The Use of Polymers to Enhance Post-Orthodontic Tooth Stability
Abstract
:1. Introduction
2. Application of Hydrogel Carbonated Hydroxyapatite-Incorporated Advanced Platelet-Rich Fibrin Improves Post-Orthodontic Tooth Stability
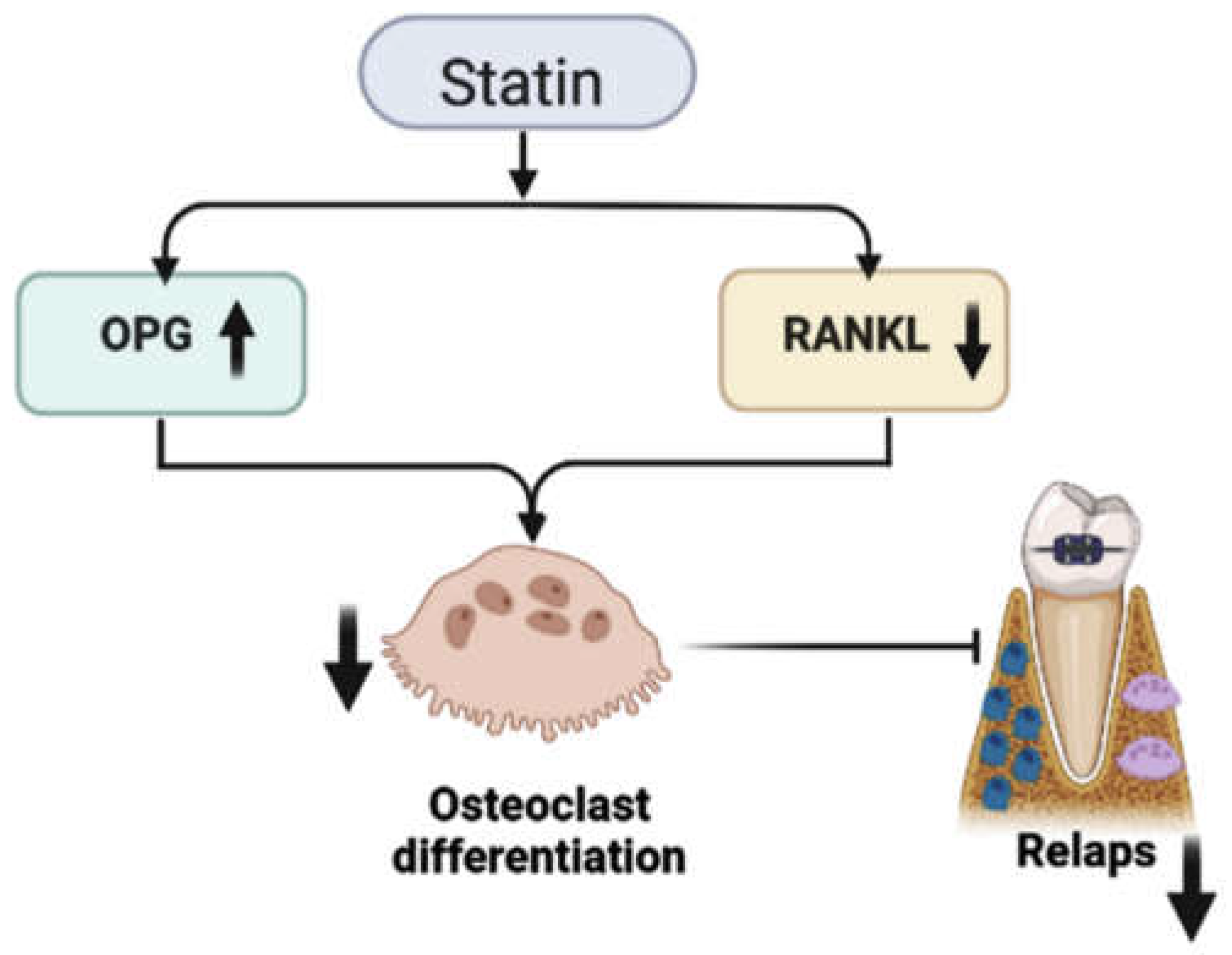
3. Statins’ Inhibitory Effect on Relapse after Orthodontic Treatment
4. Epigallocatechin Gallate-Modified Gelatin (EGCG-GL) Inhibits Bone Resorption and Tooth Movement in Rats
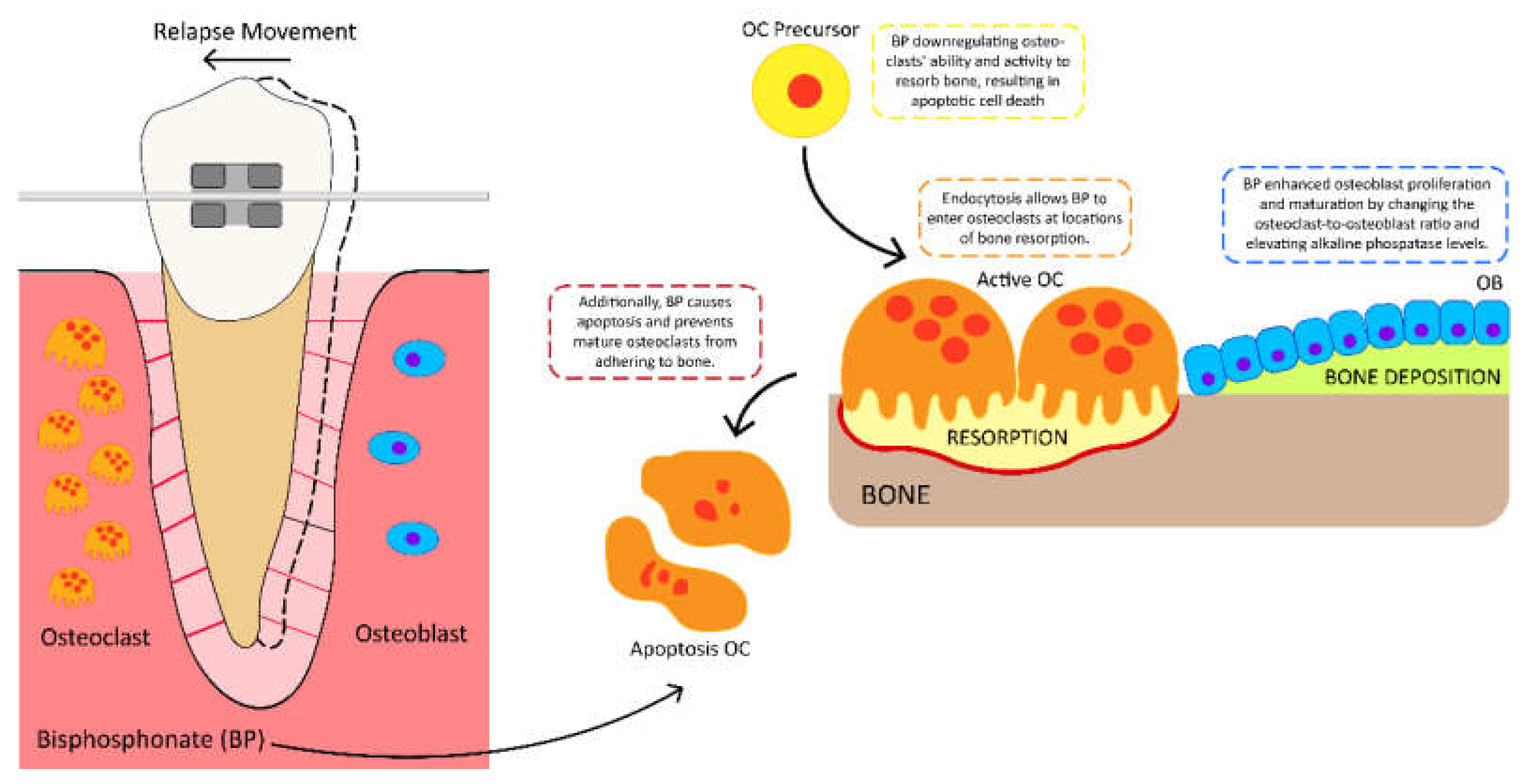
5. The Potential Benefits of Using Bisphosphonate Risedronate Hydrogel to Prevent Orthodontic Relapse Movement
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Indriasari, V.; Suparwitri, S.; Christnawati, C.; Alhasyimi, A.A. Different effects of soybean isoflavone genistein on transforming growth factor levels during orthodontic tooth movement among young and old rabbits. F1000Research 2019, 8, 2074. [Google Scholar] [CrossRef] [PubMed]
- Canuto, L.; de Freitas, M.R.; de Freitas, K.M.; Cançado, R.H.; Neves, L.S. Long-term stability of maxillary anterior alignment in non-extraction cases. Dent. Press J. Orthod. 2013, 18, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kaan, M.; Madléna, M. Retenció és recidiva az ortodonciában. Irodalmi áttekintés (Retention and relapse. Review of the literature). Fogorv. Sz. 2011, 104, 139–146. [Google Scholar] [PubMed]
- Scribante, A.; Gallo, S.; Turcato, B.; Trovati, F.; Gandini, P.; Sfondrini, M.F. Fear of the relapse: Effect of composite type on adhesion efficacy of upper and lower orthodontic fixed retainers: In vitro investigation and randomized clinical trial. Polymers 2020, 12, 963. [Google Scholar] [CrossRef] [Green Version]
- Valiathan, M.; Hughes, E. Results of a survey-based study to identify common retention practices in the United States. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 170–177. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Gallo, S.; Turcato, B.; Montasser, M.A.; Albelasy, N.F.; Vallittu, P.K.; Gandini, P.; Scribante, A. Universal Adhesive for Fixed Retainer Bonding: In Vitro Evaluation and Randomized Clinical Trial. Materials 2021, 14, 1341. [Google Scholar] [CrossRef]
- Al Yami, E.A.; Kuijpers-Jagtman, A.M.; van’t Hof, M.A. Stability of orthodontic treatment outcome: Follow-up until 10 years postretention. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 300–304. [Google Scholar] [CrossRef]
- Franzen, T.J.; Monjo, M.; Rubert, M.; Vandevska-Radunovic, V. Expression of bone markers, and micro-CT analysis of alveolar bone during orthodontic relapse. Orthod. Craniofac. Res. 2014, 17, 249–258. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.J. Bone biology and anabolic therapies for bone: Current status and future prospects. J. Bone Metab. 2014, 21, 8–20. [Google Scholar] [CrossRef]
- Schneider, D.A.; Smith, S.M.; Campbell, C.; Hayami, T.; Kapila, S.; Hatch, N.E. Locally limited inhibition of bone resorption and orthodontic relapse by recombinant osteoprotegerin protein. Orthod. Craniofac. Res. 2015, 18 (Suppl. S1), 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Abd Razak, S.I.; Javed, A.; Kadir, M.R.A. Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Nienkemper, M.; Willmann, J.H.; Becker, K.; Drescher, D. RFA measurements of survival midpalatal orthodontic mini-implants in comparison to initial healing period. Prog. Orthod. 2020, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saliba, J.; Modarres, H.P.; Bakhaty, A.; Nasajpour, A.; Mofrad, M.R.; Sanati-Nezhad, A. Micro and nanotechnologies in heart valve tissue engineering. Biomaterials 2016, 103, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Aslam Khan, M.U.; Nazir, S.; Abd Razak, S.I.; Abdul Kadir, M.R. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E1. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.-T.; Yalcin, H.C.; Marei, H.E. Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef]
- Ariful Islam, M.; Park, T.-E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.-K.; Choi, Y.-J.; Park, I.-K. Mucoadhesive chitosan derivatives as novel drug carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef]
- Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A Review on current trends of polymers in orthodontics: BPA-free and smart materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Katsumata, Y.; Kanzaki, H.; Honda, Y.; Tanaka, T.; Yamaguchi, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; et al. Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice. Polymers 2018, 10, 1384. [Google Scholar] [CrossRef]
- Utari, T.R.; Ana, I.D.; Pudyani, P.S.; Asmara, W. The intrasulcular application effect of bisphosphonate hydrogel toward osteoclast activity and relapse movement. Saudi Dent. J. 2021, 33, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Alhasyimi, A.A.; Pudyani, P.P.; Asmara, W.; Ana, I.D. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod. Craniofac. Res. 2018, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Muhammed, F.K.; Liu, Y. Simvastatin encapsulated in exosomes can enhance its inhibition of relapse after orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Tsuru, K.; Nagai, H.; Fujisawa, K.; Kudoh, T.; Ohe, G.; Ishikawa, K.; Miyamoto, Y. Fabrication and evaluation of carbonate apatite-coated calcium carbonate bone substitutes for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 2077–2087. [Google Scholar] [CrossRef]
- Alhasyimi, A.A.; Pudyani, P.S.; Asmara, W.; Ana, I.D. Effect of carbonated hydroxyapatite incorporated advanced platelet-rich fibrin intramuscular injection on the alkaline phosphatase level during orthodontic relapse. AIP Conf. Proc. 2018, 1933, 30006. [Google Scholar] [CrossRef]
- Tada, S.; Chowdhury, E.H.; Cho, C.S.; Akaike, T. pH-sensitive carbonate apatite as an intracellular protein transporter. Biomaterials 2010, 31, 1453–1459. [Google Scholar] [CrossRef]
- Maarof, N.N.N.; Abdulmalek, E.; Fakurazi, S.; Rahman, M.B.A. Biodegradable Carbonate Apatite Nanoparticle as a Delivery System to Promote Afatinib Delivery for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2022, 14, 1230. [Google Scholar] [CrossRef]
- Saito, T.; Tabata, Y. Preparation of gelatin hydrogels incorporating low-molecular-weight heparin for anti-fibrotic therapy. Acta Biomater. 2012, 8, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Galav, S.; Chandrashekar, K.T.; Mishra, R.; Tripathi, V.; Agarwal, R.; Galav, A. Comparative evaluation of platelet-rich fibrin and autogenous bone graft for the treatment of infrabony defects in chronic periodontitis: Clinical, radiological, and surgical reentry. Indian J. Dent. Res. 2016, 27, 502–507. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Aggour, R.L.; Gamil, L. Antimicrobial effects of platelet-rich plasma against selected oral and periodontal pathogens. Pol. J. Microbiol. 2017, 66, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Hokugo, A.; Ozeki, M.; Kawakami, O.; Sugimoto, K.; Mushimoto, K.; Morita, S.; Tabata, Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005, 11, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Miyamoto, M.; Ishii, Y.; Aoyama, J.; Takagi, G.; Naito, Z.; Tabata, Y.; Ochi, M.; Shimizu, K. Enhanced vascularization by controlled release of platelet-rich plasma impregnated in biodegradable gelatin hydrogel. Ann. Thorac. Surg. 2011, 92, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Lee, C.Y.; Luo, C.W.; Kuo, Y.P.; Chou, M.L.; Wu, Y.W.; Tseng, Y.H.; Su, C.Y. Human blood-derived fibrin releasates: Composition and use for the culture of cell lines and human primary cells. Biologicals 2012, 40, 21–30. [Google Scholar] [CrossRef]
- Alhasyimi, A.A.; Pudyani, P.S.; Asmara, W.; Ana, I.D. Locally Inhibition of Orthodontic Relapse by Injection of Carbonated Hydroxy Apatite-Advanced Platelet Rich Fibrin in a Rabbit Model. Key Eng. Mat. 2017, 758, 255–263. [Google Scholar] [CrossRef]
- Batra, P.; Kharbanda, O.; Duggal, R.; Singh, N.; Parkash, H. Alkaline phosphatase activity in gingival crevicular fluid during canine retraction. Orthod. Craniofac. Res. 2006, 9, 44–51. [Google Scholar] [CrossRef]
- Shetty, S.V.; Patil, A.K.; Ganeshkar, S.V. Assessment of acid phosphatase and alkaline phosphatase in gingival crevicular fluid in growing and adult orthodontic patients: An in vivo study. J. Indian Orthod. Soc. 2015, 49, 10–14. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar] [CrossRef] [Green Version]
- Alhasyimi, A.A.; Suparwitri, S.; Christnawati, C. Effect of carbonate apatite hydrogel-advanced platelet-rich fibrin injection on osteoblastogenesis during orthodontic relapse in rabbits. Eur. J. Dent. 2021, 15, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Nanjundaiah, S.M.; Astry, B.; Moudgil, K.D. Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evid. Based Complementary Altern. Med. 2013, 2013, 518094. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 26, 46009. [Google Scholar] [CrossRef] [Green Version]
- Kasagi, S.; Chen, W. TGF-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013, 15, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Narmada, I.B.; Rubianto, M.; Putra, S.T. The Role of Low-Intensity Biostimulation Laser Therapy in Transforming Growth Factor β1, Bone Alkaline Phosphatase and Osteocalcin Expression during Orthodontic Tooth Movement in Cavia porcellus. Eur. J. Dent. 2019, 13, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Han, G.H.; Jin, C.W.; Shi, R.X.; Hou, J.H. Effect of simvastatin on bone morphogenetic protein-2 expression in the periodontal tissue after rat tooth movement. Zhonghua Kou Qiang Yi Xue Za Zhi 2008, 43, 21–25. (In Chinese) [Google Scholar]
- Kamiya, N.; Ye, L.; Kobayashi, T.; Lucas, D.J.; Mochida, Y.; Yamauchi, M.; Kronenberg, H.M.; Feng, J.Q.; Mishina, Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J. Bone Miner. Res. 2008, 23, 2007–2017. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.B.; Hatch, N.; Hayami, T.; Shin, J.M.; Stolina, M.; Kostenuik, P.J.; Kapila, S. Local delivery of recombinant osteoprotegerin enhances postorthodontic tooth stability. Calcif. Tissue Int. 2012, 90, 330–342. [Google Scholar] [CrossRef]
- Enomoto, H.; Shiojiri, S.; Hoshi, K.; Furuichi, T.; Fukuyama, R.; Yoshida, C.A.; Kanatani, N.; Nakamura, R.; Mizuno, A.; Zanma, A.; et al. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2-/- mice by RANKL transgene. J. Biol. Chem. 2003, 278, 23971–23977. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wu, Z.; Sun, H.C. The effect of simvastatin on mRNA expression of transforming growth factor-beta1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int. J. Oral Sci. 2009, 1, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Ruan, F.; Zheng, Q.; Wang, J. Mechanisms of bone anabolism regulated by statins. Biosci. Rep. 2012, 32, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bradley, A.D.; Wang, D.; Reinhardt, R.A. Statins, bone metabolism and treatment of bone catabolic diseases. Pharmacol. Res. 2014, 88, 53–61. [Google Scholar] [CrossRef]
- Han, G.; Chen, Y.; Hou, J.; Liu, C.; Chen, C.; Zhuang, J.; Meng, W. Effects of simvastatin on relapse and remodeling of periodontal tissues after tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 550.e1–550.e7. [Google Scholar] [CrossRef]
- Viereck, V.; Gründker, C.; Blaschke, S.; Frosch, K.H.; Schoppet, M.; Emons, G.; Hofbauer, L.C. Atorvastatin stimulates the production of osteoprotegerin by human osteoblasts. J. Cell Biochem. 2005, 96, 1244–1253. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010, 26, 5887–5896. [Google Scholar] [CrossRef] [Green Version]
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- AlSwafeeri, H.; ElKenany, W.; Mowafy, M.; Karam, S. Effect of local administration of simvastatin on postorthodontic relapse in a rabbit model. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.S.; Portela, L.V.; Onofre de Souza, D.; Medeiros Fossati, A.C. Atorvastatin-induced osteoclast inhibition reduces orthodontic relapse. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.M.; Chaves, S.B.; Ferreira, V.M.; Freitas, K.M.; Amorim, R.F. The effect of simvastatin on relapse of tooth movement and bone mineral density in rats measured by a new method using microtomography. Acta Cir. Bras. 2015, 30, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Feizbakhsh, M.; Mortazavi, M.S.; Razavi, S.M.; Hajhashemi, V. The effects of local injection of simvastatin on tooth movement and root resorption rates under orthodontic forces in dogs. Biosci. Biotechnol. Res. Asia 2014, 11, 869–873. [Google Scholar] [CrossRef]
- MirHashemi, A.H.; Afshari, M.; Alaeddini, M.; Etemad-Moghadam, S.; Dehpour, A.; Sheikhzade, S.; Akhoundi, M.S. Effect of atorvastatin on orthodontic tooth movement in male wistar rats. J. Dent. 2013, 10, 532–539. [Google Scholar]
- Bax, B.E.; Alam, A.S.; Banerji, B.; Bax, C.M.; Bevis, P.J.; Stevens, C.R.; Moonga, B.S.; Blake, D.R.; Zaidi, M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992, 183, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Shinohara, F.; Itohiya-Kasuya, K.; Ishikawa, M.; Nakamura, Y. Nrf2 activation attenuates both orthodontic tooth movement and relapse. J. Dent. Res. 2015, 94, 787–794. [Google Scholar] [CrossRef]
- Gambari, L.; Lisignoli, G.; Cattini, L.; Manferdini, C.; Facchini, A.; Grassi, F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via nrf2-dependent mechanism. Pharmacol. Res. 2014, 87, 99–112. [Google Scholar] [CrossRef]
- Kanzaki, H.; Shinohara, F.; Itohiya, K.; Yamaguchi, Y.; Katsumata, Y.; Matsuzawa, M.; Fukaya, S.; Miyamoto, Y.; Wada, S.; Nakamura, Y. RANKL induces bach1 nuclear import and attenuates nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice. FASEB J. 2017, 31, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.; Miyaura, C.; Inada, M. Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS Open Bio 2015, 5, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Sakai, G.; Otsuka, T.; Fujita, K.; Kainuma, S.; Kuroyanagi, G.; Kawabata, T.; Matsushima-Nishiwaki, R.; Kozawa, O.; Tokuda, H. Amplification by (-)-epigallocatechin gallate of prostaglandin f2alphastimulated synthesis of osteoprotegerin in osteoblasts. Mol. Med. Rep. 2017, 16, 6376–6381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Kainuma, S.; Fujita, K.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Matsushima-Nishiwaki, R.; Harada, A.; et al. (-)-Epigallocatechin gallate synergistically potentiates prostaglandin e2-stimulated osteoprotegerin synthesis in osteoblasts. Prostaglandins Other Lipid Mediat. 2017, 128–129, 27–33. [Google Scholar] [CrossRef]
- Frith, J.C.; Mönkkönen, J.; Auriola, S.; Mönkkönen, H.; Rogers, M.J. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: Evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001, 44, 2201–2210. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 15, 6222s–6230s. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.J. New insights into the molecular mechanisms of action of bisphosphonates. Curr. Pharm. Des. 2003, 9, 2643–2658. [Google Scholar] [CrossRef]
- Krishnan, S.; Pandian, S.; Kumar, S.A. Effect of bisphosphonates on orthodontic tooth movement-an update. J. Clin. Diagn. Res. 2015, 9, ZE01–ZE05. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kitaura, H.; Yoshimatsu, M.; Eguchi, T.; Kohara, H.; Morita, Y.; Yoshida, N. Influence of bisphosphonates on orthodontic tooth movement in mice. Eur. J. Orthod. 2009, 31, 572–577. [Google Scholar] [CrossRef]
- George, E.L.; Lin, Y.L.; Saunders, M.M. Bisphosphonate-related osteonecrosis of the jaw: A mechanobiology perspective. Bone Rep. 2018, 5, 104–109. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and Pharmacodynamic Features of Nanoemulsion Following Oral, Intravenous, Topical and Nasal Route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Igarashi, K.; Mitani, H.; Shinoda, H. Effects of topical administration of a bisphosphonate (risedronate) on orthodontic tooth movements in rats. J. Dent. Res. 1994, 73, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Goya, J.A.; Paez, H.A.; Mandalunis, P.M. Effect of topical administration of monosodium olpadronate on experimental periodontitis in rats. J. Periodontol. 2006, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Manage-ment of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Jingarwar, M.M.; Bajwa, N.K.; Pathak, A. Minimal intervention dentistry—A new frontier in clinical dentistry. J. Clin. Diagn. Res. 2014, 8, ZE04–ZE08. [Google Scholar] [CrossRef]
- Utari, T.R.; Ana, I.D.; Pudyani, P.S.; Asmara, W. The Potential of Bisphosphonate Risedronate Hydrogel in Preventing Relapse Movement. Cumhur. Dent. J. 2022, 25, 103–110. [Google Scholar] [CrossRef]
- Utari, T.R.; Kurniawan, M.F.; Andewa, S.M. The controlled release profile of risedronate emulgel to inhibit relapse movement in orthodontic treatment. Padjajaran J. Dent. 2022, 34, 66–75. [Google Scholar] [CrossRef]
- Ajazuddin, A.A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–647. [Google Scholar] [CrossRef]
- Sonneville-Aubrun, O.; Simonnet, J.T.; L’Alloret, F. Nanoemulsions: A new vehicle for skincare products. Adv. Colloid Interface Sci. 2004, 20, 145–149. [Google Scholar] [CrossRef]

| Author(s) | Administration’s Route, Type, and Dose of Statin | Result |
|---|---|---|
| Chen et al. [48] | SIM systemic administration 2.5 mg/kg/day, 5.0 mg/kg/day, and 10.0 mg/kg/day | Systemic administration of SIM could reduce the incidence of orthodontic relapse in rats, and a lower dose of simvastatin appeared to be more effective. |
| Han et al. [56] | Intraperitoneal injections of SIM, 2.5 mg/kg/day | • The SIM group showed shorter relapse distances than did the control group (p < 0.01). • The percentage of relapses in the test group was significantly smaller than that in the control group (p < 0.001). |
| AlSwafeeri et al. [61] | Local injection (intraligamentous and submucosal) of SIM, 0.5 mg/480μL | Local SIM administration helps postorthodontic relapse-related bone remodeling by reducing active bone resorption and increasing bone formation, but does not significantly reduce postorthodontic relapse. |
| Dolci et al. [62] | ATO systemic administration, 15 mg/kg | Statins reduce orthodontic relapse in rats by modulating bone remodeling. Decreased osteoclastogenesis and increased OPG protein expression explain this effect. |
| Vieira et al. [63] | Oral gavage of SIM, 5 mg/kg/day | SIM did not prevent relapse movement in rats, and there was no link between bone density and orthodontic relapse. |
| Feizbakhsh et al. [64] | Local injection of 0.5 mg/kg SIM in 1 mL solution | SIM local injection can reduce the rate of tooth movement and root resorption in dogs, but the differences were not statistically significant. |
| MirHashemi et al. [65] | Daily gavage of ATO, 5 mg/kg | In rats, ATO appeared to reduce tooth movement; however, its effect on osteoclasts, particularly regarding osteoclastic activity, requires additional research. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosyida, N.F.; Ana, I.D.; Alhasyimi, A.A. The Use of Polymers to Enhance Post-Orthodontic Tooth Stability. Polymers 2023, 15, 103. https://doi.org/10.3390/polym15010103
Rosyida NF, Ana ID, Alhasyimi AA. The Use of Polymers to Enhance Post-Orthodontic Tooth Stability. Polymers. 2023; 15(1):103. https://doi.org/10.3390/polym15010103
Chicago/Turabian StyleRosyida, Niswati Fathmah, Ika Dewi Ana, and Ananto Ali Alhasyimi. 2023. "The Use of Polymers to Enhance Post-Orthodontic Tooth Stability" Polymers 15, no. 1: 103. https://doi.org/10.3390/polym15010103







