Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Protein Extraction Processes from Defatted Chickpea Flour
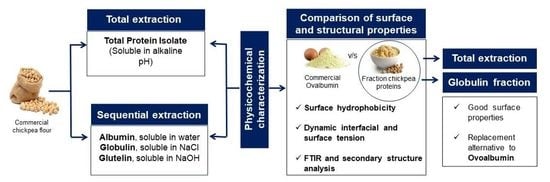
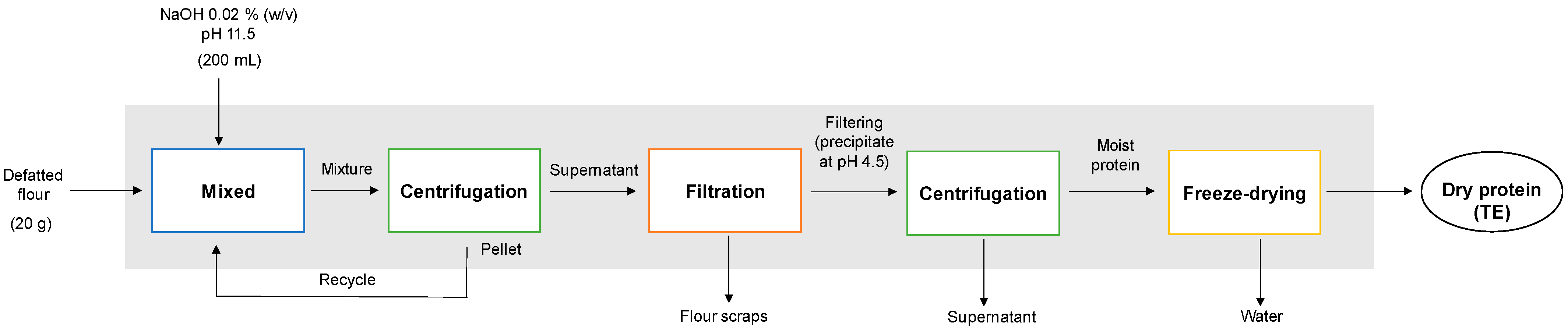
2.2.1. Total Extraction (TE)
2.2.2. Sequential Extraction (SE) of Proteins from Chickpea Flour
2.2.3. Moisture Content and Water Activity of Freeze-Dried Proteins
2.2.4. Protein Extraction Yield
2.3. Physicochemical Characterization of the Protein Fractions
2.3.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3.2. Amino Acid Analysis by High-Performance Liquid Chromatography (HPLC)
2.3.3. Zeta Potential of Protein Dispersions
2.4. Surface and Structural Properties of Protein Dispersion
2.4.1. Surface Hydrophobicity
2.4.2. Dynamic Interfacial (DIT) and Surface Tension (DST) Measurements
- (i)
- At t = 0 → * = eq and = 0
- (ii)
- At t = ∞ → * = 0 and = eq
2.4.3. Structural Characterization by Fourier Transform Infrared-Attenuated Total Reflectance (FTIR-ATR) Spectra Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Protein Extraction Processes
3.1.1. Effect of Process Parameters in Protein Fractions
3.1.2. Protein Extraction Yield
3.2. Physicochemical Characterization of Total and Sequential Protein Extracted from Chickpea Flour
3.2.1. Electrophoretic Pattern of Chickpea Protein Fraction Evaluated by SDS-PAGE
3.2.2. Amino Acid Profile of Extracted Chickpea Proteins
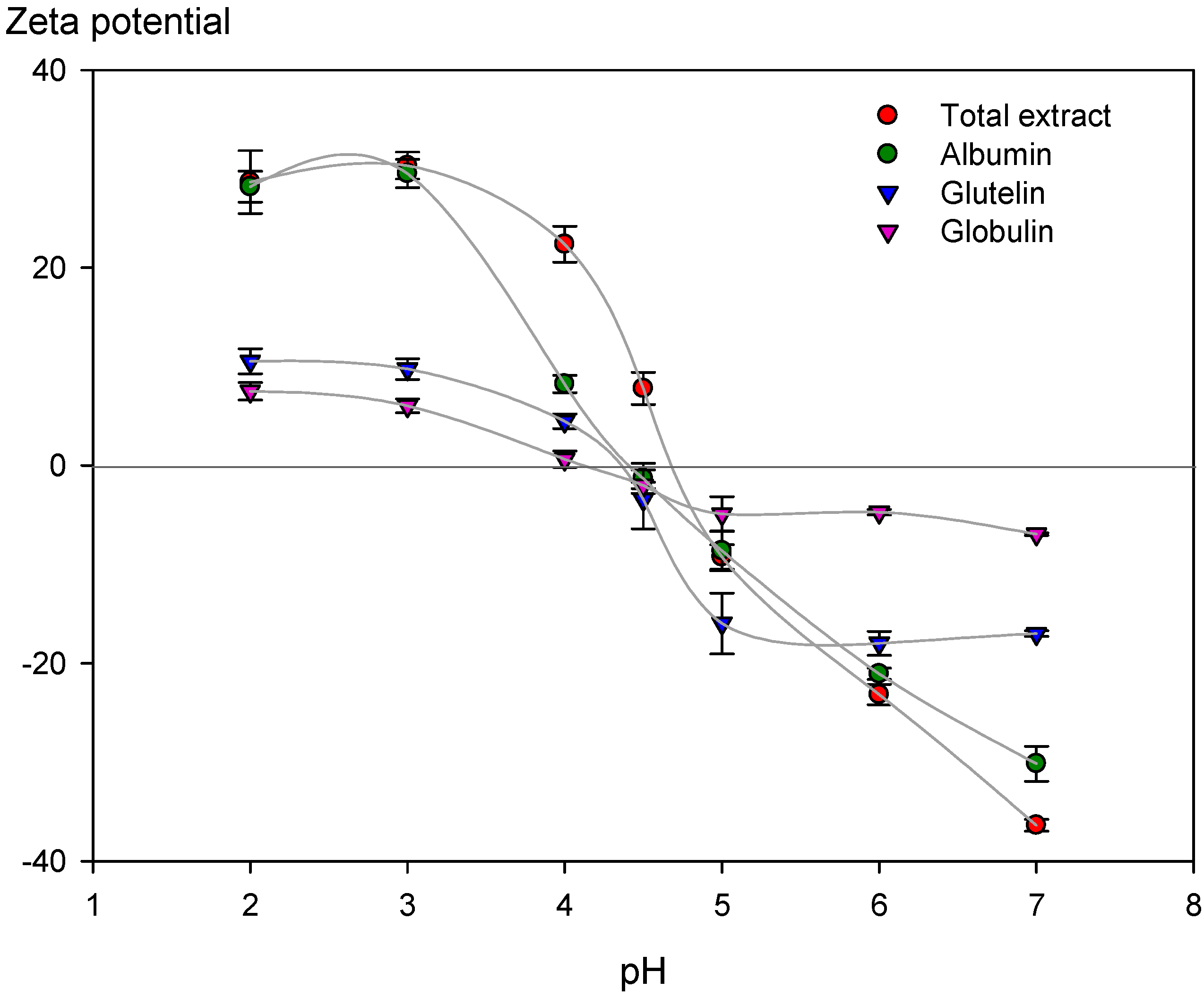
3.2.3. Zeta Potential of Extracted Chickpea Proteins
3.3. Surface and Structural Properties of Vegetable Protein Dispersion Compared to Animal Protein: Ovalbumin
3.3.1. Surface Hydrophobicity
3.3.2. Dynamic Interfacial (DIT) and Surface Tension (DST)
3.3.3. Structural Properties: Secondary Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)-A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Chang, Y.; Alli, I.; Konishi, Y.; Ziomek, E. Characterization of protein fractions from Chickpea (Cicer arietinum L.) and oat (Avena sativa L.) seeds using proteomic techniques. Food Res. Int. 2011, 44, 3094–3104. [Google Scholar] [CrossRef]
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A. Transglutaminase modifies the physical stability and digestibility of chickpea protein-stabilized oil-in-water emulsions. Food Chem. 2020, 315, 126301. [Google Scholar] [CrossRef]
- Xu, Y.; Obielodan, M.; Sismour, E.; Arnett, A.; Alzahrani, S.; Zhang, B. Physicochemical, functional, thermal and structural properties of isolated Kabuli chickpea proteins as affected by processing approaches. Int. J. Food Sci. Technol. 2017, 52, 1147–1154. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Mukesh, J.; Joshi, S.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Clemente, A.; Vioque, J.; Bautista, J.; Millán, F. Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chem. 1999, 64, 237–243. [Google Scholar] [CrossRef]
- Castellión, M.; Matiacevich, S.; Buera, P.; Maldonado, S. Protein deterioration and longevity of quinoa seeds during long-term storage. Food Chem. 2010, 121, 952–958. [Google Scholar] [CrossRef]
- Yang, J.; Sagis, L.M. Interfacial behavior of plant proteins—Novel sources and extraction methods. Curr. Opin. Colloid Interface Sci. 2021, 56, 101499. [Google Scholar] [CrossRef]
- Osborne, T.B. The vegetable proteins. In Monographs in Biochemistry; Longmans, Green and Company: London, UK, 1924. [Google Scholar]
- Agboola, S.; Ng, D.; Mills, D. Characterisation and functional properties of Australian rice protein isolates. J. Cereal Sci. 2005, 41, 283–290. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse proteins: From processing to structure-function relationships. In Grain Legumes; Kumar Goyal, A., Ed.; IntechOpen: London, UK, 2016; Volume 3, pp. 55–78. [Google Scholar]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.B.; Chen, B.; Rao, J. Plant proteins from green pea and chickpea: Extraction, fractionation, structural characterization and functional properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Lam AC, Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Thareja, P.; Saraswat, Y.C.; Oberoi, C. Ovalbumin-stabilized concentrated emulsion gels. Bull. Mater. Sci. 2020, 43, 194. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Niu, P.; Sha, X.M. Promotion of foam properties of egg white protein by subcritical water pre-treatment and fish scales gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 171–177. [Google Scholar] [CrossRef]
- Karaca, A.C.; Nickerson, M.; Low, N.H. Microcapsule production employing chickpea or lentil protein isolates and maltodextrin: Physicochemical properties and oxidative protection of encapsulated flaxseed oil. Food Chemistry 2013, 139, 448–457. [Google Scholar] [CrossRef]
- Chang, Y.W.; Alli, I.; Molina, A.T.; Konishi, Y.; Boye, J.I. Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food Bioprocess Technol. 2012, 5, 618–625. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.S.; van Nispen, J.W.; Melgers, P.A.; Van den Bogaart HW, M.; Hamelinck RL, A.E.; Goverde, B.C. HPLC analysis of phenylthiocarbamyl (PTC) amino acids. I. Evaluation and optimization of the procedure. Chromatographia 1986, 22, 345–350. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Wet-milled chickpea coproduct as an alternative to obtain protein isolates. LWT—Food Sci. Technol. 2019, 115, 108468. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. et Biophys. Acta (BBA)-Protein Struct. 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Bahtz, J.; Knorr, D.; Tedeschi, C.; Leser, M.E.; Valles-Pamies, B.; Miller, R. Adsorption of octanoic acid at the water/oil interface. Colloids Surf. B Biointerfaces 2009, 74, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Dapueto, N.; Troncoso, E.; Mella, C.; Zúñiga, R.N. The effect of denaturation degree of protein on the microstructure, rheology and physical stability of oil-in-water (O/W) emulsions stabilized by whey protein isolate. J. Food Eng. 2019, 263, 253–261. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Skurtys, O.; Osorio, F.; Aguilera, J.M.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef]
- Azuara, E.; Cortes, R.; Garcia, H.S.; Beristain, C.I. Kinetic model for osmotic dehydration and its relationship with Fick’s second law. Int. J. Food Sci. Technol. 1992, 27, 409–418. [Google Scholar] [CrossRef]
- Moyano, P.C.; Pedreschi, F. Kinetics of oil uptake during frying of potato slices: Effect of pre-treatments. LWT-Food Sci. Technol. 2006, 39, 285–291. [Google Scholar] [CrossRef]
- Lan, Y.; Xu, M.; Ohm, J.B.; Chen, B.; Rao, J. Solid dispersion-based spray-drying improves solubility and mitigates beany flavour of pea protein isolate. Food Chem. 2019, 278, 665–673. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; de Carvalho Balieiro, J.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Tang, J.; Yang, T. Dehydrated vegetables: Principles and systems. In Handbook of Vegetable Preservation and Processing, 2nd ed.; Hui, A.H., Ghazala, S., Graham, D.M., Murrell, K.D., Nip, W.K., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 335–372. [Google Scholar]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Casey, R.; Domoney, C.; Smith, A.M. Peas: Genetics, molecular biology and biotechnology. In Biochemistry and Molecular Biology of Seed Products; Casey, R., Davies, D.R., Eds.; John Innes Institute: Norwich, UK, 1993; Volume 10, pp. 121–163. [Google Scholar]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Franco, E.; Ferreira, R.B.; Teixeira, A.R. Utilization of an improved methodology to isolate Lupinus albus conglutins in the study of their sedimentation coefficients. J. Agric. Food Chem. 1997, 45, 3908–3913. [Google Scholar] [CrossRef]
- Liu, L.H.; Hung, T.V.; Bennett, L. Extraction and characterization of chickpea (Cicer arietinum) albumin and globulin. J. Food Sci. 2008, 73, C299–C305. [Google Scholar] [CrossRef]
- Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Bautista, J.; Millán, F. Purification and partial characterization of chickpea 2S albumin. J. Agric. Food Chem. 1999, 47, 1405–1409. [Google Scholar] [CrossRef]
- Clemente, A.; Vioque, J.; Sánchez-Vioque, R.; Pedroche, J.; Bautista, J.; Millán, F. Factors affecting the in vitro protein digestibility of chickpea albumins. J. Sci. Food Agric. 2000, 80, 79–84. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of drying methods on physicochemical and functional properties of chickpea protein concentrates. J. Food Eng. 2015, 165, 179–188. [Google Scholar] [CrossRef]
- FAO/WHO. Protein quality evaluation. In Report of Joint FAO/WHO Expert Consultation; FAO/WHO: Bethesda, MD, USA; Rome, Italy, 1989. [Google Scholar]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108, 63–71. [Google Scholar] [CrossRef]
- Sagis, L.M.; Yang, J. Protein-stabilized interfaces in multiphase food: Comparing structure-function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Lunkad, R.; Barroso da Silva, F.L.; Košovan, P. Both charge-regulation and charge-patch distribution can drive adsorption on the wrong side of the isoelectric point. J. Am. Chem. Soc. 2022, 144, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Vani, B.; Zayas, J.F. Wheat germ protein flour solubility and water retention. J. Food Sci. 1995, 60, 845–848. [Google Scholar] [CrossRef]
- Zhu, Z.; Mao, X.; Wu, Q.; Zhang, J.; Deng, X. Effects of oxidative modification of peroxyl radicals on the structure and foamability of chickpea protein isolates. J. Food Sci. 2021, 86, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Judge, R.A.; Johns, M.R.; White, E.T. Solubility of ovalbumin in ammonium sulfate solutions. J. Chem. Eng. Data 1996, 41, 422–424. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Giron-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of ace inhibitory peptides by digestion of chickpea legumin with alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar] [CrossRef]
- Aider, M.; Djenane, D.; Ounis, W.B. Amino acid composition, foaming, emulsifying properties and surface hydrophobicity of mustard protein isolate as affected by pH and NaCl. Int. J. Food Sci. Technol. 2012, 47, 1028–1036. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Vedantham, G.; Sparks, H.G.; Sane, S.U.; Tzannis, S.; Przybycien, T.M. A holistic approach for protein secondary structure estimation from infrared spectra in H2O solutions. Anal. Biochem. 2000, 285, 33–49. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. A distinct utility of the amide III infrared band for secondary structure estimation of aqueous protein solutions using partial least squares methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Velikov, K.P.; Velev, O.D. Stabilization of thin films, foams, emulsions and bifluid gels with surface-active solid particles. In Colloid Stability and Application in Pharmacy, 1st ed.; Tadros, T., Ed.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2007; Volume 3, pp. 277–306. [Google Scholar]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A Review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef]
- Sigma-Aldrich. IR Spectrum Table by Frequency Range. 1924. Available online: https://www.sigmaaldrich.com/CL/es/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 30 October 2022).
- Kudre, T.G.; Benjakul, S.; Kishimura, H. Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean, and Bambara groundnut. J. Sci. Food Agric. 2013, 93, 2429–2436. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. et Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Schwinte, P.; Voegel, J.C.; Picart, C.; Haikel, Y.; Schaaf, P.; Szalontai, B. Stabilizing effects of various polyelectrolyte multilayer films on the structure of adsorbed/embedded fibrinogen Molecules: An ATR−FTIR Study. J. Phys. Chem. B 2001, 105, 11906–11916. [Google Scholar] [CrossRef]
- de la Rosa-Millán, J.; Orona-Padilla, J.L.; Flores-Moreno, V.M.; Serna-Saldívar, S.O. Physicochemical, functional and ATR-FTIR molecular analysis of protein extracts derived from starchy pulses. Int. J. Food Sci. Technol. 2018, 53, 1414–1424. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, R.; Xu, X.; Zhou, G.; Liu, D. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, S.; Luo, M.; Ning, F.; Zhu, X.; Xiong, H. Preparation and self-assembly mechanism of Bovine Serum Albumin–Citrus peel pectin conjugated hydrogel: A potential delivery system for Vitamin C. J. Agric. Food Chem. 2016, 64, 7377–7384. [Google Scholar] [CrossRef] [PubMed]
- Gülseren, İ.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S.; Hu, Y.; Zhou, M.; Wang, C.; Li, D.; Li, D. Impact of hot alkali modification conditions on secondary structure of peanut protein and embedding rate of curcumin. Food Sci. Hum. Wellness 2019, 8, 283–291. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singha, P.; Singh, S.K. Comparison of hydrodynamic and acoustic cavitation’s effect on functional, rheological, and structural properties of egg white proteins. Innov. Food Sci. Emerg. Technol. 2022, 82, 103166. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, S.; Wu, C.; Qi, B.; Teng, F.; Wang, Z.; Li, Y.; Jiang, L. The investigation of protein flexibility of various soybean cultivars in relation to physicochemical and conformational properties. Food Hydrocoll. 2020, 103, 105709. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT—Food Sci. Technol. 2021, 142, 110881. [Google Scholar] [CrossRef]





| Sample | Moisture (%) | aw |
|---|---|---|
| Total extraction | 3.65 d ± 0.11 | 0.22 c ± 0.030 |
| Albumin | 1.24 a ± 0.03 | 0.05 a ± 0.004 |
| Globulin | 1.91 c ± 0.44 | 0.10 ab ± 0.020 |
| Glutelin | 1.87 bc ± 0.29 | 0.15 b ± 0.004 |
| Sample | Yield Dry Weight (%) |
|---|---|
| Total extraction | 78.0 c ± 1.0 |
| Total sequential extraction | 85.0 c ± 5.7 |
| Albumin | 69.3 b ± 4.4 |
| Globulin | 8.7 a ± 1.0 |
| Glutelin | 7.1 a ± 0.9 |
| Amino Acid | Total Extraction (g/100 g Protein) | Albumin (g/100 g Protein) | Globulin (g/100 g Protein) | Glutelin (g/100 g Protein) |
|---|---|---|---|---|
| Isoleucine | 2.95 d ± 0.01 | 2.72 c ± 0.07 | 2.23 a ± 0.01 | 2.51 b ± 0.01 |
| Leucine | 5.40 d ± 0.08 | 4.99 c ± 0.11 | 3.82 a ± 0.07 | 4.55 b ± 0.11 |
| Threonine | 2.54 ab ± 0.04 | 2.44 a ± 0.08 | 2.43 a ± 0.05 | 2.83 b ± 0.13 |
| Valine | 2.69 b ± 0.09 | 2.62 b ± 0.09 | 2.15 a ± 0.08 | 2.56 b ± 0.07 |
| Phenylalanine | 4.09 c ± 0.05 | 3.30 b ± 0.01 | 2.65 a ± 0.00 | 3.27 b ± 0.00 |
| Methionine | 0.88 b ± 0.01 | 0.71 a ± 0.02 | 0.70 a ± 0.01 | 0.76 ab ± 0.10 |
| Histidine | 1.51 b ± 0.05 | 1.53 b ± 0.08 | 1.04 a ± 0.01 | 1.37 b ± 0.16 |
| Lysine | 5.35 b ± 0.23 | 3.83 a ± 0.11 | 3.75 a ± 0.20 | 4.02 a ± 0.13 |
| Total essential amino acids | 25.4 c ± 0.10 | 22.14 b ± 0.69 | 18.76 a ± 0.06 | 21.86 b ± 0.46 |
| Essential amino acids (%) | 33.61 a ± 0.26 | 32.85 a ± 1.62 | 35.91 b ± 0.05 | 35.92 b ± 0.17 |
| Aspartic acid | 10.74 c ± 0.46 | 10.00 c ± 0.11 | 7.01 a ± 0.04 | 7.86 b ± 0.20 |
| Cysteine | 1.87 b ± 0.27 | 1.27 a ± 0.00 | 1.01 a ± 0.05 | 1.35 a ± 0.07 |
| Glutamic acid | 14.54 c ± 0.33 | 11.51 c ± 1.30 | 8.31 a ± 0.57 | 9.74 b ± 0.14 |
| Alanine | 3.20 a ± 0.13 | 3.15 a ± 0.10 | 2.92 a ± 0.02 | 3.16 a ± 0.06 |
| Serine | 4.63 c ± 0.26 | 4.38 bc ± 0.18 | 3.30 a ± 0.08 | 3.96 b ± 0.09 |
| Glycine | 3.07 a ± 0.16 | 3.11 a ± 0.18 | 2.26 a ± 0.09 | 3.14 a ± 0.03 |
| Tyrosine | 1.53 a ± 0.13 | 1.22 a ± 0.05 | 1.29 a ± 0.05 | 1.56 a ± 0.17 |
| Arginine | 7.46 b ± 0.38 | 6.91 b ± 0.02 | 4.42 a ± 0.67 | 4.91 a ± 0.13 |
| Proline | 3.14 b ± 0.29 | 3.73 c ± 0.07 | 2.61 a ± 0.08 | 3.30 bc ± 0.03 |
| Total non-essential amino acids | 50.17 d ± 0.38 | 45.28 a ± 1.93 | 33.48 b ± 0.17 | 38.99 c ± 0.53 |
| Non-essential amino acids (%) | 66.39 b ± 0.26 | 67.17 b ± 1.62 | 64.09 a ± 0.05 | 64.08 a ± 0.17 |
| Interfacial Pressure (mN/m) | Surface Pressure (mN/m) | |||||
|---|---|---|---|---|---|---|
| Sample | k (s−1) | Πeq (mN/m) | RMS (%) | k (s−1) | Πeq (mN/m) | RMS (%) |
| Ovalbumin | 0.0099 c ± 0.0003 | 12.88 a ± 0.23 | 3.37 | 0.0123 d ± 0.0010 | 26.96 a ± 0.59 | 3.40 |
| Total extraction | 0.0038 ab ± 0.0006 | 15.97 b ± 0.17 | 5.60 | 0.0040 a ± 0.0001 | 31.46 c ± 0.10 | 3.87 |
| Albumin | 0.0033 a ± 0.0004 | 16.16 b ± 0.15 | 5.31 | 0.0064 bc ± 0.0009 | 29.12 b ± 0.61 | 2.75 |
| Globulin | 0.0046 b ± 0.0007 | 19.70 c ± 0.27 | 3.89 | 0.0049 ab ± 0.0006 | 32.01 c ± 0.48 | 3.32 |
| Glutelin | 0.0029 a ± 0.0002 | 18.12 d ± 0.53 | 1.17 | 0.0071 c ± 0.0009 | 34.29 d ± 0.42 | 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Madrid, D.; Pérez, N.; Gutiérrez-Cutiño, M.; Matiacevich, S.; Zúñiga, R.N. Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions. Polymers 2023, 15, 110. https://doi.org/10.3390/polym15010110
Soto-Madrid D, Pérez N, Gutiérrez-Cutiño M, Matiacevich S, Zúñiga RN. Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions. Polymers. 2023; 15(1):110. https://doi.org/10.3390/polym15010110
Chicago/Turabian StyleSoto-Madrid, Daniela, Nicole Pérez, Marlen Gutiérrez-Cutiño, Silvia Matiacevich, and Rommy N. Zúñiga. 2023. "Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions" Polymers 15, no. 1: 110. https://doi.org/10.3390/polym15010110
APA StyleSoto-Madrid, D., Pérez, N., Gutiérrez-Cutiño, M., Matiacevich, S., & Zúñiga, R. N. (2023). Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions. Polymers, 15(1), 110. https://doi.org/10.3390/polym15010110