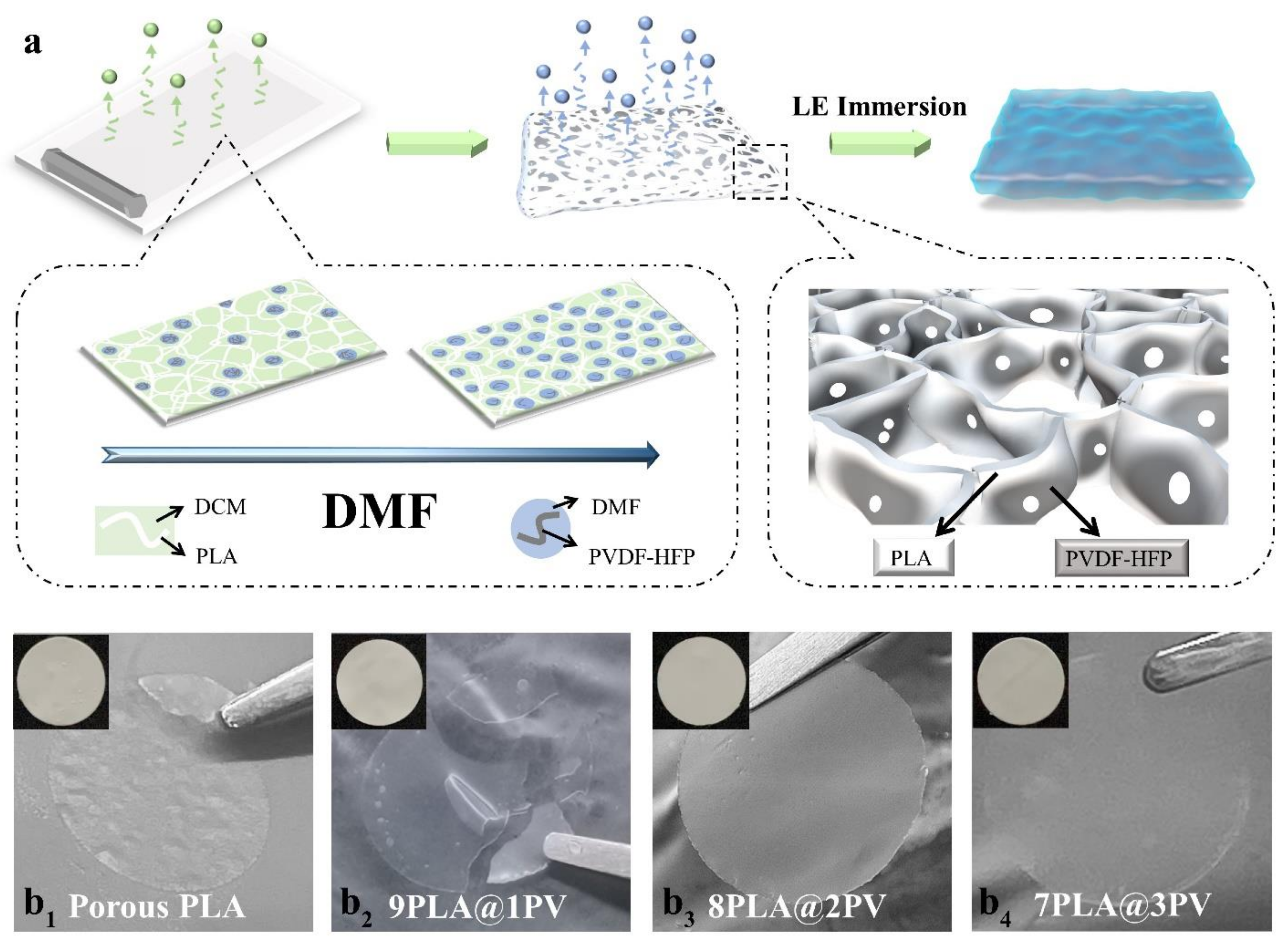
2.2. Preparation of PLA@PV Porous Biocomposite Gel Electrolyte
2.3. Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Synthesis of Porous Gel Polymer Electrolyte
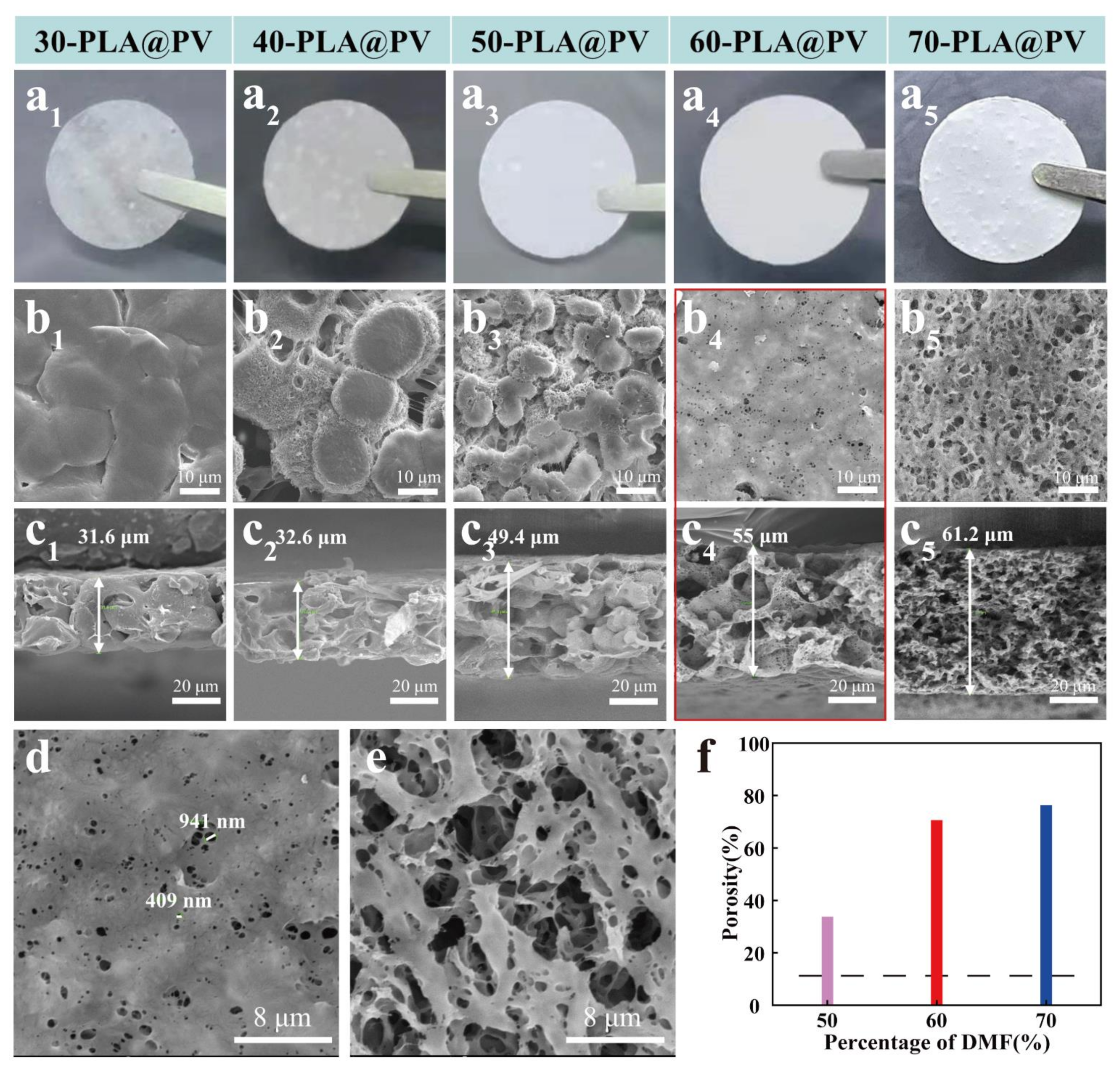
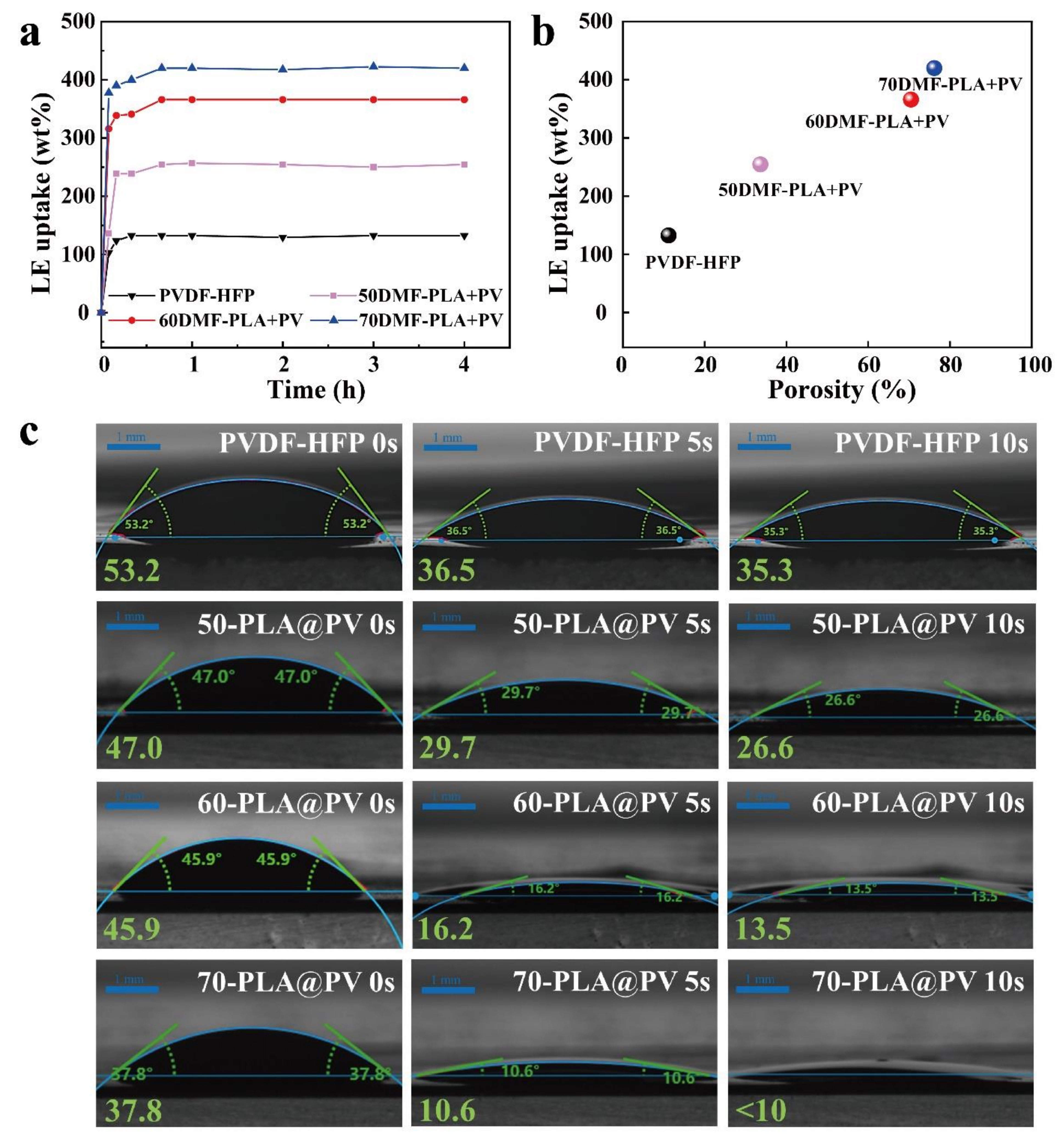
3.2. Morphology and Structure Characterization, Porosity, Liquid Uptake, and Affinity
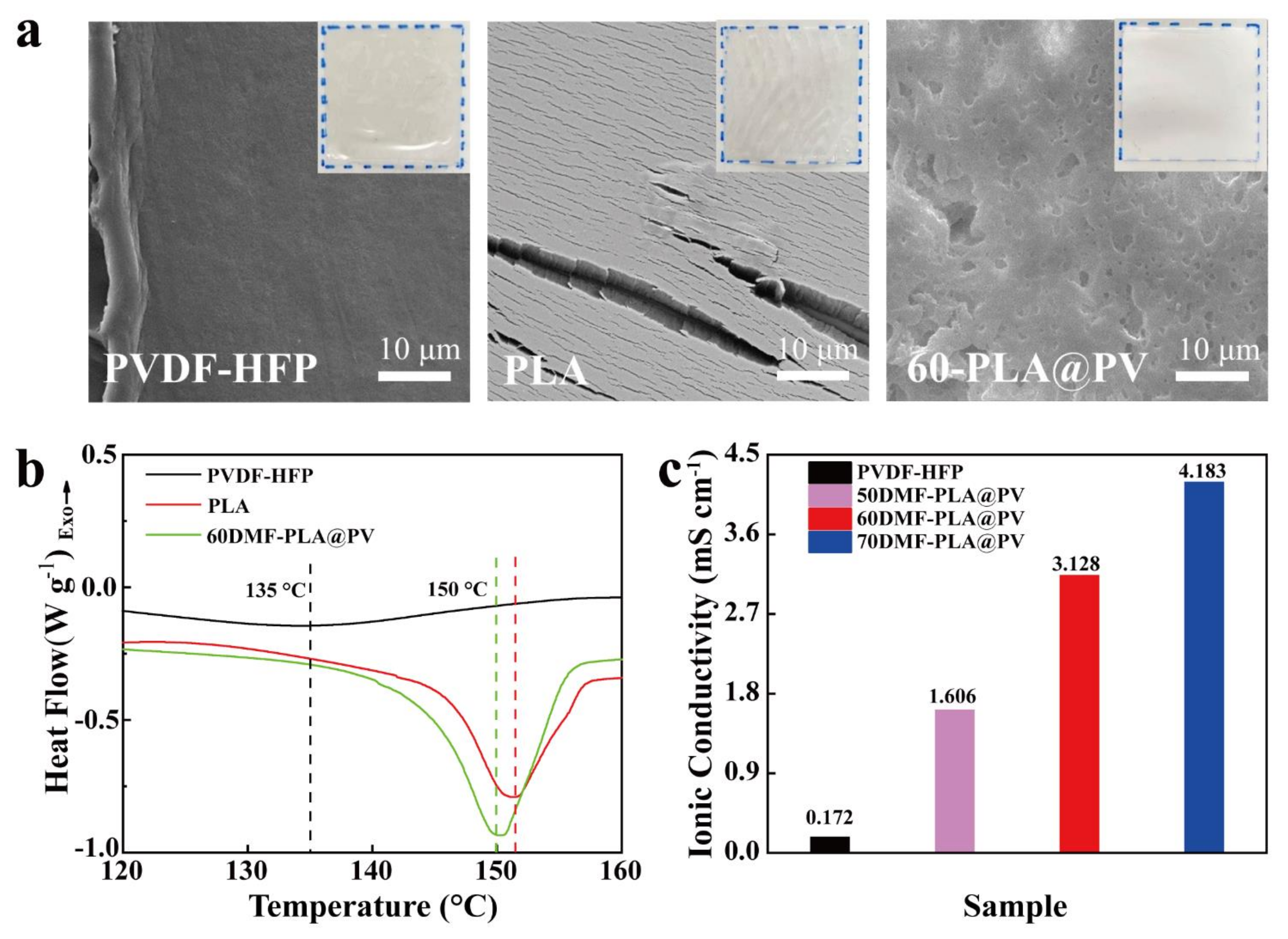
3.3. Physical and Chemical Properties
3.4. Electrochemical Performances of Coin Type SCs
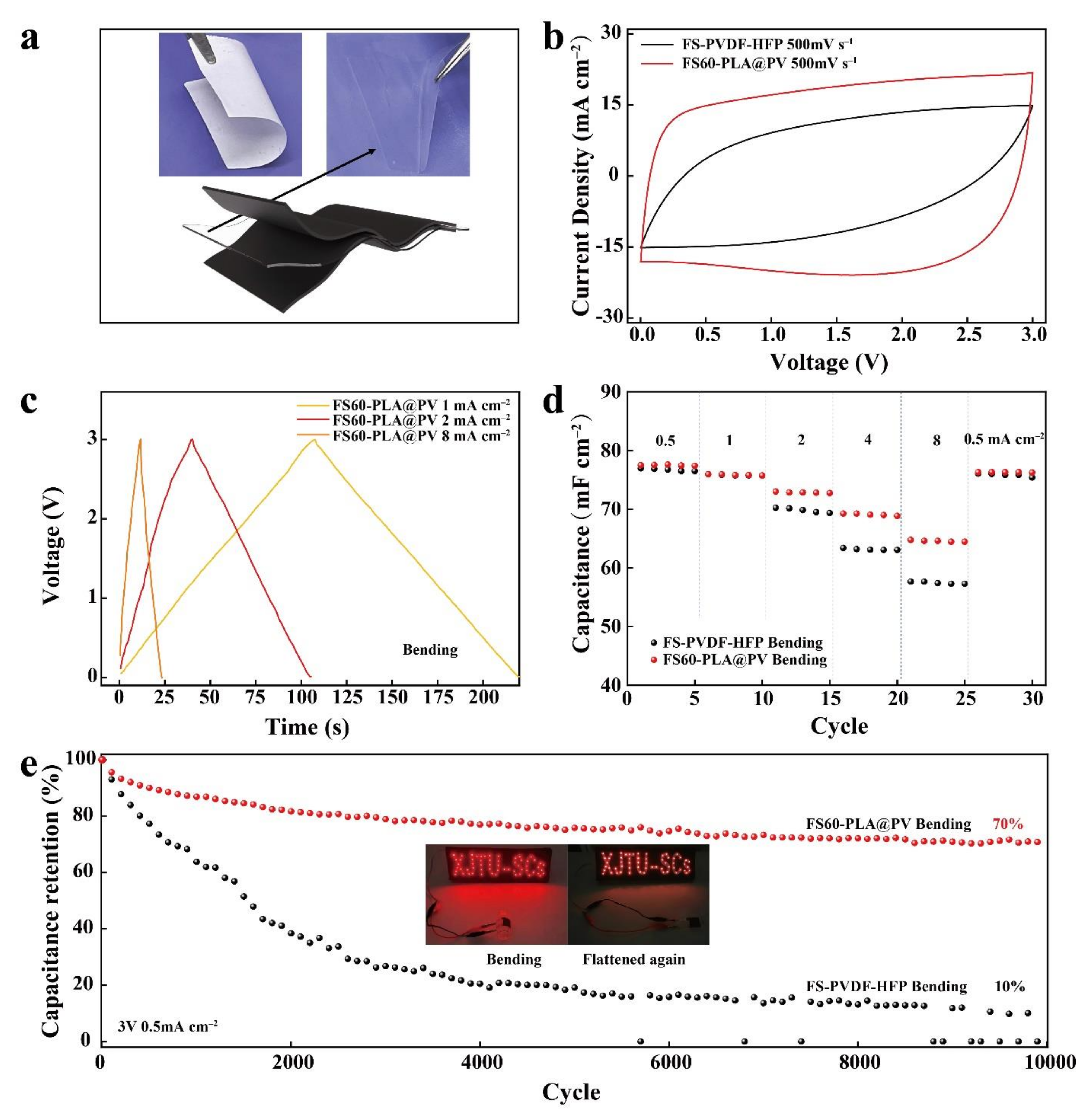
3.5. Electrochemical Performances of Flexible Supercapacitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Associated Content
References
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-Based Materials for Flexible All-Solid-State Supercapacitors. Adv. Mater. 2018, 30, e1705489. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards Flexible Solid-State Supercapacitors for Smart and Wearable Electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wan, Y.; Wei, B.; Xia, Z. Capacitive Enhancement Mechanisms and Design Principles of High-Performance Graphene Oxide-Based All-Solid-State Supercapacitors. Adv. Funct. Mater. 2018, 28, 1706721. [Google Scholar] [CrossRef]
- Zhao, Y.; Alsaid, Y.; Yao, B.; Zhang, Y.; Zhang, B.; Bhuskute, N.; Wu, S.; He, X. Wood-Inspired Morphologically Tunable Aligned Hydrogel for High-Performance Flexible All-Solid-State Supercapacitors. Adv. Funct. Mater. 2020, 30, 1909133. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. Correction to High Energy Density Asymmetric Quasi-Solid-State Supercapacitor Based on Porous Vanadium Nitride Nanowire Anode. Nano Lett. 2020, 20, 6932. [Google Scholar] [CrossRef]
- Li, X.; Shao, J.; Kim, S.-K.; Yao, C.; Wang, J.; Miao, Y.-R.; Zheng, Q.; Sun, P.; Zhang, R.; Braun, P.V. High Energy Flexible Supercapacitors Formed via Bottom-Up Infilling of Gel Electrolytes into Thick Porous Electrodes. Nat. Commun. 2018, 9, 2278. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Jiang, M.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Flexible Transparent Supercapacitors: Materials and Devices. Adv. Funct. Mater. 2020, 31, 2009136. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Wang, X.; Zhang, N.; Ma, M. Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew. Chem. Int. Ed. 2016, 55, 9196–9201. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Han, Q.; Zhao, W.; Dai, L.; Ni, Y. Screen Printing Fabricating Patterned and Customized Full Paper-Based Energy Storage Devices with Excellent Photothermal, Self-Healing, High Energy Density and Good Electromagnetic Shielding Performances. J. Mater. Sci. Technol. 2021, 97, 190–200. [Google Scholar] [CrossRef]
- Qiu, F.; Huang, Y.; Hu, X.; Li, B.; Zhang, X.; Luo, C.; Li, X.; Wang, M.; Wu, Y.; Cao, H. An Ecofriendly Gel Polymer Electrolyte Based on Natural Lignocellulose with Ultrahigh Electrolyte Uptake and Excellent Ionic Conductivity for Alkaline Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6031–6042. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, J.Y.; Suh, D.H.; Hong, Y.T.; Kim, T.-H. Electrode-Impregnable and Cross-Linkable Poly(ethylene oxide)–Poly(propylene oxide)–Poly(ethylene oxide) Triblock Polymer Electrolytes with High Ionic Conductivity and a Large Voltage Window for Flexible Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 33913–33924. [Google Scholar] [CrossRef] [PubMed]
- Le Mong, A.; Yang, S.; Kim, D. Pore-Filling Polymer Electrolyte Membrane Based on Poly (arylene ether ketone) for Enhanced Dimensional Stability and Reduced Methanol Permeability. J. Membr. Sci. 2017, 543, 133–142. [Google Scholar] [CrossRef]
- Handayani, P.L.; Nulandaya, L.; Cheon, J.Y.; Kim, T.; Yoo, S.I.; Choi, U.H. Self-Assembled Block Copolymer Electrolyte Membranes with Silica Network-Derived Nanochannels for All-Solid-State Supercapacitors. Chem. Eng. J. 2021, 429, 132273. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, X.; Liu, F.; Fan, L.-Z. Porous Polymer Electrolytes for Long-Cycle Stable Quasi-Solid-State Magnesium Batteries. J. Energy Chem. 2020, 59, 608–614. [Google Scholar] [CrossRef]
- Yang, C.; Sun, M.; Wang, X.; Wang, G. A Novel Flexible Supercapacitor Based on Cross-Linked PVDF-HFP Porous Organogel Electrolyte and Carbon Nanotube Paper@π-Conjugated Polymer Film Electrodes. ACS Sustain. Chem. Eng. 2015, 3, 2067–2076. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.; Elkoun, S.; Robert, M. Physical and Mechanical Properties of PLA Composites Reinforced by TiO2 Grafted Flax Fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Ferreira, F.; Mariano, M.; Rabelo, S.; Gouveia, R.; Lona, L. Isolation and Surface Modification of Cellulose Nanocrystals from Sugarcane Bagasse Waste: From a Micro- to a Nano-Scale View. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, C.; Li, B.; Ni, Y. Wood-Based Micro-Spring Composite Elastic Material with Excellent Electrochemical Performance, High Elasticity and Elastic Recovery Rate Applied in Supercapacitors and Sensors. Ind. Crop. Prod. 2022, 178, 114565. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Zhang, Y.; Li, B.; Han, Q.; Li, D.; Ni, Y. Li–Na Metal Compounds Inserted into Porous Natural Wood as a Bifunctional Hybrid Applied in Supercapacitors and Electrocatalysis. Int. J. Hydrogen Energy 2021, 47, 2389–2398. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Petrucci, R.; Kenny, J.; Torre, L. Processing of PLA Nanocomposites with Cellulose Nanocrystals Extracted from Posidonia Oceanica Waste: Innovative Reuse of Coastal Plant. Ind. Crop. Prod. 2015, 67, 439–447. [Google Scholar] [CrossRef]
- Jin, F.-L.; Pang, Q.-Q.; Zhang, T.-Y.; Park, S.-J. Synergistic Reinforcing of Poly(lactic acid)-Based Systems by Polybutylene Succinate and Nano-Calcium Carbonate. J. Ind. Eng. Chem. 2015, 32, 77–84. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Transparent Nanocellulosic Multilayer Thin Films on Polylactic Acid with Tunable Gas Barrier Properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Huang, J.; Lisowski, M.S.; Runt, J.; Hall, E.S.; Kean, R.T.; Buehler, N.; Lin, J.S. Crystallization and Microstructure of Poly(l-lactide-co-meso-lactide) Copolymers. Macromolecules 1998, 31, 2593–2599. [Google Scholar] [CrossRef]
- Vargun, E.; Ozaltin, K.; Fei, H.; Harea, E.; Vilčáková, J.; Kazantseva, N.; Saha, P. Biodegradable Porous Polylactic Acid Film as a Separator for Supercapacitors. J. Appl. Polym. Sci. 2020, 137, 49270. [Google Scholar] [CrossRef]
- Pandey, G.P.; Liu, T.; Hancock, C.; Li, Y.; Sun, X.S.; Li, J. Thermostable Gel Polymer Electrolyte Based on Succinonitrile and Ionic Liquid for High-Performance Solid-State Supercapacitors. J. Power Sources 2016, 328, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Khanam, Z.; Ahmed, S.; Wang, H.; Wang, T.; Song, S. A Study of Low-Temperature Solid-State Supercapacitors Based on Al-Ion Conducting Polymer Electrolyte and Graphene Electrodes. J. Power Sources 2021, 488, 229461. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, F.; Zhou, Z.; Li, J.; Tang, Y. A Flexible Dual-Ion Battery Based on PVDF-HFP-Modified Gel Polymer Electrolyte with Excellent Cycling Performance and Superior Rate Capability. Adv. Energy Mater. 2018, 8, 1801219. [Google Scholar] [CrossRef]
- Liu, H.-C.; Lee, I.-C.; Wang, J.H.; Yang, S.-H.; Young, T.-H. Preparation of PLLA Membranes with Different Morphologies for Culture of MG-63 Cells. Biomaterials 2004, 25, 4047–4056. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M. Direct Microscopic Observation of Membrane Formation by Nonsolvent Induced Phase Separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Xing, Q.; Dong, X.; Li, R.; Yang, H.; Han, C.C.; Wang, D. Morphology and Performance Control of PLLA-Based Porous Membranes by Phase Separation. Polymer 2013, 54, 5965–5973. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, Y.; Wu, A.; Yan, X.; Shen, S.; Ke, C.; Zhang, J. Preparation of Microporous Cellulose/Poly(vinylidene fluoride-hexafluoropropylene) Membrane for Lithium Ion Batteries by Phase Inversion Method. J. Power Sources 2018, 379, 197–205. [Google Scholar] [CrossRef]
- Yang, H.; Ye, Q.; Zhou, Y.; Xiang, Y.; Xing, Q.; Dong, X.; Wang, D.; Xu, W. Formation, Morphology and Control of High-Performance Biomedical Polyurethane Porous Membranes by Water Micro-Droplet Induced Phase Inversion. Polymer 2014, 55, 5500–5508. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liu, C.; Xu, Y.; Zhuang, Y.; Zhou, Y.; Gu, S.; Xu, W.; Yang, H. Effect of Temperature on the Morphology of Poly(lactic acid) Porous Membrane Prepared via Phase Inversion Induced by Water Droplets. Int. J. Biol. Macromol. 2019, 133, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, N.; Yang, C.; Wu, X.; Yang, H.; Chen, W.; Li, H.; Zhao, B.; Wang, P.-F.; Han, X. Realizing High-Voltage and Ultralong-Life Supercapacitors by a Universal Interfacial Engineering Strategy. J. Power Sources 2021, 510, 230406. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Phuong, H.A.; Ayob, N.A.I.; Blanford, C.F.; Rawi, N.F.M.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Le Mong, A.; Kim, D. Tailor-Made Pore Controlled Poly(arylene ether ketone) Membranes as a Lithium-Ion Battery Separator. J. Power Sources 2016, 304, 301–310. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Hengjing, Z.; Xia, X.; Tu, J. 3D Ultraviolet Polymerized Electrolyte Based on PEO Modified PVDF-HFP Electrospun Membrane for High-Performance Lithium-Sulfur Batteries. Electrochimica Acta 2019, 329, 135108. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Xia, Y.; Qiao, L.; Li, X.; Zhang, H. Superior Thermally Stable and Nonflammable Porous Polybenzimidazole Membrane with High Wettability for High-Power Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8742–8750. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Ding, G.Q.; Ma, J.; Lee, P.S.; Lu, X.H. Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef] [PubMed]
- Lodge, T.P. A Unique Platform for Materials Design. Science 2008, 321, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, Thermal, and Mechanical Properties of Polymers. Biosurfaces. 2015. [Google Scholar]
- Taberna, P.L.; Simon, P.; Fauvarque, J.-F. Electrochemical Characteristics and Impedance Spectroscopy Studies of Carbon-Carbon Supercapacitors. J. Electrochem. Soc. 2003, 150, A292–A300. [Google Scholar] [CrossRef]
- Choi, B.G.; Yang, M.; Hong, W.H.; Choi, J.W.; Huh, Y.S. 3D Macroporous Graphene Frameworks for Supercapacitors with High Energy and Power Densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Beguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Cao, L.; Yang, M.; Wu, D.; Lyu, F.; Sun, Z.; Zhong, X.; Pan, H.; Liu, H.; Lu, Z. Biopolymer-Chitosan Based Supramolecular Hydrogels as Solid State Electrolytes for Electrochemical Energy Storage. Chem. Commun. 2017, 53, 1615–1618. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Li, C.; Zhang, X.; Sun, X.; Han, J.; Wu, X.-L.; Li, F.; Ma, Y. Boosting Solid-State Flexible Supercapacitors by Employing Tailored Hierarchical Carbon Electrodes and a High-Voltage Organic Gel Electrolyte. J. Mater. Chem. A 2018, 6, 24979–24987. [Google Scholar] [CrossRef]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-Temperature Plasma Treatment of Poly(lactic acid) and PLA/HA Composite Material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Albuquerque, T.; Gattu, B.; Kumta, P.N. Novel Composite Polymer Electrolytes of PVdF-HFP Derived by Electrospinning with Enhanced Li-Ion Conductivities for Rechargeable Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2018, 1, 483–494. [Google Scholar] [CrossRef]
- Shalu; Singh, P.N.; Singh, R.K. Development of Ion Conducting Polymer Gel Electrolyte Membranes Based on Polymer PVdF-HFP, BMIMTFSI Ionic Liquid and the Li-Salt with Improved Electrical, Thermal and Structural Properties. J. Mater. Chem. C 2015, 3, 7305–7318. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Farooqui, U.R.; Hamid, N.A. Effect of Graphene Oxide (GO) on Poly(vinylidene fluoride-hexafluoropropylene) (PVDF- HFP) Polymer Electrolyte Membrane. Polymer 2018, 142, 330–336. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Bai, Y.; Xu, H.; Li, M.; Cong, Z.; Li, H.; Chen, W.; Zhao, B.; Han, X. Porosity Tunable Poly(Lactic Acid)-Based Composite Gel Polymer Electrolyte with High Electrolyte Uptake for Quasi-Solid-State Supercapacitors. Polymers 2022, 14, 1881. https://doi.org/10.3390/polym14091881
Yang C, Bai Y, Xu H, Li M, Cong Z, Li H, Chen W, Zhao B, Han X. Porosity Tunable Poly(Lactic Acid)-Based Composite Gel Polymer Electrolyte with High Electrolyte Uptake for Quasi-Solid-State Supercapacitors. Polymers. 2022; 14(9):1881. https://doi.org/10.3390/polym14091881
Chicago/Turabian StyleYang, Chao, Yuge Bai, Huan Xu, Manni Li, Zhi Cong, Hongjie Li, Weimeng Chen, Bin Zhao, and Xiaogang Han. 2022. "Porosity Tunable Poly(Lactic Acid)-Based Composite Gel Polymer Electrolyte with High Electrolyte Uptake for Quasi-Solid-State Supercapacitors" Polymers 14, no. 9: 1881. https://doi.org/10.3390/polym14091881






