CD34 Antibody-Coated Biodegradable Fiber Membrane Effectively Corrects Atrial Septal Defect (ASD) by Promoting Endothelialization
Abstract
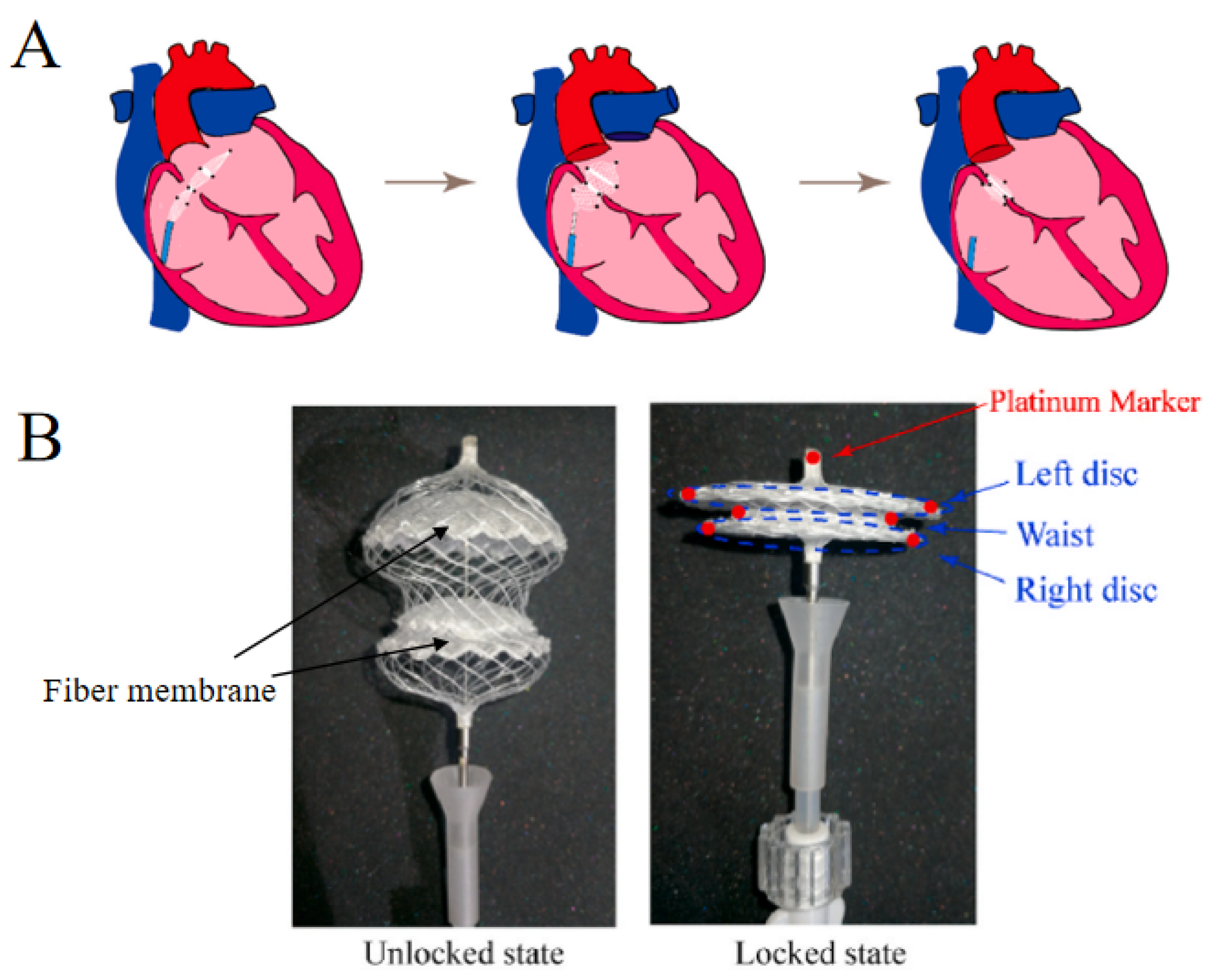
:1. Introduction
2. Materials and Methods
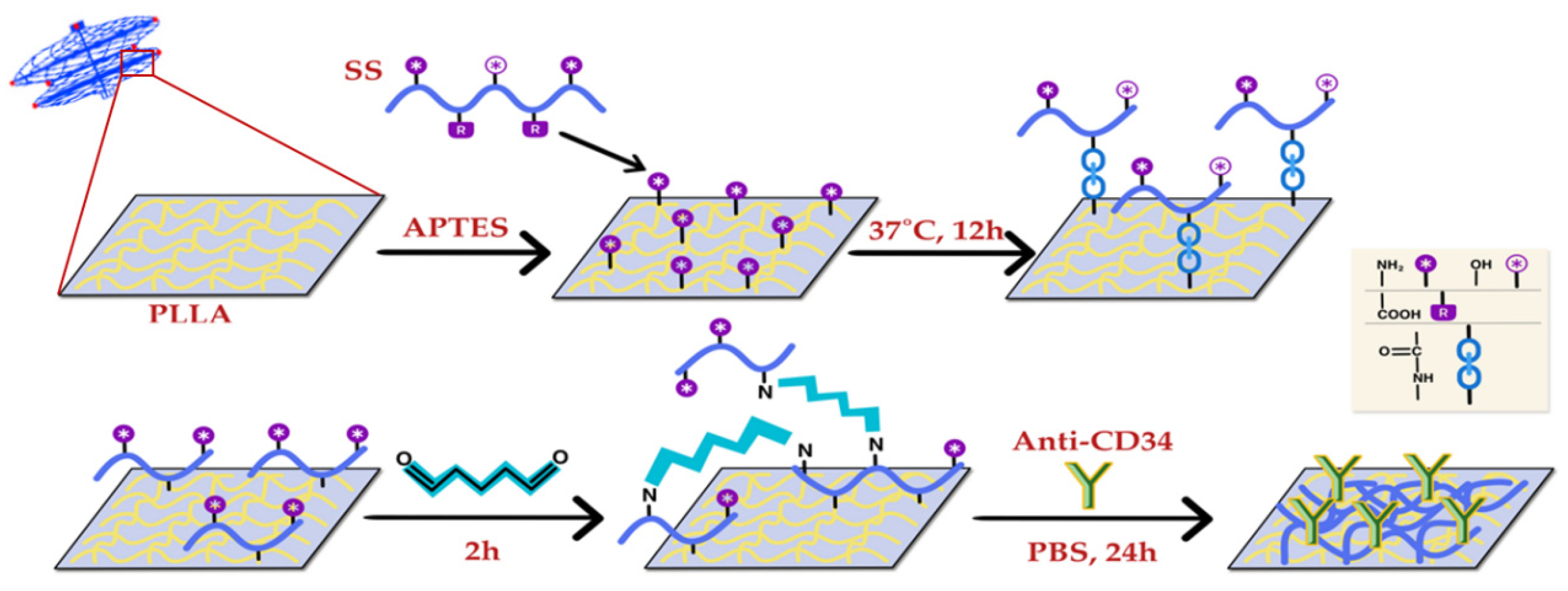
2.1. Purification of Sericin
2.2. Preparation of the Sericin-Modified PLLA Membrane (PLLA-NH2)
2.3. Preparation of the CD34 Antibody-Modified PLLA Membrane
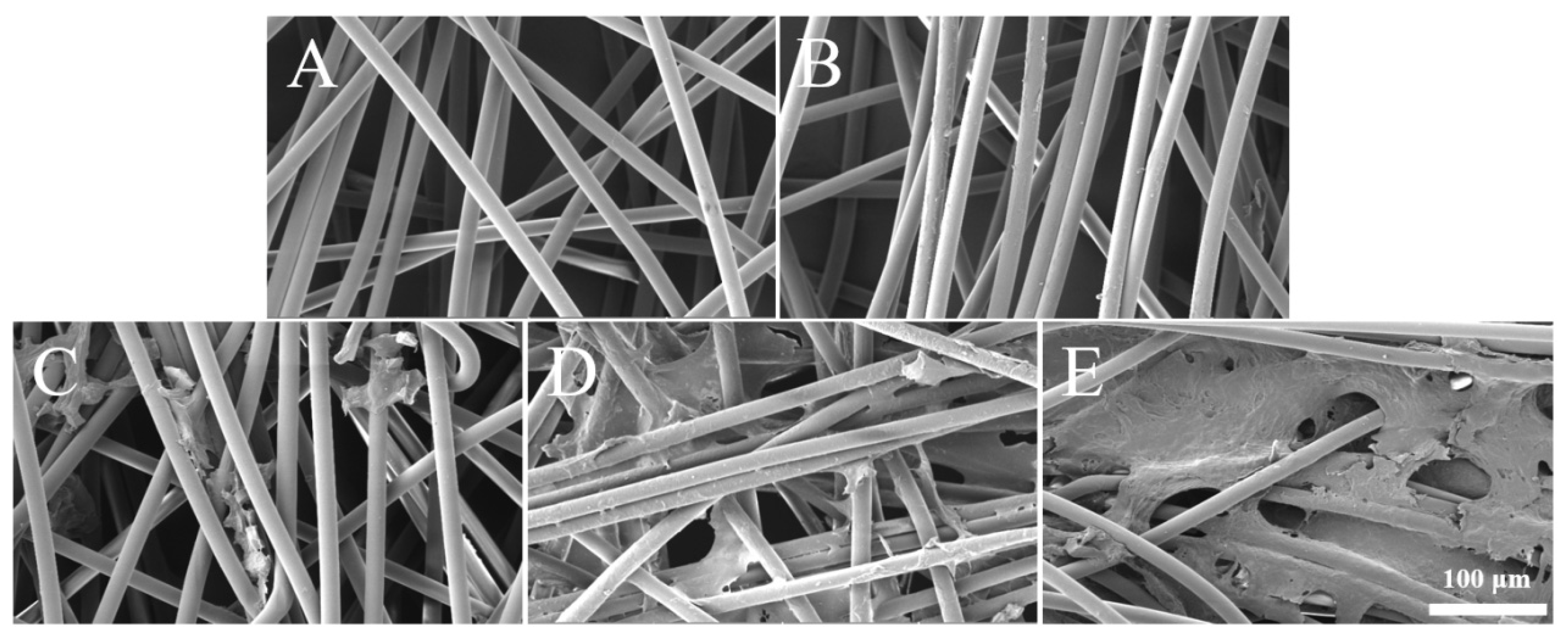
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Contact Angle Measurement
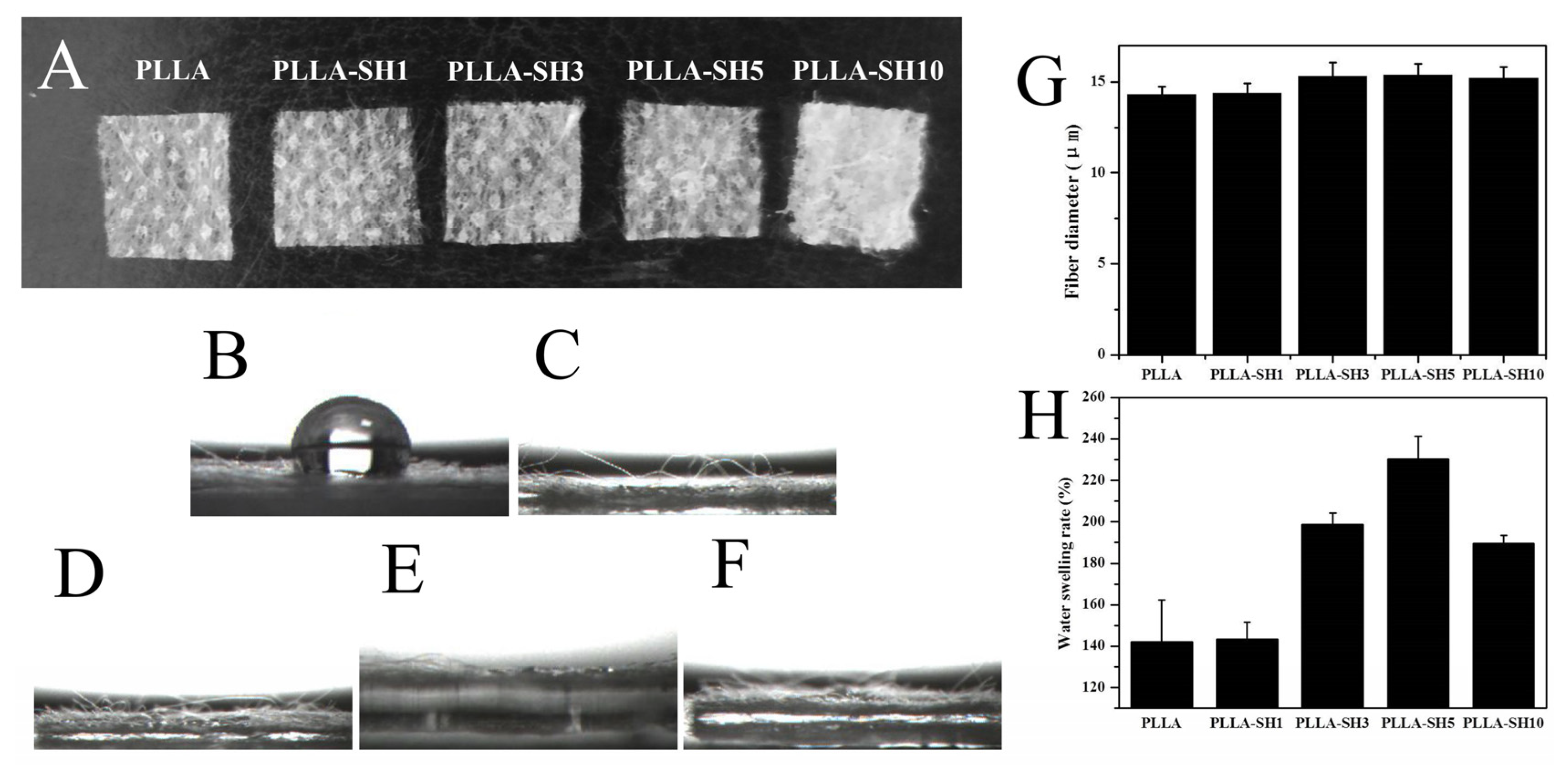
2.6. Water Swelling Rate
2.7. Mechanical Property Test
2.8. FT-IR
2.9. XPS
2.10. Cytocompatibility of Fiber Membranes
2.11. Statistical Analysis
3. Results
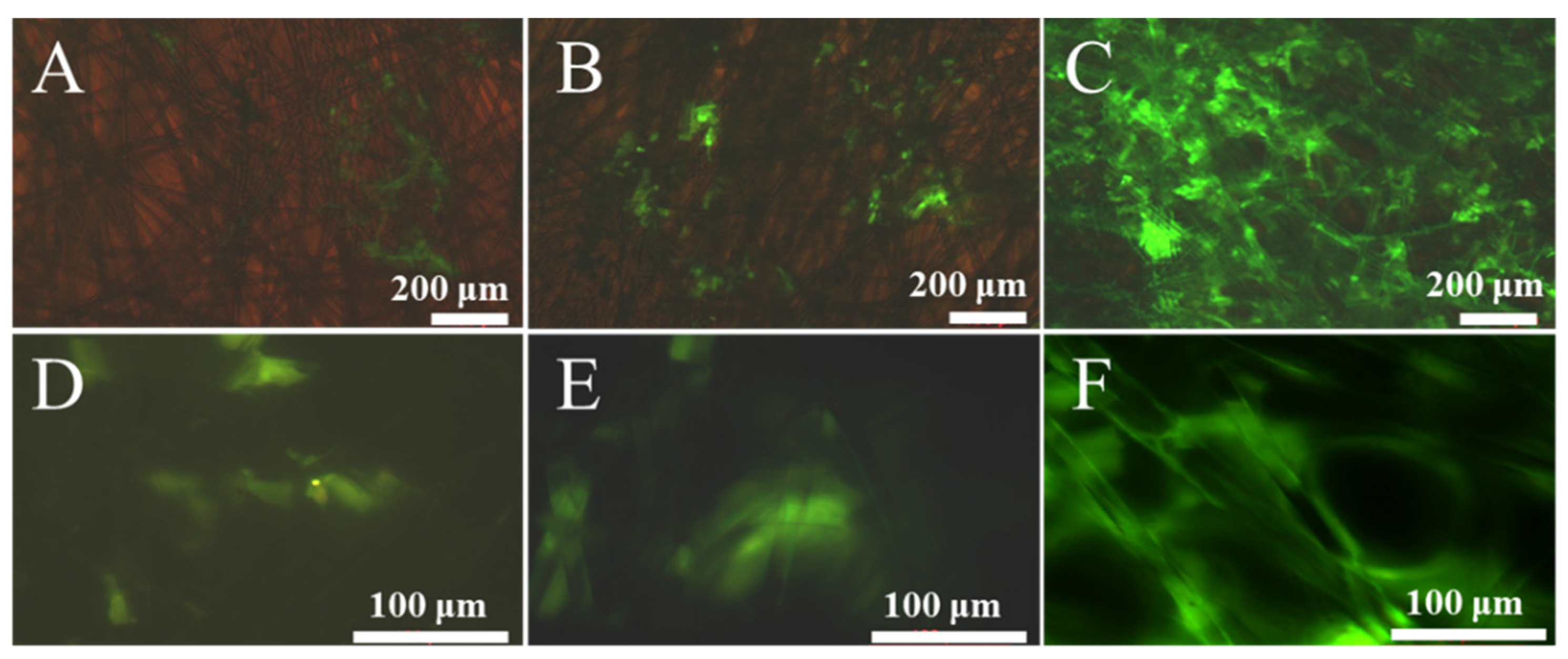
3.1. Characteristics of Sericin-Modified Fiber Membranes
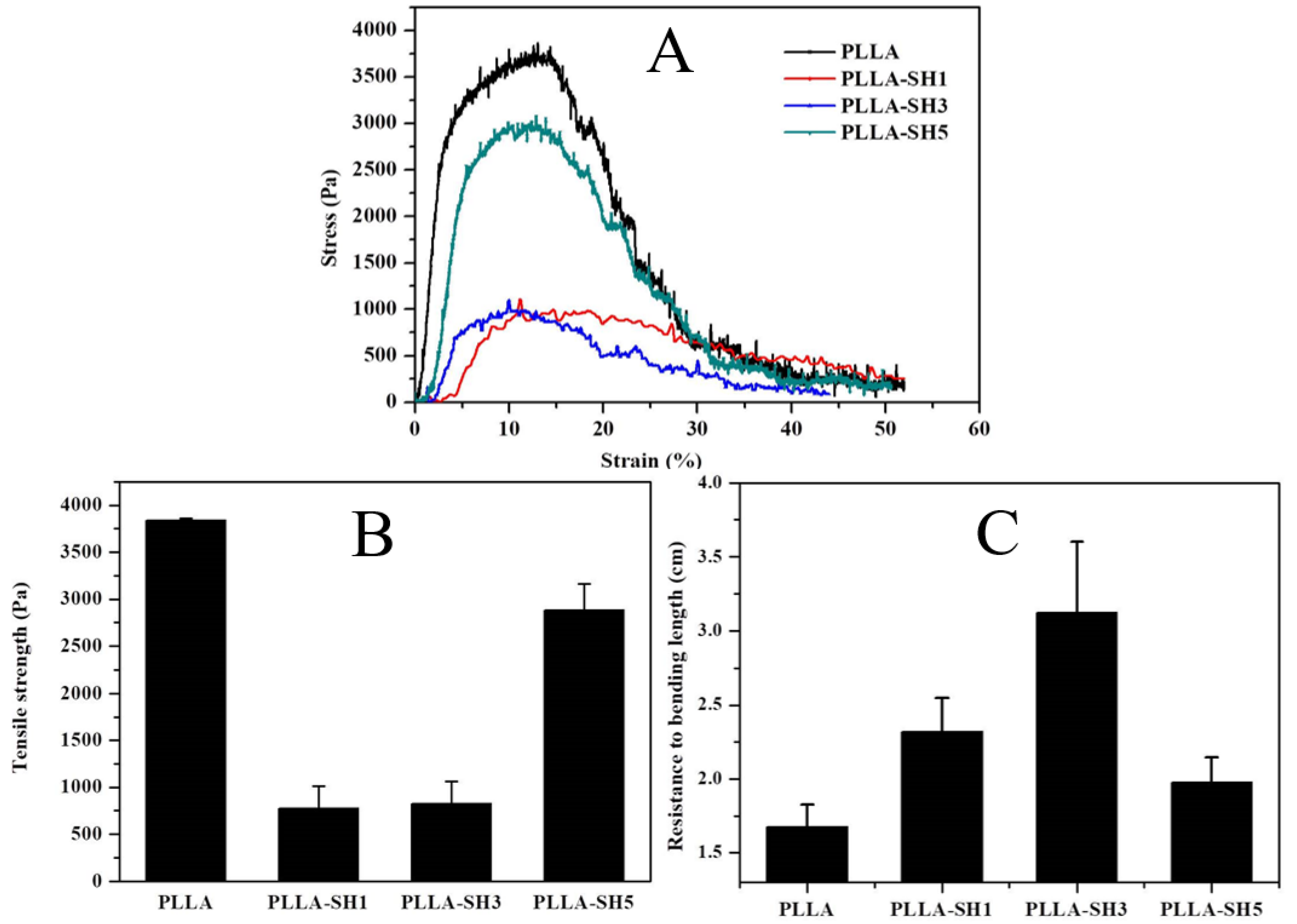
3.2. Mechanical Property Analysis
3.3. Characterization of PLLA, PLLA-NH2, and PLLA-SH5
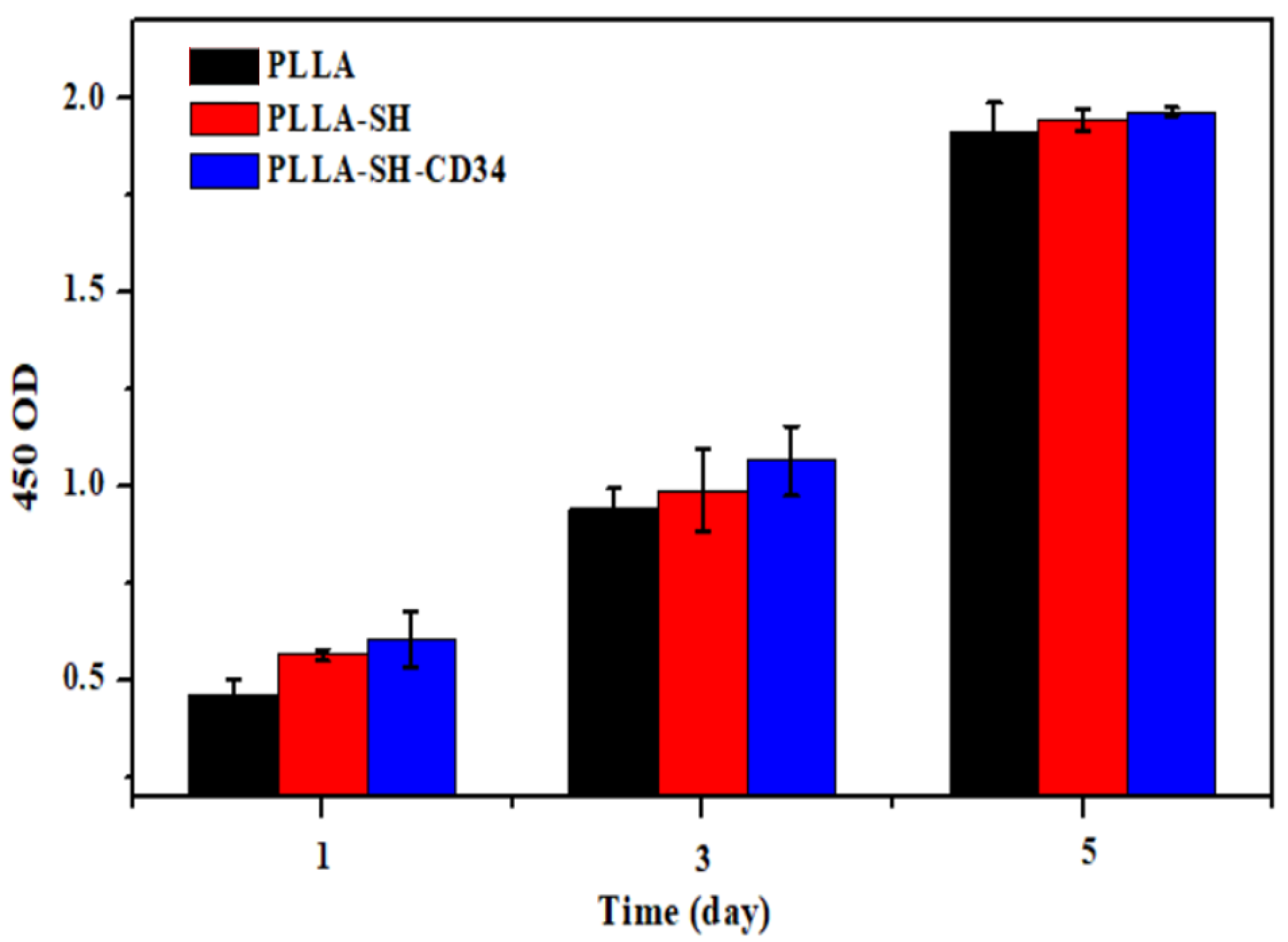
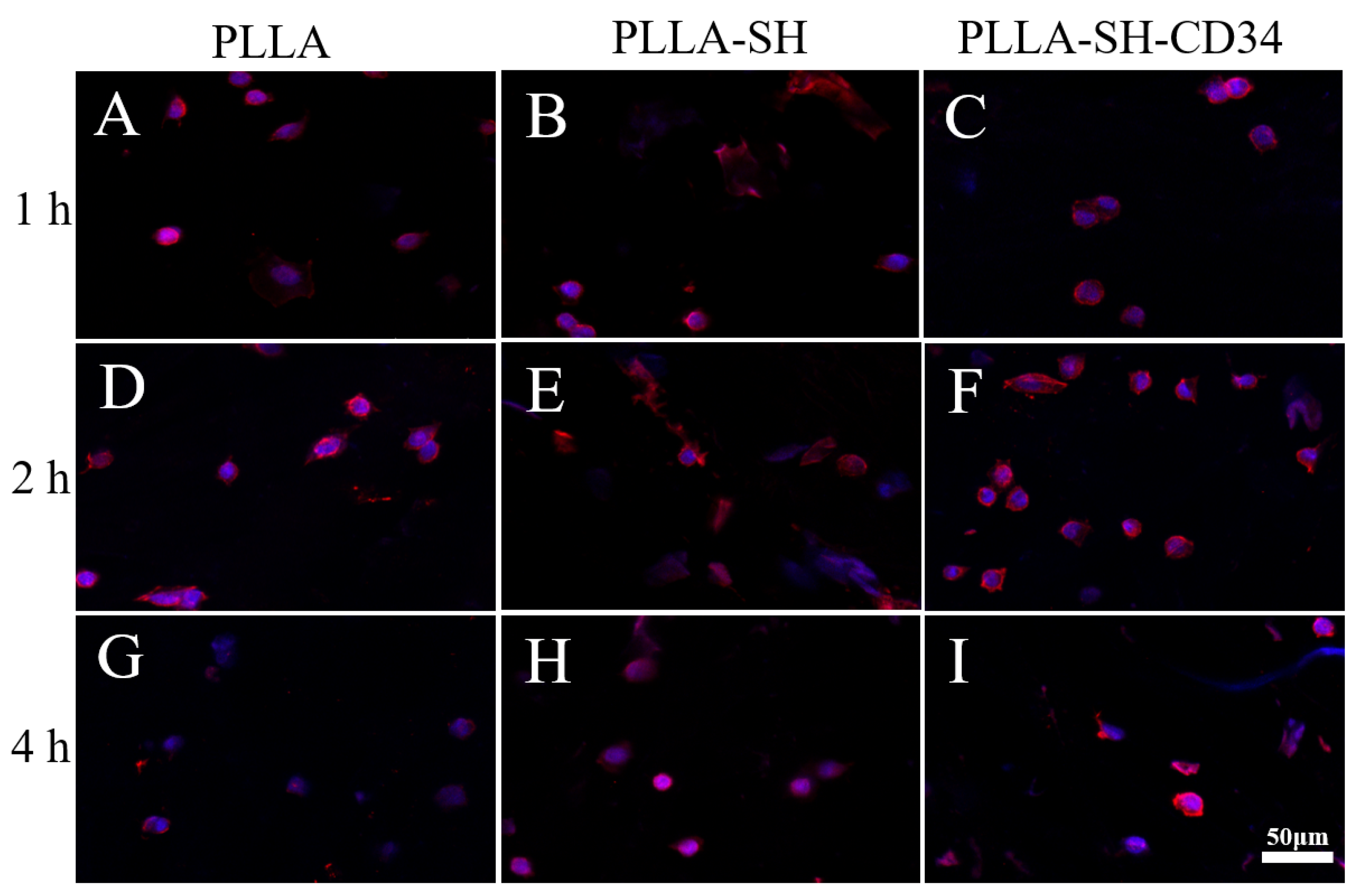
3.4. Cytocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Bialkowski, J.; Szkutnik, M.; Rusingiza, E. Report from a visit to the cardiology centres in Kigali, Rwanda. Adv. Interv. Cardiol./Postępy Kardiol. Interwencyjnej 2022, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.B.; Brindis, R.G.; Krucoff, M.W.; Mansalis, B.P.; Carroll, J.D. Percutaneous atrial septal occluder devices and cardiac erosion: A review of the literature. Catheter. Cardiovasc. Interv. 2012, 80, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, Z.; Wang, Q.; Chen, X.; Liu, Q.; Wang, W.; Shen, Y.; Liu, J.; Li, A.; Li, Y. Biodegradable polymeric occluder for closure of atrial septal defect with interventional treatment of cardiovascular disease. Biomaterials 2021, 274, 120851. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-based biocompatible nanoparticles for next-generation tolerogenic vaccines against autoimmune disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Hu, J.; Wang, F.; Fu, D.; Luo, R.; Zhang, F.; Hu, C.; Chen, J.; Pan, X.; Yang, L. A fully degradable transcatheter ventricular septal defect occluder: Towards rapid occlusion and post-regeneration absorption. Biomaterials 2022, 291, 121909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Yang, L.; Luo, R.; Guo, G. Development of Innovative Biomaterials and Devices for the Treatment of Cardiovascular Diseases. Adv. Mater. 2022, 34, 2201971. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen I: An in vitro study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Popelka, A.; Abdulkareem, A.; Mahmoud, A.A.; Nassr, M.G.; Al-Ruweidi, M.K.A.; Mohamoud, K.J.; Hussein, M.K.; Lehocky, M.; Vesela, D.; Humpolíček, P. Antimicrobial modification of PLA scaffolds with ascorbic and fumaric acids via plasma treatment. Surf. Coat. Technol. 2020, 400, 126216. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Wu, H.; He, Q.; Li, L.; Li, L.; Zhou, Z.; Chen, N.; Yang, M.; Luo, Q.; Zhang, B.; Luo, R. A facile and versatile superhydrophilic coating on biodegradable PLA stent with stepwise assembly of metal/phenolic networks for mimicking endothelium function. Chem. Eng. J. 2022, 427, 130932. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Li, W.; Di, Y.; Xing, Q.; Cao, Q. 3D printing and biocompatibility study of a new biodegradable occluder for cardiac defect. J. Cardiol. 2019, 74, 182–188. [Google Scholar] [CrossRef]
- Jiang, N.; Jia, B. Progress of biodegradable materials for occlusion devices. Ann. Med. Surg. 2022, 103745. [Google Scholar] [CrossRef]
- Abreu-Rejón, A.D.; Herrera-Kao, W.A.; May-Pat, A.; Ávila-Ortega, A.; Rodríguez-Fuentes, N.; Uribe-Calderón, J.A.; Cervantes-Uc, J.M. Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts. Polymers 2022, 14, 4912. [Google Scholar] [CrossRef]
- Lin, Q.; Ding, X.; Qiu, F.; Song, X.; Fu, G.; Ji, J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin–collagen multilayer. Biomaterials 2010, 31, 4017–4025. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing risk of failure from ceramic-on-ceramic total hip prosthesis by selecting ceramic materials based on tresca stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Yin, M.; Yuan, Y.; Liu, C.; Wang, J. Combinatorial coating of adhesive polypeptide and anti-CD34 antibody for improved endothelial cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2009, 20, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, M.; Saeinasab, M.; Zhang, W.; Zhang, X.; Min Tsui, K.; Maasoumi Sarvestani, A.; Nawaz, S.; Coates, P.; Youseffi, M.; Elies, J. Investigation of Cell Adhesion and Cell Viability of the Endothelial and Fibroblast Cells on Electrospun PCL, PLGA and Coaxial Scaffolds for Production of Tissue Engineered Blood Vessel. J. Funct. Biomater. 2022, 13, 282. [Google Scholar] [CrossRef]
- Baiguera, S.; Ribatti, D. Endothelialization approaches for viable engineered tissues. Angiogenesis 2013, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Kinstlinger, I.S.; Calderon, G.A.; Royse, M.K.; Means, A.K.; Grigoryan, B.; Miller, J.S. Perfusion and endothelialization of engineered tissues with patterned vascular networks. Nat. Protoc. 2021, 16, 3089–3113. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T. Isolation of putative endothelial progenitor cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Khakoo, A.Y.; Finkel, T. Endothelial progenitor cells. Annu. Rev. Med. 2005, 56, 79. [Google Scholar] [CrossRef]
- Nakazawa, G.; Granada, J.F.; Alviar, C.L.; Tellez, A.; Kaluza, G.L.; Guilhermier, M.Y.; Parker, S.; Rowland, S.M.; Kolodgie, F.D.; Leon, M.B. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 2010, 3, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-X.; Wan, C.-X.; Chen, H.-Q. Preparation and endothelialization of decellularised vascular scaffold for tissue-engineered blood vessel. J. Mater. Sci. Mater. Med. 2008, 19, 319–326. [Google Scholar]
- Cardinal, K.O.H.; Bonnema, G.T.; Hofer, H.; Barton, J.K.; Williams, S.K. Tissue-engineered vascular grafts as in vitro blood vessel mimics for the evaluation of endothelialization of intravascular devices. Tissue Eng. 2006, 12, 3431–3438. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Su, Y.; Ma, L.; Zhu, S.; Zheng, Y.; Guan, S. Sol-gel coating loaded with inhibitor on ZE21B Mg alloy for improving corrosion resistance and endothelialization aiming at potential cardiovascular application. Colloids Surf. B Biointerfaces 2021, 207, 111993. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, K.; Wojasiński, M.; Eichler, M.; Genç, H.; Friedrich, R.P.; Stein, R.; Singh, R.; Alexiou, C.; Hlawaty, H.; Ciach, T. Polydopamine and gelatin coating for rapid endothelialization of vascular scaffolds. Biomater. Adv. 2022, 134, 112544. [Google Scholar] [CrossRef] [PubMed]



| Samples | C (%) | O (%) | N (%) |
|---|---|---|---|
| PLLA | 67.8 | 30.38 | 1.83 |
| PLLA-NH2 | 68.04 | 20.38 | 11.58 |
| PLLA-SH5 | 67.24 | 24.23 | 8.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, B.; Li, X.; Fan, S.; He, J.; Ge, X.; Li, H.; Chen, C.; Wang, Z.; Wang, S.; Li, B. CD34 Antibody-Coated Biodegradable Fiber Membrane Effectively Corrects Atrial Septal Defect (ASD) by Promoting Endothelialization. Polymers 2023, 15, 108. https://doi.org/10.3390/polym15010108
Chu B, Li X, Fan S, He J, Ge X, Li H, Chen C, Wang Z, Wang S, Li B. CD34 Antibody-Coated Biodegradable Fiber Membrane Effectively Corrects Atrial Septal Defect (ASD) by Promoting Endothelialization. Polymers. 2023; 15(1):108. https://doi.org/10.3390/polym15010108
Chicago/Turabian StyleChu, Bin, Xiaoli Li, Shiqiang Fan, Jinmei He, Xiaohong Ge, Hui Li, Changsheng Chen, Zhen Wang, Song Wang, and Boning Li. 2023. "CD34 Antibody-Coated Biodegradable Fiber Membrane Effectively Corrects Atrial Septal Defect (ASD) by Promoting Endothelialization" Polymers 15, no. 1: 108. https://doi.org/10.3390/polym15010108





