Wastewater Treatment by Polymeric Microspheres: A Review
Abstract
:1. Introduction
2. Synthetic Polymer
2.1. Vinylic Polymer
2.2. Polydopamine
3. Natural Polymer
3.1. Cellulose
3.2. Alginate
3.3. Chitosan
4. Photocatalytic Degradation
4.1. Alginate
4.2. Chitosan
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Xing, J.; Xu, P.; Chang, J.; Zhang, Q.; Usman, K.M. Activated carbon microsphere from sodium lignosulfonate for Cr(VI) adsorption evaluation in wastewater treatment. Polymers 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhou, Z. Citrus pectin-derived carbon microspheres with superior adsorption ability for methylene blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Dai, Y.; Situ, Y.; Liu, D.; Huang, H. Preparation of nanosheet-based spherical Ti/SnO2-Sb electrode by in-situ hydrothermal method and its performance in the degradation of methylene blue. Electrochim. Acta 2021, 398, 139335. [Google Scholar] [CrossRef]
- Dai, K.; Zhao, G.; Wang, Z.; Peng, X.; Wu, J.; Yang, P.; Li, M.; Tang, C.; Zhuang, W.; Ying, H. Novel Mesoporous Lignin-Calcium for Efficiently Scavenging Cationic Dyes from Dyestuff Effluent. ACS Omega 2021, 6, 816–826. [Google Scholar] [CrossRef]
- Bao, C.; Mingxin, C.; Liu, G.; Jin, X.; Huang, Q. Efficient adsorption/reduction of aqueous hexavalent chromium using oligoaniline hollow microspheres fabricated by a template-free method. J. Chem. Technol. Biotechnol. 2018, 93, 1147–1158. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; He, C. Photocatalytic activity of π-conjugated conducting polymer microspheres from ultrasonic spray pyrolysis. High Perform. Polym. 2017, 29, 616–621. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, F.; Ke, Z.; Wang, Q.; Chen, C.; Qian, G.; Yu, Y. A novel porous hollow carboxyl-polysulfone microsphere for selective removal of cationic dyes. Chemosphere 2022, 289, 133205. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Shi, X.; Liu, Z.; Zhou, G. P123 assisted morphology-engineered and hierarchical TiO2 microspheres for enhanced photocatalytic activity. J. Porous Mater. 2017, 24, 1425–1436. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Bekhit, M.; El Fadl, F.I.A.; Sokary, R. Synthesis and Characterization of Magnetically Retrievable Fe3O4/Polyvinylpyrrolidone/Polystyrene Nanocomposite Catalyst for Efficient Catalytic Oxidation Degradation of Dyes Pollutants. J. Inorg. Organomet. Polym. Mater. 2021, 32, 383–398. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, Z.; Bi, L.; Kang, J.; Zhang, X.; Zhao, S.; Wu, Y.; Tong, Y.; Shen, J. Adsorption property and mechanism of polyacrylate-divinylbenzene microspheres for removal of trace organic micropollutants from water. Sci. Total Environ. 2021, 781, 146635. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Zhang, L.; Li, Y.; Zhou, Y.; Peng, J. Selective recovery of silver from aqueous solutions by poly (glycidyl methacrylate) microsphere modified with trithiocyanuric acid. J. Mol. Liq. 2018, 254, 340–348. [Google Scholar] [CrossRef]
- Yu, B.; Yang, B.; Li, G.; Cong, H. Preparation of monodisperse cross-linked poly(glycidyl methacrylate)@Fe3O4@diazoresin magnetic microspheres with dye removal property. J. Mater. Sci. 2018, 53, 6471–6481. [Google Scholar] [CrossRef]
- Cui, K.; Yan, B.; Xie, Y.; Qian, H.; Wang, X.; Huang, Q.; He, Y.; Jin, S.; Zeng, H. Regenerable urchin-like Fe3O4@PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes. J. Hazard. Mater. 2018, 350, 66–75. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Maurelli, A.M.; Ingrosso, C.; Lupone, F.; Catucci, L. Easy preparation of liposome@pda microspheres for fast and highly efficient removal of methylene blue from water. Int. J. Mol. Sci. 2021, 22, 11916. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zuo, G.; Su, T.; Cheng, S.; Gu, Y.; Qi, X.; Dong, W. Polycarboxylic magnetic polydopamine sub-microspheres for effective adsorption of malachite green. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 106–113. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, X.; Liu, P.; Zhao, L.; Zou, W. Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: Kinetic, equilibrium and adsorption mechanism studies. Sci. Total Environ. 2018, 639, 428–437. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Q.; Phanlavong, P.; Li, Y.; Wang, Z.; Jiao, T.; Peng, Q. Highly Efficient Lead(II) Sequestration Using Size-Controllable Polydopamine Microspheres with Superior Application Capability and Rapid Capture. ACS Sustain. Chem. Eng. 2017, 5, 4161–4170. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, Y.; Jiang, X.; Lin, F.; Liu, X.; Lu, B. Removal of bisphenol A from aqueous solution via host-guest interactions based on beta-cyclodextrin grafted cellulose bead. Int. J. Biol. Macromol. 2019, 140, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Andrews, M.P. Carboxylated Cellulose Nanocrystal Microbeads for Removal of Organic Dyes from Wastewater: Effects of Kinetics and Diffusion on Binding and Release. ACS Appl. Nano Mat. 2020, 3, 11217–11228. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloy. Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Q.; Gao, T.; Dong, K.; Wei, G.; Yao, J. Facile Preparation of Tannic Acid-Poly(vinyl alcohol)/Sodium Alginate Hydrogel Beads for Methylene Blue Removal from Simulated Solution. ACS Omega 2018, 3, 7523–7531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, X.; He, Y.; Luo, X. Phenolic hydroxyl derived copper alginate microspheres as superior adsorbent for effective adsorption of tetracycline. Int. J. Biol. Macromol. 2019, 136, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wang, T.; Liao, Y.; Du, K. Macroporous chitin microspheres prepared by surfactant micelle swelling strategy for rapid capture of lead (II) from wastewater. Carbohydr. Polym. 2022, 276, 118775. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of highly efficient porous inorganic polymer microspheres for the adsorptive removal of Pb2+ from wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- An, Y.; Xiao, P.; Zheng, H.; Zhao, R.; Han, M.; Mao, W.; Li, Y. Multi-functionalized self-floating microspheres for dyes capture: Amphoteric adsorption and rapid surface solid-liquid separation. J. Clean. Prod. 2021, 296, 126535. [Google Scholar] [CrossRef]
- Nie, Z.J.; Guo, Q.F.; Xia, H.; Song, M.M.; Qiu, Z.J.; Fan, S.T.; Chen, Z.H.; Zhang, S.X.; Zhang, S.; Li, B.J. Cyclodextrin self-assembled graphene oxide aerogel microspheres as broad-spectrum adsorbent for removing dyes and organic micropollutants from water. J. Environ. Chem. Eng. 2021, 9, 104749. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Wang, Q.; Liu, J.; Yang, J.; Huang, Y. Synthesis of novel low-cost porous gangue microsphere/geopolymer composites and their adsorption properties for dyes. Int. J. Appl. Ceram. Technol. 2018, 15, 1602–1614. [Google Scholar] [CrossRef]
- Yu, B.; He, L.; Wang, Y.; Cong, H. Multifunctional PMMA@Fe3O4@DR magnetic materials for efficient adsorption of dyes. Materials 2017, 10, 1239. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Guo, S.; Zeng, C.; Wang, C.; Zhang, L. Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres. J. Hazard. Mater. 2015, 292, 90–97. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Li, L.S.; Xu, L. Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere. Chem. Eng. J. 2017, 320, 501–509. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Preparation of titanium dioxide modified biomass polymer microspheres for photocatalytic degradation of rhodamine-B dye and tetracycline. J. Taiwan Inst. Chem. Eng. 2021, 122, 157–167. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Liu, L.; Zhang, P.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Electroconductive nanofibrous membranes with nanosheet-based microsphere-threaded heterostructures enabling oily wastewater remediation. J. Mater. Chem. A 2021, 9, 15310–15320. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.; Hu, B.; Li, W.; Jia, M.; Shi, Y.; Xiong, S.; Bai, J.; Yin, H. Synergic effect of adsorption and biodegradation by microsphere immobilizing Bacillus velezensis for enhanced removal organics in slaughter wastewater. Processes 2021, 9, 1145. [Google Scholar] [CrossRef]
- Özdemir, İ.; Kara, A.; Tekin, N.; Olgun, A. Synthesis and characterization of polymer microspheres and its application for phenol adsorption. Desalin. Water Treat. 2019, 159, 290–303. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Zhang, B.; Zhang, X.; Wu, Y.; Wang, T.; Qiu, J. Tailoring the structure and property of microfiltration carbon membranes by polyacrylonitrile-based microspheres for oil-water emulsion separation. J. Water Process. Eng. 2019, 32, 100973. [Google Scholar] [CrossRef]
- Hridya, T.; Varghese, E.; Harikumar, P.S. Removal of heavy metals from aqueous solution using porous (Styrene-divinylbenzene)/CuNi bimetallic nanocomposite microspheres. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100606. [Google Scholar] [CrossRef]
- Podkościelna, B.; Kołodyńska, D.; Podkościelny, P. Chemical modification of commercial St-DVB microspheres and their application for metal ions removal. Adsorption 2019, 25, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, K.; Gong, B.; Yin, Y.; Zhou, S.; Xiao, K. Scalable synthesis of monodisperse and recyclable sulphonated polystyrene microspheres for sustainable elimination of heavy metals in wastewater. Environ. Technol. 2021, 1–13. [Google Scholar] [CrossRef]
- Bosacka, A.; Zienkiewicz-Strzalka, M.; Wasilewska, M.; Derylo-Marczewska, A.; Podkościelna, B. Physicochemical and adsorption characteristics of divinylbenzene-co-triethoxyvinylsilane microspheres as materials for the removal of organic compounds. Molecules 2021, 26, 2396. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Preparation of Pyridine Polyionic Liquid Porous Microspheres and Their Application in Organic Dye Adsorption. J. Polym. Environ. 2021, 30, 385–400. [Google Scholar] [CrossRef]
- Feng, M.; Yu, S.; Wu, P.; Wang, Z.; Liu, S.; Fu, J. Rapid, high-efficient and selective removal of cationic dyes from wastewater using hollow polydopamine microcapsules: Isotherm, kinetics, thermodynamics and mechanism. Appl. Surf. Sci. 2021, 542, 148633. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Li, Y.; Yang, Q.; Chen, H.; Chen, X.; Jiao, T.; Peng, Q. Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard. Mater. 2018, 342, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Cha, Y.L.; Alam, A.M.; Park, S.M.; Moon, Y.H.; Kim, K.S.; Lee, J.E.; Kwon, D.E.; Kang, Y.G. Hydrothermal-process-based direct extraction of polydisperse lignin microspheres from black liquor and their physicochemical characterization. Bioresour. Technol. 2020, 297, 122399. [Google Scholar] [CrossRef]
- Zhang, H.; Yong, X.; Zhou, J.; Deng, J.; Wu, Y. Biomass Vanillin-Derived Polymeric Microspheres Containing Functional Aldehyde Groups: Preparation, Characterization, and Application as Adsorbent. ACS Appl. Mater. Interfaces 2016, 8, 2753–2763. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, D.; Kong, L.; Tsang, D.C.W.; Su, M. Rapid and effective removal of uranium (VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J. Hazard. Mater. 2019, 371, 397–405. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Tun, T.; Liu, S.; Hu, J.; Zhou, G. Cu(II) and Cd(II) capture using novel thermosensitive hydrogel microspheres: Adsorption behavior study and mechanism investigation. J. Chem. Technol. Biotechnol. 2021, 96, 2382–2389. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, L.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z.; Ji, H. PEI modified magnetic porous cassava residue microspheres for adsorbing Cd(II) from aqueous solution. Eur. Polym. J. 2021, 159, 110741. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Y.; Hui, A.; Wang, A. Preparation of porous microspherical adsorbent via pine pollen stabilized O1/W/O2 double emulsion for high-efficient removal of cationic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124997. [Google Scholar] [CrossRef]
- Yang, H.R.; Li, S.S.; An, Q.D.; Zhai, S.R.; Xiao, Z.Y.; Zhang, L.P. Facile transformation of carboxymethyl cellulose beads into hollow composites for dye adsorption. Int. J. Biol. Macromol. 2021, 190, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, S.; Pei, Y.; Luo, X. Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. Int. J. Biol. Macromol. 2021, 166, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, L.; Song, Y.; Yu, X.; Li, X.; Liu, Y.; Zhang, Z.; Yuan, Y.; Yan, S.; Zhang, J. Green fabrication of porous microspheres containing cellulose nanocrystal/MnO2 nanohybrid for efficient dye removal. Carbohydr. Polym. 2021, 270, 118340. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, R.; Ren, B.; Yaseen, M.; Hursthouse, A. Enhancing the removal of Sb (III) from water: A Fe3O4@HCO composite adsorbent caged in sodium alginate microbeads. Processes 2020, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Yu, D.; Wang, Y.; Wang, W.; Wu, M. Hyperbranched polyamide–functionalized sodium alginate microsphere as a novel adsorbent for the removal of antimony(III) in wastewater. Environ. Sci. Pollut. Res. 2019, 26, 27372–27384. [Google Scholar] [CrossRef]
- El hotaby, W.; Bakr, A.M.; Ibrahim, H.S.; Ammar, N.S.; Hani, H.A.; Mostafa, A.A. Eco-friendly zeolite/alginate microspheres for Ni ions removal from aqueous solution: Kinetic and isotherm study. J. Mol. Struct. 2021, 1241, 130605. [Google Scholar] [CrossRef]
- Ablouh, E.H.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan microspheres/sodium alginate hybrid beads: An efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Envir. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, H.; Chen, T.; Li, H.; Meng, C.; Xia, Y.; Guo, J. Preparation and characterization of polyacrylamide/sodium alginate microspheres and its adsorption of MB dye. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 184–192. [Google Scholar] [CrossRef]
- Song, T.; Tian, W.; Zhao, J.; Qiao, K.; Zou, M.; Chu, M. N-doped Reduced Graphene Oxide nanocomposites encapsulated sodium alginate/polyvinyl alcohol microspheres for anthracene and its oxygenated-PAH removal in aqueous solution. J. Taiwan Inst. Chem. Eng. 2021, 125, 168–175. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, D.; Ouyang, X.K.; Yang, L.Y. Fabrication of porous polyethyleneimine-functionalized chitosan/Span 80 microspheres for adsorption of diclofenac sodium from aqueous solutions. Sustain. Chem. Pharm. 2021, 21, 100418. [Google Scholar] [CrossRef]
- Ali, N.; Uddin, S.; Khan, A.; Khan, S.; Khan, S.; Ali, N.; Khan, H.; Khan, H.; Bilal, M. Regenerable chitosan-bismuth cobalt selenide hybrid microspheres for mitigation of organic pollutants in an aqueous environment. Int. J. Biol. Macromol. 2020, 161, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, A.; Ali, N.; Ahmad, S.; Ahmad, W.; Malik, S.; Ali, N.; Khan, H.; Shah, S.; Bilal, M. Degradation of Congo red dye using ternary metal selenide-chitosan microspheres as robust and reusable catalysts. Environ. Technol. Innov. 2021, 22, 101402. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Chen, X.; He, L. Microwave Irradiation Assisted Preparation of Chitosan Composite Microsphere for Dye Adsorption. Int. J. Polym. Sci. 2017, 2017, 2672597. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Xu, K.; Cui, J.; Li, X.; Wang, G. Preparation of Quaternary Ammonium Salt-Modified Chitosan Microspheres and Their Application in Dyeing Wastewater Treatment. ACS Omega 2020, 5, 24700–24707. [Google Scholar] [CrossRef]
- Men, J.; Shi, H.; Dong, C.; Yang, Y.; Han, Y.; Wang, R.; Zhang, Y.; Zhao, T.; Li, J. Preparation of poly(sodium 4-styrene sulfonate) grafted magnetic chitosan microspheres for adsorption of cationic dyes. Int. J. Biol. Macromol. 2021, 181, 810–823. [Google Scholar] [CrossRef]
- Song, W.; Xu, J.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of freeze-dried porous chitosan microspheres for the removal of hexavalent chromium. Appl. Sci. 2021, 11, 4217. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, Q.; Liu, C.; Cha, D.; Yu, Z.; Zhu, W.; Fan, B. Uptake of Pb(II) and Cd(II) on Chitosan Microsphere Surface Successively Grafted by Methyl Acrylate and Diethylenetriamine. ACS Appl. Mater. Interfaces 2017, 9, 11144–11155. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, P.; Yang, Z.; Lv, L.; Tang, S.; Liang, B. Successive grafting of poly(hydroxyethyl methacrylate) brushes and melamine onto chitosan microspheres for effective Cu(II) uptake. Int. J. Biol. Macromol. 2018, 109, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Bui, T.S.; Lee, B.K. Potential applications of ASR fly ash in photo-Fenton like process for the degradation of tetracycline at neutral pH: Fixed-bed approach. Chem. Eng. J. 2020, 391, 123509. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A.; Talwar, S. Detoxification of real pharmaceutical wastewater by integrating photocatalysis and photo-Fenton in fixed-mode. Chem. Eng. J. 2018, 349, 838–848. [Google Scholar] [CrossRef]
- Gong, J.; Yao, K.; Liu, J.; Jiang, Z.; Chen, X.; Wen, X.; Mijowska, E.; Tian, N.; Tang, T. Striking influence of Fe2O3 on the “catalytic carbonization” of chlorinated poly(vinyl chloride) into carbon microspheres with high performance in the photo-degradation of Congo red. J. Mater. Chem. A 2013, 1, 5247–5255. [Google Scholar] [CrossRef]
- Mandor, H.; El-Ashtoukhy, E.S.Z.; Abdelwahab, O.; Amin, N.K.; Kamel, D.A. A flow-circulation reactor for simultaneous photocatalytic degradation of ammonia and phenol using N-doped ZnO beads. Alex. Eng. J. 2022, 61, 3385–3401. [Google Scholar] [CrossRef]
- Talwar, S.; Verma, A.K.; Sangal, V.K. Synergistic degradation employing photocatalysis and photo-Fenton process of real industrial pharmaceutical effluent utilizing the Iron-Titanium dioxide composite. Process. Saf. Environ. Prot. 2021, 146, 564–576. [Google Scholar] [CrossRef]
- De Oliveira Lopes, E.; Dalponte Dallabona, I.; Weinschutz, R.; Matos Jorge, R.M. Fe/polymer-based photocatalyst synthesized by sono-sorption method applied to wastewater treatment. J. Photochem. Photobiol. A Chem. 2020, 396, 112545. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Lan, Y.; Cui, Y.; Zhang, Y.; Feng, Y.; Pan, J.; Meng, M.; Wu, C. A controllable floating pDA-PVDF bead for enhanced decomposition of H2O2 and degradation of dyes. Chem. Eng. J. 2020, 385, 123907. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, D.; Im, S.; Jang, A. Optimization of alginate bead size immobilized with Chlorella vulgaris and Chlamydomonas reinhardtii for nutrient removal. Bioresour. Technol. 2020, 302, 122891. [Google Scholar] [CrossRef]
- Du, Z.; Liu, F.; Xiao, C.; Dan, Y.; Jiang, L. Fabrication of poly(vinyl alcohol)/sodium alginate hydrogel beads and its application in photo-Fenton degradation of tetracycline. J. Mater. Sci. 2021, 56, 913–926. [Google Scholar] [CrossRef]
- Nematpour Sedaghati, M.; Aminian, M.; Dadvand koohi, A. Heterogeneous photo-fenton oxidation degradation of methyl orange using Alg-m, Alg-HAp, and Alg-mHAp catalysts. Int. J. Environ. Anal. Chem. 2021, 1–23. [Google Scholar] [CrossRef]
- Xiong, D.; Zhao, W.; Guo, J.; Li, S.; Ye, Y.; Lei, E.; Yang, X. Highly efficient and reusable BiOCl photocatalyst modulating by hydrogel immobilization and oxygen vacancies engineering. Sep. Purif. Technol. 2021, 277, 119628. [Google Scholar] [CrossRef]
- Solano, R.A.; De León, L.D.; De Ávila, G.; Herrera, A.P. Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environ. Technol. Innov. 2021, 21, 101378. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manage. 2021, 283, 111987. [Google Scholar] [CrossRef]
- Lima Santos Klienchen Dalari, B.; Lisboa Giroletti, C.; Dalri-Cecato, L.; Gonzaga Domingos, D.; Nagel Hassemer, M.E. Application of heterogeneous photo-fenton process using chitosan beads for textile wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103893. [Google Scholar] [CrossRef]

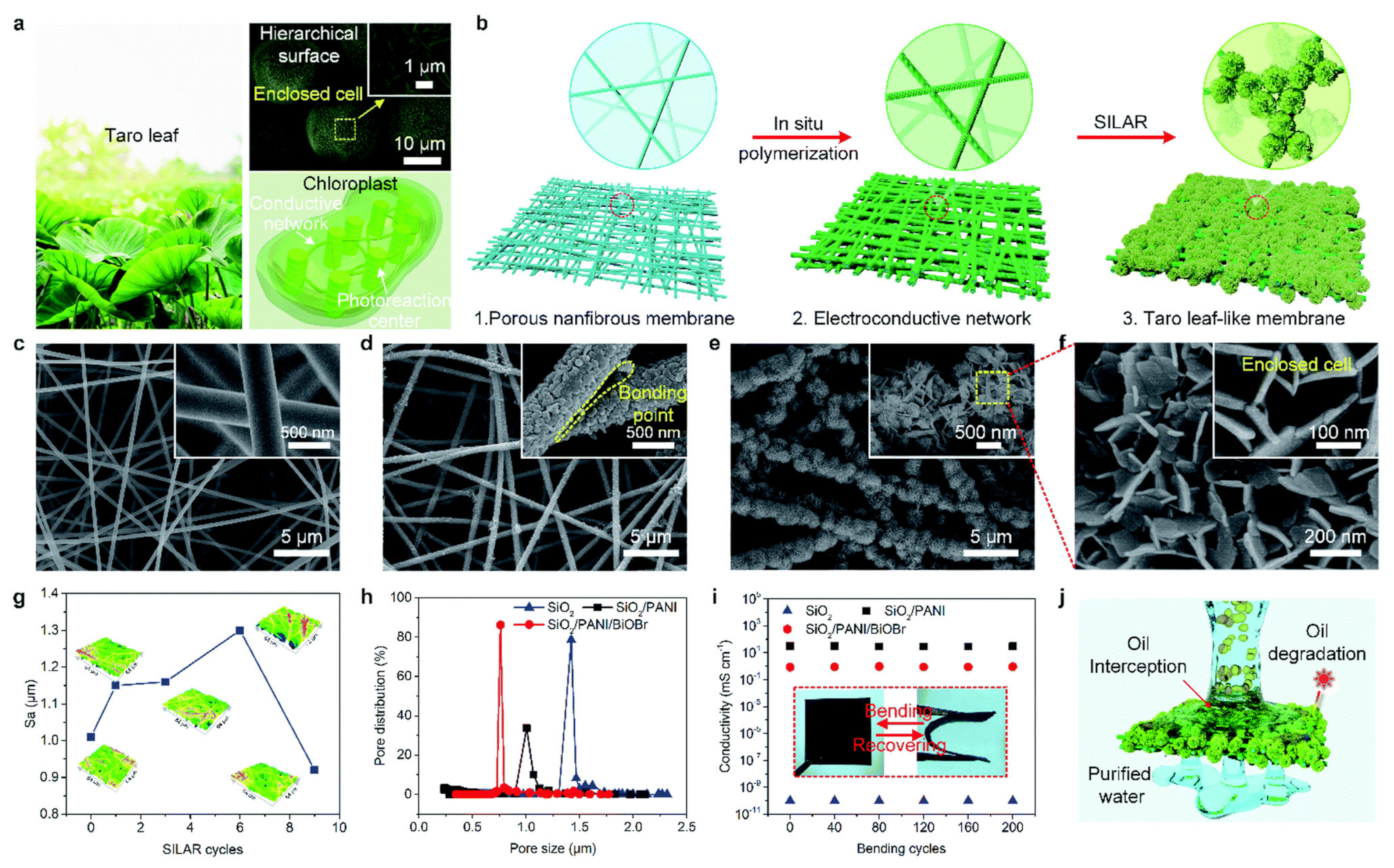

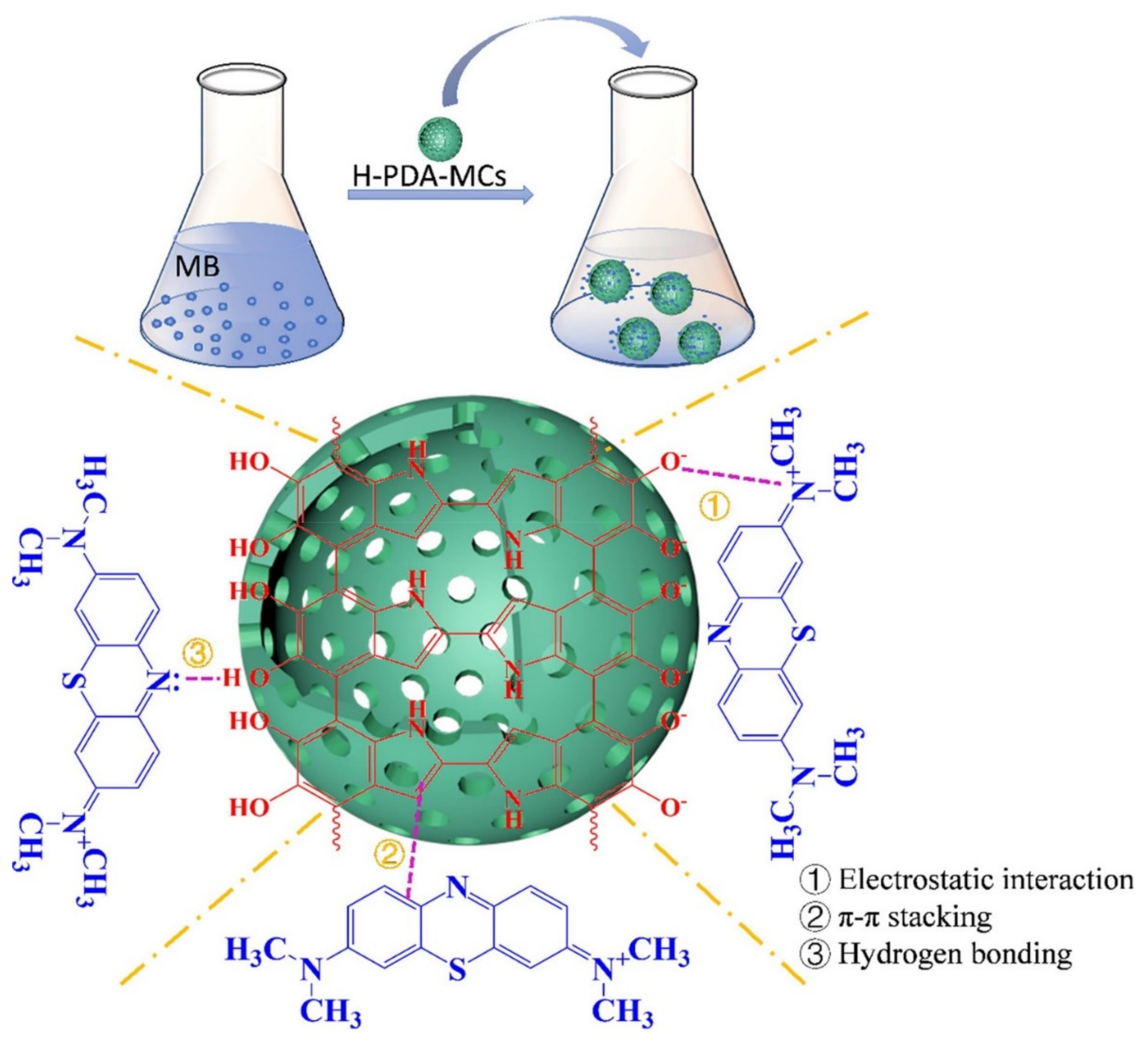
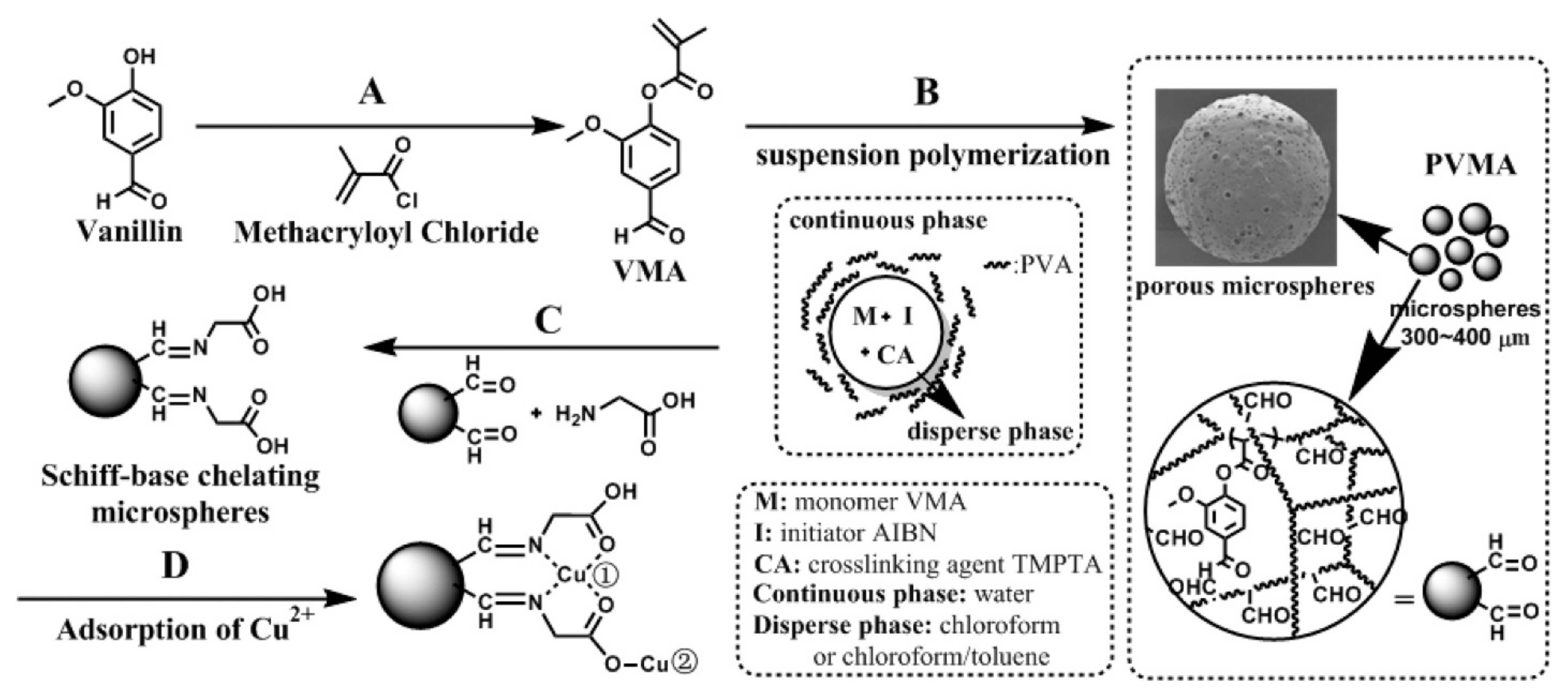
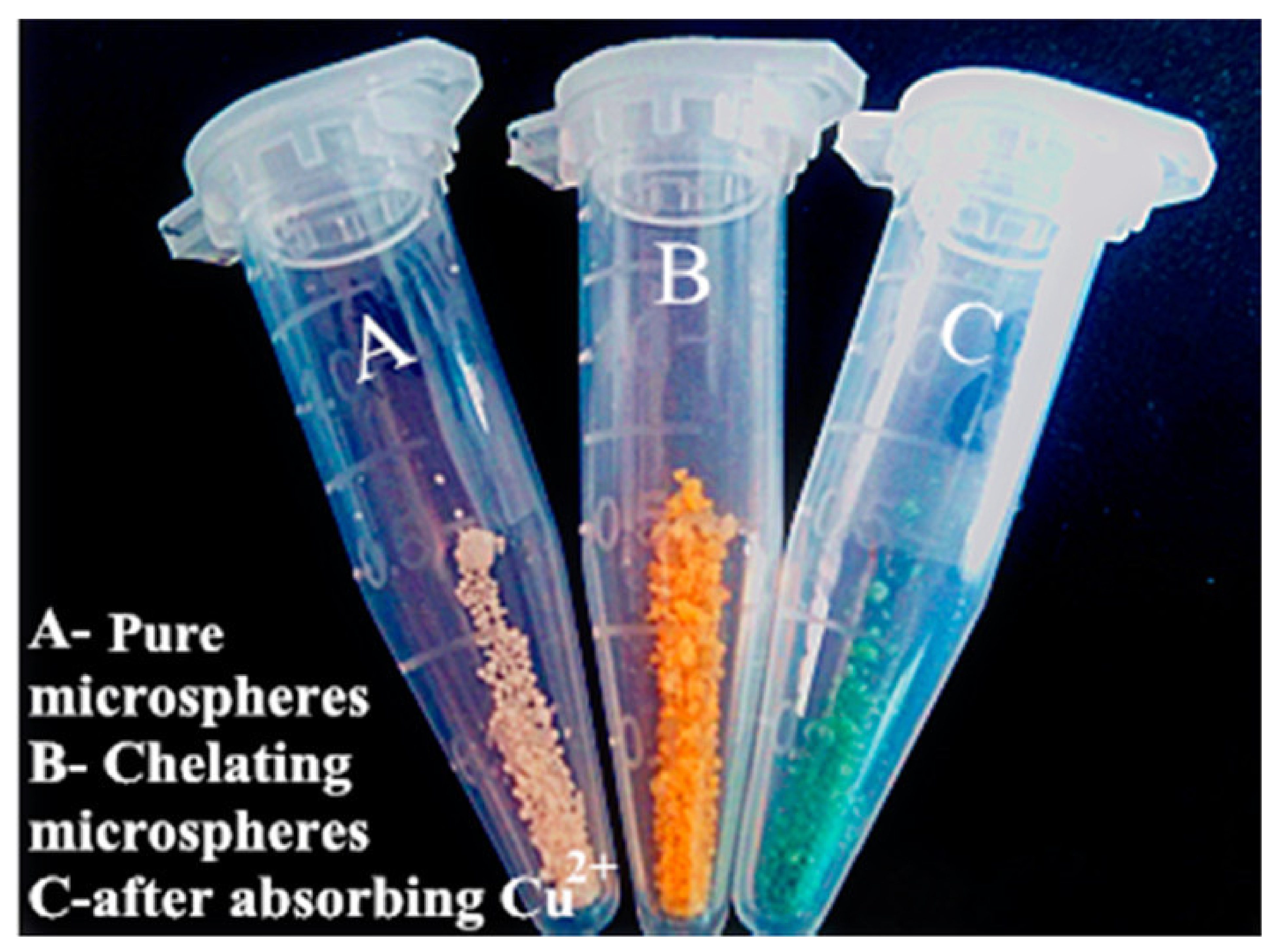

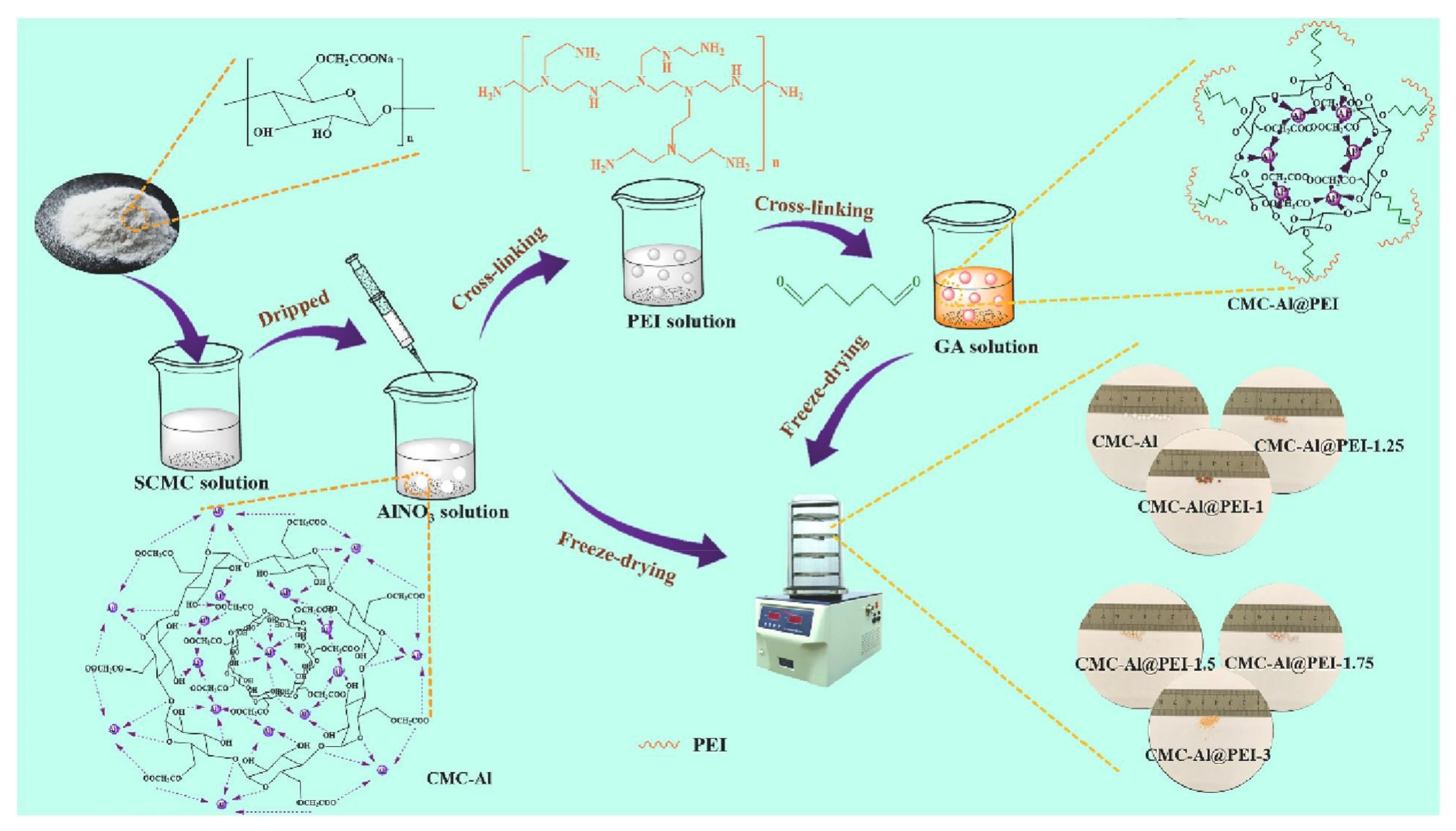
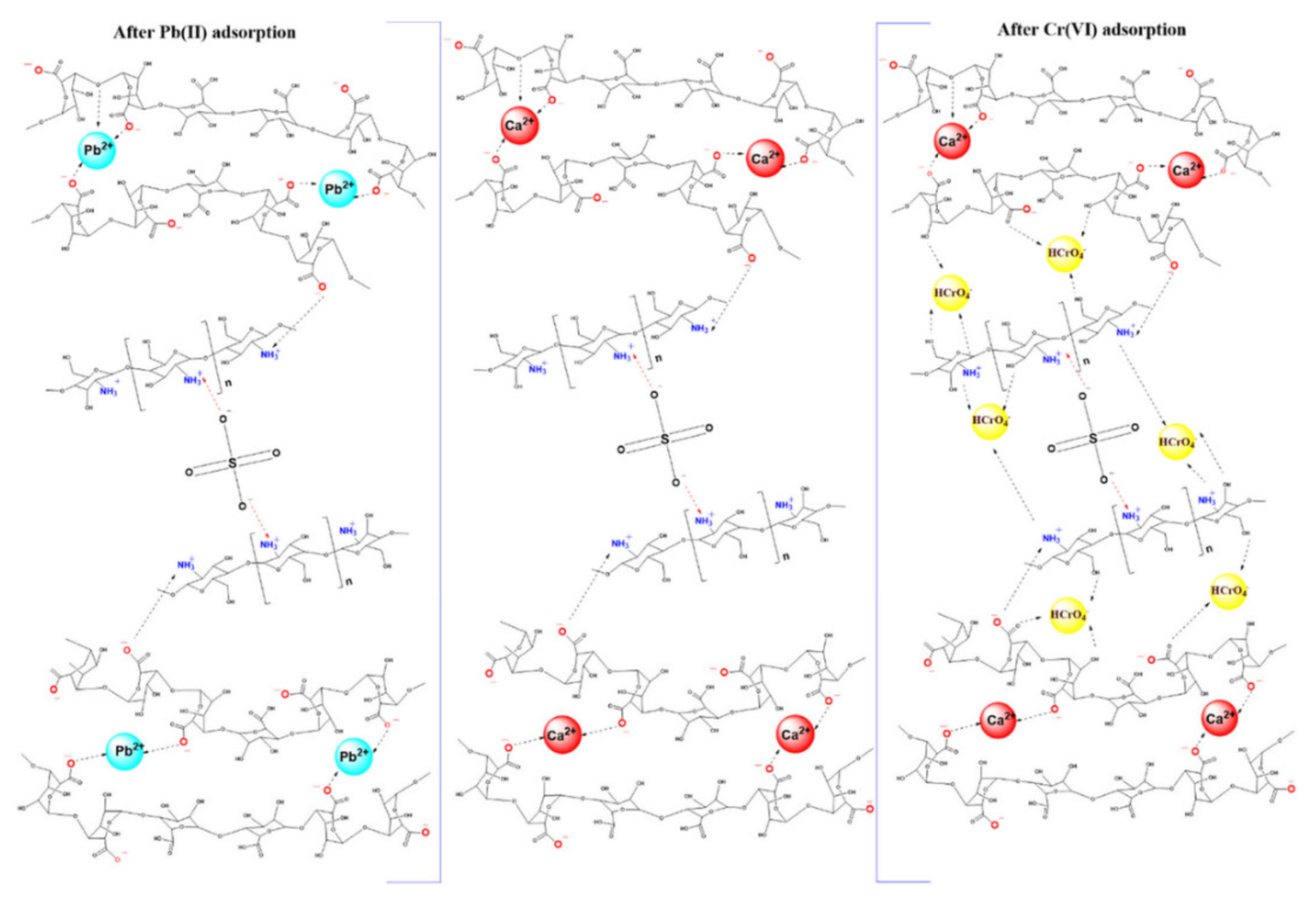
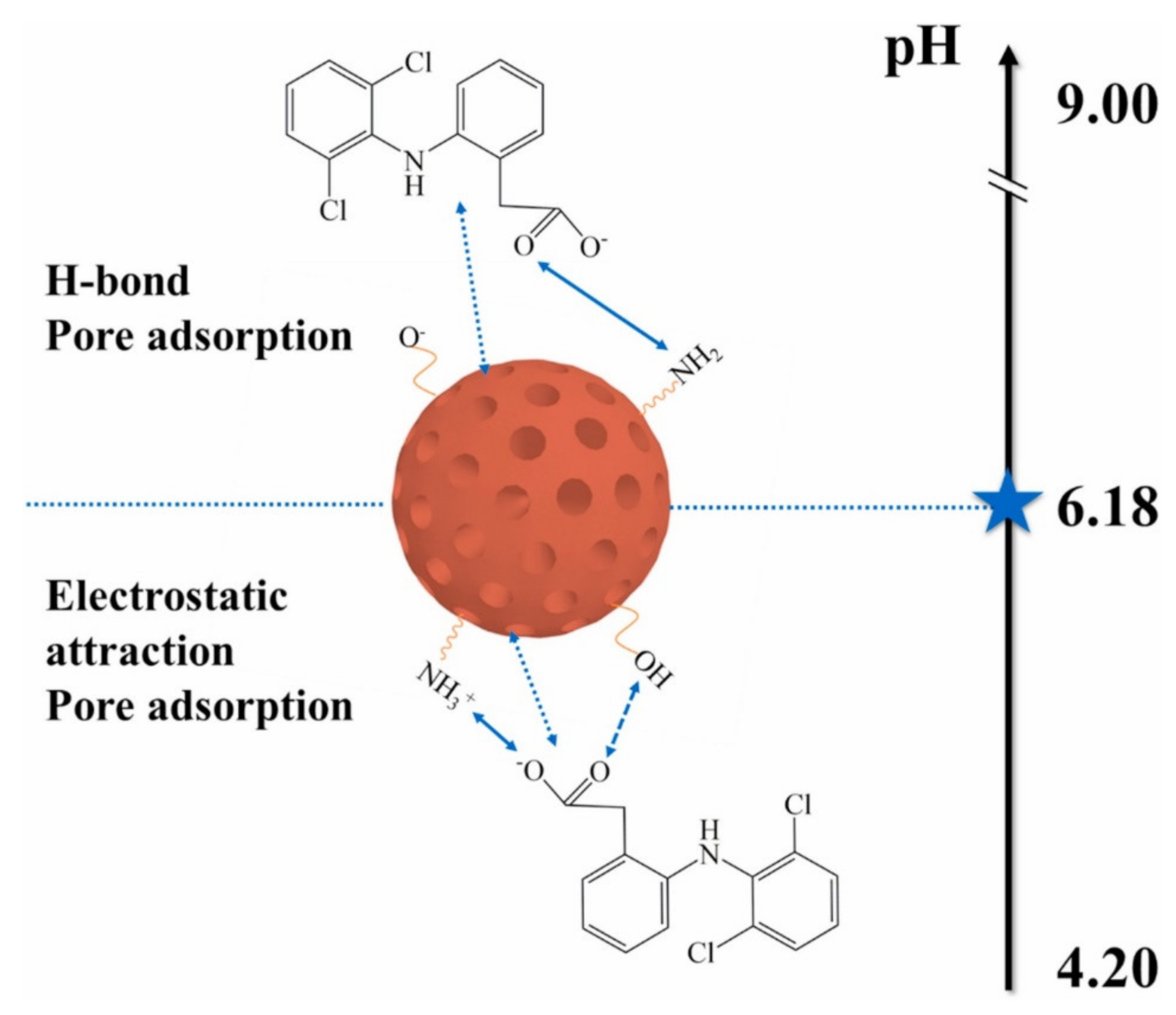
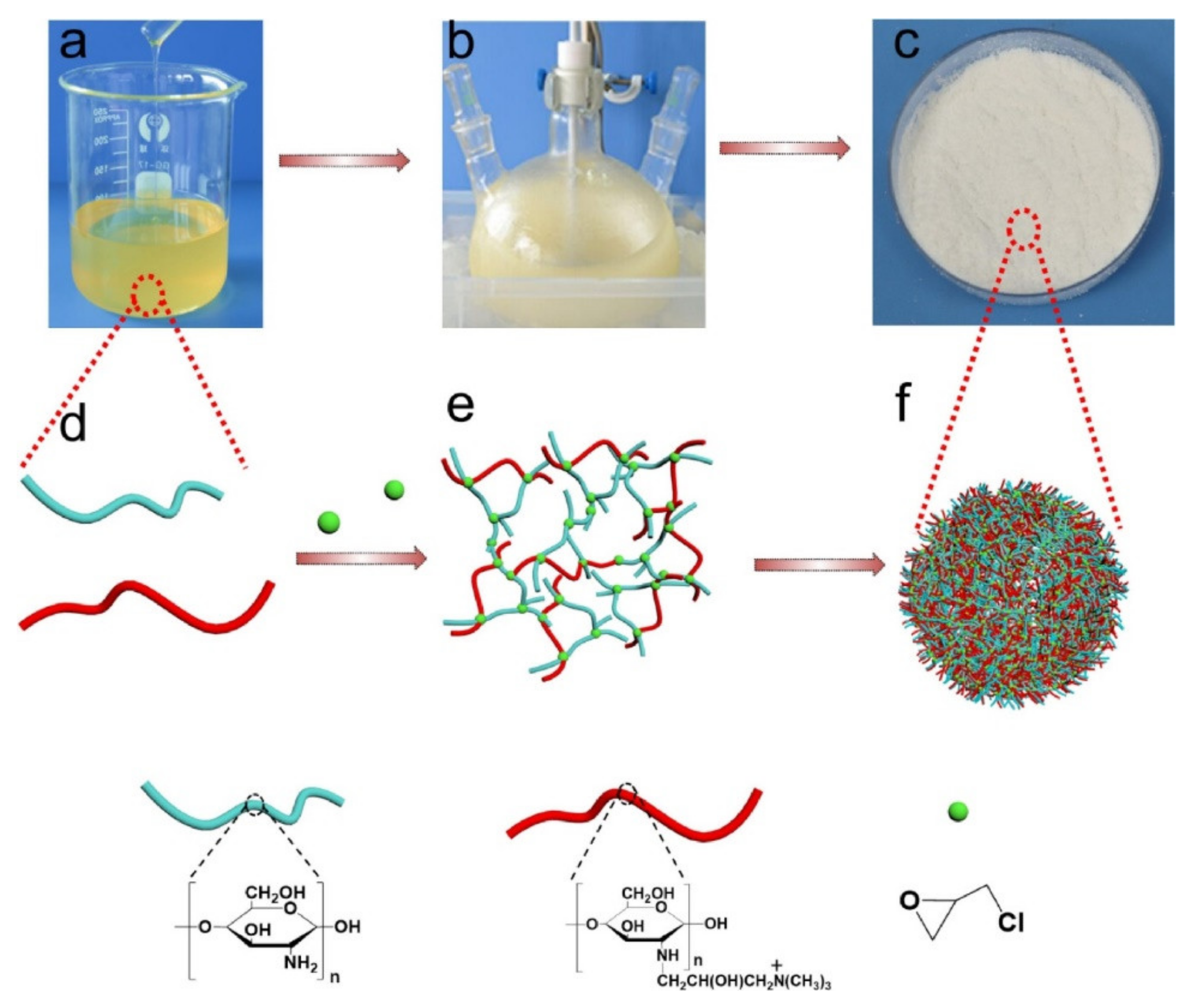
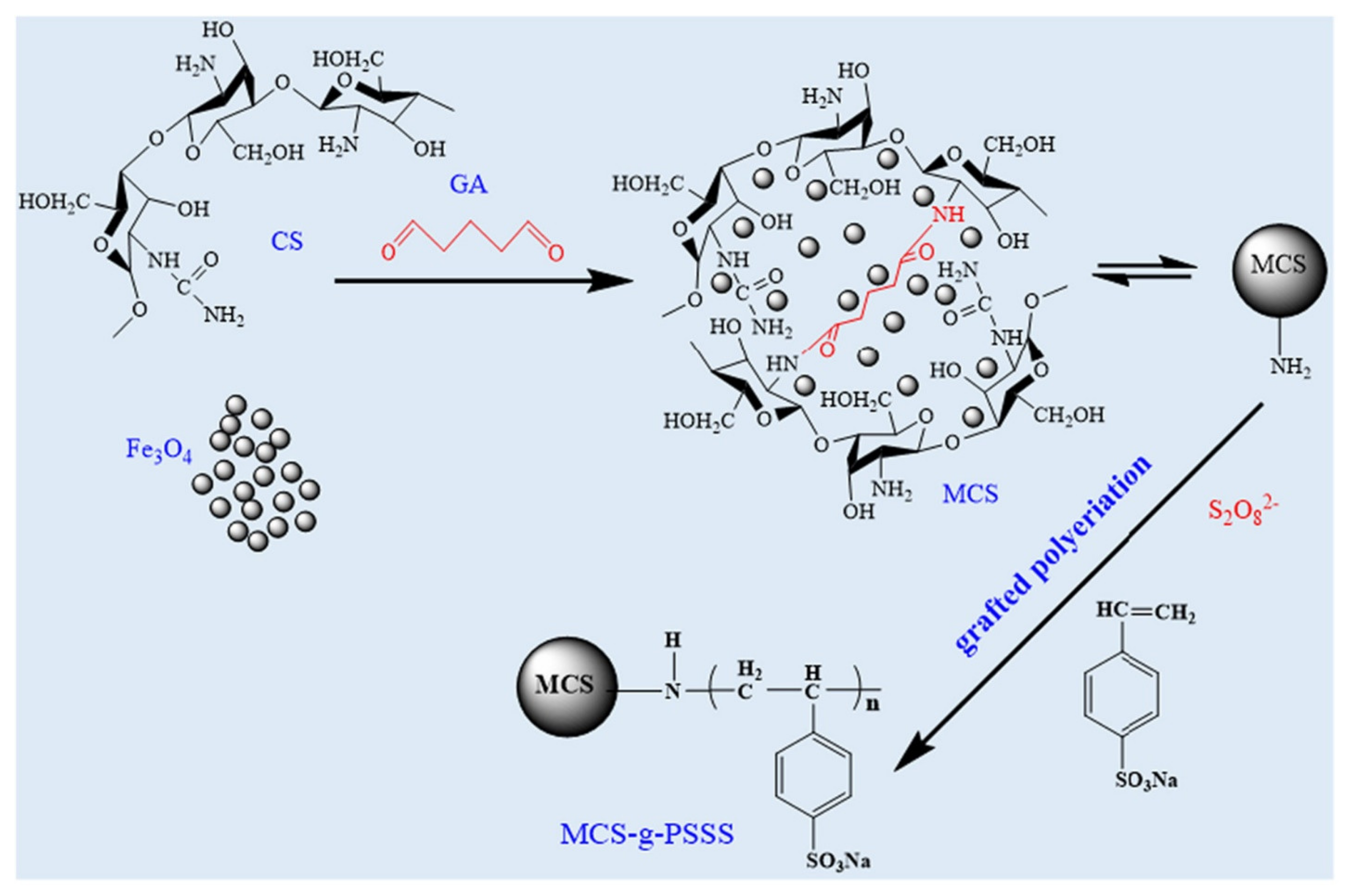



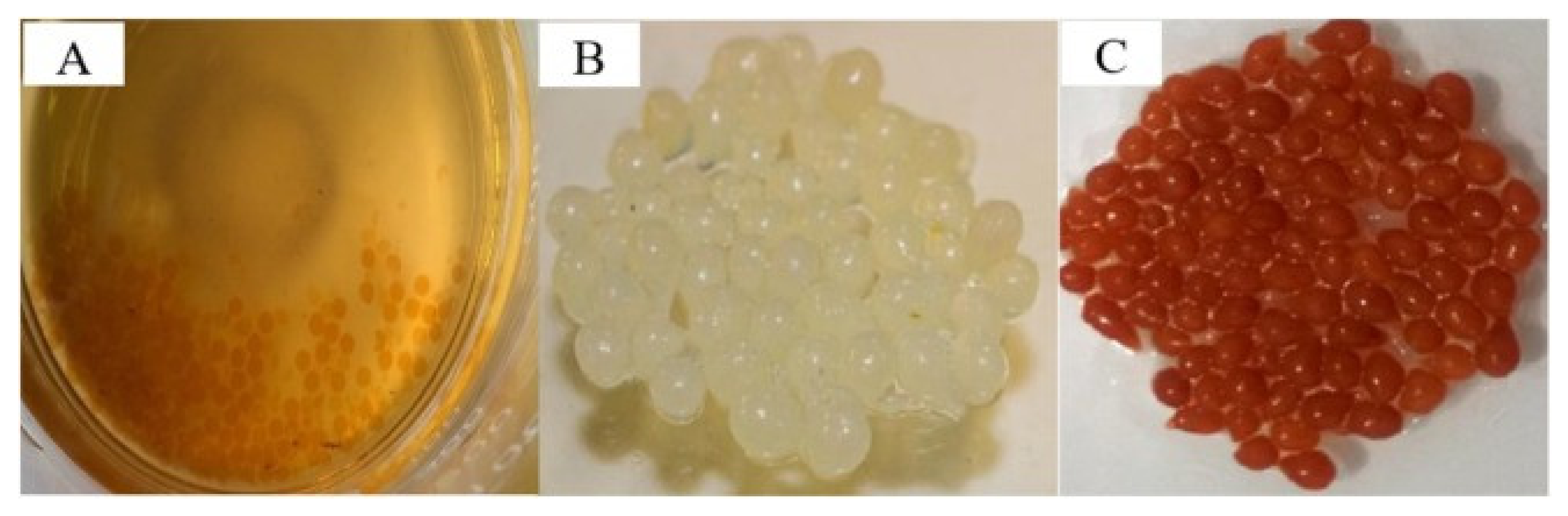



| S. No | Polymer | Adsorbate | Results | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | Sodium silicate | Pb(II) | 629.21 mg/g | PSO | Langmuir, D-R | [24] |
| 2 | HGM@MPD-ALS | AG25, BF dyes | 454.55 mg/g, 588.24 mg/g | PSO | Langmuir | [25] |
| 3 | β-CD-GO aerogel | MB, RhB, AR87, MO dyes, 2,4-DCP, PRO, EO, 4BPA | 439, 388, 234, 167, 17, 49, 19, 38 mg/g | - | - | [26] |
| 4 | CG/KGP | MB dye | 24.6 mg/g | PSO | Langmuir | [27] |
| 5 | PMMA@Fe3O4@DR | RhB, ST dye | 99%, 98% | [28] | ||
| 6 | PAM | MB, NR, GV dye | 1990, 1937, 1850 mg/g | Langmuir | [29] | |
| 7 | MF resin | PFOA | 1.18 mM/g | PSO | Freundlich, Langmuir | [30] |
| 8 | Poly[ANE + N-PMI]-TiO2 | RhB, TC dye | 95% for 50 mg/L RhB, 97% for 100 mg/L TC | PFO | L-H | [31] |
| 9 | SiO2/PANI/BiOBr | Oil-water emulsion separation | TOC content < 5 mg/L Flux recovery ratio 99.8% | - | - | [32] |
| S. No | Polymer | Adsorbate | Result | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | PVA | CODMn removal rate | 16.99% | - | - | [33] |
| 2 | poly(EGMDA-VIM) | phenol | 34.7441 mg/g | PSO | Freundlich | [34] |
| 3 | PAN, phenolic resin | oil removal efficiency | 93.6% | - | - | [35] |
| 4 | P(St-DVB)/CuNi | Pb(II), Cd(II), Mn(II), Zn(II) | 15.60, 5.28, 22.42, 20.57 mg/g | PFO | Langmuir | [36] |
| 5 | St-DBV-SH | Cu(II), Zn(II), Cd(II), Pb(II), Ni(II) | 45.26, 32.42, 62.77, 135.85, 49.88 mg/g | PSO | Langmuir | [37] |
| 6 | SPS | Pb(II), Zn(II), Cu(II) | 49.16, 15.38, 13.89 mg/g | PSO | Langmuir | [38] |
| 7 | DVB, TEVS | nitrobenzene, 4-nitrophenol, phenol | - | - | - | [39] |
| 8 | P(VP-DVB)-6 | SY, BPB dye | 261.78, 277.01 mg/g | PSO | Langmuir | [40] |
| S. No | Polymer | Adsorbate | Adsorbent Capacity | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | PDA | MB dye | 191.55 mg/g | PSO | Langmuir, Temkin | [41] |
| 2 | PDA | MB dye | 90.7 mg/g | PSO | Langmuir | [42] |
| 3 | PDA | MB, MG, NR dye | 93.86, 91.98, 88.58 mg/g | PSO | Langmuir | [43] |
| 4 | PAM/PA/PDA | Cu(II) | 231.36 mg/g | - | - | [44] |
| 5 | PDA | Cr(VI) | 199.6 mg/g | PFO | Langmuir | [45] |
| 6 | PDA@ZIF-8 | Cr(VI) | 136.56 mg/g | PSO | Langmuir | [46] |
| S. No | Polymer | Adsorbate | Adsorbent Capacity | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | Lignin | Lignin extraction efficiency | 21.62 g/L | - | - | [47] |
| 2 | PVMA | Cu(II) | 135 mg/g | PSO | Freundlich | [48] |
| 3 | HAP | U(VI) | 2659 mg/g | PSO | Freundlich | [49] |
| 4 | NH2-SA/PNIPA | Cu(II), Cd(II) | 57.5 mg/g, 100.5 mg/g | PSO | Langmuir | [50] |
| 5 | PEI/HMPCR | Cd(II) | 143.6 mg/g | PSO | Langmuir | [51] |
| 6 | PAM, MBA, pine pollen | MB, MV dye | 668.96, 749.69 mg/g | PSO | Langmuir | [52] |
| S. No | Polymer | Adsorbate | Adsorbent Capacity | Functional Group | Reference |
|---|---|---|---|---|---|
| 1 | CMC-Al@PEI-1.75 | MB dye | 235 mg/g | Amino | [53] |
| 2 | Pd NP/cellulose | MB dye | 99.8% | Hydroxyl | [54] |
| 3 | CNC/MnO2/SA | MB dye | 95.4%, 114 mg/g | Carboxyl | [55] |
| S. No | Polymer | Adsorbate | Adsorbent Capacity | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | Fe3O4@HCO in SA microbead | Sb(III) | 15.368 mg/g | PSO | Langmuir | [56] |
| 2 | HA@SA | Sb(III) | 195.7 mg/g | PSO | Langmuir | [57] |
| 3 | zeolite/alginate | Ni(II) | 98% | PFO | Freundlich | [58] |
| 4 | CSM/SA hybrid bead | Pb(II), Cr(VI) | 189, 16 mg/g | PFO, PSO | Langmuir | [59] |
| 5 | PAM/SA | MB dye | 1070.54 mg/g | PFO | Langmuir | [60] |
| 6 | NRGO/SA/PVA | ANT, 2-MAQ | 0.72, 0.70 mg/g | PSO | Freundlich, D-R | [61] |
| S. No | Polymer | Adsorbate | Adsorbent Capacity | Kinetic Model | Isotherm Model | Reference |
|---|---|---|---|---|---|---|
| 1 | (CYCTS/Span80)-@PEI | DS | 572.28 mg/g | PSO | Langmuir, Freundlich | [62] |
| 2 | BSCM-CM | CR dye degradation efficiency | 85% | first order | - | [63] |
| 3 | ZBiSe-CM | CR dye degradation efficiency | 99.63% | PFO | - | [64] |
| 4 | CCQM | CR, MO dye, Cu(II), Fe(II) | 1500, 179.4, 687.6, 398.8 5 mg/g | PSO | Langmuir | [65] |
| 5 | chitosan-activated carbon composite | MO dye | 35.64 mg/g | PSO | Freundlich | [66] |
| 6 | CTA-CSM | MO dye | 131.9 mg/g, 98.8% | PSO | Langmuir | [67] |
| 7 | CS-PSSS | MB dye | 854 mg/g | PSO | Langmuir | [68] |
| 8 | CS | Cr(VI) | 945.2 mg/g | PSO | [69] | |
| 9 | CS-MA-DETA | Pb(II), Cd(II) | 239.2, 201.6 mg/g | PSO | Langmuir | [70] |
| 10 | CM with PHEMA brushes | Cu(II) | 299 mg/g | PSO | Langmuir | [71] |
| S. No | Polymer | Catalyst | Adsorbate | Degradation Efficiency (%) | Reaction Time (min) | Reference |
|---|---|---|---|---|---|---|
| 1 | Chitosan | Fe | MB | 98.8 | 6 | [77] |
| 2 | PDA-PVDF | Fe species | MO | ~100 | 60 | [78] |
| 3 | PVA/SA | SA-FeCl3 | TC | 90.5 | 60 | [80] |
| 4 | Alg-HAP, Alg-m, Alg-mHAP | HAP, Fe3O4 | MO | 71.42, 79.41, 84.28 | 90 | [81] |
| 5 | CA | BiOCl | RhB | 100 | 75 | [82] |
| 6 | Chitosan | ZnFeO4 | CDM | ~100 | 7 | [84] |
| 7 | Chitosan | FeSO4 | Strong purple coloration, aromatic compound | 91.92, 70 | 105 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Patel, R. Wastewater Treatment by Polymeric Microspheres: A Review. Polymers 2022, 14, 1890. https://doi.org/10.3390/polym14091890
Lee J, Patel R. Wastewater Treatment by Polymeric Microspheres: A Review. Polymers. 2022; 14(9):1890. https://doi.org/10.3390/polym14091890
Chicago/Turabian StyleLee, Jiwon, and Rajkumar Patel. 2022. "Wastewater Treatment by Polymeric Microspheres: A Review" Polymers 14, no. 9: 1890. https://doi.org/10.3390/polym14091890
APA StyleLee, J., & Patel, R. (2022). Wastewater Treatment by Polymeric Microspheres: A Review. Polymers, 14(9), 1890. https://doi.org/10.3390/polym14091890







