Recent Trends in the Design, Synthesis and Biomedical Applications of Covalent Organic Frameworks
Abstract
:1. Introduction
2. Chemistry, Design Principle and Topology of COFs
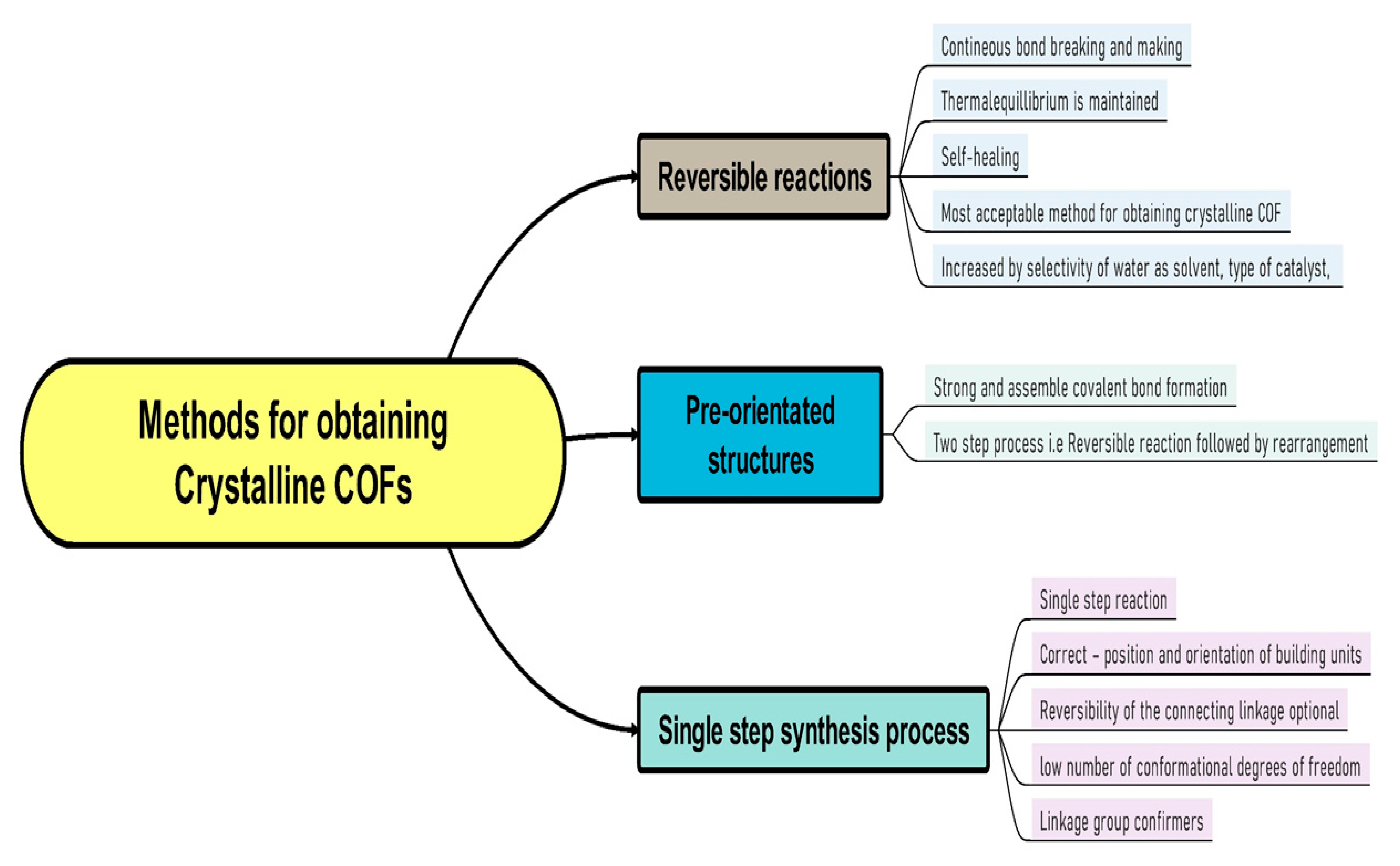
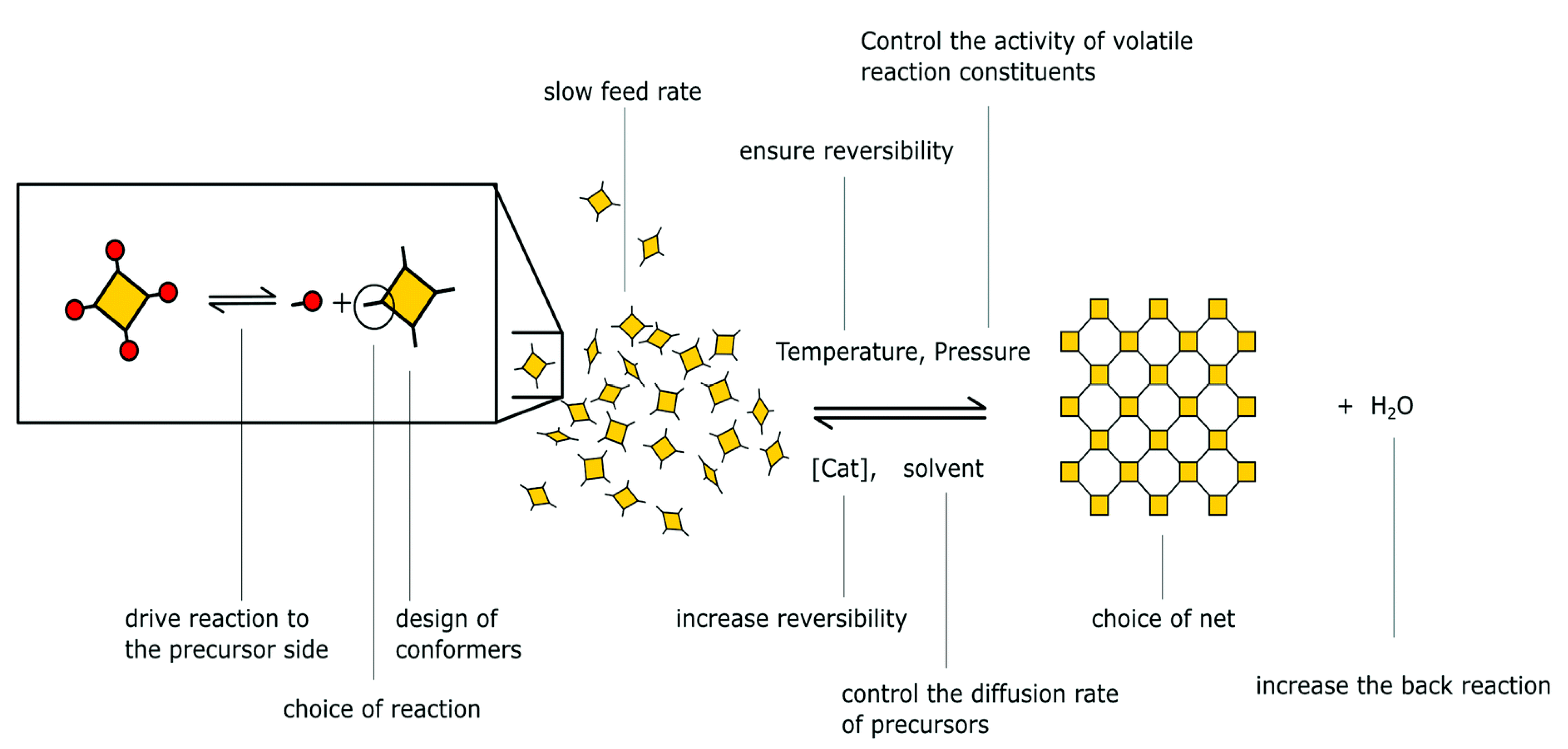
2.1. Chemistry of COFs (Reticular Chemistry and Dynamic Covalent Chemistry)
2.2. Design Principle and Topology of COFs
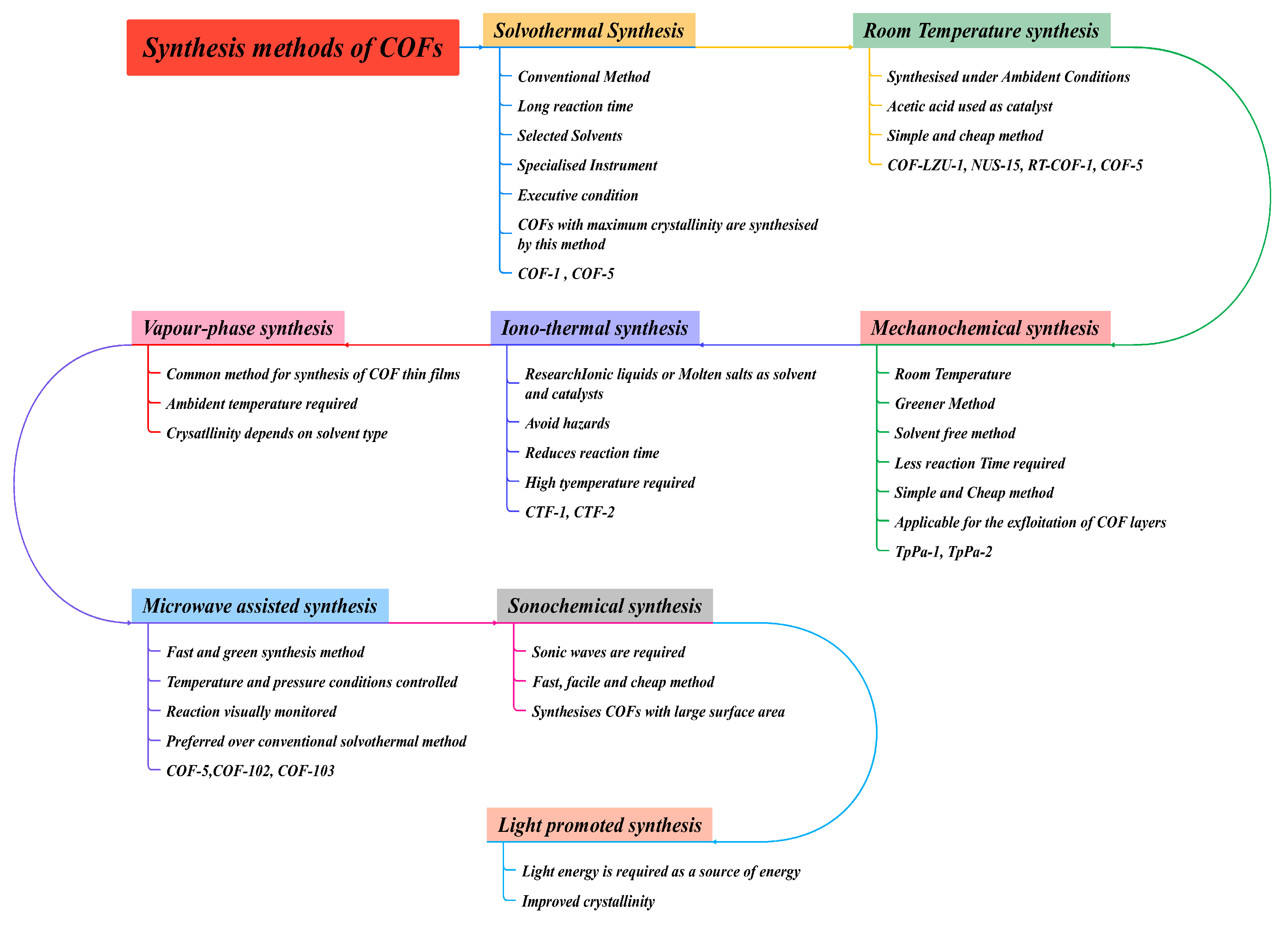
3. Synthesis Method of COFs
3.1. Solvothermal Synthesis
3.2. Ionothermal Process
3.3. Microwave Assisted Synthesis
3.4. Mechanochemical Synthesis
3.5. Sonochemical Method
3.6. Vapour-Assisted Method
3.7. Light Promoted Synthesis
| S. No. | COF | Synthesis Method | Monomers | Reaction Conditions | References |
|---|---|---|---|---|---|
| 1. | COF-5 | Microwave assisted | BDBA + HHTP | 1,4-Dioxane/mesitylene, 100 °C, 20 min | [24] |
| 2. | COF-102 | Microwave assisted | TBPM | 1,4-Dioxane/mesitylene, 200 W, 100 °C, 20 min | [24] |
| 3. | TpPa-COF | Microwave assisted | Pa + Tp | 1,4-Dioxane/mesitylene, acetic acid, 100 °C, 60 min | [25] |
| 4. | TfpTP-H | Microwave assisted | TP-H + Tp | DCE/1,4-dioxane, acetic acid, 250 W, 175 °C, 10 min | [26] |
| 5. | TfpTP-OEt | Microwave assisted | TP-OEt + Tp | DCE/mesitylene, acetic acid, 250 W, 175 °C,15 min | [26] |
| 6. | TfpTP-OMEG | Microwave assisted | TP-OMEG + Tp | DCB/prop, acetic acid, 250 W, 175 °C, 20 min | [26] |
| 7. | TfpTP-ODEG | Microwave assisted | TP-ODEG + Tp | DCB/prop, acetic acid, 250 W, 175 °C, 20 min | [26] |
| 8. | CTF-1 | Ionothermal | DCB | ZnCl2, DCB, 120–210 W, 400 °C, 0.5–2 h | [24] |
| 9. | TpBD | Room temperature synthesis | Tp + DABP | EtOH, RT for 30 min | [27] |
| 10. | COF-LZU-1 | Room temperature synthesis | BTCA + Pa | 1,4-dioxane, acetic acid, RT, 72 h | [28] |
| 11. | TpPa-1 | Room temperature synthesis | Tp + Pa | 1,4-dioxane, acetic acid, RT, 72 h | [28] |
| 12. | N3–COF | Room temperature synthesis | HZ + TFPT | DCE/EtOH, acetic acid, RT, 72 h | [28] |
| 13. | TFPB-HZ | Room temperature synthesis | HZ + TPB | EtOH/acetic acid, RT, 72 h | [28] |
| 14. | CAF-1 | Aqueous phase-mediated synthesis | TMA + CHDA | DMF/H2O, 250 °C, 72 h | [29] |
| 15. | CAF-2 | Aqueous phase-mediated synthesis | MTAB + CHDA | DMF/H2O, 250 °C, 72 h | [29] |
| 16. | HCOF-2 | Aqueous phase-mediated synthesis | B1 + HZ | H2O, 120 °C, 12 h | [30] |
| 17. | TpPa-1 (MC) | Mechanochemical synthesis | Tp + Pa | 1,4-Dioxane/mesitylene, 45 min | [31] |
| 18. | TpPa-2 (MC) | Mechanochemical synthesis | Tp + MPA | 1,4-Dioxane/mesitylene, 45 min | [31] |
| 19. | TpBD (MC) | Mechanochemical synthesis | Tp + DABP | 1,4-Dioxane/mesitylene, 45 min | [31] |
| 20. | TpBD-Me2 | Mechanochemical synthesis | Tp + DABP-Me2 | PTSA-H2O, 170 °C, 60 s | [32] |
| 21. | TpAzo | Mechanochemical synthesis | Tp + Azo | PTSA-H2O, 170 °C, 60 s | [32] |
4. Biological Applications of COFs
4.1. Drug Delivery
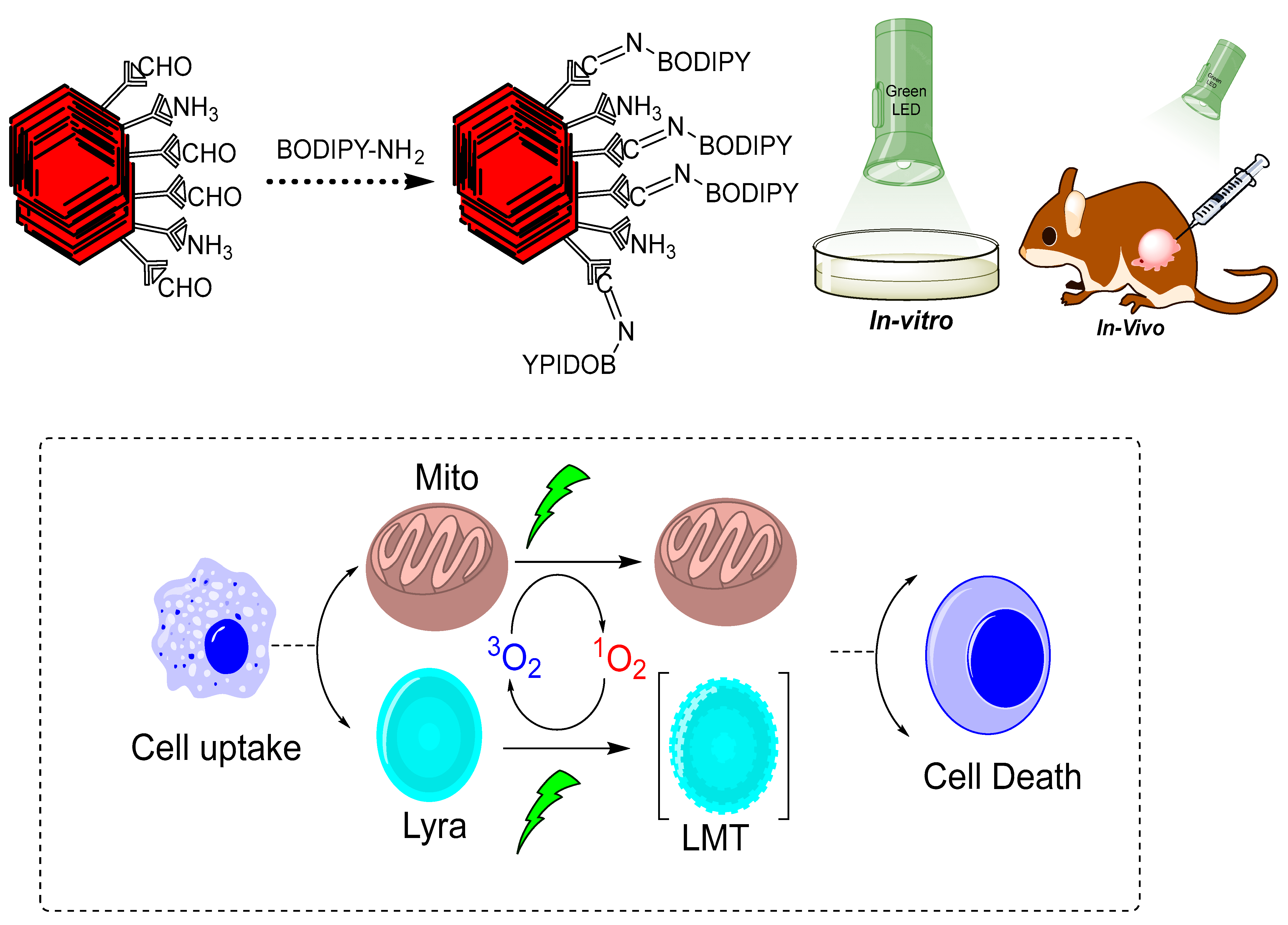
4.2. Photodynamic (PDT) and Photothermal Therapy (PTT)
4.3. Biosensing and Bioimaging
5. Miscellaneous Applications
5.1. 3D Printing Technology Applications
5.2. Gas Storage and Transport
5.3. Solar Cells
5.4. Super-Capacitors
5.5. Photocatalysts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.X.; Yang, Y.W. Applications of covalent organic frameworks (COFs): From gas storage and separation to drug delivery. Chin. Chem. Lett. 2017, 28, 1135–1143. [Google Scholar] [CrossRef]
- Guan, X.; Fang, Q.; Yan, Y.; Qiu, S. Functional Regulation and Stability Engineering of Three-Dimensional Covalent Organic Frameworks. Acc. Chem. Res. 2022, 55, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Grenu, B.; Torres, J.; García-González, J.; Muñoz-Pina, S.; de Los Reyes, R.; Costero, A.M.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef] [PubMed]
- Rungtaweevoranit, B.; Diercks, C.S.; Kalmutzki, M.J.; Yaghi, O.M. Spiers Memorial Lecture: Progress and prospects of reticular chemistry. Faraday Discuss. 2017, 201, 9–45. [Google Scholar] [CrossRef] [PubMed]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; George, N.; Singh, R.; Singh, G.; Kaur, J.D.; Kaur, G.; Singh, H.; Singh, J. CuAAC-Derived Selective Fluorescent Probe as a Recognition Agent for Pb (II) and Hg (II): DFT and Docking Studies. ACS Omega 2022, 7, 39159–39168. [Google Scholar] [CrossRef]
- Singh, G.; George, N.; Singh, R.; Singh, G.; Kaur, G.; Singh, H.; Singh, J. Ion recognition by 1, 2, 3-triazole moieties synthesized via “click chemistry”. Appl. Organomet. Chem. 2022, e6897. [Google Scholar] [CrossRef]
- Feng, L.; Qian, C.; Zhao, Y. Recent advances in covalent organic framework-based nanosystems for bioimaging and therapeutic applications. ACS Mater. Lett. 2020, 9, 1074–1092. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.L.; Li, Y.A.; Li, W.Y.; Wang, S.; Song, C.; Dong, Y.B. Nanoscale covalent organic framework for combinatorial antitumor photodynamic and photothermal therapy. ACS Nano 2019, 13, 13304–13316. [Google Scholar] [CrossRef]
- Vyas, V.S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. Lett. 2016, 28, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Fu, D.D.; Li, Y.A.; Kong, X.M.; Wei, Z.Y.; Li, W.Y.; Zhang, S.J.; Dong, Y.B. BODIPY-decorated nanoscale covalent organic frameworks for photodynamic therapy. IScience 2019, 14, 180–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghi, O.M. Reticular Chemistry—Construction, Properties, and Precision Reactions of Frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Reticular Chemistry for Highly Porous Metal–Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhu, X.; Yang, C.; Liu, M. Stacked Reticular Frame Boosted Circularly Polarized Luminescence of Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2022, 61, 202113979. [Google Scholar] [CrossRef]
- Hu, J.; Gupta, S.K.; Ozdemir, J.; Beyzavi, M.H. Applications of Dynamic Covalent Chemistry Concept toward Tailored Covalent Organic Framework Nanomaterials: A Review. ACS Appl. Nano Mater. 2020, 3, 6239–6269. [Google Scholar] [CrossRef]
- Chen, X.; Geng, K.; Liu, R.; Tan, K.T.; Gong, Y.; Li, Z.; Tao, S.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Chemical Approaches to Designer Structures and Built-In Functions. Angew. Chem. Int. Ed. 2020, 59, 5050–5091. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19. [Google Scholar] [CrossRef]
- Gui, B.; Lin, G.; Ding, H.; Gao, C.; Mal, A.; Wang, C. Three-dimensional covalent organic frameworks: From topology design to applications. Acc. Chem. Res. 2020, 53, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, J.; Gao, Y. Synthesis, Properties, and Their Potential Application of Covalent Organic Frameworks (COFs). In Mesoporous Materials-Properties and Applications; IntechOpen: London, UK, 2018; pp. 1–27. [Google Scholar]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Wang, D.; Wan, L.J. Synthesis of covalent organic framework films at interfaces. Bull. Chem. Soc. Jpn. 2021, 94, 1090–1098. [Google Scholar] [CrossRef]
- Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. [Google Scholar] [CrossRef]
- Vazquez-Molina, D.A.; Pope, G.M.; Ezazi, A.A.; Mendoza-Cortes, J.L.; Harper, J.K.; Uribe-Romo, F.J. Framework vs. side-chain amphidynamic behaviour in oligo-(ethylene oxide) functionalised covalent-organic frameworks. Chem. Commun. 2018, 54, 6947–6950. [Google Scholar] [CrossRef]
- Yang, C.X.; Liu, C.; Cao, Y.M.; Yan, X.P. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 2015, 51, 12254–12257. [Google Scholar] [CrossRef]
- Peng, Y.; Wong, W.K.; Hu, Z.; Cheng, Y.; Yuan, D.; Khan, S.A.; Zhao, D. Room temperature batch and continuous flow synthesis of water-stable covalent organic frameworks (COFs). Chem. Mater. 2016, 28, 5095–5101. [Google Scholar] [CrossRef]
- Stewart, D.; Antypov, D.; Dyer, M.S.; Pitcher, M.J.; Katsoulidis, A.P.; Chater, P.A.; Blanc, F.; Rosseinsky, M.J. Stable and ordered amide frameworks synthesised under reversible conditions which facilitate error checking. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Lin, F.; Wen, Q.; Qi, Q.Y.; Xu, J.Q.; Zhao, X. Large-scale synthesis of azine-linked covalent organic frameworks in water and promoted by water. New J. Chem. 2019, 43, 6116–6120. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing ultraporous covalent organic frameworks in seconds via an organic terracotta process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; He, B.; Lin, Y.; Wang, J.; Li, M. 8-Hydroxyquinoline functionalized covalent organic framework as a pH sensitive carrier for drug delivery. Mater. Sci. Eng. C 2020, 117, 111243. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chedid, G.; Yassin, A. Recent trends in covalent and metal organic frameworks for biomedical applications. Nanomaterials 2018, 8, 916. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Peng, Y.; Yan, F.; Li, C.; He, Y.; Lou, Y.; Ma, D.; Li, Y.; Shi, Z.; Feng, S. A cage-based covalent organic framework for drug delivery. New J. Chem. 2021, 45, 3343–3348. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, Z.; Su, H.; Jing, X.; Sun, F.; Zhu, G. Targeted synthesis of a 2D ordered porous organic framework for drug release. Chem. Comm. 2011, 47, 6389–6391. [Google Scholar] [CrossRef]
- Rengaraj, A.; Puthiaraj, P.; Haldorai, Y.; Heo, N.S.; Hwang, S.K.; Han, Y.K.; Kwon, S.; Ahn, W.S.; Huh, Y.S. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Appl. Mater. Interfaces 2016, 8, 8947–8955. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Liu, Y.; Lyu, Y. Porphyrin-based covalent triazine frameworks: Porosity, adsorption performance, and drug delivery. J. Polym. Sci. A Polym. Chem. 2017, 55, 2594–2600. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, P. Ibuprofen tagged imine RT-COF-1 as customisable vehicle for controlled drug delivery application. Inorg. Chem. Commun. 2022, 145, 110043. [Google Scholar]
- Bai, L.; Phua, S.Z.F.; Lim, W.Q.; Jana, A.; Luo, Z.; Tham, H.P.; Zhao, L.; Gao, Q.; Zhao, Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016, 52, 4128–4131. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yao, C.; Yang, W.L.; Zhang, L.; Zhang, D.W.; Wang, H.; Zhang, F.; Liu, Y.; Li, Z.T. In situ-prepared homogeneous supramolecular organic framework drug delivery systems (sof-DDSs): Overcoming cancer multidrug resistance and controlled release. Chin. Chem. Lett. 2017, 28, 798–806. [Google Scholar] [CrossRef]
- Mitra, S.; Sasmal, H.S.; Kundu, T.; Kandambeth, S.; Illath, K.; Díaz Díaz, D.; Banerjee, R. Targeted Drug Delivery in Covalent Organic Nanosheets (CONs) via Sequential Postsynthetic Modification. J. Am. Chem. Soc. 2017, 139, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.C.; Vella-Zarb, L. Evolution of Nanocarrier Drug-Delivery Systems and Recent Advancements in Covalent Organic Framework-Drug Systems. ACS Appl. Nano Mater. 2020, 3, 3097–3115. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Liu, Y.; Zhao, X.; Pang, M.; Lin, J. One-Pot Synthesis of DOX@ Covalent Organic Framework with Enhanced Chemotherapeutic Efficacy. Chem. Eur. J. 2019, 25, 4315–4319. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Mathe, S.D.; Dong, A.; Zhang, J. Covalent organic frameworks: From materials design to biomedical application. Nanomaterials 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, Y.; Hu, C.; Zhao, X.; Pang, M. Boosting the antitumor efficacy over a nanoscale porphyrin-based covalent organic polymer via synergistic photodynamic and photothermal therapy. Chem. Commun. 2019, 55, 6269–6272. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Liu, Y.; Sun, C.; Chang, M.; Zhao, X.; Hu, C.; Pang, M. Facile fabrication of nanoscale porphyrinic covalent organic polymers for combined photodynamic and photothermal cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 12321–12326. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Zhang, C.; Yin, S.Y.; Teng, L.; Chen, L.; Hu, X.X.; Liu, H.W.; Yin, X.; Zhang, X.B. Ultrathin two-dimensional covalent organic framework nanoprobe for interference-resistant two-photon fluorescence bioimaging. Chem. Sci. 2018, 9, 8402–8408. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Bhunia, S.; Deo, K.A.; Gaharwar, A.K. 2D covalent organic frameworks for biomedical applications. Adv. Funct. Mater. 2020, 30, 2002046. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. MOF/COF-based materials using 3D printing technology: Applications in water treatment, gas removal, biomedical, and electronic industries. New J. Chem. 2021, 45, 13247–13257. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Pang, Y.H.; Shen, X.F.; Jiang, R.; Wang, Y.Y. Covalent organic framework DQTP modified pencil graphite electrode for simultaneous determination of bisphenol A and bisphenol S. Talanta 2022, 236, 122859. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lim, G.J.; Wang, Y.; Zhang, L.; Mullangi, D.; Wu, Y.; Zhao, D.; Ding, J.; Cheetham, A.K.; Wang, J. Binder-free 3D printing of covalent organic framework (COF) monoliths for CO2 adsorption. Chem. Eng. J. 2021, 403, 126333. [Google Scholar] [CrossRef]
- Martinez-Bulit, P.; Sorrenti, A.; Rodriguez-San-Miguel, D.; Mattera, M.; Belce, Y.; Xia, Y.; Ma, S.; Huang, M.H.; Pané, S.; Puigmartí-Luis, J. In flow-based technologies: A new paradigm for the synthesis and processing of covalent-organic frameworks. Chem. Eng. J. 2022, 435, 135117. [Google Scholar] [CrossRef]
- Han, S.S.; Furukawa, H.; Yaghi, O.M.; Goddard Iii, W.A. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 2008, 130, 11580–11581. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.; Chen, Z.; Zhang, Q. Recent progress in two-dimensional COFs for energy-related applications. J. Mater. Chem. A 2017, 5, 14463–14479. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Hao, D.; Shi, Q.; Dong, B.; Leng, W.; Wang, C.; Gao, Y. Target Synthesis of an Azo (N=N) Based Covalent Organic Framework with High CO2-over-N2 Selectivity and Benign Gas Storage Capability. J. Chem. Eng. Data 2016, 61, 1904–1909. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dogru, M.; Handloser, M.; Auras, F.; Kunz, T.; Medina, D.; Hartschuh, A.; Knochel, P.; Bein, T. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. 2013, 52, 2920–2924. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly conjugated three-dimensional covalent organic frameworks based on spirobifluorene for perovskite solar cell enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Lee, C.C.; EL-Mahdy, A.F.; Lüder, J.; Yu, M.H.; Li, Z.; Zhu, Z.; Chueh, C.C.; Kuo, S.W. Exploitation of two-dimensional conjugated covalent organic frameworks based on tetraphenylethylene with bicarbazole and pyrene units and applications in perovskite solar cells. J. Mater. Chem. 2020, 8, 11448–11459. [Google Scholar] [CrossRef]
- Chang, P.H.; Sil, M.C.; Reddy, K.S.K.; Lin, C.H.; Chen, C.M. Polyimide-Based Covalent Organic Framework as a Photocurrent Enhancer for Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 25466–25477. [Google Scholar] [CrossRef]
- Song, S.; Kranthiraja, K.; Heo, J.; Kim, T.; Walker, B.; Jin, S.H.; Kim, J.Y. Efficiency exceeding 11% in tandem polymer solar cells employing high open-circuit voltage wide-bandgap π-conjugated polymers. Adv. Energy Mater. 2017, 7, 1700782. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Lu, Y.; Hua, X.; Liu, J.; Yang, Z.; Wei, H.; Mai, Y. All-organic covalent organic framework/polyaniline composites as stable electrode for high-performance supercapacitors. Mater. Lett. 2019, 236, 354–357. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.D.; Liu, Y.; Li, Y.; Cheng, C.; Zhu, H.; Yan, X.; Li, Z.; Gu, Z.G. A two-dimensional semiconducting covalent organic framework with nickel (II) coordination for high capacitive performance. J. Mater. Chem. 2019, 7, 19676–19681. [Google Scholar] [CrossRef]
- Kandambeth, S.; Jia, J.; Wu, H.; Kale, V.S.; Parvatkar, P.T.; Czaban-Jóźwiak, J.; Zhou, S.; Xu, X.; Ameur, Z.O.; Abou-Hamad, E.; et al. Covalent organic frameworks as negative electrodes for high-performance asymmetric supercapacitors. Adv. Energy Mater. 2020, 10, 2001673. [Google Scholar] [CrossRef]
- Iqbal, R.; Badshah, A.; Ma, Y.J.; Zhi, L.J. An electrochemically sTable 2D covalent organic framework for high-performance organic supercapacitors. Chin. J. Polym. Sci. 2020, 38, 558–564. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Xue, R.; Ma, B.; Li, Q.; Wu, N.; Liu, H.; Yao, W.; Guo, H.; Yang, W. Ultrastable triazine-based covalent organic framework with an interlayer hydrogen bonding for supercapacitor applications. ACS Appl. Mater. Interfaces 2019, 11, 26355–26363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, T.; Gottschling, K.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V. H2 evolution with covalent organic framework photocatalysts. ACS Energy Lett. 2018, 3, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegbauer, L.; Zech, S.; Savasci, G.; Banerjee, T.; Podjaski, F.; Schwinghammer, K.; Ochsenfeld, C.; Lotsch, B.V. Tailor-Made Photoconductive Pyrene-Based Covalent Organic Frameworks for Visible-Light Driven Hydrogen Generation. Adv. Energy Mater. 2018, 8, 1703278. [Google Scholar] [CrossRef]
- Kong, D.; Han, X.; Xie, J.; Ruan, Q.; Windle, C.D.; Gadipelli, S.; Shen, K.; Bai, Z.; Guo, Z.; Tang, J. Tunable covalent triazine-based frameworks (CTF-0) for visible-light-driven hydrogen and oxygen generation from water splitting. ACS Catal. 2019, 9, 7697–7707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhu, X.; Huang, L.; Zhang, X.; Zhang, F.; Zhu, W. Azine-based covalent organic frameworks as metal-free visible light photocatalysts for CO2 reduction with H2O. Appl. Catal. B 2018, 239, 46–51. [Google Scholar] [CrossRef]
- Stegbauer, L.; Hahn, M.W.; Jentys, A.; Savasci, G.; Ochsenfeld, C.; Lercher, J.A.; Lotsch, B.V. Tunable water and CO2 sorption properties in isostructural azine-based covalent organic frameworks through polarity engineering. Chem. Mater. 2015, 27, 7874–7881. [Google Scholar] [CrossRef]







| S. No | Topology Design Diagram | Condensation | Monomer Symmetry | Topology Type | Reference |
|---|---|---|---|---|---|
| 1. | [C3 + C2] design | Poly-condensation | C3 and C2 symmetric monomers | Hexagonal | [16] |
| 2. | [C4 + C2] design | Poly-condensation | C4 and C2 symmetric monomers | Tetragonal | [16] |
| 3. | [C2 +C2] design | Self-condensation | C2 symmetric monomers | Rhombic | [16] |
| 4. | [C6 + C2] design | Poly-condensation | C6 and C2 symmetric monomers | Triangular (microporous) | [16] |
| 5. | [C3 + C3] design | Self-condensation | C3 symmetric monomers | Hexagonal (microporous) | [17] |
| 6. | [C2 + C2] design | Self-condensation | C2 symmetric monomers | Hexagonal (microporous) | [17] |
| 7. | [C4 + C4] design | Self-condensation | C4 symmetric monomers | Tetragonal (microporous) | [17] |
| 8. | [C3 + C1] design | Poly-condensation | C3 and C1 symmetric monomers | Hexagonal | [18] |
| 9. | [Td +Td] design | Self-condensation | Td monomers | Dia | [18] |
| 10. | [Td + C3] design | Poly-condensation | Td and C3 symmetric monomer | bor, ctn, srs | [18] |
| 11. | [Td + C2] design | Poly-condensation | Td and C2 symmetric monomer | Dia | [19] |
| 12. | [Td + C4] design | Poly-condensation | Td and C4 symmetric monomer | Pts | [19] |
| S. No. | Covalent Organic Framework | Organic Linkers | Therapeutics | Drug Loading | Drug Release | Reference |
|---|---|---|---|---|---|---|
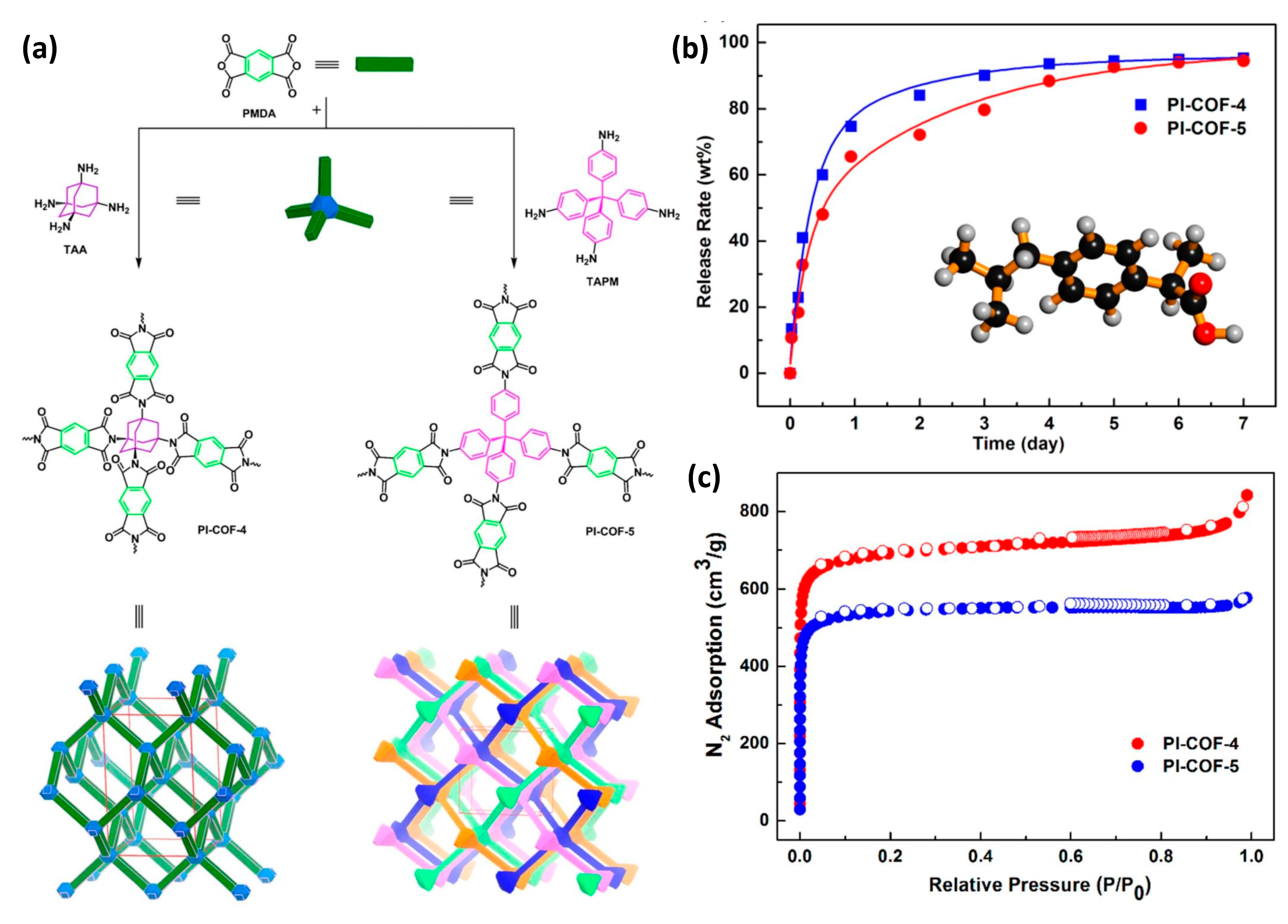
| 1. | Polyimide-covalent organic framework-4 PI-COF-4 | Pyromellitic dianhydride (PMDA) + Tetrahedral 1,3,5,7-tetraaminoadamantane (TAA) | IBU (ibuprofen) | 24 wt% | 60% after 12 h | [33] |
| 2. | Polyimide-covalent organic framework-5 PI-COF-5 | Pyromellitic dianhydride (PMDA) + Tetra (4-aminophenyl) methane (TAPM) | IBU (ibuprofen) | 20 wt% | 49% after 12 h | [33] |
| 3. | t PEG-CCM@APTES-COF-1 | Polyethylene-glycol-modified monofunctional curcumin derivatives (PEG-CCM) + Amine-functionalized COFs (APTES-COF-1) | Doxorubicin | 9.71 ± 0.13 wt% | - | [35] |
| 4. | Porous covalent triazine framework PCTF | 5,10,15,20-Tetraphenylporphyrin + Cyanuric chloride | IBU (ibuprofen) | 19% | 90% after 48 h | [36] |
| 5. | t Cage-COF-TT (TT = triammonia–terephthalaldehyde), | Bis (tetraoxacalix (2) arene (2) triazine + Terephthalaldehyde | IBU (ibuprofen) | 17.1 wt% | 93% after 52 h | [37] |
| 6. | t Cage-COF-TT (TT = triammonia–terephthalaldehyde), | Bis (tetraoxacalix (2) arene (2) triazine + terephthalaldehyde | FLU (fluorouracil) | 21.4 wt% | 93% after 52 h | [37] |
| 7. | t Cage-COF-TT (TT = triammonia–terephthalaldehyde), | Bis (tetraoxacalix (2) arene (2) triazine + Terephthalaldehyde | CAP | 22.3 wt% | 94% after 52 h | [37] |
| 8. | Porous aromatic frameworks PAF-6 | Cyanuric chloride + Piperazine | IBU (ibuprofen) | 0.35 gm | 75% within 10 h | [38] |
| 9. | Nanoscale covalent triazine polymer NCTP | Cyanuric chloride + Biphenyl | Doxorubicin | 200 mg/g | 80% at pH 4.8 over 48 h | [39] |
| 10. | Porous covalent triazine framework PCTF-Mn | 5,10,15,20-Tetraphenylporphyrin-Mn + Cyanuric chloride | IBU (ibuprofen) | 23% | 95% after 48 h | [40] |
| 11. | Room temperature covalent organic framework-1 RT-COF-1 | 1,3,5-Tris(4-aminophenyl) benzene (TAPB) + 1,3,5-Benzenetricarbaldehyde (BTCA) | IBU | - | 33% within 105 min | [41] |
| 12. | Polyimide covalent organic framework-2 PI-2-COF | 1,3,5-Triformylbenzene + 4,4′-Biphenyldiamine | 5-FU | 60 µg/mL | 85% of initially loaded drug | [2,42] |
| 13. | Polyimide covalent organic framework-3 PI-3-COF | 1,3,5-Triformylbenze + 2,4,6-Tris (4-aminophenyl)-s-triazine | 5-FU | 32 µg/mL | 85% of initially loaded drug | [2,42] |
| 14. | Pemetrexed supramolecular framework (PMX@SOF) | Pyridinium-based tetracationic monomer (variable composition) + Cucurbit (8) uril ring | Pemetrexed | 23 | 60 h | [43] |
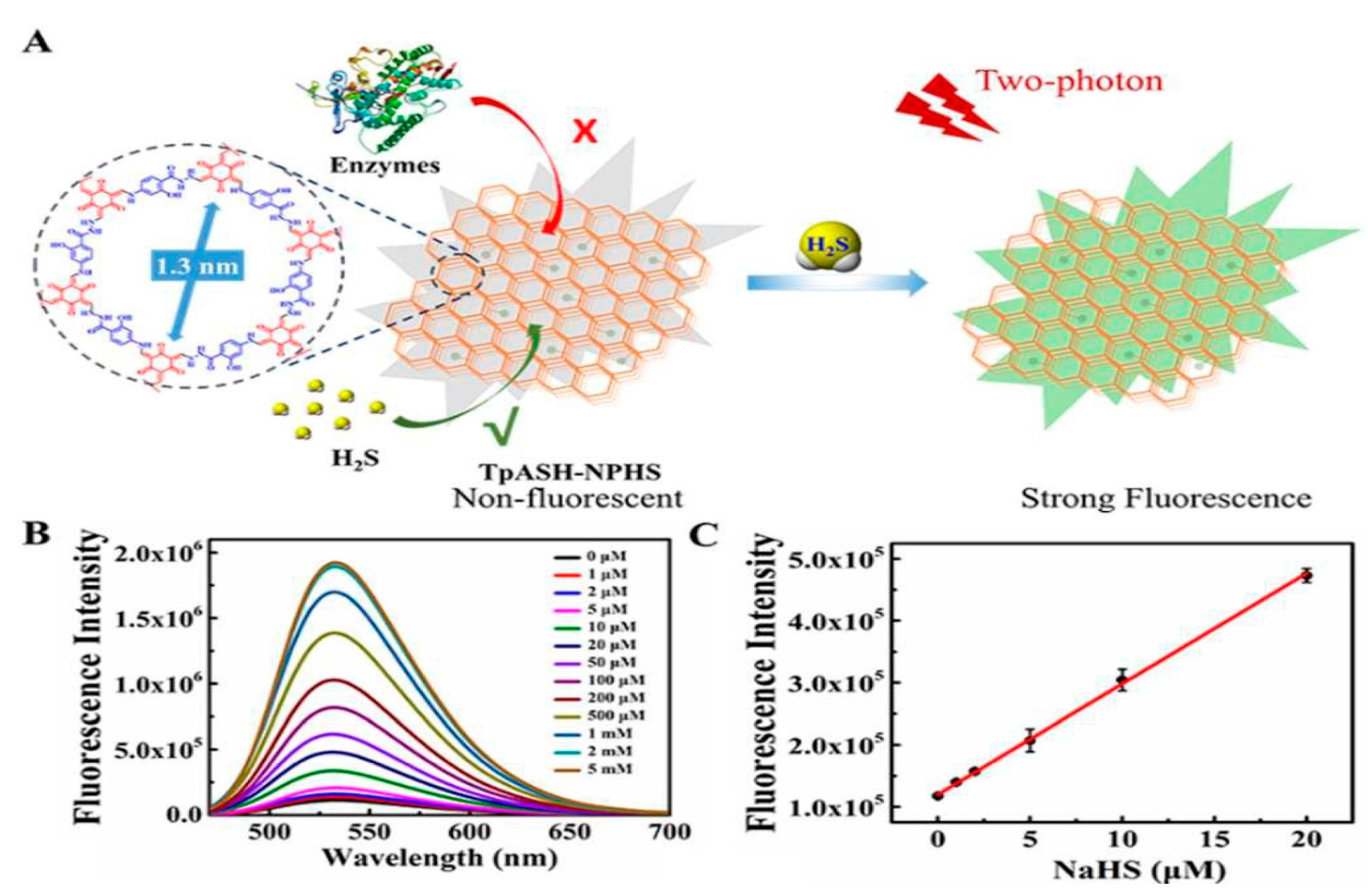
| 15. | 1,3,5-triformylphloroglu 4-amino salicyl hydride (TpASH) | 1,3,5-triformylphloroglu (Tp) + 4-Amino salicyl hydride (ASH) | 5-FU Fluorouracil | 12 | 72 h | [44] |
| 16. | 1,3,5-triformylphloroglu 4 amino benzo hydride (TpAPH) | 1,3,5-triformylphloroglu (Tp) + 4-Amino benzo hydrides (APH) | 5-FU Fluorouracil | 12 | 72 h | [44] |
| 17. | 2,5-dihydroxyterephthalaldehyde 1,3,5-tris(4-aminophenyl) benzene COF (COF-DhaTab) | 2,5-dihydroxyterephthalaldehyde+ 1,3,5-tris(4-aminophenyl) benzene | DOX Doxorubicin | 35 | Less than 7 days | [45] |
| 18. | 1,3,5-tris(4-aminophenyl) benzene -2,5-dimethoxyterephthaldehyde-COF (TAPB-DMTP-COF) | 2,5-dimethoxyterephthaldehyde (DMTP) + 1,3,5-tris(4-aminophenyl) benzene (TAPB) | DOX Doxorubicin | - | - | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Kumar, D.; Sundarrajan, S.; Ramakrishna, S.; Kumar, P. Recent Trends in the Design, Synthesis and Biomedical Applications of Covalent Organic Frameworks. Polymers 2023, 15, 139. https://doi.org/10.3390/polym15010139
Kaur G, Kumar D, Sundarrajan S, Ramakrishna S, Kumar P. Recent Trends in the Design, Synthesis and Biomedical Applications of Covalent Organic Frameworks. Polymers. 2023; 15(1):139. https://doi.org/10.3390/polym15010139
Chicago/Turabian StyleKaur, Gagandeep, Dinesh Kumar, Subramanian Sundarrajan, Seeram Ramakrishna, and Pawan Kumar. 2023. "Recent Trends in the Design, Synthesis and Biomedical Applications of Covalent Organic Frameworks" Polymers 15, no. 1: 139. https://doi.org/10.3390/polym15010139






