Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
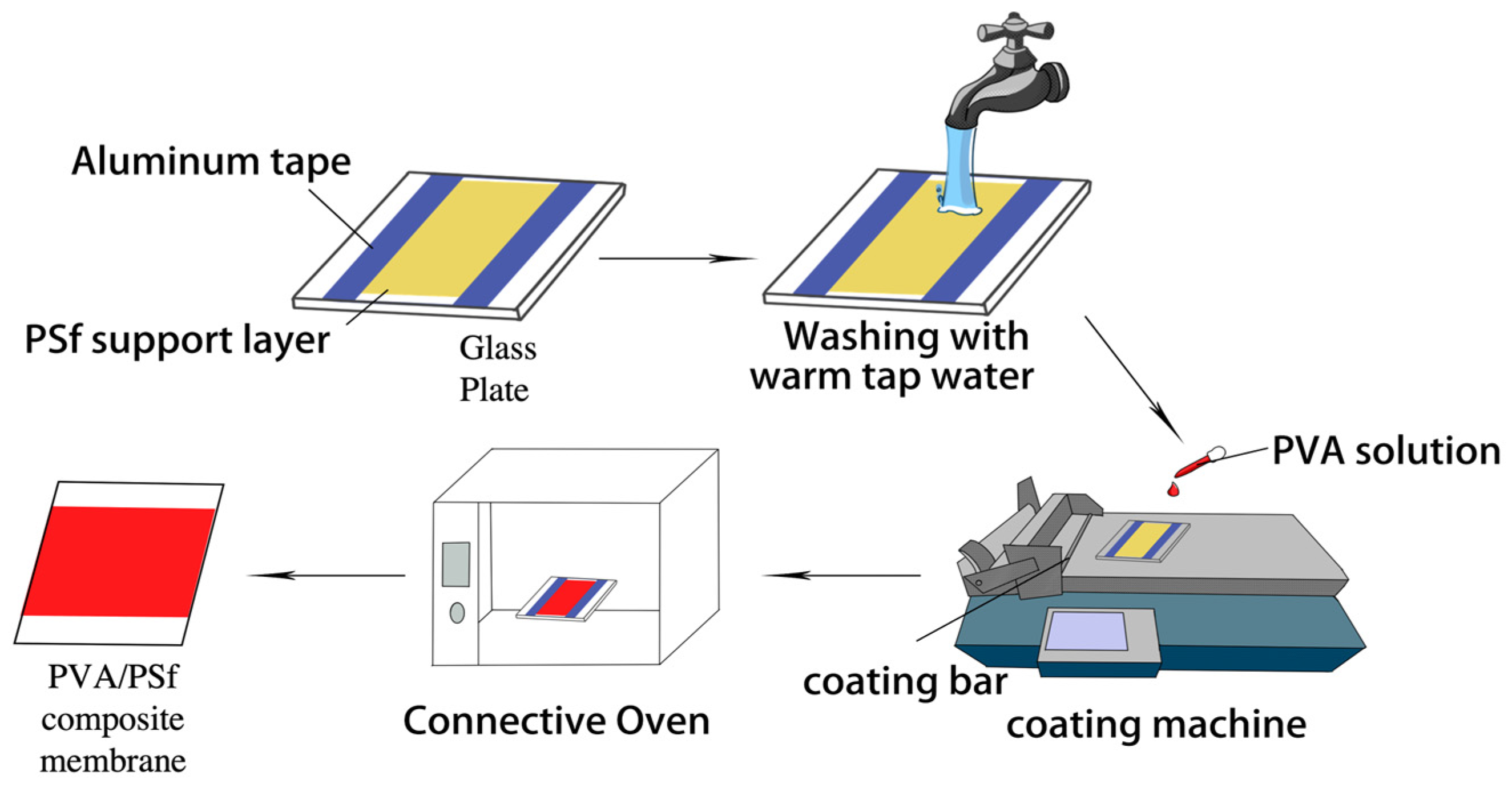
2.2. Fabrication of Composite Membranes
2.3. Membrane Characterization
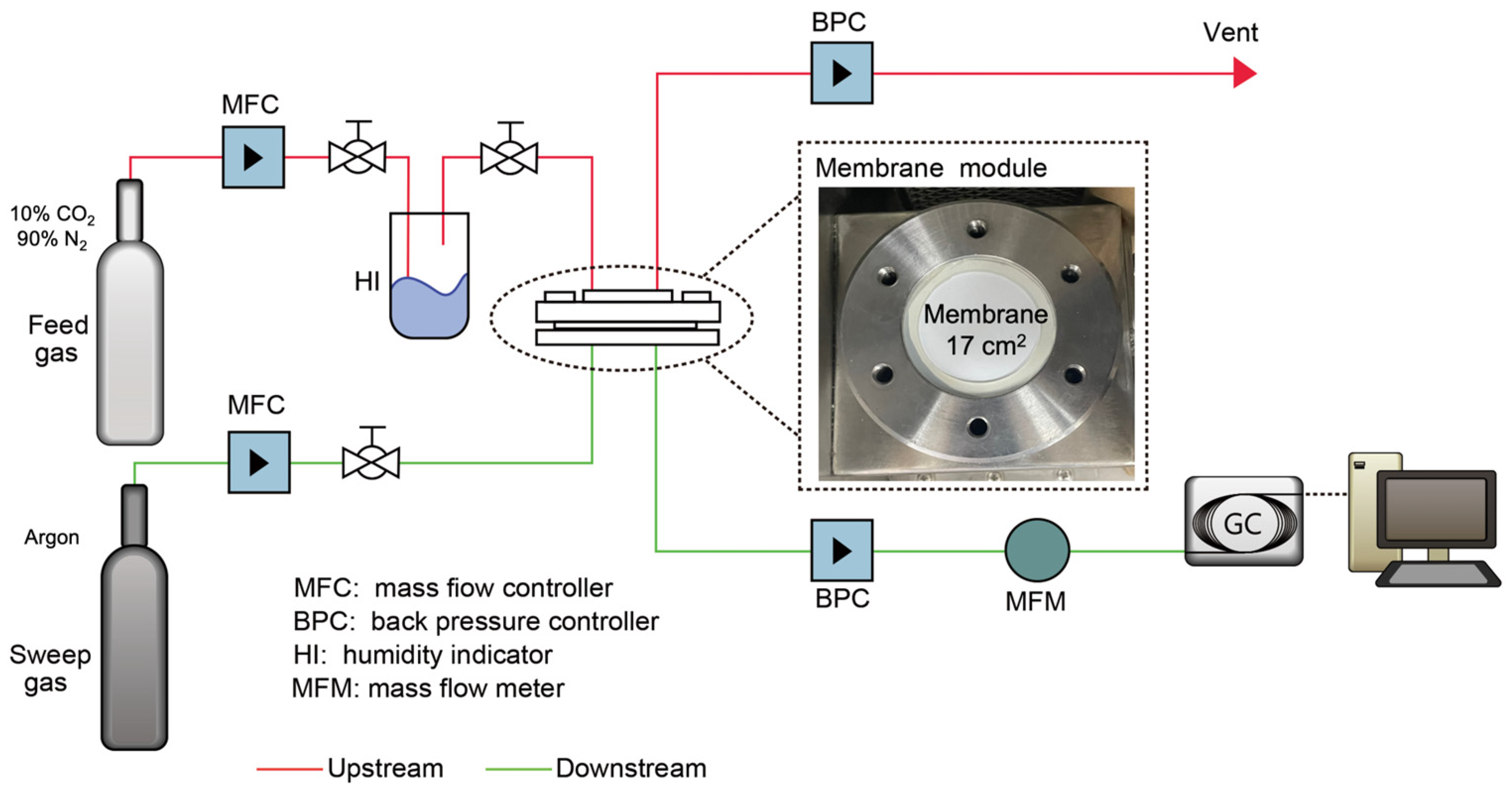
2.4. Membrane Performance Testing for CO2/N2 Separation
3. Results and Discussion
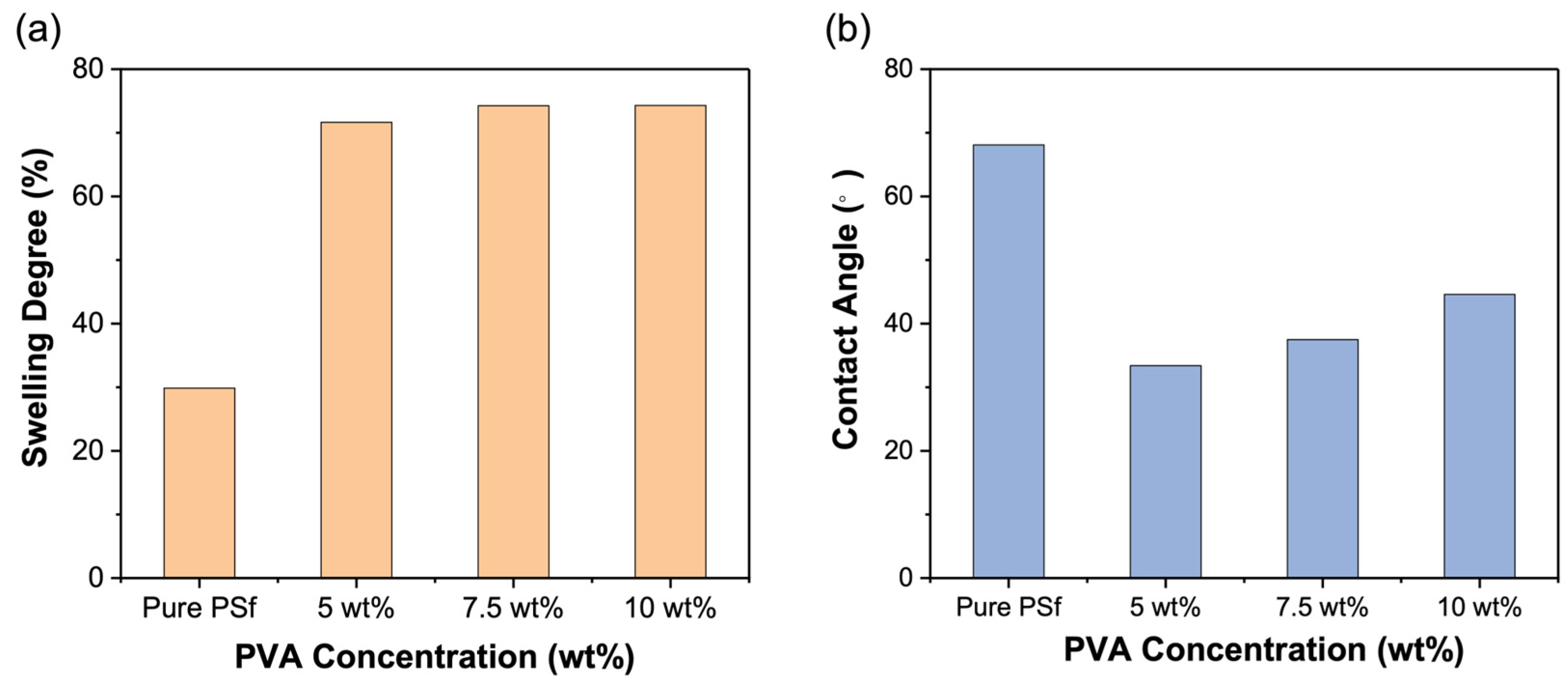
3.1. Membrane Swelling Behavior
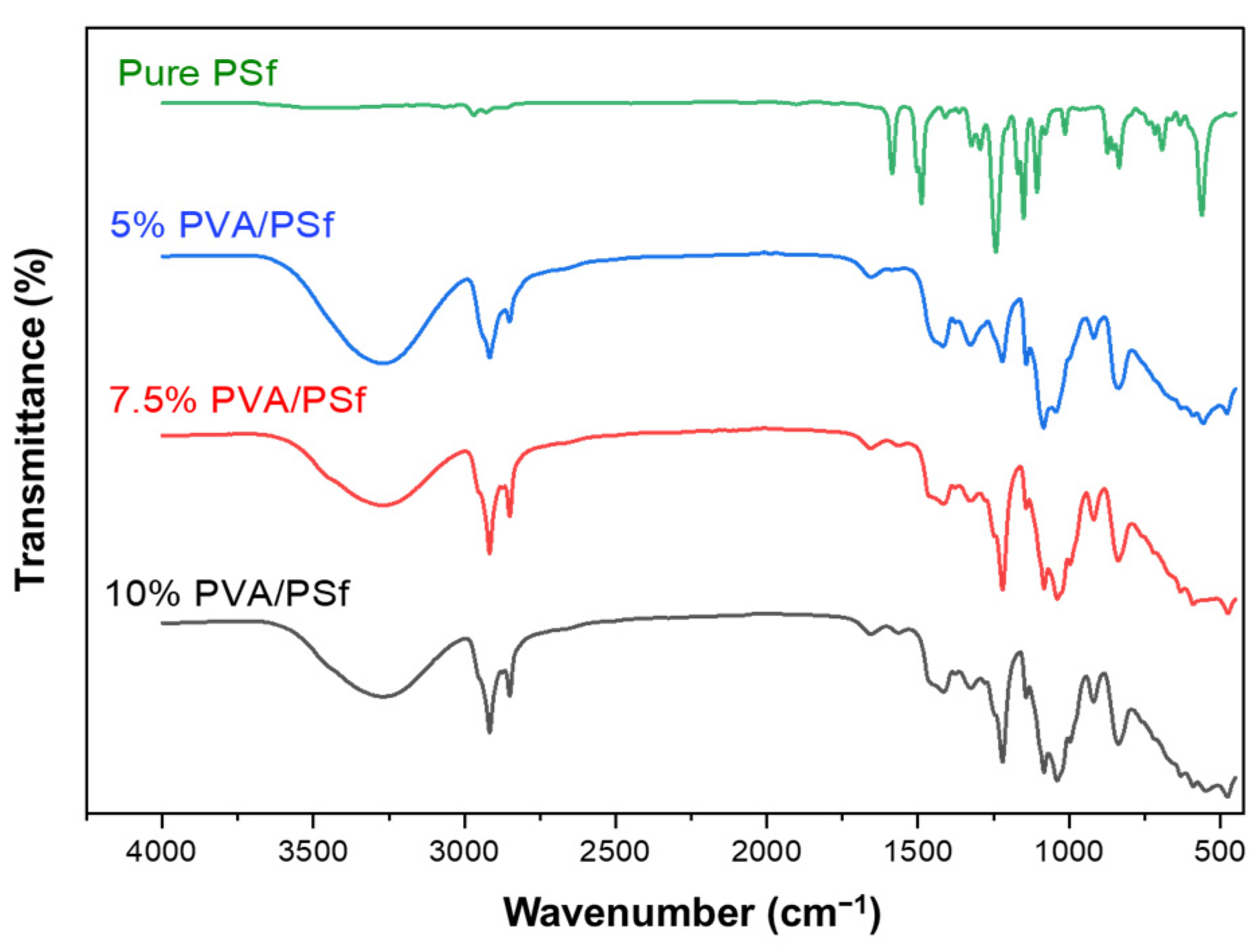
3.2. FTIR Spectra
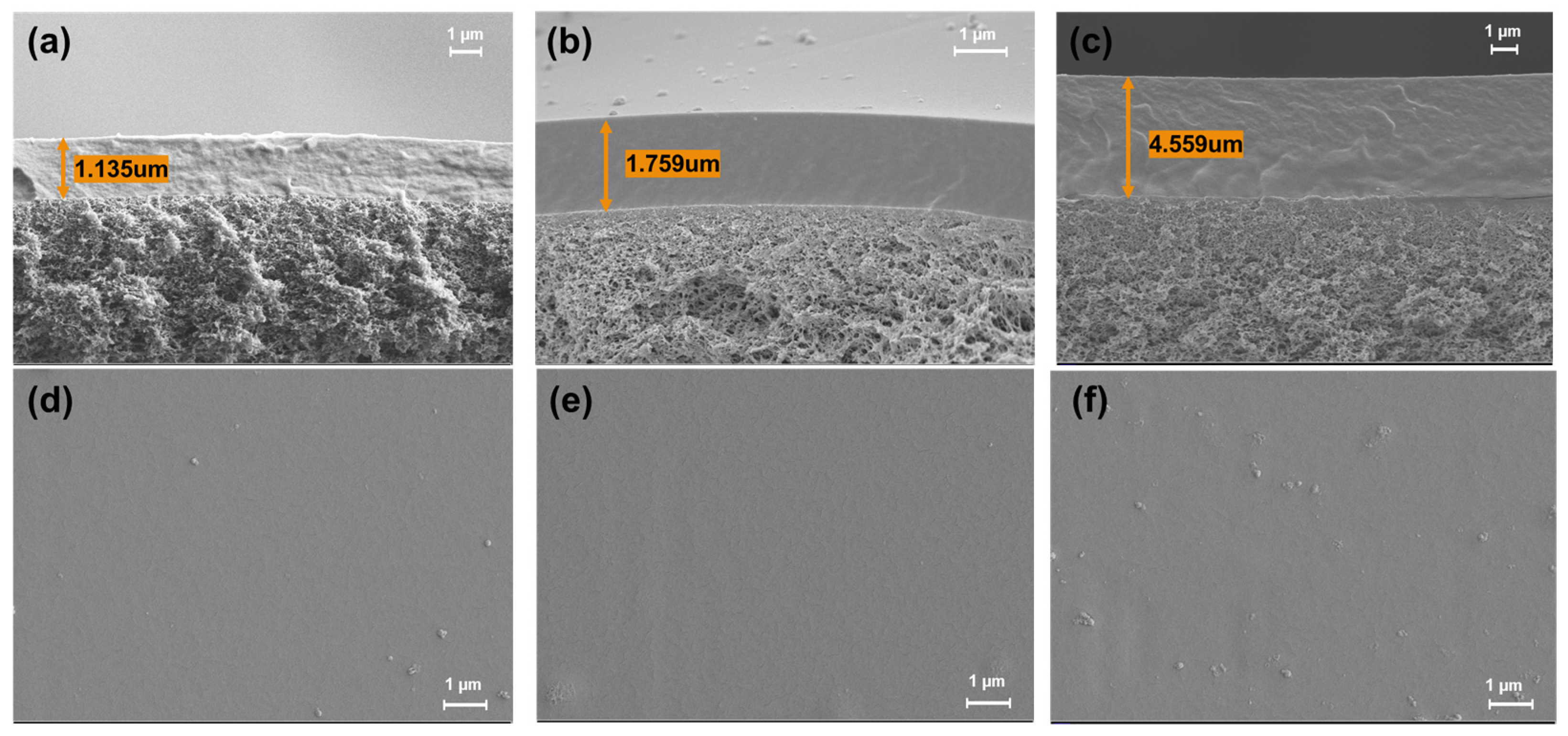
3.3. Membrane Morphology and Structure
3.4. CO2/N2 Mixed Gas Separation Performance
3.4.1. Effect of the PVA Concentration
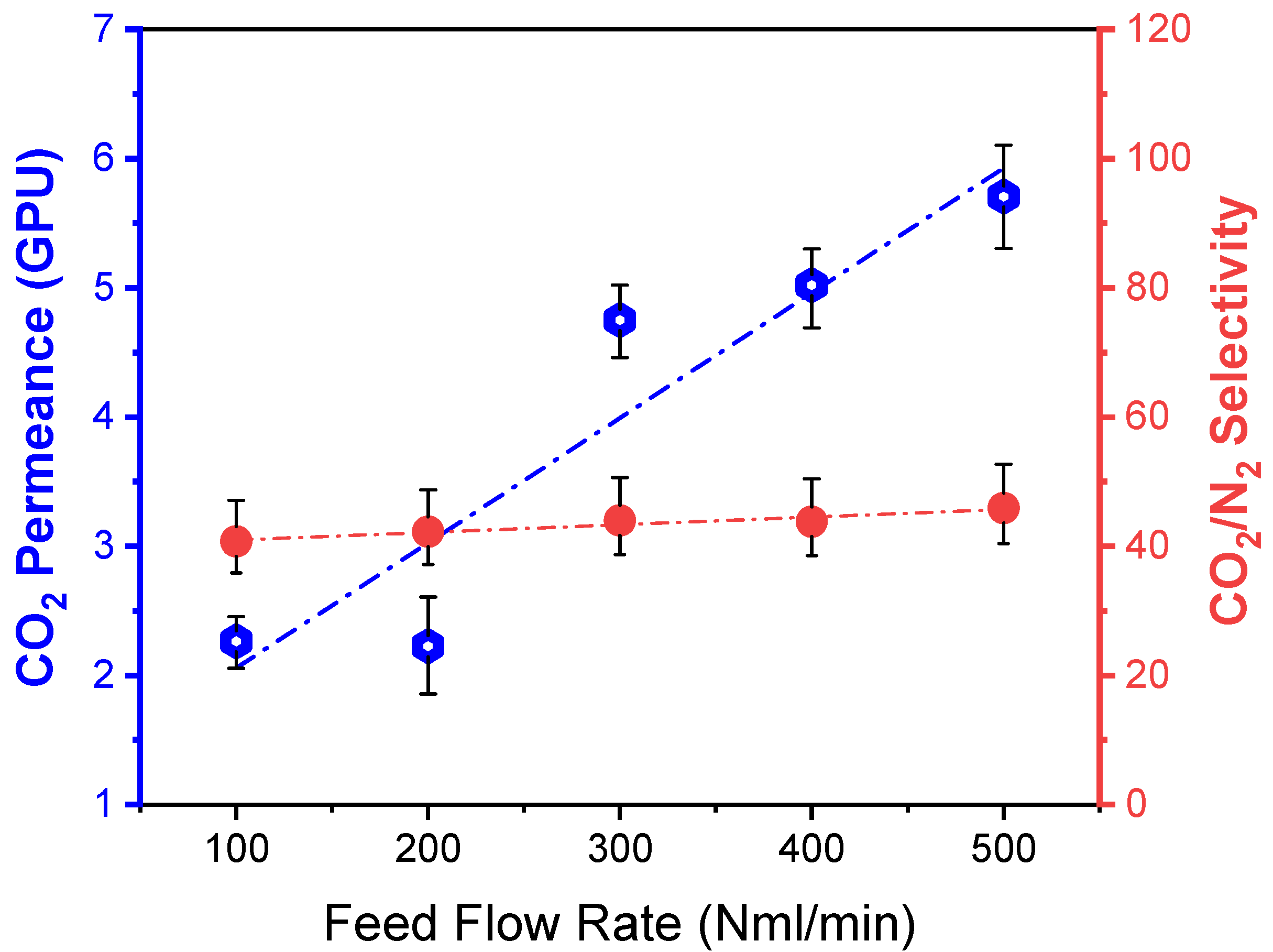
3.4.2. Effect of Feed Flow Rate
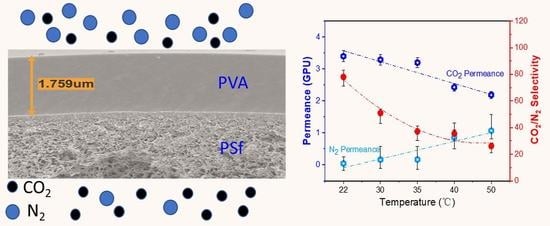
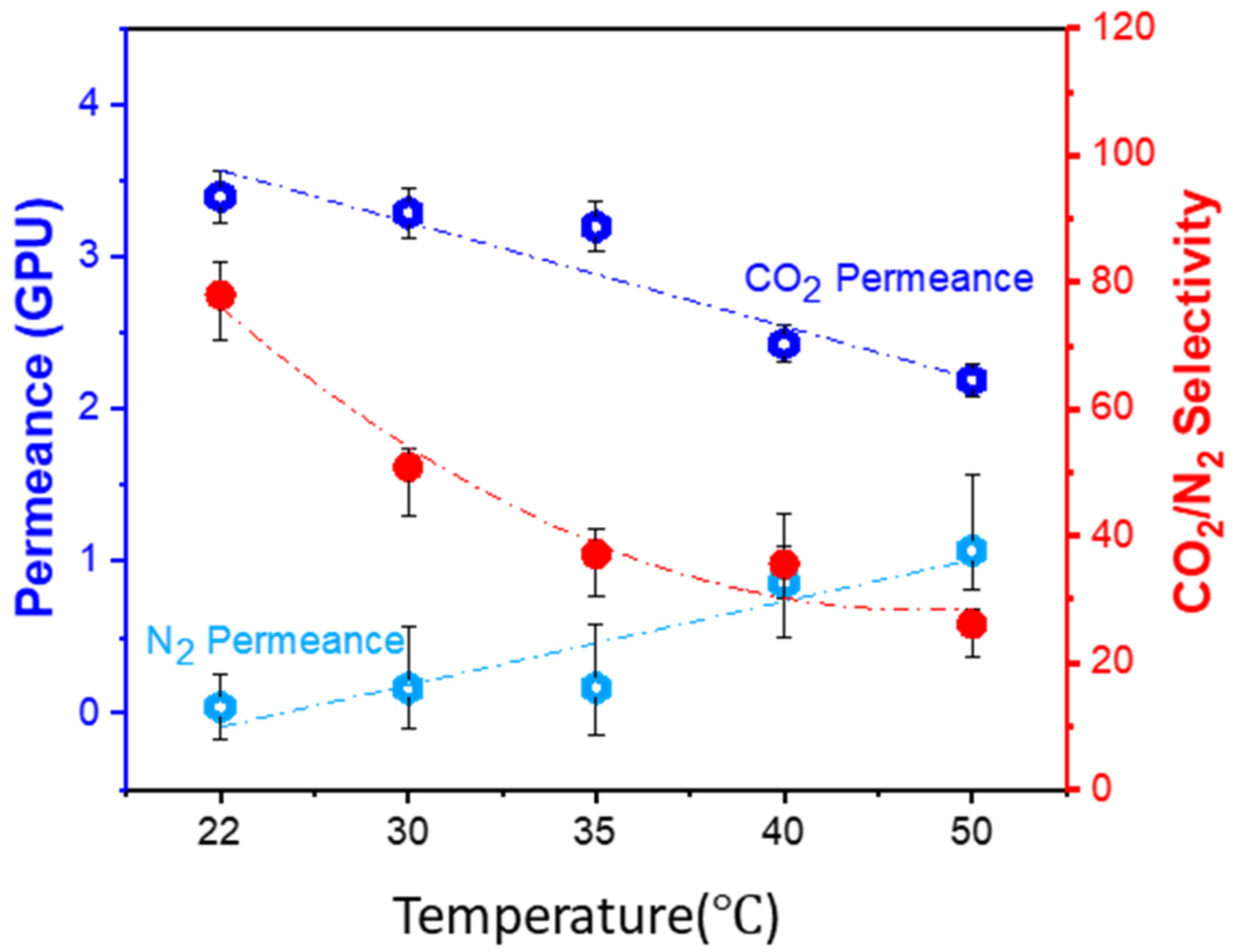
3.4.3. Effect of Operating Temperature
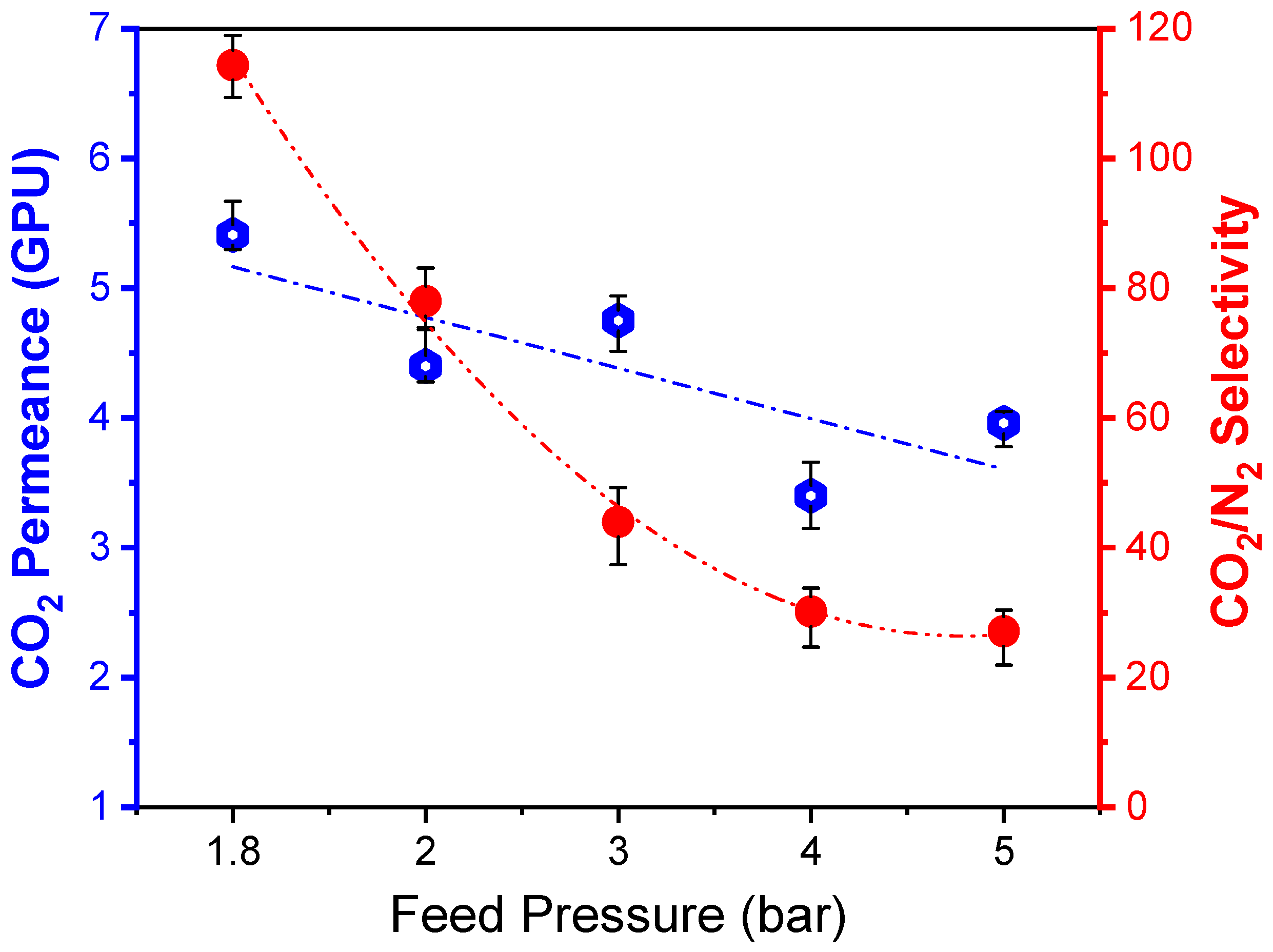
3.4.4. Effect of Feed Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, K.; Ashworth, P. The Development of Carbon Capture Utilization and Storage (CCUS) Research in China: A Bibliometric Perspective. Renew. Sustain. Energy Rev. 2021, 138, 110521. [Google Scholar] [CrossRef]
- Peilan, S. Research Progress of Carbon Dioxide Membrane Separation Materials. Energy Chem. Ind. 2021, 42, 27–32. [Google Scholar]
- Meisen, A.; Shuai, X. Research and Development Issues in CO2 Capture. Energy Convers. Manag. 1997, 38, 37–42. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of Process Parameters on Power Requirements of Vacuum Swing Adsorption Technology for CO2 Capture from Flue Gas. Energy Convers. Manag. 2008, 49, 346–356. [Google Scholar] [CrossRef]
- de Meis, D. Overview on Porous Inorganic Membranes for Gas Separation. In Proceedings of the 7° Convegno Nazionale di Viticoltura, Piacenza, Italy, 9–11 July 2017; pp. 9–11. [Google Scholar]
- He, X. Polyvinylamine-Based Facilitated Transport Membranes for Post-Combustion CO2 Capture: Challenges and Perspectives from Materials to Processes. Engineering 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Wang, J.; Xuehua, R.; Gaohong, H.E. Research Progress of Membrane Separation Materials for Different Industrial CO2-Containing Mixtures. Chem. J. 2022, 73, 3417–3432. [Google Scholar] [CrossRef]
- Mancuso, R.; Amuso, R.; Armentano, B.; Grasso, G.; Rago, V.; Cappello, A.R.; Galiano, F.; Figoli, A.; de Luca, G.; Hoinkis, J.; et al. Synthesis and Antibacterial Activity of Polymerizable Acryloyloxyalkyltriethyl Ammonium Salts. ChemPlusChem 2017, 82, 1233–1234. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent Progress on Fabrication Methods of Polymeric Thin Film Gas Separation Membranes for CO2 Capture. J. Membr. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, 1137. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent Advances in Multi-Layer Composite Polymeric Membranes for CO2 Separation: A Review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef]
- Linh, N.T.B.; Lee, K.H.; Lee, B.T. Fabrication of Photocatalytic PVA-TiO2 Nano-Fibrous Hybrid Membrane Using the Electro-Spinning Method. J. Mater. Sci. 2011, 46, 5615–5620. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.B. Membranes for Environmentally Friendly Energy Processes. Membranes 2012, 2, 706–726. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, B.; Zhou, J. Research Status and Development Trend of Carbon Dioxide Separation by Membrane Method. Hebei Chem. Ind. 2006, 29, 8–10. [Google Scholar]
- Deng, L.; Kim, T.J.; Hägg, M.B. PVA/PVAm Blend FSC Membrane for CO2-Capture. Desalination 2006, 199, 523–524. [Google Scholar] [CrossRef]
- Zamani Pedram, M.; Omidkhah, M.; Ebadi Amooghin, A. Synthesis and Characterization of Diethanolamine-Impregnated Cross-Linked Polyvinylalcohol/Glutaraldehyde Membranes for CO2/CH4 Separation. J. Ind. Eng. Chem. 2014, 20, 74–82. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Lavanya, C.; Geetha Balakrishna, R. Naturally Derived Polysaccharides-Modified PSF Membranes: A Potency in Enriching the Antifouling Nature of Membranes. Sep. Purif. Technol. 2020, 230, 115887. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Lasseuguette, E.; Janakiram, S.; Deng, L.; Ferrari, M.C. Analysis of CO2 Facilitation Transport Effect through a Hybrid Poly (Allyl Amine) Membrane: Pathways for Further Improvement. Membranes 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-S.; Shieh, J.-J.; Lau, W.W.Y.; Srinivasan, M.P.; Paul, D.R. Fabrication of Multi-Layer Composite Hollow Fiber Membranes for Gas Separation. J. Membr. Sci. 1999, 152, 211–225. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Z.; Hoek, E.M.V. Tuning the Molecular Structure, Separation Performance and Interfacial Properties of Poly(Vinyl Alcohol)-Polysulfone Interfacial Composite Membranes. J. Membr. Sci. 2011, 368, 26–33. [Google Scholar] [CrossRef]
- Peng, F.; Huang, X.; Jawor, A.; Hoek, E.M.V. Transport, Structural, and Interfacial Properties of Poly(Vinyl Alcohol)-Polysulfone Composite Nanofiltration Membranes. J. Membr. Sci. 2010, 353, 169–176. [Google Scholar] [CrossRef]
- Shieh, J.J.; Chung, T.S.; Paul, D.R. Study on Multi-Layer Composite Hollow Fiber Membranes for Gas Separation. Chem. Eng. Sci. 1999, 54, 675–684. [Google Scholar] [CrossRef]
- Turco, R.; Zannini, D.; Mallardo, S.; Dal Poggetto, G.; Tesser, R.; Santagata, G.; Malinconico, M.; di Serio, M. Biocomposites Based on Poly(Lactic Acid), Cynara Cardunculus Seed Oil and Fibrous Presscake: A Novel Eco-Friendly Approach to Hasten PLA Biodegradation in Common Soil. Polym. Degrad. Stab. 2021, 188, 109576. [Google Scholar] [CrossRef]
- Weltrowski, M.; Dolez, P.I. Compatibilizer Polarity Parameters as Tools for Predicting Organoclay Dispersion in Polyolefin Nanocomposites. J. Nanotechnol. 2019, 2019, 1404196. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Z.; Li, Q.; ud Din, Z.; Xiong, H. Sodium Dodecyl Sulfate Improves the Properties of Bio-Based Wood Adhesive Derived from Micronized Starch: Microstructure and Rheological Behaviors. Int. J. Biol. Macromol. 2019, 140, 1026–1036. [Google Scholar] [CrossRef]
- Dai, S.; Tam, K.C. Effect of Cosolvents on the Binding Interaction between Poly(Ethylene Oxide) and Sodium Dodecyl Sulfate. J. Phys. Chem. B 2006, 110, 20794–20800. [Google Scholar] [CrossRef]
- Gu, J.; Li, J. Research Progress on Modification and Application of Polyvinyl Alcohol Membranes. Chem. Ind. Eng. Prog. 2013, 32, 1074–1080. [Google Scholar]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water Absorption and States of Water in Semicrystalline Poly(Vinyl Alcohol) Films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Deng, L.; Hägg, M.B. Swelling Behavior and Gas Permeation Performance of PVAm/PVA Blend FSC Membrane. J. Membr. Sci. 2010, 363, 295–301. [Google Scholar] [CrossRef]
- Li, F.; Xia, H. Melt-Processable and Self-Healing Poly(Vinyl Alcohol) Elastomer Containing Diol Groups in the Side Chain. J. Appl. Polym. Sci. 2018, 135, 46050. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Wang, Y.; Liu, C.; Ling, J. Advances of Modified Polyvinyl Alcohol Membrane on separation of Carbon Dioxide. Appl. Chem. Ind. 2019, 48, 12605–12612. [Google Scholar]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Lei, L.; Lindbråthen, A.; Hillestad, M.; He, X. Carbon Molecular Sieve Membranes for Hydrogen Purification from a Steam Methane Reforming Process. J. Membr. Sci. 2021, 627, 119241. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Sjölin, M.; Rodrigues, G.; Bildyukevich, A.V.; Lipnizki, F.; Ulbricht, M. Development of Antifouling Polysulfone Membranes by Synergistic Modification with Two Different Additives in Casting Solution and Coagulation Bath: Synperonic F108 and Polyacrylic Acid. Materials 2022, 15, 359. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O.; Gomaa, M.M. Physical and Electrochemical Properties of PVA/TiO2 Nanocomposite Membrane. Adv. Polym. Technol. 2018, 37, 3842–3853. [Google Scholar] [CrossRef]
- Sherrington, D.C. Polymers for Advanced Technologies. React. Polym. 1989, 11, 191. [Google Scholar] [CrossRef]
- He, X.; Lindbråthen, A.; Kim, T.J.; Hägg, M.B. Pilot Testing on Fixed-Site-Carrier Membranes for CO2 Capture from Flue Gas. Int. J. Greenh. Gas Control 2017, 64, 323–332. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, D.; He, X. Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation. Polymers 2023, 15, 124. https://doi.org/10.3390/polym15010124
Li Y, Chen D, He X. Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation. Polymers. 2023; 15(1):124. https://doi.org/10.3390/polym15010124
Chicago/Turabian StyleLi, Ying, Danlin Chen, and Xuezhong He. 2023. "Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation" Polymers 15, no. 1: 124. https://doi.org/10.3390/polym15010124
APA StyleLi, Y., Chen, D., & He, X. (2023). Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation. Polymers, 15(1), 124. https://doi.org/10.3390/polym15010124