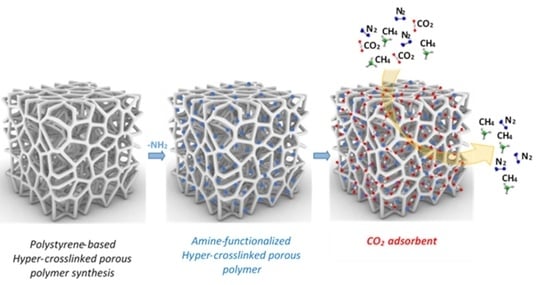
Synthesis, Characterization, and Gas Adsorption Performance of Amine-Functionalized Styrene-Based Porous Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
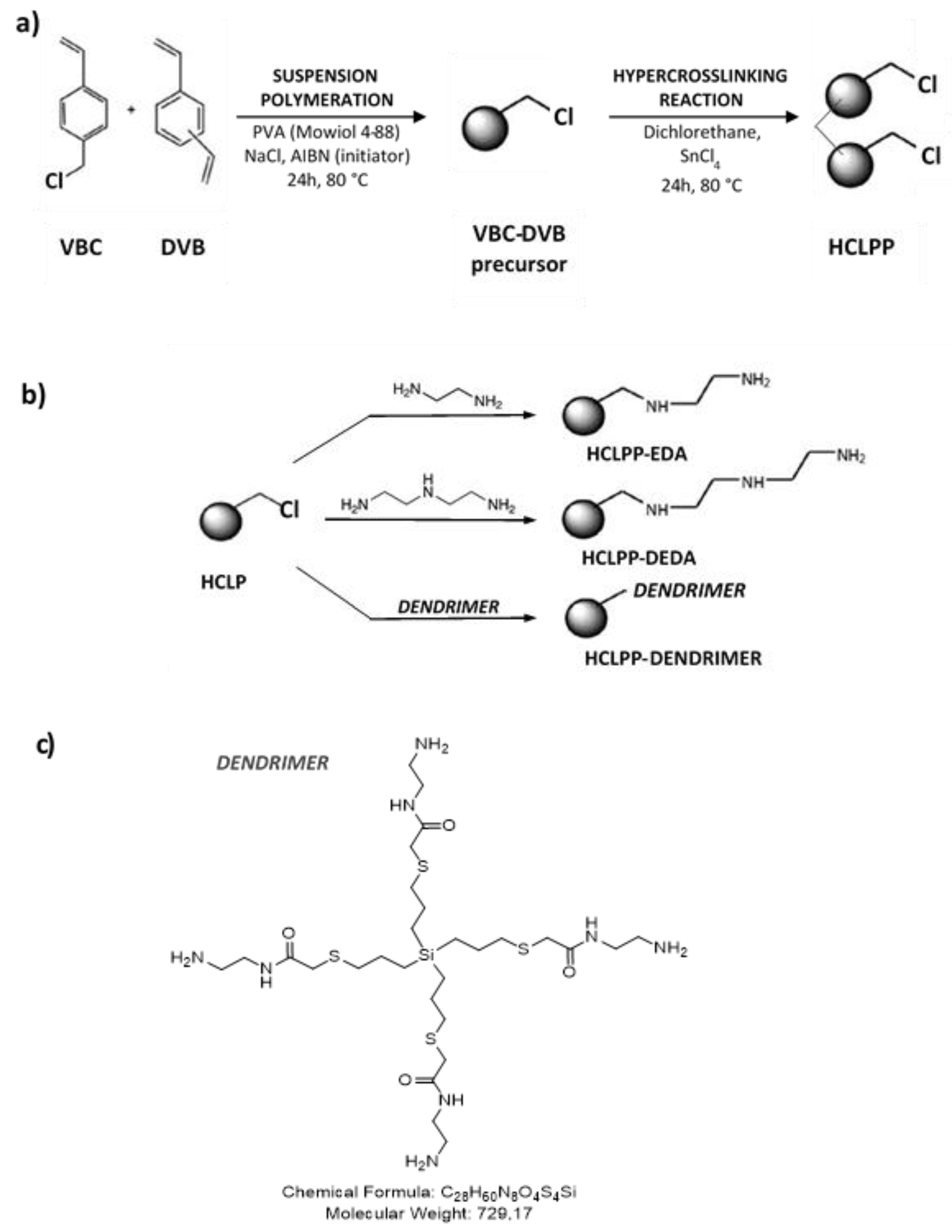
2.2. Synthesis of Porous Polymer
2.2.1. Preparation of Hyper-Crosslinked Porous Polymer Sample (HCLPP)
2.2.2. Preparation of Dendrimer
2.2.3. Functionalization of HCLPP
2.3. Characterization
2.3.1. Properties of the HCLPPs
2.3.2. Characterization of Dendrimers
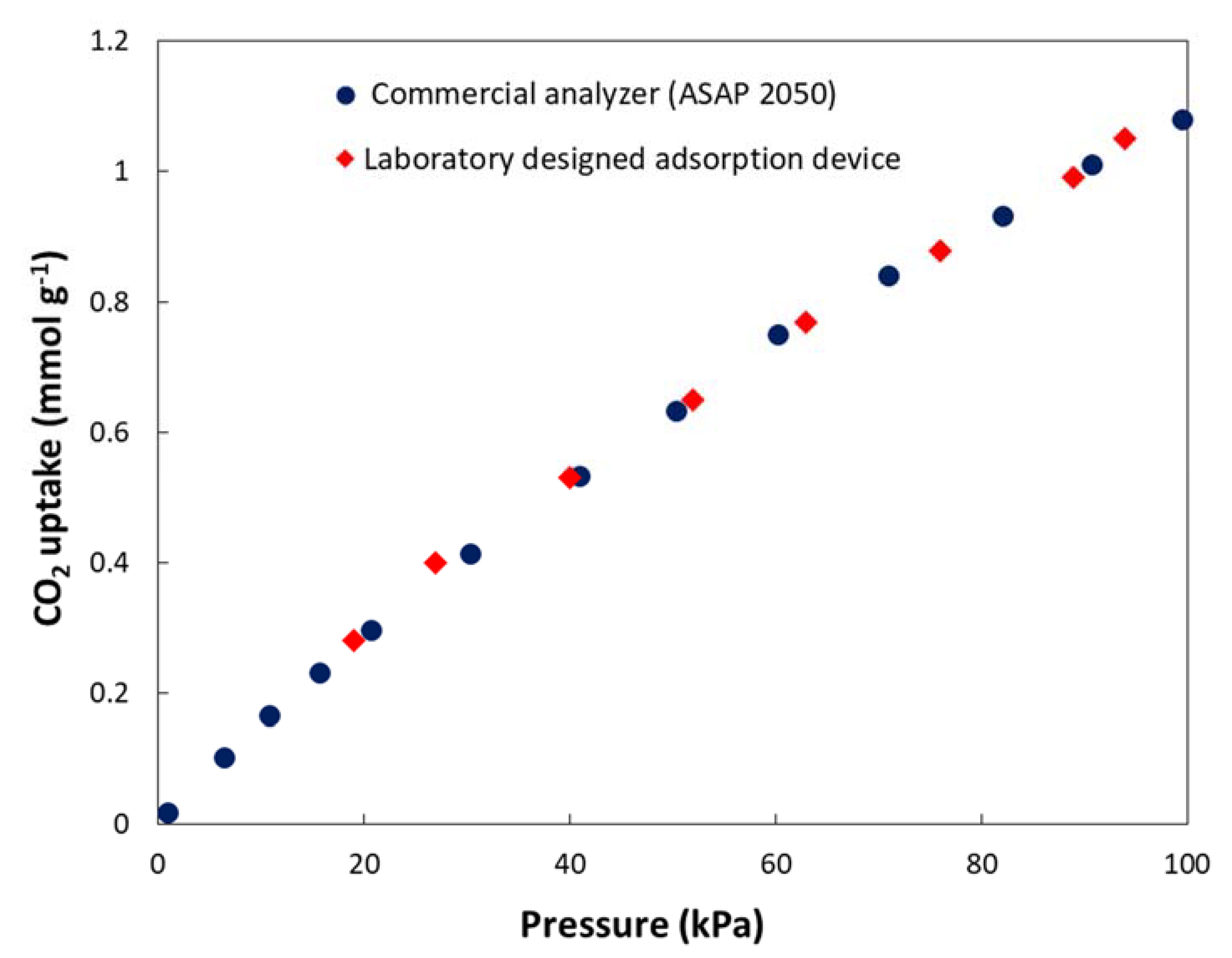
2.4. Gas Adsorption Measurement
3. Results and Discussion
3.1. Properties of HCLPPs
3.2. Gas Adsorption and Selectivity
| Sample | SBET, (m2 g−1) | CO2, (μmol g−1) 1 bar, 273 K, 298 K | CO2/N2 Selectivity | Ref. | |
|---|---|---|---|---|---|
| HCP-3A | 179 | 932 | 9 | ||
| HCP-6A | 299 | 1604 | 22 | ||
| HCP-12A | 406 | 1987 | 273 K | 23 | [27] |
| HCP-24A | 548 | 1715 | 20 | ||
| HCP-12B | 488 | 1771 | 20 | ||
| HCP-12C | 573 | 1846 | 15 | ||
| HCP-0 | 425 | 1201 | 17 | ||
| HCP-A | 777 | 1473 | 38 | ||
| HCP-B | 768 | 1178 | 298 K | 25 | [28] |
| HCP-C | 611 | 1258 | 15 | ||
| HCP-D | 673 | 1359 | 15 | ||
| P(DVB-VBC)-31 | 744 | 390 | 20 | ||
| P(DVB-VBC)-31-EDA | 389 | 1150 | 152 | ||
| P(DVB-VBC)-31-DETA | 376 | 1440 | 298 K | 223 | [32] |
| P(DVB-VBC)-11 | 134 | 180 | - | ||
| P(DVB-VBC)-11-EDA | 91 | 1150 | - | ||
| P(DVB-VBC)-11-DETA | 48 | 750 | - | ||
| HCLPP | 757 | 1010 | 28 | ||
| HCLPP-EDA | 283 | 910 | 298 K | 81 | This work |
| HCLPP-DETA | 277 | 820 | 71 | ||
| HCLPP-DENDRIMER | 616 | 1100 | 29 | ||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mikkelsen, M.; Jorgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2015. [Google Scholar]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Sun, Y.; Che, S.; Yang, X.; Wang, X.; Bosch, M.; Wang, Q.; Li, H.; Smith, M.; Yuan, S.; et al. Porous organic polymers for post-combustion carbon capture. Adv. Mater. 2017, 29, 1700229. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A. State-of-the-art Adsorption and Membrane Separation Processes for Carbon Dioxide Production from Carbon Dioxide Emitting Industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Gray, M.L.; Champagne, K.J.; Fauth, D.; Baltrus, J.P.; Pennline, H. Performance of immobilized tertiary amine solid sorbents for the capture of carbon dioxide. Int. J. Greenh. Gas Control 2008, 2, 3–8. [Google Scholar] [CrossRef]
- Darunte, L.A.; Walton, K.S.; Sholl, D.S.; Jones, C.W. CO2 Capture via Adsorption in Amine-Functionalized Sorbents. Curr. Opin. Chem. Eng. 2016, 12, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. Review of the latest developments in polyimide-based membranes for CO2 separation. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Dynamic CO2 capture by carbon adsorbents: Kinetics, isotherm, and thermodynamic studies. Sep. Purif. Technol. 2017, 181, 107–122. [Google Scholar] [CrossRef]
- Teo, J.Y.Q.; Ong, A.; Tan, T.T.Y.; Li, X.; Loh, X.J.; Lim, J.Y.C. Materials from waste plastics for CO2 capture and utilization. Green Chem. 2022, 24, 6086. [Google Scholar] [CrossRef]
- Zhao, X.; Boruah, B.; Chin, K.F.; Đokić, M.; Modak, J.M.; Soo, H.S. Upcycling to sustainably reuse plastics. Adv. Mater. 2021, 34, 2100843. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.W.S.; Teo, J.Y.Q.; Loh, X.J.; Lim, J.Y.C. Polyolefins and Polystyrene as Chemical Resources for a Sustainable Future: Challenges, Advances, and Prospects. ACS Mater. Lett. 2021, 3, 1660–1676. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131, 39931. [Google Scholar] [CrossRef]
- Yeung, C.W.S.; Loh, W.W.; Lau, H.H.; Loh, X.J.; Lim, J.Y.C. Catalysts developed from waste plastics: A versatile system for biomass conversion. Mater. Today Chem. 2021, 21, 100524. [Google Scholar] [CrossRef]
- Williamson, J.B.; Lewis, S.E.; Johnson, R.R., III; Manning, I.M.; Leibfarth, F.A. C–H Functionalization of Commodity Polymers. Angew. Chem. Int. Ed. 2019, 58, 8654–8668. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Designed from Recycled: Turning Polyethylene Waste to Covalently Attached Polylactide Plasticizers. ACS Sustain. Chem. Eng. 2019, 7, 11004–11013. [Google Scholar] [CrossRef] [Green Version]
- Gazi, S.; Đokić, M.; Chin, K.; Ng, P.R.; Soo, H.S. Visible light-driven cascade carbon–carbon bond formation for organic transformations and plastic recycling. Adv. Sci. 2019, 6, 1902020. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, B.; Tan, B.; Chen, C.F.; Xu, H.B.; Yang, X.L. Triptycene-based microporous polymers: Synthesis and their gas storage properties. ACS Macro. Lett. 2012, 1, 190–193. [Google Scholar] [CrossRef]
- Ma, H.; Chen, J.J.; Tan, L.; Bu, J.H.; Zhu, Y.; Tan, B.; Zhang, C. Nitrogen-rich triptycene-based porous polymer for gas storage and iodine enrichment. ACS Macro. Lett. 2016, 5, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Hankova, L.; Holub, L.; Jerabek, K. Formation of porous polymer morphology by microsyneresis during divinylbenzene polymerization. J. Polym. Sci. B Polym. Phys. 2015, 53, 774–781. [Google Scholar] [CrossRef]
- Veverka, P.; Jeřábek, K. Mechanism of hypercrosslinking of chloromethylated styrene–divinylbenzene copolymers. React. Funct. Polym. 1999, 41, 21. [Google Scholar] [CrossRef]
- Plastics: The Facts 2015: An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2015.
- Kaliva, M.; Armatas, G.S.; Vamvakaki, M. Microporous Polystyrene Particles for Selective Carbon Dioxide Capture. Langmuir 2012, 28, 2690–2695. [Google Scholar] [CrossRef]
- Fu, Z.; Jia, J.; Li, J.; Liu, C. Transforming waste expanded polystyrene foam into hypercrosslinked polymers for carbon dioxide capture and separation. Chem. Eng. J. 2017, 323, 557–564. [Google Scholar] [CrossRef]
- Fu, Z.; Mohamed, I.M.A.; Li, J.; Liu, C. Novel adsorbents derived from recycled waste polystyrene via cross-linking reaction for enhanced adsorption capacity and separation selectivity of CO2. J. Taiwan Inst. Chem. Eng. 2019, 97, 381–388. [Google Scholar] [CrossRef]
- Flouraki, C.; Kaliva, M.; Papadas, I.T.; Armatas, G.S.; Vamvakaki, M. Nanoporous polystyrene–porphyrin nanoparticles for selective gas separation. Polym. Chem. 2016, 7, 3026–3033. [Google Scholar] [CrossRef]
- Sneddon, G.; McGlynn, J.C.; Neumann, M.S.; Aydin, H.M.; Yiu, H.H.P.; Ganin, A.Y. Aminated poly(vinyl chloride) solid-state adsorbents with hydrophobic function for post-combustion CO2 capture. J. Mater. Chem. A 2017, 5, 11864–11872. [Google Scholar] [CrossRef] [Green Version]
- Jo, D.H.; Lee, C.H.; Jung, H.; Jeon, S.; Kim, S.H. Effect of amine surface density on CO2 adsorption behaviors of amine-functionalized polystyrene. Bull. Chem. Soc. Jpn. 2015, 88, 1317–1322. [Google Scholar] [CrossRef]
- Lee, D.Y.; Zhang, C.Y.; Gao, H.F. Amine-functionalized porous polymer network for highly selective absorption of CO2 over N2. Macromol. Chem. Phys. 2015, 216, 489–494. [Google Scholar] [CrossRef]
- Yaumi, A.L.; Abu Bakar, M.Z.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Li, Q.; Jin, W.; Li, X.; Hameed, A.; Qiao, S. Rational skeletal rigidity of conjugated microporous polythiophenes for gas uptake. Chem. Polym. Chem. 2017, 8, 6733–6740. [Google Scholar] [CrossRef]
- Davankov, V.; Tsyurupa, M.P. Hypercrosslinked Polymeric Networks and Adsorbing Materials: Synthesis, Properties, Structure, and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wu, Y.; Li, L.; Yang, W.; Feng, S.; Liu, H. Hybrid nanoporous polystyrene derived from cubic octavinylsilsesquioxane and commercial polystyrene via the Friedel–Crafts reaction. RSC Adv. 2015, 5, 12987–12993. [Google Scholar] [CrossRef]
- Heer, P.K.S.; Khot, K.M.; Gaikar, V.G. Development of polystyrene adsorbents functionalized with heterocyclic ligands for selective adsorption of CO2 from CH4 and N2. Sep. Purif. Technol. 2016, 158, 212–222. [Google Scholar] [CrossRef]
- Merchán-Arenas, D.R.; Murcia-Patiño, A.F. Synthesis of polyamino styrene from post-consumption expanded polystyrene and analysis of its CO2 scavenging capacity. Int. J. Environ. Sci. Technol. 2021, 18, 2519–2532. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. Surfaces of solids. XIII. A vapor adsorption method is used to determine the area of a solid without the assumption of a molecular area and the areas occupied by nitrogen and other molecules on the surface of a solid. J. Am. Chem. Soc. 1944, 66, 1366–1373. [Google Scholar] [CrossRef]
- Zhuang, G.L.; Tseng, H.H.; Wey, M.Y. Feasibility of using waste polystyrene as a membrane material for gas separation. Chem. Eng. Res. Des. 2016, 111, 204–217. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Yang, H.; Liu, S.; Chen, L.; Tang, Z.; Fu, R.; Wu, D. Stepwise crosslinking: A facile yet versatile conceptual strategy to nanomorphology-persistent porous organic polymers. Adv. Mater. 2017, 29, 1700723. [Google Scholar] [CrossRef] [PubMed]



| Sample | SBET a (m2 g−1) | Smeso b (m2 g−1) | Vtot c (mm3liq g−1) | Vmicro d (mm3liq g−1) |
|---|---|---|---|---|
| HCLPP | 757 | 310 | 408 | 226 |
| HCLPP-EDA | 283 | 93 | 157 | 97 |
| HCLPP-DETA | 277 | 132 | 159 | 76 |
| HCLPP-DENDRIMER | 616 | 194 | 341 | 218 |
| Sample | C (%) | Cl (%) | N (%) | S (%) | Si (%) |
|---|---|---|---|---|---|
| HCLPP | 97.79 | 0.42 | 1.74 | - | 0.05 |
| HCLPP-EDA | 90.62 | 0.08 | 9.21 | - | 0.10 |
| HCLPP-DETA | 90.55 | 0.05 | 9.32 | - | 0.07 |
| HCLPP-DENDRIMER | 99.93 | 0.30 | 5.37 | 0.20 | 0.21 |
| Sample | CO2 (μmol g−1) | CH4 (μmol g−1) | N2 (μmol g−1) | H2 (μmol g−1) | O2 (μmol g−1) |
|---|---|---|---|---|---|
| HCLPP | 1010 | 310 | 90 | 21 | 12 |
| HCLPP-EDA | 910 | 130 | 33 | 15 | 47 |
| HCLPP-DETA | 820 | 130 | 29 | 12 | 42 |
| HCLPP-DENDRIMER | 1100 | 240 | 63 | 17 | 89 |
| Sample | CO2 (μmol m2) | CH4 (μmol m2) | N2 (μmol m2) | H2 (μmol m2) | O2 (μmol m2) |
|---|---|---|---|---|---|
| HCLPP | 1.33 | 0.41 | 0.12 | 0.03 | 0.02 |
| HCLPP-EDA | 3.22 | 0.46 | 0.12 | 0.05 | 0.17 |
| HCLPP-DETA | 2.96 | 0.47 | 0.10 | 0.04 | 0.15 |
| HCLPP-DENDRIMER | 1.79 | 0.39 | 0.10 | 0.03 | 0.14 |
| Sample | CO2/CH4 (-) | CO2/N2 (-) | CO2/H2 (-) | O2/N2 (-) | CH4/N2 (-) |
|---|---|---|---|---|---|
| HCLPP | 5.8 | 27.6 | 74.2 | 0.1 | 4.7 |
| HCLPP-EDA | 16.0 | 81.0 | 188.9 | 1.4 | 5.1 |
| HCLPP-DETA | 12.8 | 71.4 | 172.6 | 1.5 | 5.6 |
| HCLPP-DENDRIMER | 6.2 | 28.9 | 109.3 | 1.4 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setnickova, K.; Jerabek, K.; Strasak, T.; Mullerova, M.; Jandova, V.; Soukup, K.; Petrickovic, R.; Tseng, H.-H.; Uchytil, P. Synthesis, Characterization, and Gas Adsorption Performance of Amine-Functionalized Styrene-Based Porous Polymers. Polymers 2023, 15, 13. https://doi.org/10.3390/polym15010013
Setnickova K, Jerabek K, Strasak T, Mullerova M, Jandova V, Soukup K, Petrickovic R, Tseng H-H, Uchytil P. Synthesis, Characterization, and Gas Adsorption Performance of Amine-Functionalized Styrene-Based Porous Polymers. Polymers. 2023; 15(1):13. https://doi.org/10.3390/polym15010013
Chicago/Turabian StyleSetnickova, Katerina, Karel Jerabek, Tomas Strasak, Monika Mullerova, Vera Jandova, Karel Soukup, Roman Petrickovic, Hui-Hsin Tseng, and Petr Uchytil. 2023. "Synthesis, Characterization, and Gas Adsorption Performance of Amine-Functionalized Styrene-Based Porous Polymers" Polymers 15, no. 1: 13. https://doi.org/10.3390/polym15010013
APA StyleSetnickova, K., Jerabek, K., Strasak, T., Mullerova, M., Jandova, V., Soukup, K., Petrickovic, R., Tseng, H.-H., & Uchytil, P. (2023). Synthesis, Characterization, and Gas Adsorption Performance of Amine-Functionalized Styrene-Based Porous Polymers. Polymers, 15(1), 13. https://doi.org/10.3390/polym15010013