Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis Methodology and Equipment
2.2. Screening Phase for Extraction of Hemicellulose from Wheat Straw
2.3. Experimental Design of Alkaline Extraction of Hemicelluloses from Freeze–Thaw Pretreated Wheat Straw
2.4. Separation and Characterization of Extracted Hemicelluloses
3. Results and Discussion
3.1. Wheat Straw Chemical Composition
3.2. Screening Phase for Extraction of Hemicellulose from Wheat Straw
3.3. The Influence of Extraction Parameters
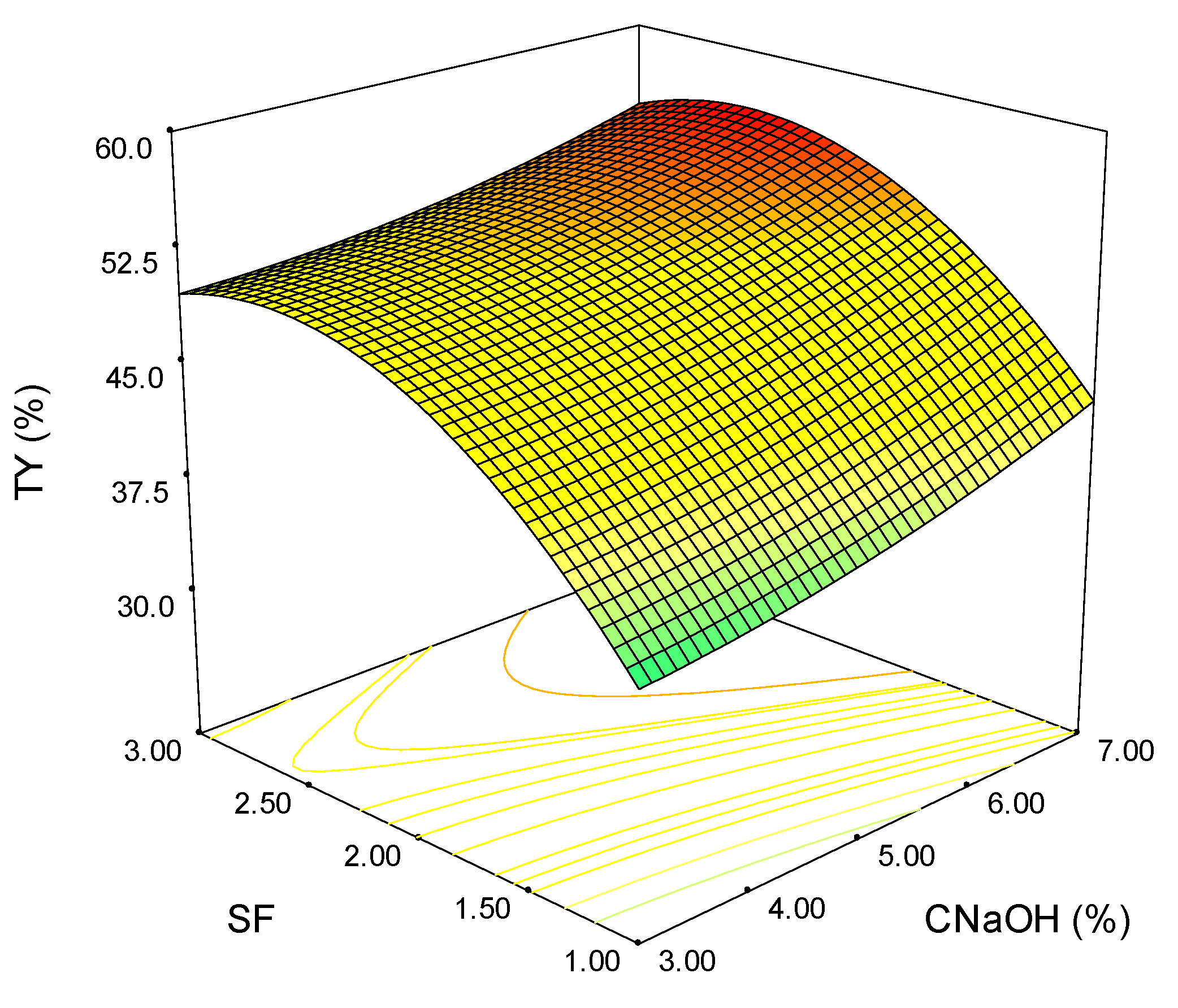
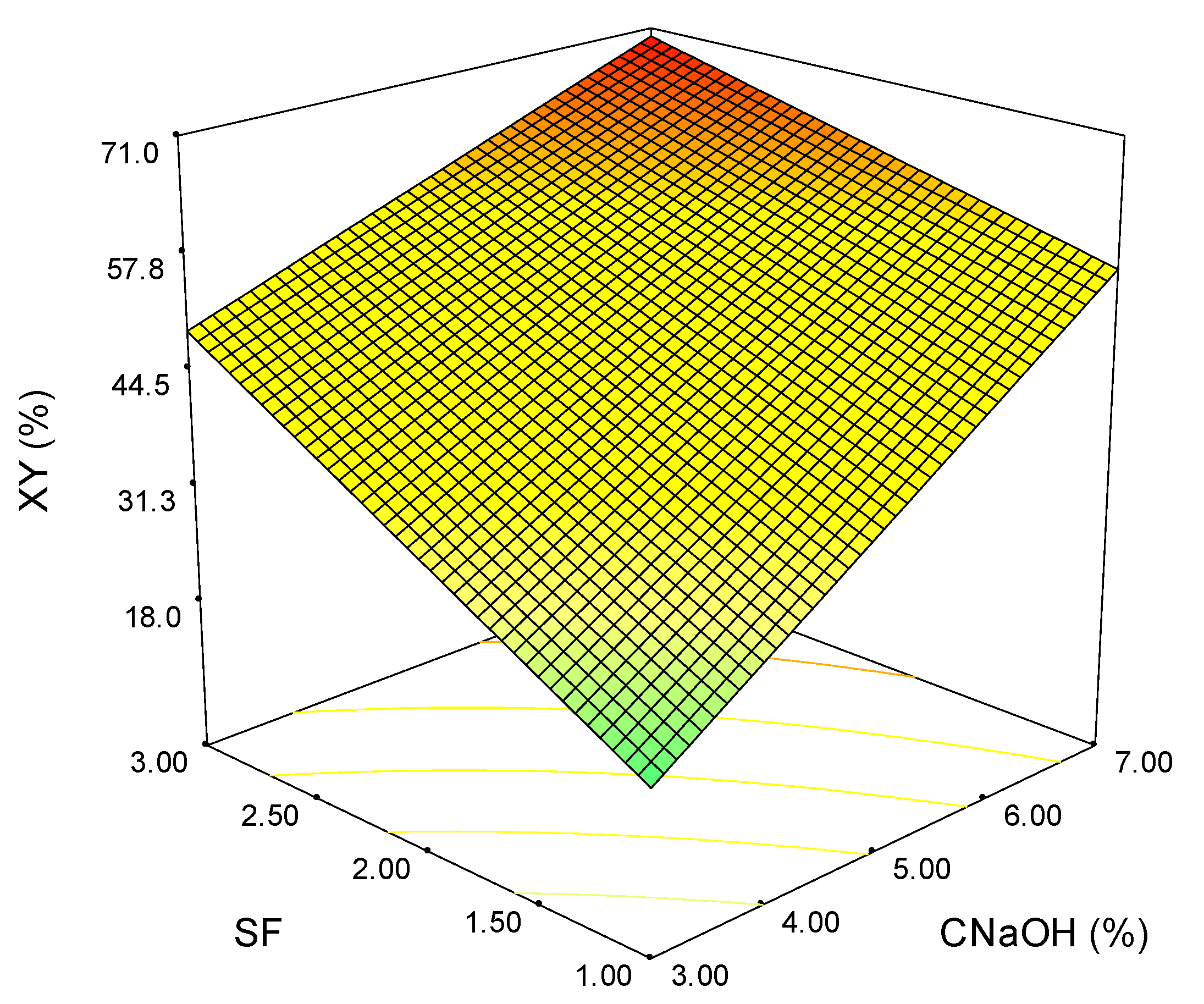
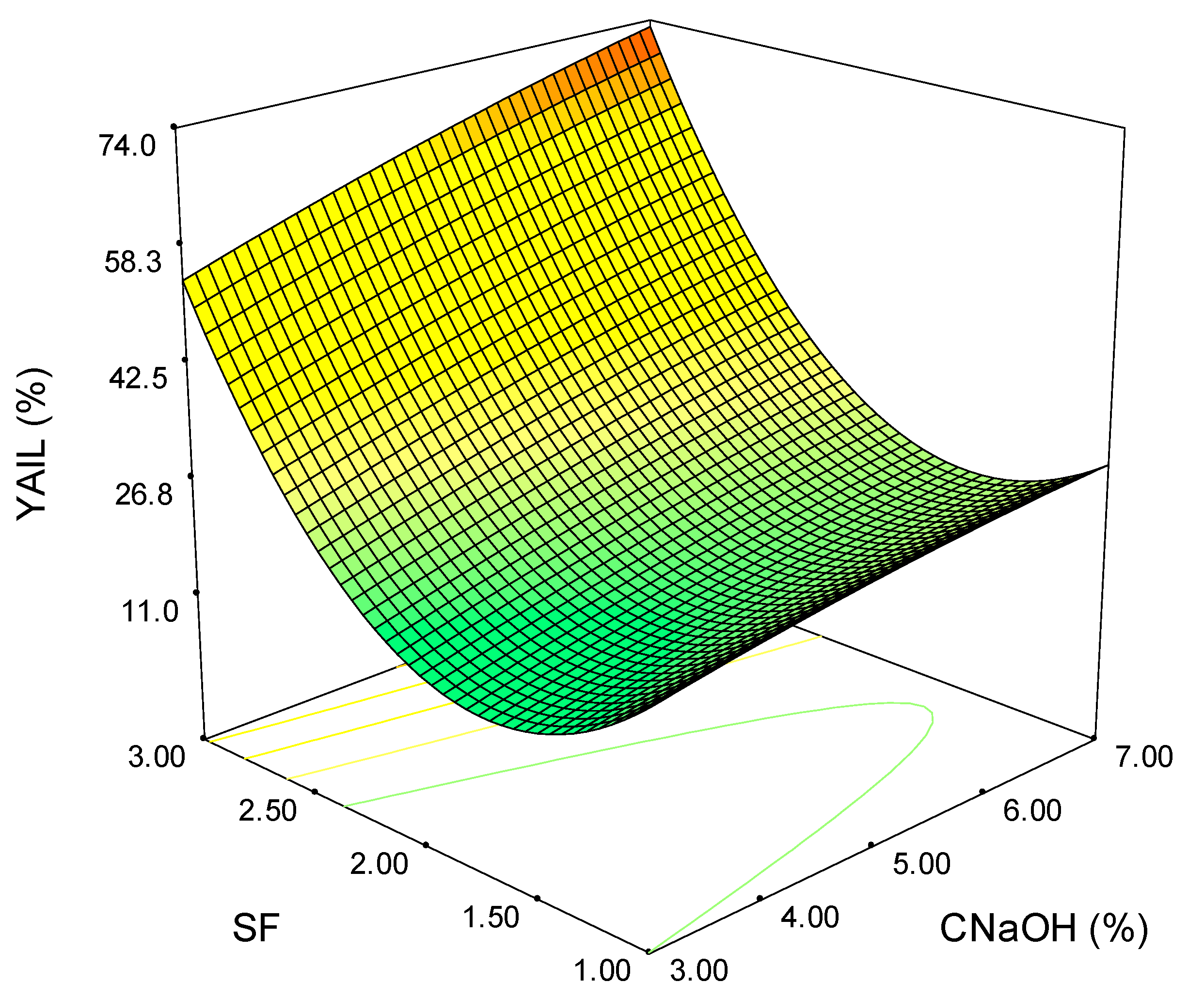
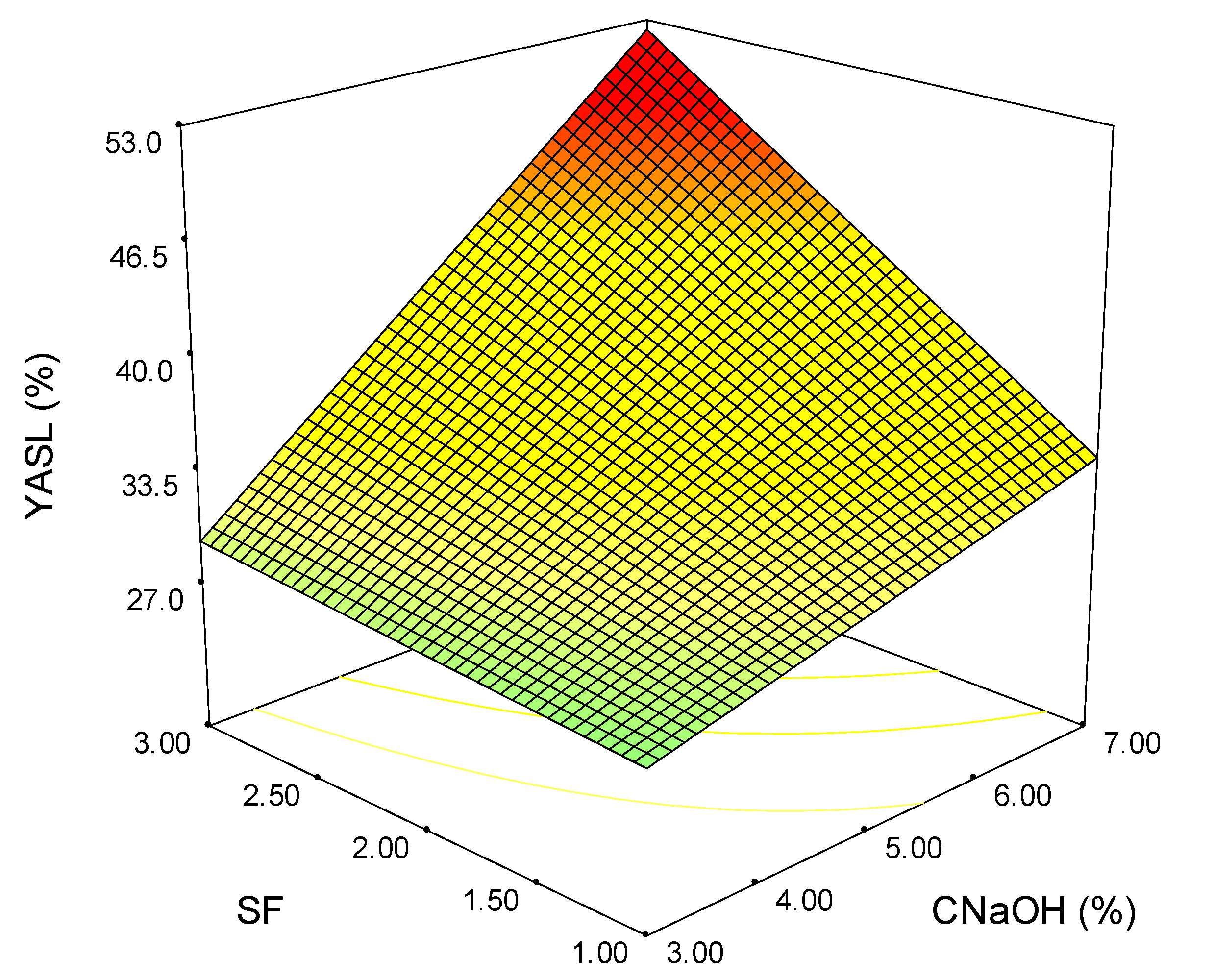
3.4. Process Optimization
3.5. Product Characterization
3.5.1. Component Identification
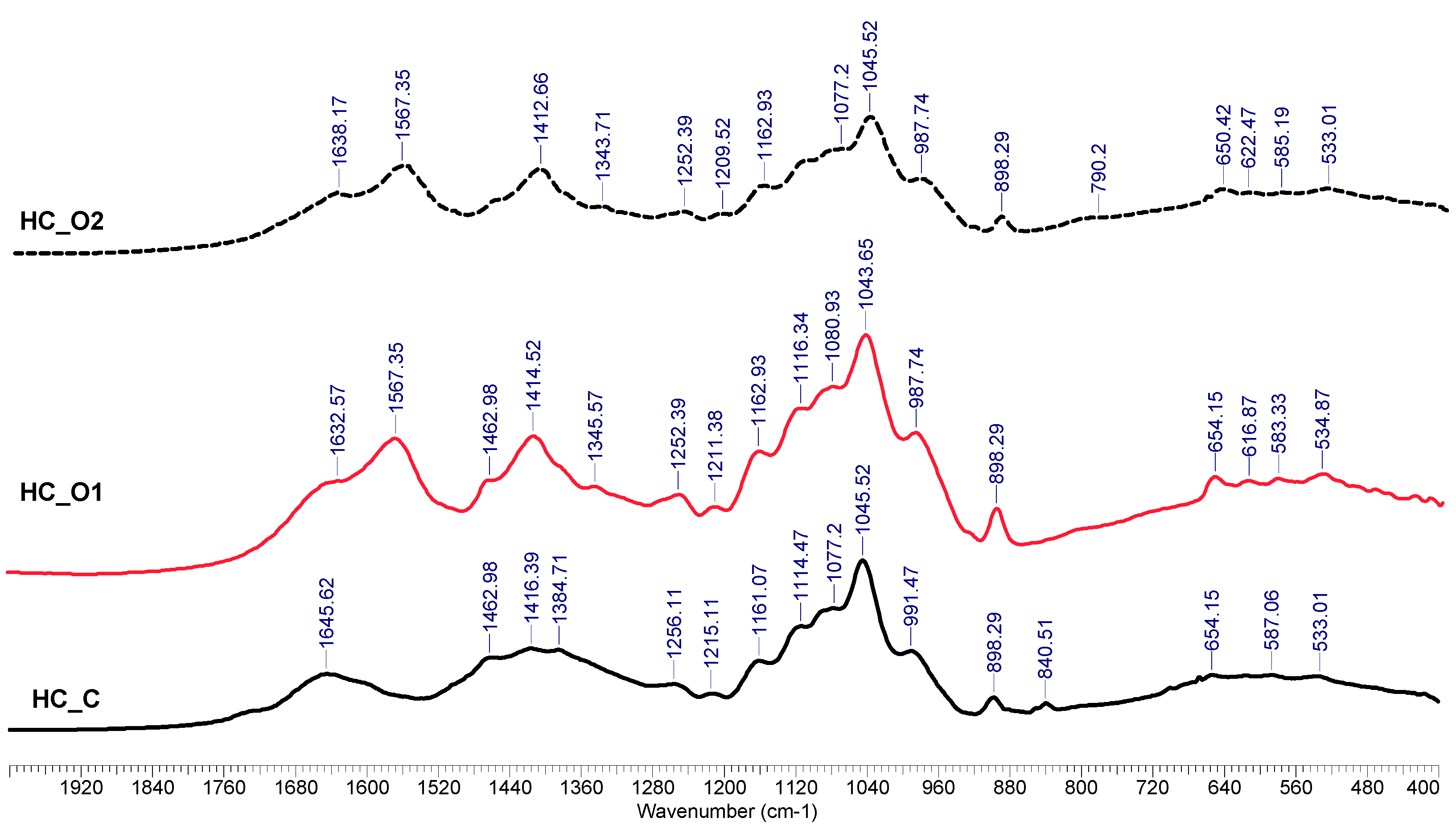
3.5.2. FTIR Analysis
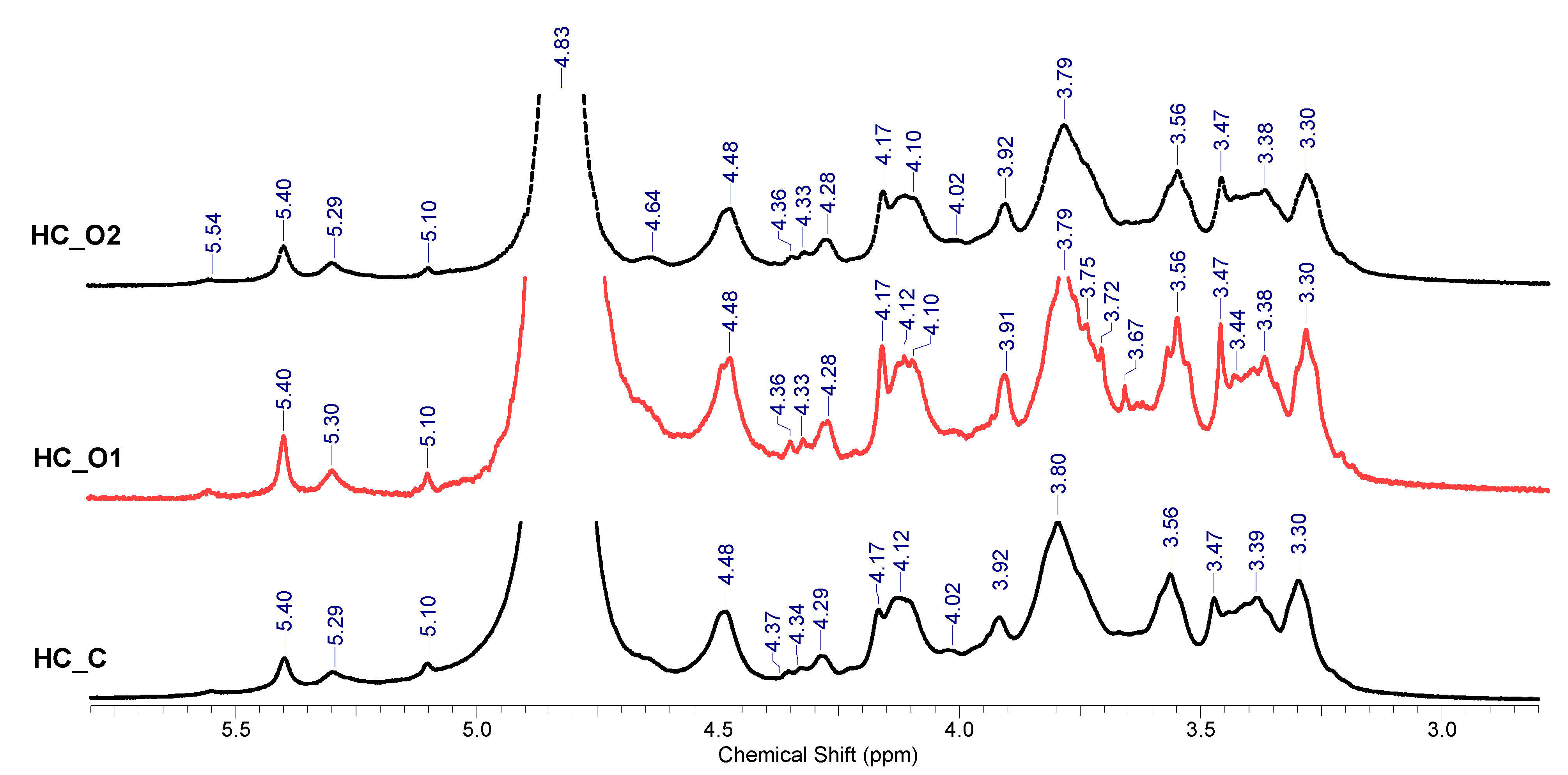
3.5.3. 1H-NMR Spectroscopy Analysis
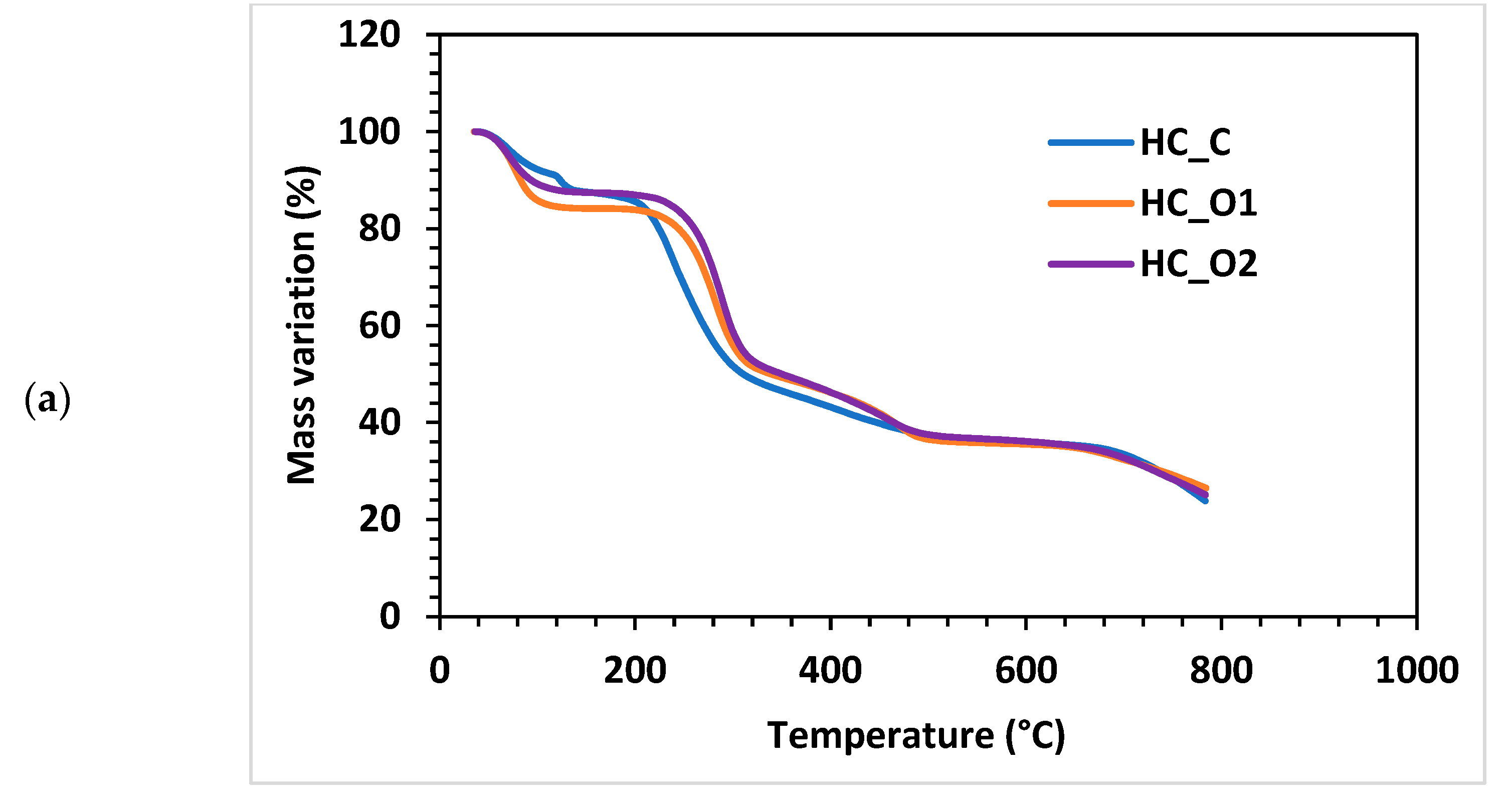
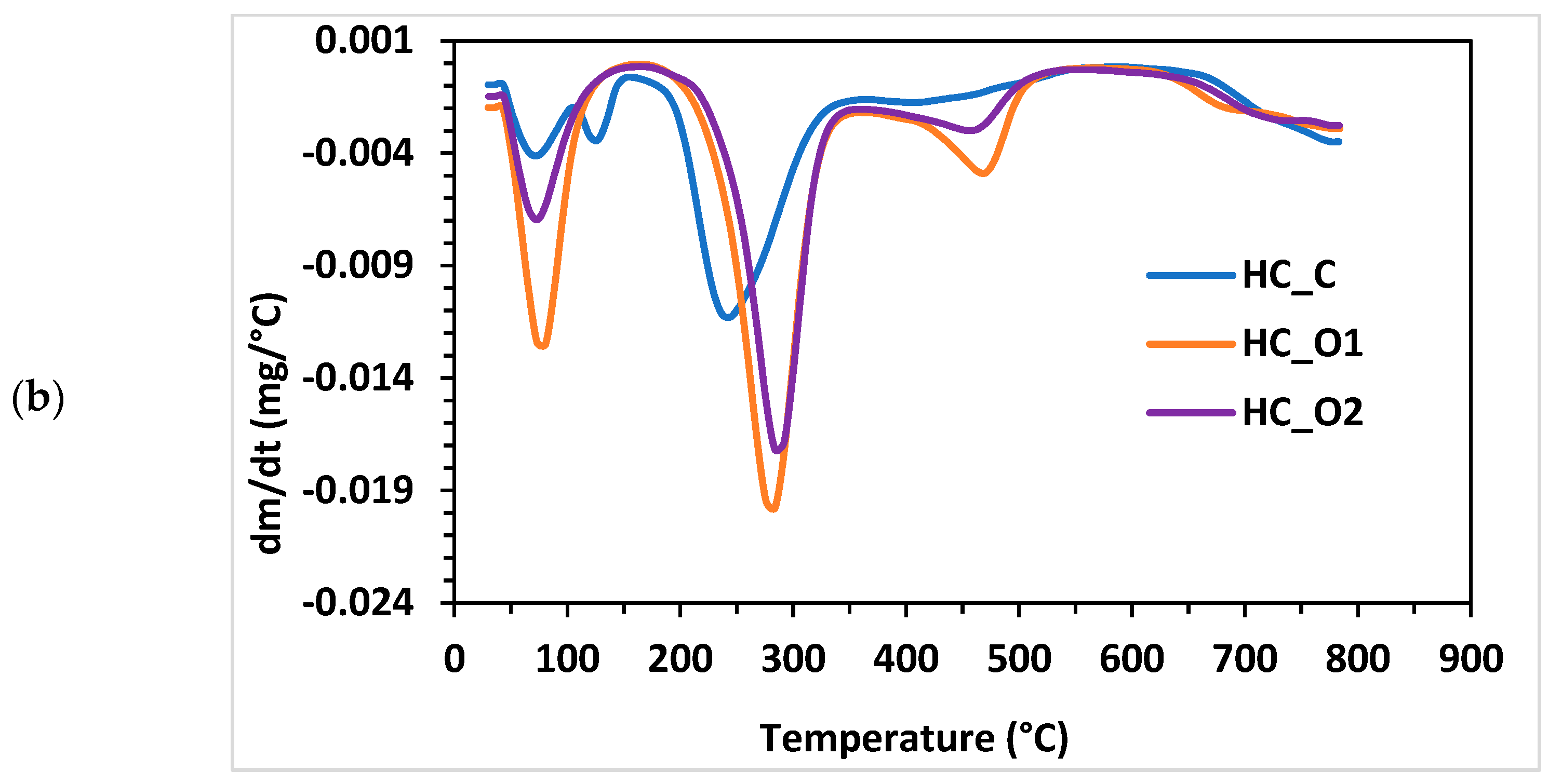
3.5.4. Thermogravimetric Analysis
3.6. Mechanism of Complementary Pretreatments and Comparison with Similar Studies
3.6.1. Freeze–Thaw Cycles
3.6.2. Microwave Pretreatments
3.6.3. Ultrasound Pretreatments
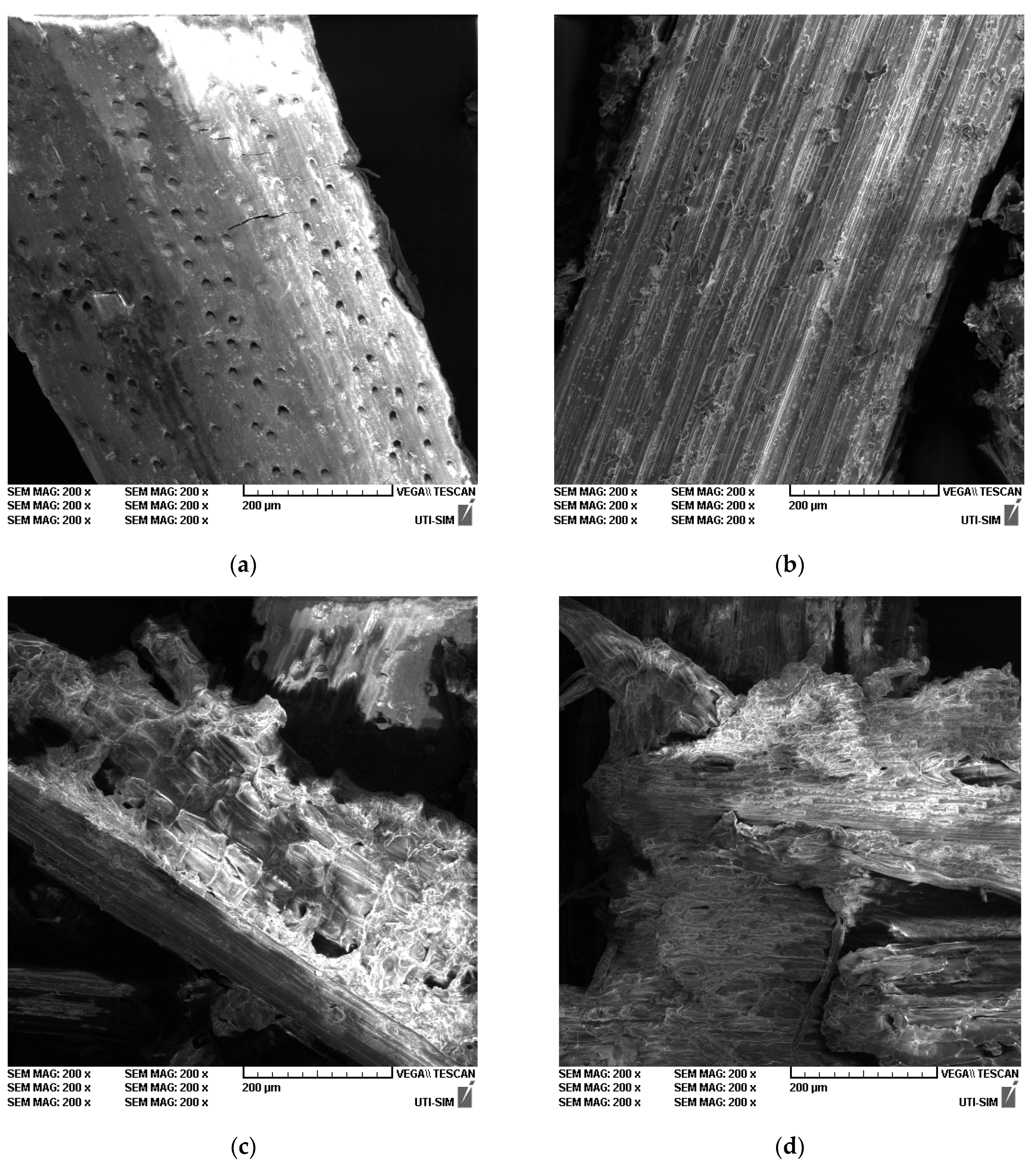
3.6.4. SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbarian, A.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Asgari, S.; Cheshmeh, Z.A. Challenges and opportunities of lignocellulosic biomass gasification in the path of circular bioeconomy. Bioresour. Technol. 2022, 362, 127774. [Google Scholar] [CrossRef]
- Gil, A. Challenges on waste-to-energy for the valorization of industrial wastes: Electricity, heat and cold, bioliquids and biofuels. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100615. [Google Scholar] [CrossRef]
- Babu, S.; Singh Rathore, S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.K.; Yadav, V.; Raj, R.; Yadav, D.; Shekhawat, K.; et al. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresour. Technol. 2022, 360, 127566. [Google Scholar] [CrossRef]
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and biomass-based energy supply and demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Qian, H.; Chen, W.; Zhu, W.; Liu, C.; Lu, X.; Guo, X.; Huang, D.; Liang, X.; Kontogeorgis, G.M. Simulation and evaluation of utilization pathways of biomasses based on thermodynamic data prediction. Energy 2019, 173, 610–625. [Google Scholar] [CrossRef]
- Bian, H.; Yang, Y.; Tu, P.; Chen, J.Y. Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film. Membranes 2022, 12, 475. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, L.J.; Yu, M.; Chen, H. Freeze-Thaw and Sulfuric Acid Pretreatment of Wheat Straw for Fermentable Sugar Release. Adv. Mater. Res. 2013, 724–725, 257–260. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Tyagi, V.K.; Gunjyal, N.; Kazmi, A.A.; Ojha, C.S.P.; Moustakas, K. Hydrothermal and thermal-alkali pretreatments of wheat straw: Co-digestion, substrate solubilization, biogas yield and kinetic study. Environ. Res. 2023, 216, 114436. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, A.; Fuciños, C.; Torrado, A.M.; Rúa, M.L. Extraction of the wheat straw hemicellulose fraction assisted by commercial endo-xylanases. Role of the accessory enzyme activities. Ind. Crops Prod. 2022, 179, 114655. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Ingrao, C.; Matarazzo, A.; Gorjian, S.; Adamczyk, J.; Failla, S.; Primerano, P.; Huisingh, D. Wheat-straw derived bioethanol production: A review of Life Cycle Assessments. Sci. Total Environ. 2021, 781, 146751. [Google Scholar] [CrossRef]
- Jaffar, M.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Korai, R.M.; Li, X. Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Chin. J. Chem. Eng. 2016, 24, 404–409. [Google Scholar] [CrossRef]
- Townsend, T.J.; Sparkes, D.L.; Ramsden, S.J.; Glithero, N.J.; Wilson, P. Wheat straw availability for bioenergy in England. Energy Policy 2018, 122, 349–357. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Sui, Y.; Cui, Y.; Wang, Y.; Zeb, S.; Sun, G. A green and efficient way to improve sugar recovery of wheat straw by ultrasonic-assisted xylanase pretreatment. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Zhong, C.; Jia, H.; Wei, P. Enhanced saccharification of wheat straw with the application of ultrasonic-assisted quaternary ammonium hydroxide pretreatment. Process Biochem. 2017, 53, 180–187. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Adamcyk, J.; Beisl, S.; Miltner, M.; Friedl, A. Sequential Pretreatment of Wheat Straw: Liquid Hot Water Followed by Organosolv for the Production of Hemicellulosic Sugars, Lignin, and a Cellulose-Enriched Pulp. Waste Biomass Valorization 2022, 13, 4771–4784. [Google Scholar] [CrossRef]
- Tian, S.-Q.; Zhao, R.-Y.; Chen, Z.-C. Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew. Sustain. Energy Rev. 2018, 91, 483–489. [Google Scholar] [CrossRef]
- Gou, G.; Meng, F.; Wang, H.; Jiang, M.; Wei, W.; Zhou, Z. Wheat straw-derived magnetic carbon foams: In-situ preparation and tunable high-performance microwave absorption. Nano Res. 2019, 12, 1423–1429. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Huang, Y. Structure and morphology of cellulose in wheat straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Gao, C.; Yang, J.; Zhang, H.; Xiao, W.; Han, L. Quantitative and qualitative characterization of dual scale mechanical enhancement on cellulosic and crystalline-structural variation of NaOH treated wheat straw. Bioresour. Technol. 2020, 312, 123535. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, M.; Wang, D.; Zhao, D.; Wang, M. Advances in extraction, purification, structural characteristics and biological activities of hemicelluloses: A review. Int. J. Biol. Macromol. 2022, 15, 467–483. [Google Scholar] [CrossRef]
- Mohammad Rahmani, A.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Shekhar Prasad Ojha, C.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Huang, C.; Xu, C.; Meng, X.; Wang, L.; Zhou, X. Isolation, Modification, and Characterization of the Constituents (Cellulose, Hemicellulose, Lignin, et al.) in Biomass and Their Bio-Based Applications. Front. Bioeng. Biotechnol. 2022, 10, 866531. [Google Scholar] [CrossRef]
- Goodman, B.A. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H. Hemicellulose degradation: An overlooked issue in acidic deep eutectic solvents pretreatment of lignocellulosic biomass. Ind. Crops Prod. 2022, 187, 115335. [Google Scholar] [CrossRef]
- Lou, R.; Zhang, X. Evaluation of pretreatment effect on lignin extraction from wheat straw by deep eutectic solvent. Bioresour. Technol. 2022, 344, 126174. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, K.-N.; Ma, C.-Y.; Bian, J.; Wen, J.-L.; Yuan, T.-Q. Assessing the availability of bamboo (Phyllostachys pubescens) fibers and parenchyma cells for producing lignin nanoparticles and fermentable sugars by rapid carboxylic acid-based deep eutectic solvents pretreatment. Ind. Crops Prod. 2023, 193, 116204. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, D.; Chen, L.; Qi, H.; Liu, A.; Deng, M.; Wu, X.; Wang, K. Recyclable choline chloride-based deep eutectic solvent pretreatment for accelerated enzymatic digestibility of Triarrhena lutarioriparia. Ind. Crops Prod. 2022, 187, 115542. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 2019, 12, 159. [Google Scholar] [CrossRef]
- Tan, J.; Tiwari, S.K.; Ramakrishna, S. Single-Use Plastics in the Food Services Industry: Can It Be Sustainable? Mater. Circ. Econ. 2021, 3, 7. [Google Scholar] [CrossRef]
- Sitch, D.A.; Marshall, H.B. The effect of hemicelluloses on the papermaking properties of white birch. Can. J. Res. 1950, 28f, 376–389. [Google Scholar] [CrossRef]
- Fahmy, Y.; Fahmy, T.Y.; Mobarak, F.; El-Sakhawy, M.; Fadl, M. Agricultural residues (wastes) for manufacture of paper, board, and miscellaneous products: Background overview and future prospects. Int. J. ChemTech Res. 2017, 10, 424–448. [Google Scholar]
- Lima, D.U.; Oliveira, R.C.; Buckeridge, M.S. Seed storage hemicelluloses as wet-end additives in papermaking. Carbohydr. Polym. 2003, 52, 367–373. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.-L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Stevanato, L.; Cravotto, G.; Marjamaa, K.; Pihlajaniemi, V.; Koivula, A.; Aro, N.; Uusitalo, J.; et al. Optimization of ultrasound pretreatment and enzymatic hydrolysis of wheat straw: From lab to semi-industrial scale. J. Clean. Prod. 2022, 380, 134897. [Google Scholar] [CrossRef]
- Ouahabi, Y.R.; Bensadok, K.; Ouahabi, A. Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration. Sustainability 2021, 13, 7202. [Google Scholar] [CrossRef]
- Liu, X.; Zicari, S.M.; Liu, G.; Li, Y.; Zhang, R. Improving the bioenergy production from wheat straw with alkaline pretreatment. Biosyst. Eng. 2015, 140, 59–66. [Google Scholar] [CrossRef]
- Yang, C.; Qian, J.; Zhang, H. Cyclic Freeze-Thaw Treatment on Enzymatic Saccharification of Wheat Straw and its Synergism Effect with Alkali. BioEnergy Res. 2023, 1–11. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, R.C. Chapter 19 - Recent Advances in Alkaline Pretreatment of Lignocellulosic Biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–459. [Google Scholar]
- Liu, Q.; He, W.-Q.; Aguedo, M.; Xia, X.; Bai, W.-B.; Dong, Y.-Y.; Song, J.-Q.; Richel, A.; Goffin, D. Microwave-assisted alkali hydrolysis for cellulose isolation from wheat straw: Influence of reaction conditions and non-thermal effects of microwave. Carbohydr. Polym. 2021, 253, 117170. [Google Scholar] [CrossRef]
- Sasaki, C.; Sumitomo, Y.; Odashima, K.; Asada, C.; Nakamura, Y. Microwave-Assisted Hydrolysis of Cellulose in Towel and Wheat Straw Using Freeze-Thawing with NaOH. Waste Biomass Valorization 2021, 12, 3331–3339. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Delignification efficiency of various types of biomass using microwave-assisted hydrotropic pretreatment. Sci. Rep. 2022, 12, 4561. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Mu, X.; Zhang, D. Ultrasonic Pretreatment of Wheat Straw in Oxidative and Nonoxidative Conditions Aided with Microwave Heating. Ind. Eng. Chem. Res. 2013, 52, 12514–12522. [Google Scholar] [CrossRef]
- Dedhia, B.S.; Csoka, L.; Rathod, V.K. Xylanase and Ultrasound Assisted Pulping of Wheat Straw. Appl. Biochem. Biotechnol. 2012, 168, 731–741. [Google Scholar] [CrossRef]
- Korai, R.M.; Wachemo, A.C.; Yue, L.; Jaffar, M.; Li, Z.; Shahbaz, M.; Yuan, H.; Li, X. Effect of ultrasonic application during KOH pretreatment and anaerobic process on digestion performance of wheat straw. RSC Adv. 2020, 10, 9290–9298. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.J.; Wang, X.M. Preliminary Study on Natural Freeze-Thaw Pretreatment of Wheat Straw. Adv. Mater. Res. 2012, 550–553, 472–475. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry. 211 om-02. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; TAPPI: Atlanta, GA, USA, 2002; Volume 5. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. Water solubility of wood and pulp; TAPPI: Atlanta, GA, USA, 1988. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. T 204 cm-97: Solvent extractives of wood and pulp. In TAPPI Test Methods; TAPPI: Atlanta, GA, USA, 1997. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. 2008, 9, 1–6. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Serna-Loaiza, S.; Zikeli, F.; Adamcyk, J.; Friedl, A. Towards a wheat straw biorefinery: Combination of Organosolv and Liquid Hot Water for the improved production of sugars from hemicellulose and lignin hydrolysis. Bioresour. Technol. Rep. 2021, 14, 100667. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sun, R.C.; Tompkinson, J. Comparative study of organic solvent and water-soluble lipophilic extractives from wheat straw I: Yield and chemical composition. J. Wood Sci. 2003, 49, 0047–0052. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Lu, Y.; Lu, Z.-L.; Wei, X.-Y. Detailed Componential Characterization of Extractable Species with Organic Solvents from Wheat Straw. Int. J. Anal. Chem. 2017, 2017, 7305682. [Google Scholar] [CrossRef]
- Kuchelmeister, C.; Bauer, S. Rapid Small-Scale Determination of Extractives in Biomass. BioEnergy Res. 2015, 8, 68–76. [Google Scholar] [CrossRef]
- Hickey, D.T.; Hayes, D.J.; Pembroke, J.T.; Ryan, M.P.; Leahy, J.J. The Importance of Extraction Protocol on the Analysis of Novel Waste Sources of Lignocellulosic Biomass. Energies 2021, 14, 6406. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xiao, L.-P.; Sun, Y.-C.; Sun, S.-N.; Xu, F.; Sun, R.-C.; Zhang, X.-L. Comparative study of alkali-soluble hemicelluloses isolated from bamboo (Bambusa rigida). Carbohydr. Res. 2011, 346, 111–120. [Google Scholar] [CrossRef]
- Vincent, P.; Ham-Pichavant, F.; Michaud, C.; Mignani, G.; Mastroianni, S.; Cramail, H.; Grelier, S. Extraction and Characterization of Hemicelluloses from a Softwood Acid Sulfite Pulp. Polymers 2021, 13, 2044. [Google Scholar] [CrossRef]
- Duan, J.; Karaaslan, M.A.; Cho, M.; Liu, L.-Y.; Johnson, A.M.; Renneckar, S. Investigation into electrospinning water-soluble xylan: Developing applications from highly absorbent and hydrophilic surfaces to carbonized fiber. Cellulose 2019, 26, 413–427. [Google Scholar] [CrossRef]
- Peng, F.; Bian, J.; Peng, P.; Guan, Y.; Xu, F.; Sun, R.-C. Fractional separation and structural features of hemicelluloses from sweet sorghum leaves. BioResources 2012, 7, 4744–4759. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Lanjewar, R.; Thakur, L.; Parmar, H.; Verma, A.; Hinge, V. A Review on Physicochemical Characterization and Pyrolysis Kinetics of Biomass. Int. J. Manag. Technol. Eng. 2019. [Google Scholar]
- Peng, Y.; Wu, S. The structural and thermal characteristics of wheat straw hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Lazdovica, K.; Kampars, V.; Liepina, L.; Vilka, M. Comparative study on thermal pyrolysis of buckwheat and wheat straws by using TGA-FTIR and Py-GC/MS methods. J. Anal. Appl. Pyrolysis 2017, 124, 1–15. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Y.; Li, S.; Xu, W.; Liu, G. Enhanced efficiency of enzymatic hydrolysis of wheat straw via freeze–thaw pretreatment. Environ. Sci. Pollut. Res. 2022, 29, 56696–56704. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Bian, H.; Qin, C.; Yao, S. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, S.; Liang, J.; Cao, L.; Liu, X.; Qin, C.; Liang, C.; Si, C.; Yu, Z.; Yao, S. High-efficiency separation of hemicellulose from bamboo by one-step freeze–thaw-assisted alkali treatment. Bioresour. Technol. 2022, 361, 127735. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Bichot, A.; Lerosty, M.; Radoiu, M.; Méchin, V.; Bernet, N.; Delgenès, J.-P.; García-Bernet, D. Decoupling thermal and non-thermal effects of the microwaves for lignocellulosic biomass pretreatment. Energy Convers. Manag. 2020, 203, 112220. [Google Scholar] [CrossRef]
- Janker-Obermeier, I.; Sieber, V.; Faulstich, M.; Schieder, D. Solubilization of hemicellulose and lignin from wheat straw through microwave-assisted alkali treatment. Ind. Crops Prod. 2012, 39, 198–203. [Google Scholar] [CrossRef]
- Iskalieva, A.; Yimmou, B.M.; Gogate, P.R.; Horvath, M.; Horvath, P.G.; Csoka, L. Cavitation assisted delignification of wheat straw: A review. Ultrason. Sonochemistry 2012, 19, 984–993. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical technologies in the catalytic conversion of biomass. Chem. Soc. Rev. 2021, 50, 1785–1812. [Google Scholar] [CrossRef]
- Sun, R.; Sun, X.F.; Xu, X.P. Effect of ultrasound on the physicochemical properties of organosolv lignins from wheat straw. J. Appl. Polym. Sci. 2002, 84, 2512–2522. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Jashina, L.; Telysheva, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus Chim. 2018, 21, 563–571. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J. Separation and Characterization of Cellulose from Wheat Straw. Sep. Sci. Technol. 2005, 39, 391–411. [Google Scholar] [CrossRef]

| Independent Variables | Units | Label | Range | Symbol | |
|---|---|---|---|---|---|
| From | To | ||||
| Severity factor | - | X1 | 1 | 3 | SF |
| NaOH concentration | % wt. | X2 | 3 | 7 | CNaOH |
| Variety | Cellulose % (Std) | Xylan % (Std) | HC % (Std) | AIL % (Std) | ASL % (Std) | AE % (Std) | HWT % (Std) | Ash % (Std) |
|---|---|---|---|---|---|---|---|---|
| Otilia | 39.64 (0.98) | 21.62 (1.14) | 24.97 (0.38) | 18.67 (1.12) | 1.95 (0.12) | 2.17 (0.03) | 15.47 (0.02) | 5.57 (0.06) |
| Sorial | 39.73 (0.45) | 18.79 (1.88) | 26.01 (0.24) | 15.8 (0.48) | 2.45 (0.11) | 4.09 (0.04) | 18.08 (0.41) | 6.44 (0.17) |
| Izvor | 40.9 (0.59) | 20.56 (1.37) | 27.27 (0.31) | 20.6 (0.11) | 1.83 (0.16) | 2.49 (0.05) | 13.6 (0.5) | 4.88 (0.14) |
| Mixture of WS (1:1:1) | 40.1 (1.12) | 20.23 (1.1) | 26.2 (1.78) | 16.36 (1.1) | 1.48 (0.22) | 2.91 (0.15) | 15.66 (0.18) | 5.61 (0.35) |
| Sample Treatment | TY (%) | XY (%) | HCY (%) | YAIL (%) | YASL (%) |
|---|---|---|---|---|---|
| HA 40-90 | 42.21 | 24.16 | 34.03 | 25.9 | 27.5 |
| HA 40-100 | 44.2 | 36.1 | 35.8 | 32.08 | 35.1 |
| FT2 | 7.50 | 3.72 | 3.08 | 2.68 | 0.89 |
| US10-30 | 31.33 | 4.38 | 9.28 | 19.16 | 11.22 |
| US20-45 | 34.40 | 21.67 | 21.96 | 24.85 | 18.9 |
| MW 10-100 | 52.18 | 31.51 | 31.39 | 29.40 | 19.91 |
| FT 1 HA 40-90 | 45.2 | 58.41 | 60.02 | 23.69 | 37.81 |
| FT 2 HA 40-90 | 47.3 | 61.75 | 63.43 | 25.66 | 39.8 |
| FT 3 HA 40-90 | 46.6 | 58.19 | 56.51 | 23.66 | 38.2 |
| FT 4 HA 40-90 | 45.1 | 56.68 | 54.80 | 24.23 | 38.9 |
| Exp. | SF | CNaOH (%) | TY (%) | XY (%) | HCY (%) | YAIL (%) | YASL (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 3 | 34.86 | 15.24 | 19.24 | 19.67 | 28.42 |
| 2 | 2 | 3 | 49.79 | 34.75 | 34.87 | 13.65 | 24.66 |
| 3 | 3 | 3 | 49.58 | 50.15 | 48.28 | 56.05 | 28.77 |
| 4 | 1 | 7 | 41.60 | 52.57 | 50.72 | 27.55 | 32.19 |
| 5 | 2 | 7 | 56.01 | 63.84 | 61.70 | 26.24 | 45.89 |
| 6 | 3 | 7 | 54.21 | 70.84 | 70.98 | 75.46 | 48.56 |
| 7 | 1 | 5 | 40.22 | 49.65 | 49.06 | 38.59 | 31.44 |
| 8 | 3 | 5 | 51.43 | 55.60 | 57.23 | 60.21 | 44.86 |
| 9 | 2 | 5 | 49.76 | 48.44 | 46.98 | 20.97 | 36.30 |
| 10 | 2 | 5 | 50.55 | 54.32 | 52.23 | 21.03 | 34.93 |
| 11 | 2 | 5 | 49.73 | 49.68 | 42.46 | 20.77 | 39.04 |
| 12 | 2 | 5 | 50.36 | 45.11 | 47.10 | 25.94 | 35.62 |
| 113 | 2 | 5 | 50.22 | 49.18 | 48.45 | 19.67 | 36.30 |
| TY (%) | XY (%) | HCY (%) | YAIL (%) | YASL (%) | ||
|---|---|---|---|---|---|---|
| O1 | Predicted model parameters | CNaOH = 7%; SF = 1.63 | ||||
| Predicted results | 51.56 | 59.97 | 57.07 | 21.58 | 39.60 | |
| Experimental validation | 49.94 | 62.56 | 61.25 | 20.72 | 38.63 | |
| O2 | Predicted model parameters | CNaOH = 9%; SF = 1.44 | ||||
| Predicted result | 55.9 | 67.71 | 63.69 | 24.10 | 23.62 | |
| Experimental validation | 50.34 | 52.20 | 52.05 | 19.64 | 19.70 | |
| Sample | Glucan (%) | Xylan (%) | Arabinan (%) | Purity (%) |
|---|---|---|---|---|
| HC_C (control) | 3.61 | 77.50 | 13.14 | 94.25 |
| HC_O1 | 3.40 | 85.51 | 7.89 | 96.80 |
| HC_O2 | 2.61 | 83.92 | 10.02 | 96.55 |
| Biomass Type | Extraction Procedure | No. of FT Cycles | Temperature (°C) | Freezing Time (h) | Reported Extraction Yields * | Reference No., Year |
|---|---|---|---|---|---|---|
| wheat straw | enzymatic hydrolysis | 1 | −10 | 12, 24, 48, 96 | 57.06% cellulose, 70.66% hemicelluloses | [75], 2022 |
| −20 | ||||||
| −40 | ||||||
| −80 | ||||||
| wheat straw | enzymatic hydrolysis | 1 | −20 | 48 | 75.95% cellulose and hemicelluloses | [52], 2012 |
| wheat straw | enzymatic hydrolysis | 2 | −20 | 12 | 67% cellulose and hemicelluloses | [10], 2013 |
| bamboo chips | alkaline extraction | 1 | −30 | 12 | 64.71% hemicelluloses | [76], 2021 |
| bamboo chips | alkaline extraction | 1 | −20 | 12 | 73.26% hemicelluloses | [77], 2022 |
| −30 | ||||||
| −40 | ||||||
| −50 | ||||||
| −60 | ||||||
| −70 | ||||||
| wheat straw | FT standalone | 2 | −22 | 1 | 3.8% hemicelluloses | this work, 2023 |
| alkaline extraction | 34.03% hemicelluloses | |||||
| FT + alkaline extraction | 63.43% hemicelluloses |
| Alkali Concentration Range (%wt) | Temperature Range (°C) | Time Range (Minutes) | Reported Yields * | Reference No., Year |
|---|---|---|---|---|
| 0.5–2.5 | 120–200 | 5–25 | 69.48% of lignin solubilized | [79], 2019 |
| 1–5 | 60–140 | 5–80 | 90.66% purity of cellulose | [46], 2021 |
| 2–5 | 60–140 | 10–60 | 80% of hemicelluloses 90% of lignin | [81], 2012 |
| 5 | 100 | 10 | 31.39% hemicelluloses | this work, 2023 |
| Extraction Procedure | US Frequency (kHz) | Power (W) | Time, (Minutes) | Solvent | Reported Extraction Yields * | Reference No., Year |
|---|---|---|---|---|---|---|
| enzymatic hydrolysis | 40 | 200 | 60, 120 | water, diluted H2SO4, diluted NaOH | 59.2% lignin removal | [41], 2022 |
| 25 | 2000 | |||||
| 3000 | ||||||
| organosolv | 20 | 100 | 5, 10, 15, 20, 25, 30, 35 | solution NaOH/methanol/water | 78.5% lignin removal | [84], 2002 |
| deep eutectic solvents | 40 | 200 | 60, 120 | acetic acid/glycerol/ choline chloride | 27% delignification | [85], 2018 |
| γ-valerolactone/ water | ||||||
| ammonia | 20 | 180, 270, 450, 540, 650 | 15, 30, 45, 60, 90 | water | 92% saccharification | [19], 2017 |
| alkaline extraction | 20 | 100 | 5, 10, 15, 20, 25, 30, 35 | KOH | solubilization 91.6% hemicelluloses, 91.4% lignin | [86], 2005 |
| standalone ultrasonic treatment | 40 | 50 | 10 | NaOH | 9.28% hemicelluloses | this work, 2023 |
| 20 | 21.96% hemicelluloses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puițel, A.C.; Suditu, G.D.; Drăgoi, E.N.; Danu, M.; Ailiesei, G.-L.; Balan, C.D.; Chicet, D.-L.; Nechita, M.T. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers 2023, 15, 1038. https://doi.org/10.3390/polym15041038
Puițel AC, Suditu GD, Drăgoi EN, Danu M, Ailiesei G-L, Balan CD, Chicet D-L, Nechita MT. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers. 2023; 15(4):1038. https://doi.org/10.3390/polym15041038
Chicago/Turabian StylePuițel, Adrian Cătălin, Gabriel Dan Suditu, Elena Niculina Drăgoi, Maricel Danu, Gabriela-Liliana Ailiesei, Cătălin Dumitrel Balan, Daniela-Lucia Chicet, and Mircea Teodor Nechita. 2023. "Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles" Polymers 15, no. 4: 1038. https://doi.org/10.3390/polym15041038
APA StylePuițel, A. C., Suditu, G. D., Drăgoi, E. N., Danu, M., Ailiesei, G.-L., Balan, C. D., Chicet, D.-L., & Nechita, M. T. (2023). Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers, 15(4), 1038. https://doi.org/10.3390/polym15041038










