Extraction of Corn Bract Cellulose by the Ammonia-Coordinated Bio-Enzymatic Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
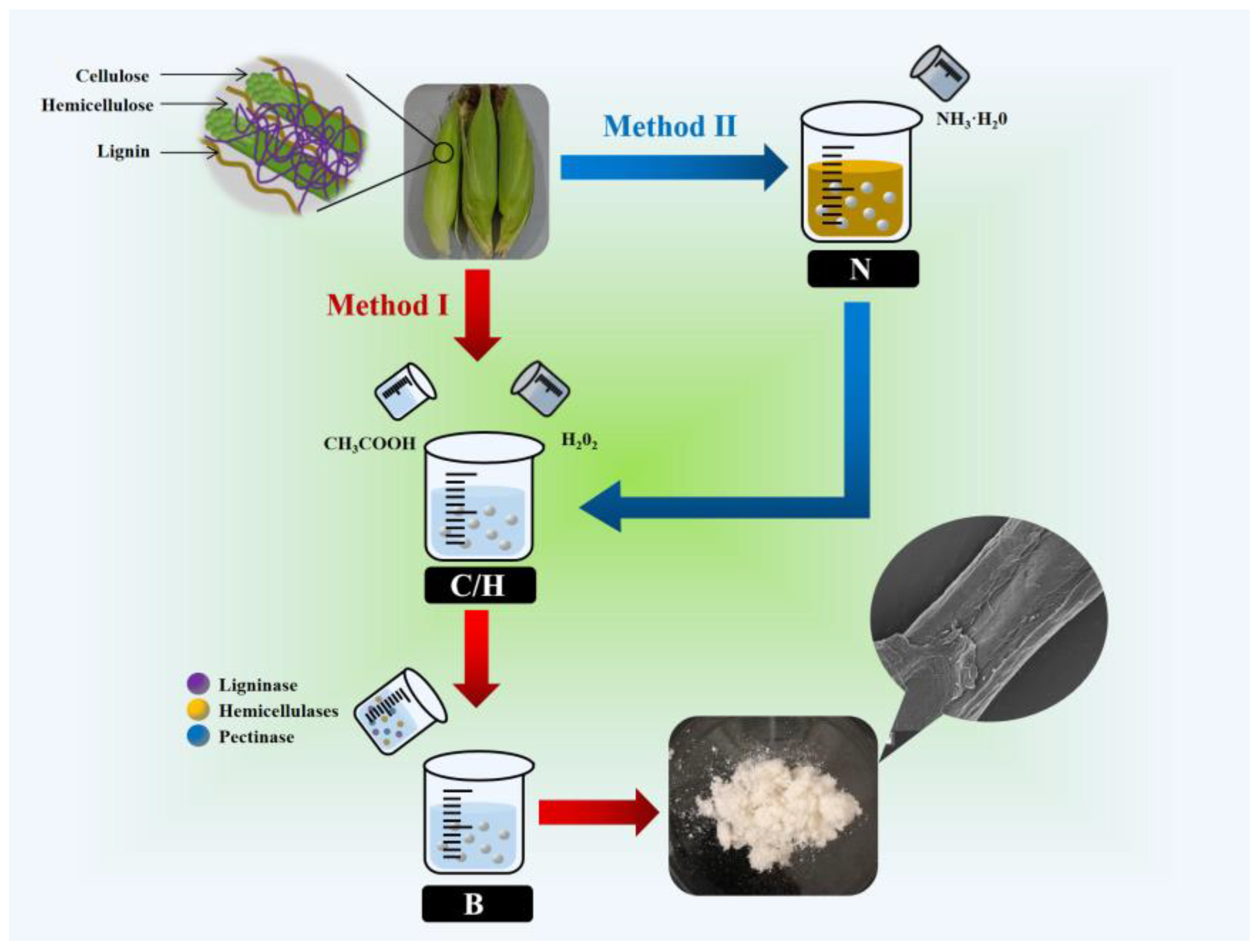
2.2. Extraction of Cellulose from Corn Bracts
2.2.1. Method I: CHB (CH3COOH/H2O2/Bio-Enzyme)
2.2.2. Method II: N-CHB (NH3·H2O-CH3COOH/H2O2/Bio-Enzyme)
2.3. Properties of Cellulose
2.3.1. Appearance Morphology Analysis
2.3.2. The Content of the Three Major Elements (Cellulose, Hemicellulose, Lignin)
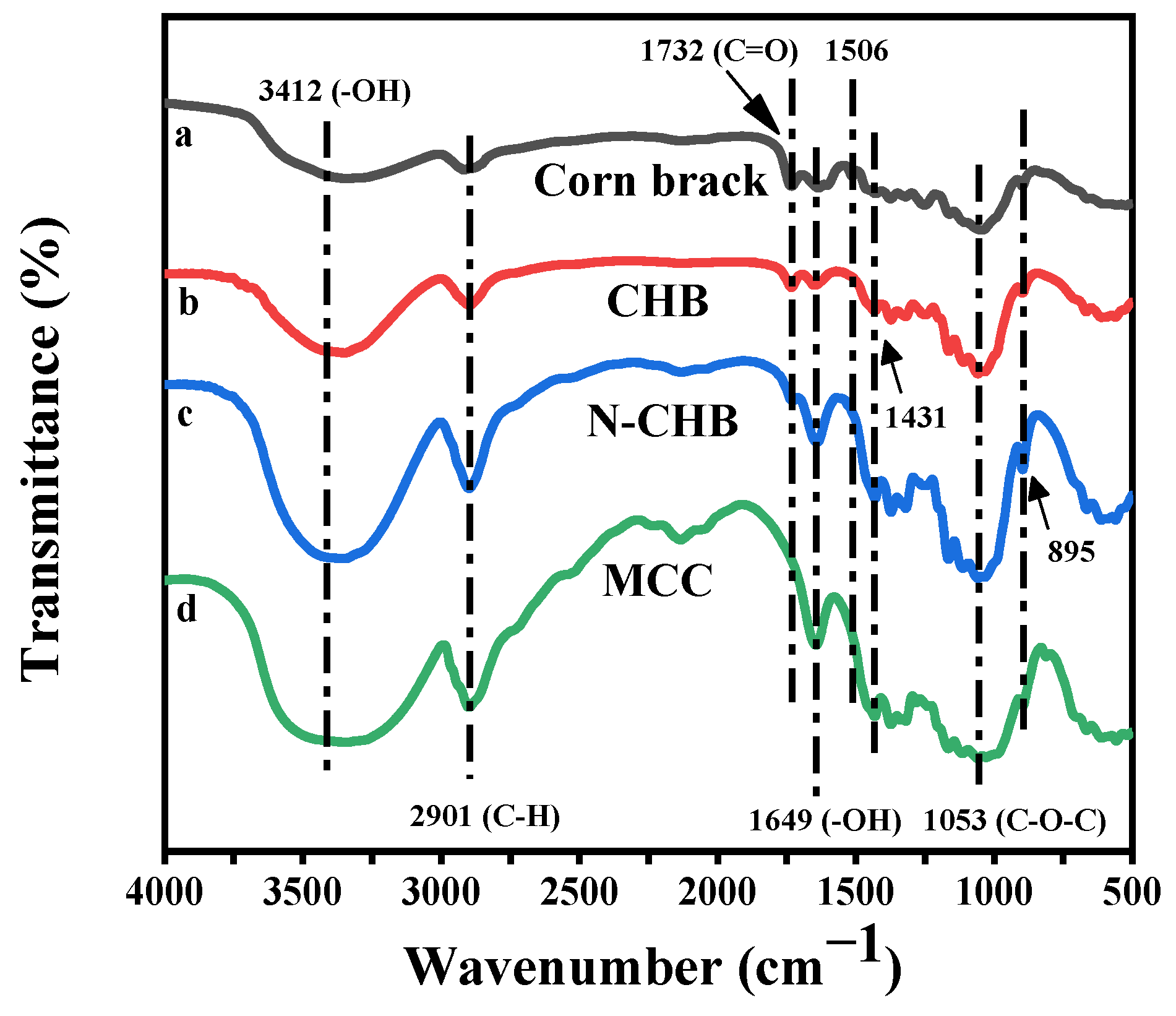
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
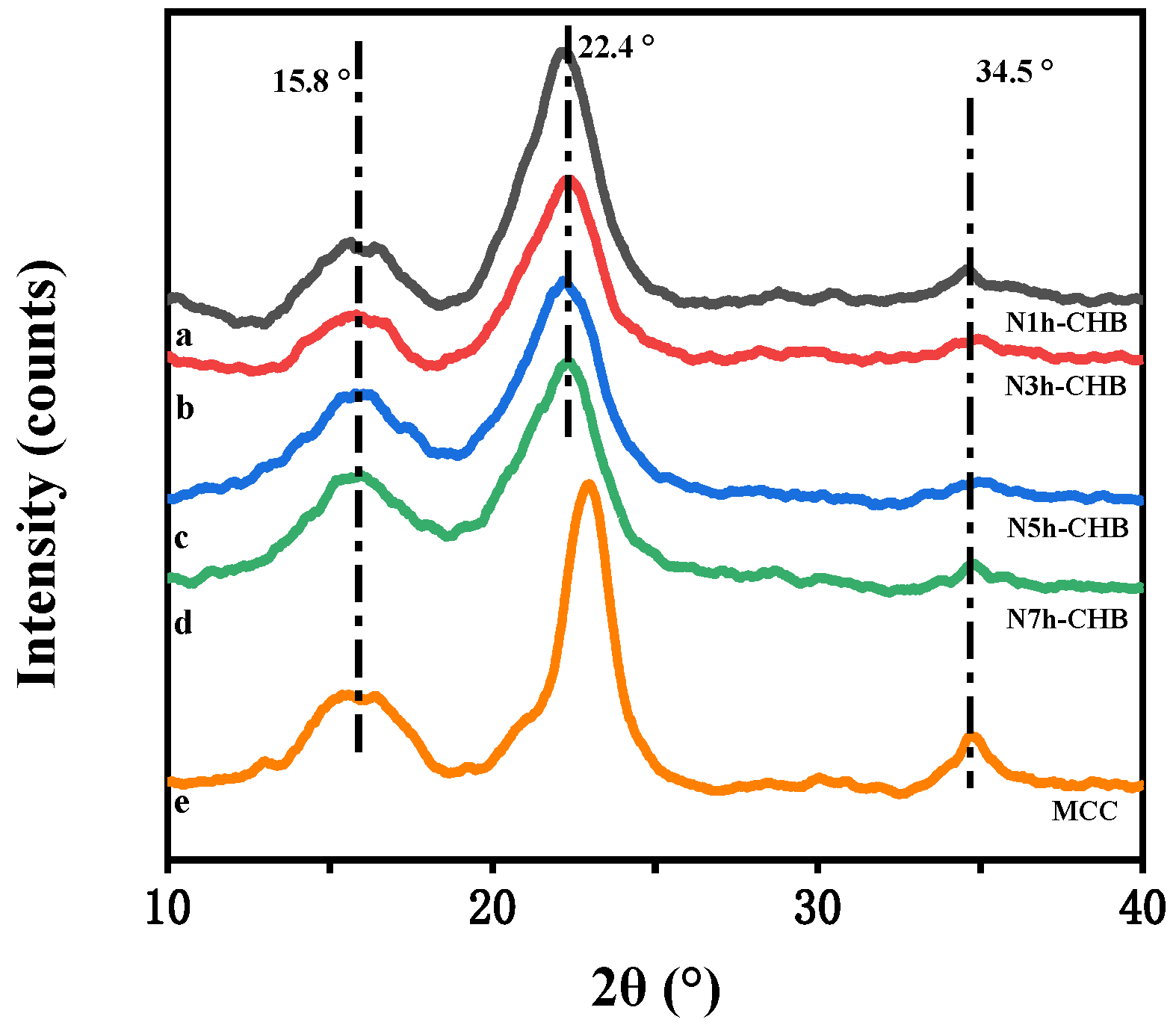
2.3.4. X-ray Diffraction (XRD) Analysis
2.3.5. Thermogravimetric (TG) Analysis
2.3.6. Sample Microstructure (SEM) Analysis
3. Results and Discussion
3.1. Extraction Method
3.1.1. Corn Bract Powder and Cellulose
3.1.2. The Contents of the Three Major Elements
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.4. Sample Microstructure
3.2. Exploration of the Pretreatment Time of Ammonia (NH3·H2O)
3.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.2. X-ray Diffraction (XRD) Analysis
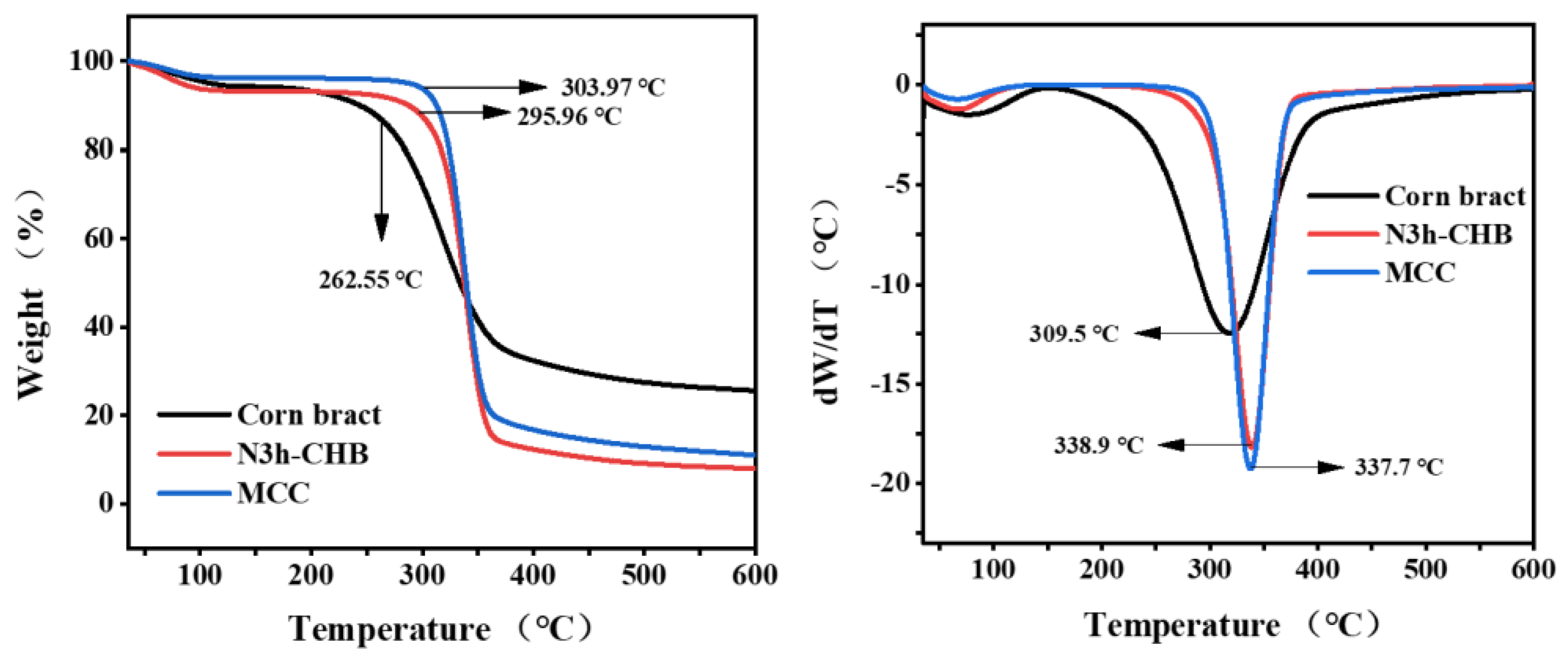
3.2.3. Thermogravimetric (TG) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Tang, Z.; Sun, Z.; Simonsen, J.; Luo, Z.; Li, X.; Morrell, J.J. Chemical and Enzymatic Fiber Modification to Enhance the Mechanical Properties of CMC Composite Films. Polymers 2022, 14, 4127. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Lee, S.Y.; Wei, T.; Li, J.; Fan, Z.J. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Khan, I.; Usmani, M.A.; Umapathi, R.; Al-Kindy, S.M.Z. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Xu, C.Y.; Ma, F.Y.; Zhang, X.Y.; Chen, S.L. Biological pretreatment of corn stover by Irpex lacteus for enzymatic hydrolysis. J. Agric. Food Chem. 2010, 58, 10893–10898. [Google Scholar] [CrossRef]
- Gomez, L.D.; Vanholme, R.; Bird, S.; Goeminne, G.; Trindade, L.M.; Polikarpov, I.; Simister, R.; Morreel, K.; Boerjan, W.; McQueen-Mason, S.J. Side by Side Comparison of Chemical Compounds Generated by Aqueous Pretreatments of Maize Stover, Miscanthus and Sugarcane Bagasse. BioEnergy Res. 2014, 7, 1466–1480. [Google Scholar] [CrossRef] [Green Version]
- Flores-Velazquez, V.; Cordova-Perez, G.E.; Silahua-Pavon, A.A.; Torres-Torres, J.G.; Sierra, U.; Fernandez, S.; Godavarthi, S.; Ortiz-Chi, F.; Espinosa-Gonzalez, C.G. Cellulose obtained from banana plant waste for catalytic production of 5-HMF: Effect of grinding on the cellulose properties. Fuel 2020, 265, 11. [Google Scholar] [CrossRef]
- Yang, M.X.; Zhou, R. Research on Degumming experiment of Corn Bracts. In Proceedings of the 2nd International Conference on Chemical Engineering and Advanced Materials (CEAM 2012), Guangzhou, China, 13–15 July 2012; pp. 1242–1247. [Google Scholar]
- Lu, P.; Hsieh, Y.L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Rahimi, M.; Behrooz, R. Effect of Cellulose Characteristic and Hydrolyze Conditions on Morphology and Size of Nanocrystal Cellulose Extracted from Wheat Straw. Int. J. Polym. Mater. 2011, 60, 529–541. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sust. Energ. Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Wan, C.X.; Zhou, Y.G.; Li, Y.B. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.J.; Borch, K.; Westh, P. Xylan oligosaccharides and cellobiohydrolase I (TrCeI7A) interaction and effect on activity. Biotechnol. Biofuels 2011, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulbaran-de-Ferrer, B.; Aristiguieta, M.; Dale, B.E.; Ferrer, A.; Ojeda-de-Rodriguez, G. Enzymatic hydrolysis of ammonia-treated rice straw. Appl. Biochem. Biotechnol. 2003, 105, 155–164. [Google Scholar] [CrossRef]
- Ren, H.W.; Shen, J.L.; Pei, J.W.; Wang, Z.Y.; Peng, Z.P.; Fu, S.F.; Zheng, Y. Characteristic microcrystalline cellulose extracted by combined acid and enzyme hydrolysis of sweet sorghum. Cellulose 2019, 26, 8367–8381. [Google Scholar] [CrossRef]
- Yilmaz, N.D. Effects of enzymatic treatments on the mechanical properties of corn husk fibers. J. Text. Inst. 2013, 104, 396–406. [Google Scholar] [CrossRef]
- Kim, I.; Han, J.I. Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y. Pretreatment of corn stover by soaking in aqueous ammonia. Appl. Biochem. Biotechnol. 2005, 121, 1119–1131. [Google Scholar] [CrossRef]
- Truong, N.P.V.; Kim, T.H. Effective saccharification of corn stover using low-liquid aqueous ammonia pretreatment and enzymatic hydrolysis. Molecules 2018, 23, 1050. [Google Scholar] [CrossRef] [Green Version]
- Zulkiple, N.; Maskat, M.Y.; Hassan, O. Pretreatment of Oil Palm Empty Fruit Fiber (OPEFB) with Aquaeous Ammonia for High Production of Sugar. In Proceedings of the International Seminar on Molecular and Cellular Life Sciences—Infectious Diseases, Biochemistry and Structural Biology (MCLS), Surabaya, Indonesia, 7–8 May 2015; pp. 155–161. [Google Scholar]
- Kim, J.S.; Lee, Y.Y.; Park, S.C. Pretreatment of wastepaper and pulp mill sludge by aqueous ammonia and hydrogen peroxide. Appl. Biochem. Biotechnol. 2000, 84–86, 129–139. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y.; Sunwoo, C.; Kim, J.S. Pretreatment of corn stover by low-liquid ammonia recycle percolation process. Appl. Biochem. Biotechnol. 2006, 133, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norvell, K.L.; Nghiem, N.P. Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions. Fermentation 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Hoover, A.N.; Tumuluru, J.S.; Teymouri, F.; Moore, J.; Gresham, G. Effect of pelleting process variables on physical properties and sugar yields of ammonia fiber expansion pretreated corn stover. Bioresour. Technol. 2014, 164, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Lee, Y.Y. Pretreatment of Hybrid Poplar by Aqueous Ammonia. Biotechnol. Prog. 2009, 25, 357–364. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Kim, T.H.; Lee, Y.Y.; Chen, R.G.; Elander, R.T. Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Appl. Biochem. Biotechnol. 2006, 130, 586–598. [Google Scholar] [CrossRef]
- Kamm, B.; Leiss, S.; Schonicke, P.; Bierbaum, M. Biorefining of Lignocellulosic Feedstock by a Modified Ammonia Fiber Expansion Pretreatment and Enzymatic Hydrolysis for Production of Fermentable Sugars. ChemSusChem 2017, 10, 48–52. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madadlou, A.; Sabouri, A.A. Isolation of micro- and nano-crystalline cellulose particles and fabrication of crystalline particles-loaded whey protein cold-set gel. Food Chem. 2015, 174, 97–103. [Google Scholar] [CrossRef]
- Chen, J.H.; Xu, J.K.; Wang, K.; Cao, X.F.; Sun, R.C. Cellulose acetate fibers prepared from different raw materials with rapid synthesis method. Carbohydr. Polym. 2016, 137, 685–692. [Google Scholar] [CrossRef]
- Ma, X.J.; Yang, X.F.; Zheng, X.; Lin, L.; Chen, L.H.; Huang, L.L.; Cao, S.L. Degradation and dissolution of hemicelluloses during bamboo hydrothermal pretreatment. Bioresour. Technol. 2014, 161, 215–220. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.G.; Wang, L.S.; Chao, Y.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of Lignin Aromatic Structure in Wood Fractions Based on IR Spectroscopy. J. Wood Chem. Technol. 2016, 36, 377–382. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Agwuncha, S.C.; Owonubi, S.; Fapojuwo, D.P.; Abdulkarim, A.; Okonkwo, T.P.; Makhatha, E.M. Evaluation of mercerization treatment conditions on extracted cellulose from shea nut shell using FTIR and thermogravimetric analysis. In Proceedings of the International Symposium on Nanostructured, Nanoengineered and Advanced Materials (ISNNAM), Johannesburg, South Africa, 30 April–3 May 2020; pp. 958–963. [Google Scholar]
- Tokoh, C.; Takabe, K.; Fujita, M.; Saiki, H. Cellulose synthesized by Acetobacter xylinum in the presence of acetyl glucomannan. Cellulose 1998, 5, 249–261. [Google Scholar] [CrossRef]
- Kuthi, F.; Badri, K.H. Effect of Cooking Temperature on the Crystallinity of Acid Hydrolysed-oil Palm Cellulose. In Proceedings of the 14th Post-Graduate Colloquium of Faculty-of-Science-and-Technology of Universiti-Kebangsaan-Malaysia, Bangi, Malaysia, 9–11 April 2014; pp. 456–462. [Google Scholar]
- Kim, T.H.; Lee, Y.Y. Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresour. Technol. 2005, 96, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stabil. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Sun, J.; Sun, X.; Zhao, H.; Sun, R. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stabil. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Tsujimoto, Y.; Zarubin, M.J.; Krutov, S.M.; Hatakeyama, T. Thermal decomposition and glass transition of industrial hydrolysis lignin. J. Therm. Anal. Calorim. 2010, 101, 289–295. [Google Scholar] [CrossRef]



| Name | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Hemicellulose Removal Rate (%) | Lignin Removal Rate (%) |
|---|---|---|---|---|---|
| Corn bract powder | 36.28 (0.41) | 39.43 (0.19) | 14.78 (0.37) | - | - |
| CHB cellulose | 52.16 (0.34) | 35.16 (0.42) | 3.98 (0.93) | 10.83 (0.24) | 73.07 (0.37) |
| N-CHB cellulose | 75.66 (0.52) | 12.95 (0.63) | 2.12 (0.29) | 67.16 (0.31) | 85.66 (0.50) |
| Test No. | Test Name | NH3·H2O Treatment Time/h | NH3·H2O Treatment Temperature/°C |
|---|---|---|---|
| 1 | N1h-CHB | 1 | 50 |
| 2 | N3h-CHB | 3 | 50 |
| 3 | N5h-CHB | 5 | 50 |
| 4 | N7h-CHB | 7 | 50 |
| Test Name | IMax | IAm | DOC (%) |
|---|---|---|---|
| N1h-CHB | 1334.465 | 337.691 | 74.695 |
| N3h-CHB | 1042.954 | 201.698 | 80.661 |
| N5h-CHB | 1358.574 | 650.962 | 52.085 |
| N7h-CHB | 1360.979 | 685.597 | 49.625 |
| MCC | 2546.406 | 717.176 | 71.836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Zhao, J.; Wu, X.; Yao, W.; Guo, H.; Ji, D.; Yu, Q.; Luo, L.; Li, X.; Zhang, L. Extraction of Corn Bract Cellulose by the Ammonia-Coordinated Bio-Enzymatic Method. Polymers 2023, 15, 206. https://doi.org/10.3390/polym15010206
Yuan X, Zhao J, Wu X, Yao W, Guo H, Ji D, Yu Q, Luo L, Li X, Zhang L. Extraction of Corn Bract Cellulose by the Ammonia-Coordinated Bio-Enzymatic Method. Polymers. 2023; 15(1):206. https://doi.org/10.3390/polym15010206
Chicago/Turabian StyleYuan, Xushuo, Jiaxin Zhao, Xiaoxiao Wu, Wentao Yao, Haiyang Guo, Decai Ji, Qingkai Yu, Liwen Luo, Xiaoping Li, and Lianpeng Zhang. 2023. "Extraction of Corn Bract Cellulose by the Ammonia-Coordinated Bio-Enzymatic Method" Polymers 15, no. 1: 206. https://doi.org/10.3390/polym15010206
APA StyleYuan, X., Zhao, J., Wu, X., Yao, W., Guo, H., Ji, D., Yu, Q., Luo, L., Li, X., & Zhang, L. (2023). Extraction of Corn Bract Cellulose by the Ammonia-Coordinated Bio-Enzymatic Method. Polymers, 15(1), 206. https://doi.org/10.3390/polym15010206





