Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors
Abstract
1. Introduction
2. Experimental
2.1. Materials Synthesis
2.1.1. Materials and Equipment
2.1.2. Preparation of Phenolic Resin Fibers
2.1.3. Preparation of MPCNFs
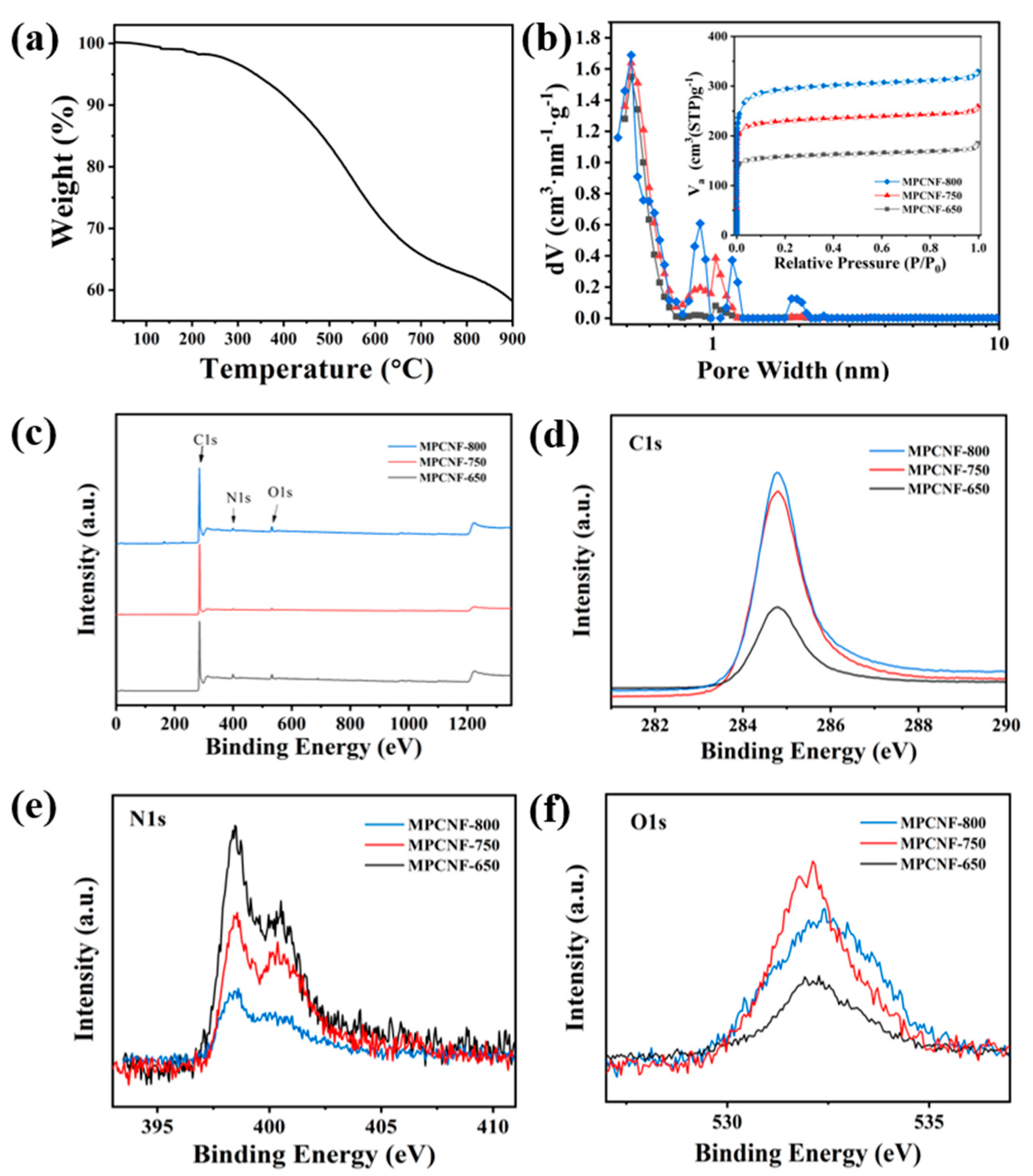
2.2. Characterization
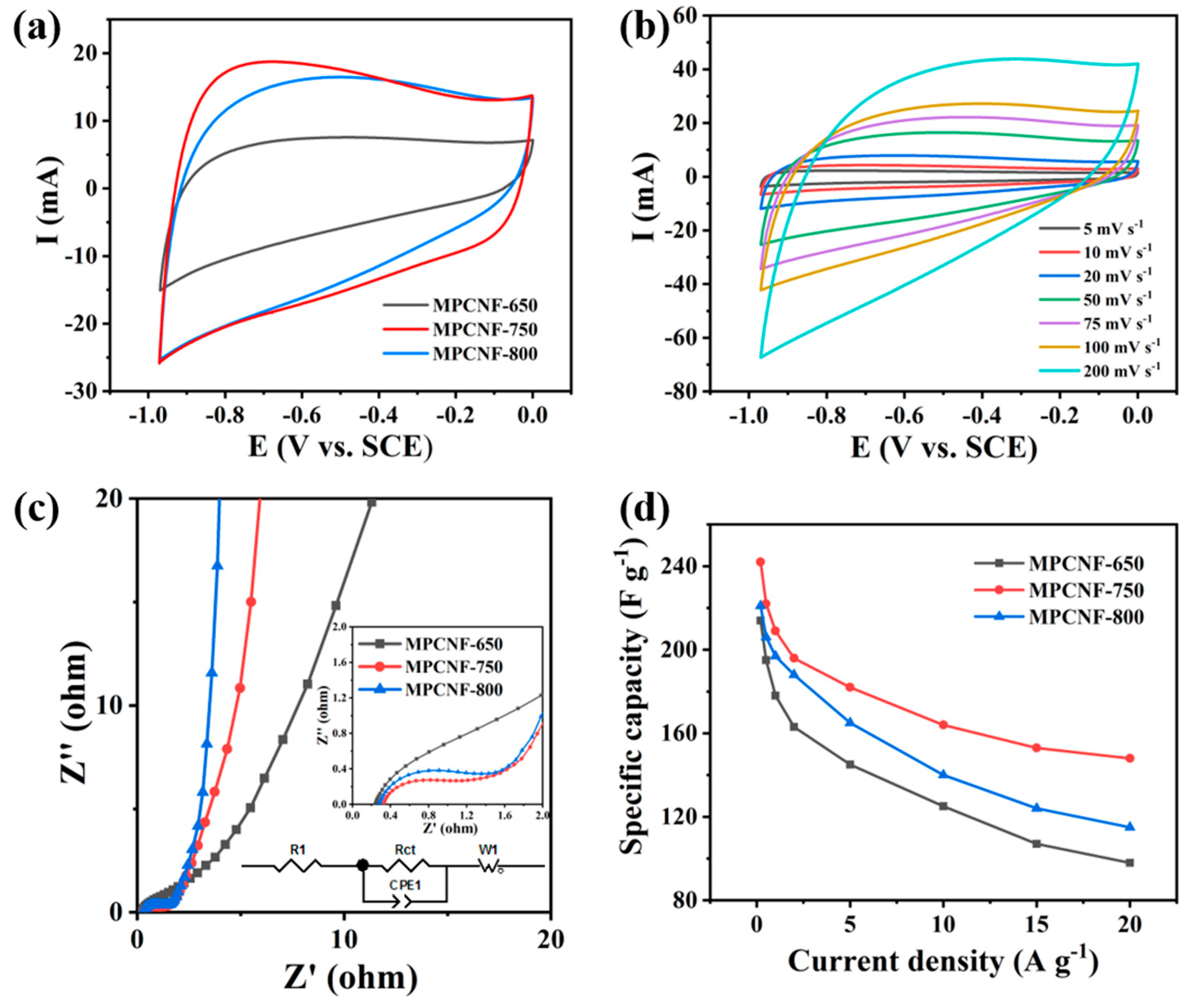
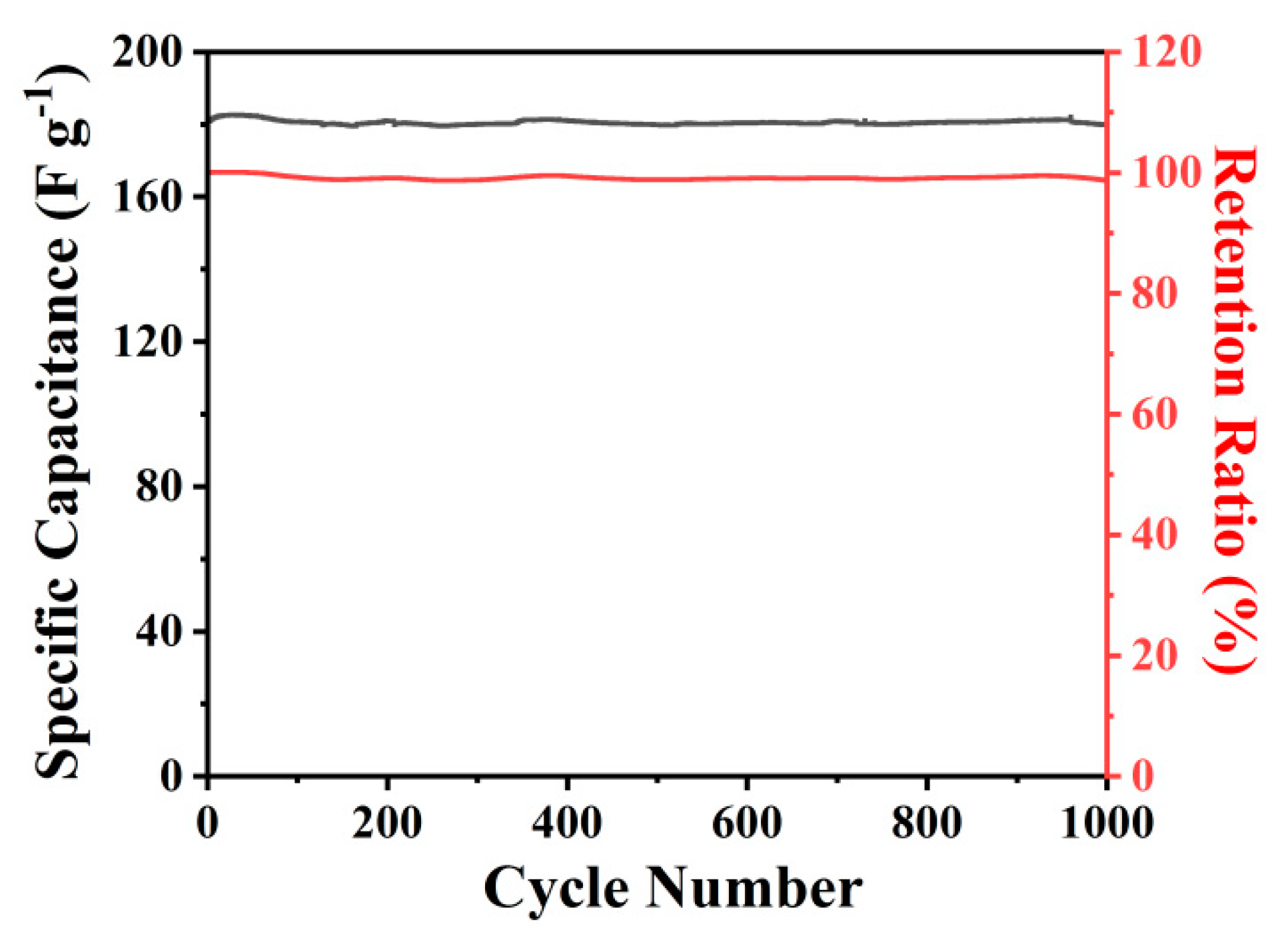
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herbert, G.J.; Iniyan, S.; Sreevalsan, E.; Rajapandian, S. A review of wind energy technologies. Renew. Sustain. Energy Rev. 2007, 11, 1117–1145. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Rattner, A.; Garimella, S. Energy harvesting, reuse and upgrade to reduce primary energy usage in the USA. Energy 2011, 36, 6172–6183. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hesarian, M.S.; Tavoosi, J.; Alanazi, T.I. Model development of a hybrid battery–piezoelectric fiber system based on a new control method. Polymers 2022, 14, 5428. [Google Scholar] [CrossRef]
- Han, C.; Tong, J.; Tang, X.; Zhou, D.; Duan, H.; Li, B.; Wang, G. Boost Anion Storage Capacity Using Conductive Polymer as a Pseudocapacitive Cathode for High-Energy and Flexible Lithium Ion Capacitors. ACS Appl. Mater. Interfaces 2020, 12, 10479–10489. [Google Scholar] [CrossRef]
- Duay, J.; Gillette, E.; Liu, R. Highly flexible pseudocapacitor based on freestanding heterogeneous MnO2/conductive polymer nanowire arrays. Phys. Chem. Chem. Phys. 2012, 14, 3329–3337. [Google Scholar] [CrossRef]
- Naoi, K.; Naoi, W.; Aoyagi, S. New generation “nanohybrid supercapacitor”. Acc. Chem. Res. 2013, 46, 1075–1083. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Fares, A.M.; Kippke, M.; Rashed, M.; Klumpner, C.; Bozhko, S. Development of a Smart Supercapacitor Energy Storage System for Aircraft Electric Power Systems. Energies 2021, 14, 8056. [Google Scholar] [CrossRef]
- Çınar, H.; Kandemir, I. Active energy management based on meta-heuristic algorithms of fuel cell/battery/supercapacitor energy storage system for aircraft. Aerospace 2021, 8, 85. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Lv, Z. Scalable combustion synthesis of graphene-welded activated carbon for high-performance superca-pacitors. Chem. Eng. J. 2021, 414, 128781. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhang, D.W.; Liu, S. Ultrathin nanosheet-assembled nickel-based metal–organic framework microflowers for supercapacitor applications. Chem. Commun. 2022, 58, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; Van Tran, C.; Lee, J. Nitrogen and boron Co-doped densified laser-induced graphene for supercapacitor applications. Chem. Eng. J. 2022, 428, 131119. [Google Scholar] [CrossRef]
- Sadeghinia, M.; Shayeh, J.S.; Fatemi, F.; Rahmandoust, M.; Ehsani, A.; Rezaei, M. Electrochemical study of perlite-barium ferrite/conductive polymer nano composite for super capacitor applications. Int. J. Hydrogen Energy 2019, 44, 28088–28095. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; Qin, Y.; Wang, T.; He, C.; Shao, Q.; Zhu, H.; Cao, N. Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction. Nano-Micro Lett. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1704449. [Google Scholar] [CrossRef]
- Futaba, D.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Zhao, X.; Sajjad, M.; Zheng, Y.; Zhao, M.; Li, Z.; Wu, Z.; Kang, K.; Qiu, L. Covalent organic framework templated ordered nanoporous C60 as stable energy efficient supercapacitor electrode material. Carbon 2021, 182, 144–154. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid superca-pacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Dou, Y. A self-template strategy for the synthesis of mesoporous carbon nanofibers as advanced superca-pacitor electrodes. Adv. Energy Mater. 2011, 1, 382–386. [Google Scholar] [CrossRef]
- Daraghmeh, A.; Hussain, S.; Saadeddin, I.; Servera, L.; Xuriguera, E.; Cornet, A.; Cirera, A. A Study of Carbon Nanofibers and Active Carbon as Symmetric Supercapacitor in Aqueous Electrolyte: A Comparative Study. Nanoscale Res. Lett. 2017, 12, 639. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Chen, L.; Zhang, P.; Fu, W.; Zhao, H.; Ma, Y.; Pan, X.; Zhang, Z.; Han, W.; et al. Highly Flexible Freestanding Porous Carbon Nanofibers for Electrodes Materials of High-Performance All-Carbon Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23515–23520. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Imaizumi, S.; Matsumoto, H.; Suzuki, K.; Minagawa, M.; Kimura, M.; Tanioka, A. Phenolic Resin-Based Carbon Thin Fibers Prepared by Electrospinning: Additive Effects of Poly(vinyl butyral) and Electrolytes. Polym. J. 2009, 41, 1124–1128. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-performance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. Graphite nanoparticles paper supercapacitor based on gel electrolyte. Mater. Renew. Sustain. Energy 2018, 7, 29. [Google Scholar] [CrossRef]
- Mirzaee, M.; Pour, G.B. Design and fabrication of ultracapacitor based on paper substrate and BaTiO3/PEDOT: PSS separator film. Recent Pat. Nanotech. 2018, 12, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Pour, G.B.; Aval, L.F.; Mirzaee, M. CNTs Supercapacitor Based on the PVDF/PVA Gel Electrolytes. Recent Pat. Nanotech. 2020, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.; Sharma, Y.; Archana, P.S.; Jose, R.; Ramakrishna, S.; Mhaisalkar, S.; Srinivasan, M. Electrospun polyaniline nanofibers web electrodes for supercapacitors. J. Appl. Polym. Sci. 2012, 129, 1660–1668. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2013, 245, 101–106. [Google Scholar] [CrossRef]
- Xu, L.; Shi, R.; Li, H.; Han, C.; Wu, M.; Wong, C.-P.; Kang, F.; Li, B. Pseudocapacitive anthraquinone modified with reduced graphene oxide for flexible symmetric all-solid-state supercapacitors. Carbon 2018, 127, 459–468. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Mao, X. All-solid state symmetric supercapacitors based on compressible and flexible free-standing 3D carbon nanotubes (CNTs)/poly (3,4-ethylenedioxythiophene)(PEDOT) sponge electrodes. J. Power Sources 2018, 376, 138–146. [Google Scholar] [CrossRef]



| TSSA a (m2 g−1) | MSSA b (m2 g−1) | TPV c (cm3 g−1) | MPV d (cm3 g−1) | C Content (at.%) | N Content (at.%) | O Content (at.%) | |
|---|---|---|---|---|---|---|---|
| MPCNF-650 | 632 | 603 | 0.277 | 0.236 | 92.2 | 4.9 | 2.9 |
| MPCNF-750 | 923 | 869 | 0.393 | 0.335 | 93.0 | 3.8 | 3.3 |
| MPCNF-800 | 1165 | 1089 | 0.501 | 0.424 | 93.6 | 3.5 | 3.0 |
| Materials | Current Density (A g−1) | Specific Capacity (F g−1) | Energy Density (Wh kg−1) | Power Density (W kg−1) | Cycle Retention (Cycle Number) | References |
|---|---|---|---|---|---|---|
| MPCNF-750 | 0.2 | 242 | 33.6 | 295.6 | 99.5% (1000) | This work |
| PANI/PEO | 0.35 | 267 | - | - | 86.0% (1000) | [33] |
| Fe3O4/graphene | 0.5 | 220 | 30.6 | 550.8 | 98.4% (3000) | [34] |
| Tetrahydroanthraquinone/reduced graphene oxide | 0.2 | 234.8 | - | - | 95.6% (3000) | [35] |
| 3D carbon nanotubes/poly (3, 4-ethylenedioxythiophene) | 0.5 | 147 | 20.4 | 245.0 | 93.0% (200) | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Wang, H.; Bai, Y.; Yang, H.; Song, H.; Li, B. Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors. Polymers 2023, 15, 213. https://doi.org/10.3390/polym15010213
Zhao D, Wang H, Bai Y, Yang H, Song H, Li B. Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors. Polymers. 2023; 15(1):213. https://doi.org/10.3390/polym15010213
Chicago/Turabian StyleZhao, Donghui, Hui Wang, Yu Bai, Hao Yang, Hongfang Song, and Baohua Li. 2023. "Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors" Polymers 15, no. 1: 213. https://doi.org/10.3390/polym15010213
APA StyleZhao, D., Wang, H., Bai, Y., Yang, H., Song, H., & Li, B. (2023). Preparation of Advanced Multi-Porous Carbon Nanofibers for High-Performance Capacitive Electrodes in Supercapacitors. Polymers, 15(1), 213. https://doi.org/10.3390/polym15010213





