Periodic Self-Assembly of Poly(ethyleneimine)–poly(4-styrenesulfonate) Complex Coacervate Membranes
Abstract
:1. Introduction
2. Materials and Methods
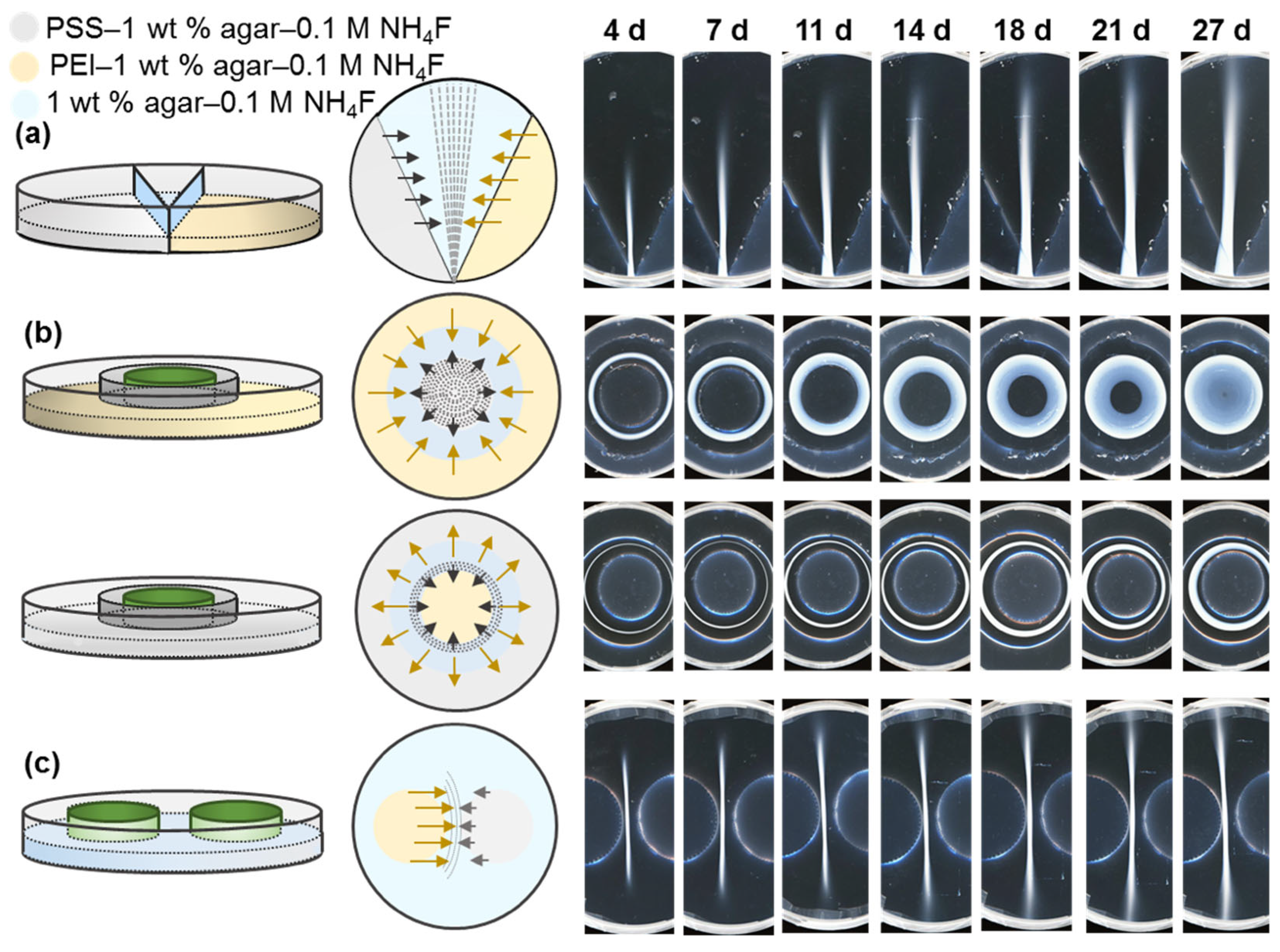
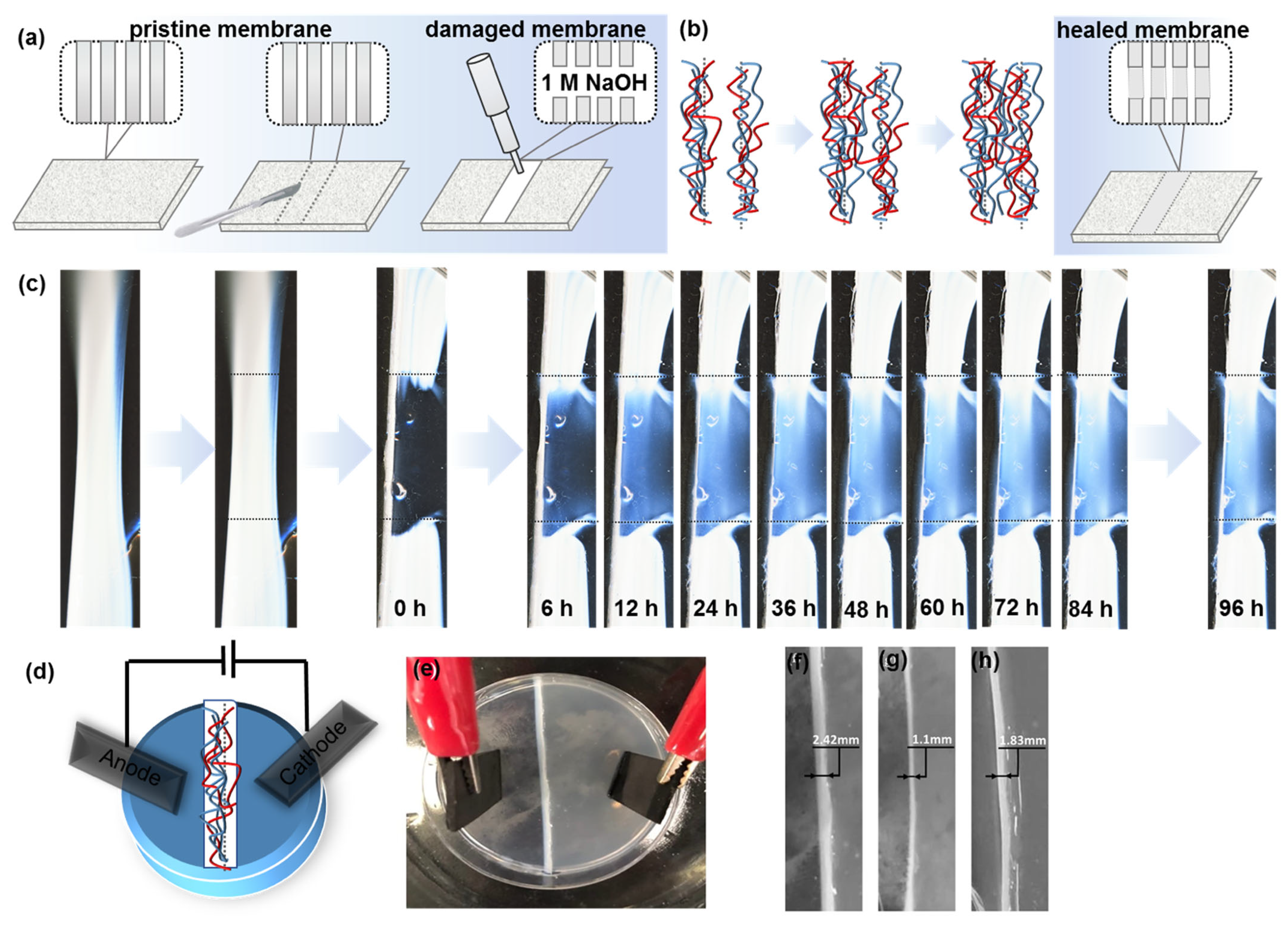
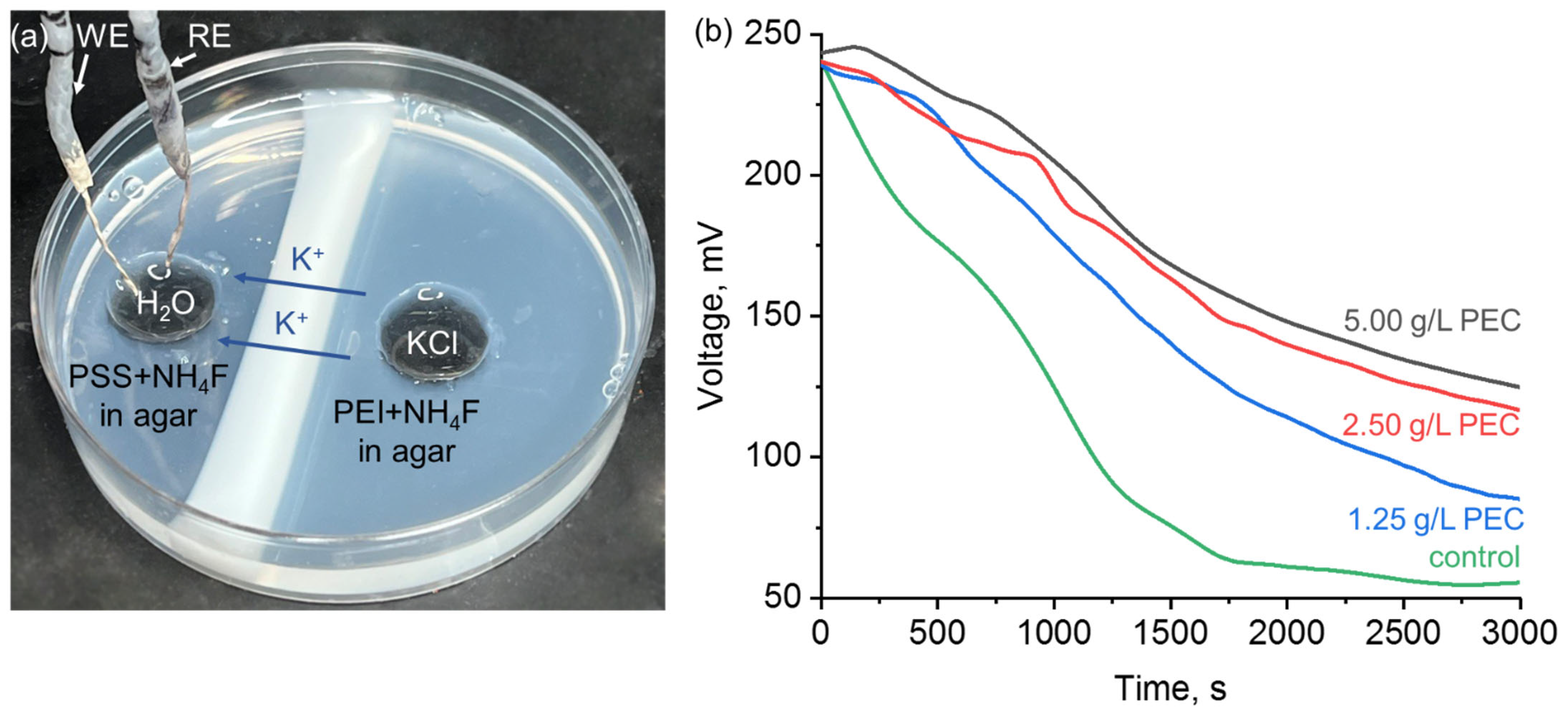
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skorb, E.V.; Andreeva, D.V. Layer-by-Layer approaches for formation of smart self-healing materials. Polym. Chem. 2013, 4, 4834–4845. [Google Scholar] [CrossRef]
- Nabika, H.; Sato, M.; Unoura, K. Liesegang patterns engineered by a chemical reaction assisted by complex formation. Langmuir 2014, 30, 5047–5051. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.J.; Baker, E.; Fialkowski, M.; Bitner, A.; Smoukov, S.K.; Grzybowski, B.A. Self-organization of planar microlenses by periodic precipitation. J. Appl. Phys. 2005, 97, 126102. [Google Scholar] [CrossRef] [Green Version]
- Nabika, H.; Itatani, M.; Lagzi, I. Pattern formation in precipitation reactions: The Liesegang phenomenon. Langmuir 2020, 36, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Fitzner, K.; Paczesny, J.; Granick, S. From dynamic self-assembly to networked chemical systems. Chem. Soc. Rev. 2017, 46, 5647–5678. [Google Scholar] [CrossRef]
- Lagzi, I.; Kowalczyk, B.; Grzybowski, B.A. Liesegang rings engineered from charged nanoparticles. J. Am. Chem. Soc. 2010, 132, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Babicheva, T.S.; Konduktorova, A.A.; Shmakov, S.L.; Shipovskaya, A.B. Formation of Liesegang structures under the conditions of the spatiotemporal reaction of polymer-analogous transformation (salt –> base) of chitosan. J. Phys. Chem. B 2020, 124, 9255–9266. [Google Scholar] [CrossRef]
- Douaihy, R.Z.; Al-Ghoul, M.; Hmadeh, M. Liesegang banding for controlled size and growth of zeolitic-imidazolate frameworks. Small 2019, 15, 1901605. [Google Scholar] [CrossRef]
- Eltantawy, M.M.; Belokon, M.A.; Belogub, E.V.; Ledovich, O.I.; Skorb, E.V.; Ulasevich, S.A. Self-assembled Liesegang rings of hydroxyapatite for cell culturing. Adv. NanoBiomed Res. 2021, 1, 2000048. [Google Scholar] [CrossRef]
- Farkas, S.; Fonyi, M.S.; Holló, G.; Német, N.; Valletti, N.; Kukovecz, Á.; Schuszter, G.; Rossi, F.; Lagzi, I. Periodic precipitation of zeolitic imidazolate frameworks in a gelled medium. J. Phys. Chem. C 2022, 126, 9580–9586. [Google Scholar] [CrossRef]
- Park, J.H.; Paczesny, J.; Kim, N.; Grzybowski, B.A. Shaping microcrystals of metal-organic frameworks by reaction-diffusion. Angew. Chem. Int. Ed. Engl. 2020, 59, 10301–10305. [Google Scholar] [CrossRef]
- Ackroyd, A.J.; Holló, G.; Mundoor, H.; Zhang, H.; Gang, O.; Smalyukh, I.I.; Lagzi, I.; Kumacheva, E. Self-organization of nanoparticles and molecules in periodic Liesegang-type structures. Sci. Adv. 2021, 7, 3801. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lv, Z.Y.; Sun, Y.F.; Chi, Z.G.; Qing, G.Y. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Surface nanoarchitecture for bio-applications: Self-regulating intelligent interfaces. Adv. Funct. Mater. 2013, 23, 4483–4506. [Google Scholar] [CrossRef]
- Feng, E.K.; Gao, W.; Yan, Z.; Li, J.J.; Li, Z.L.; Ma, X.X.; Ma, L.H.; Yang, Z.M. A multifunctional hydrogel polyelectrolyte based flexible and wearable supercapacitor. J. Power Sources 2020, 479, 229100. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Ping, Z.H.; Nguyen, T.; Rigal, P. Simple method for immobilization of bio-macromolecules onto membranes of different types. J. Membr. Sci. 2003, 213, 85–95. [Google Scholar] [CrossRef]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z.G. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Nikolaev, K.G.; Stekolshchikova, A.A.; Tesfatsion, W.T.; Yurchenko, S.O.; Novoselov, K.S.; Andreeva, D.V.; Rubtsova, M.Y.; Vorovitch, M.F.; Ishmukhametov, A.A.; et al. Tick-borne encephalitis electrochemical detection by multilayer perceptron on liquid-metal interface. ACS Appl. Bio Mater. 2020, 3, 7352–7356. [Google Scholar] [CrossRef]
- Baldina, A.A.; Pershina, L.V.; Noskova, U.V.; Nikitina, A.A.; Muravev, A.A.; Skorb, E.V.; Nikolaev, K.G. Uricase crowding via polyelectrolyte layers coacervation for carbon fiber-based electrochemical detection of uric acid. Polymers 2022, 14, 5145. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Nikolaev, K.G.; Novikov, A.S.; Yurchenko, S.O.; Novoselov, K.S.; Andreeva, D.V.; Skorb, E.V. Programmable soft-matter electronics. J. Phys. Chem. Lett. 2021, 12, 2017–2022. [Google Scholar] [CrossRef]
- Fares, H.M.; Schlenoff, J.B. Diffusion of sites versus polymers in polyelectrolyte complexes and multilayers. J. Am. Chem. Soc. 2017, 139, 14656–14667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, T.S.; Yang, S.M.; Kim, S.H. Dynamic designing of microstructures by chemical gradient-mediated growth. Nat. Commun. 2015, 6, 6584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.M.B.; Binks, B.P.; Sekine, T. Emulsion stabilisation by complexes of oppositely charged synthetic polyelectrolytes. Soft Matter 2018, 14, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Wang, C.; Jiang, H.W.; Dawadi, M.B.; Vogt, B.D.; Modarelli, D.A.; Zacharia, N.S. Polyelectrolyte-micelle coacervates: Intrapolymer-dominant vs. interpolymer-dominant association, solute uptake and rheological properties. Soft Matter 2019, 15, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Spruijt, E. Multiphase complex coacervate droplets. J. Am. Chem. Soc. 2020, 142, 2905–2914. [Google Scholar] [CrossRef] [Green Version]
- van Stevendaal, M.H.M.E.; Vasiukas, L.; Yewdall, N.A.; Mason, A.F.; van Hest, J.C.M. Engineering of biocompatible coacervate-based synthetic cells. ACS Appl. Mater. Interfaces 2021, 13, 7879–7889. [Google Scholar] [CrossRef]
- Last, M.G.F.; Deshpande, S.; Dekker, C. pH-Controlled coacervate-membrane interactions within liposomes. ACS Nano 2020, 14, 4487–4498. [Google Scholar] [CrossRef]
- Mateos-Maroto, A.; Abelenda-Nunez, I.; Ortega, F.; Rubio, R.G.; Guzman, E. Polyelectrolyte multilayers on soft colloidal nanosurfaces: A new life for the layer-by-layer method. Polymers 2021, 13, 1221. [Google Scholar] [CrossRef]
- Ryzhkov, N.V.; Ledovich, O.; Eggert, L.; Bund, A.; Paszuk, A.; Hannappel, T.; Klyukin, K.; Alexandrov, V.; Skorb, E.V. Layer-by-layer polyelectrolyte assembly for the protection of GaP surfaces from photocorrosion. ACS Appl. Nano Mater. 2021, 4, 425–431. [Google Scholar] [CrossRef]
- Gai, M.Y.; Frueh, J.; Kudryavtseva, V.L.; Mao, R.; Kiryukhin, M.V.; Sukhorukov, G.B. Patterned microstructure fabrication: Polyelectrolyte complexes vs polyelectrolyte multilayers. Sci. Rep. 2016, 6, 37000. [Google Scholar] [CrossRef]
- Nikitina, A.A.; Ulasevich, S.A.; Kassirov, I.S.; Bryushkova, E.A.; Koshel, E.I.; Skorb, E.V. Nanostructured layer-by-layer polyelectrolyte containers to switch biofilm fluorescence. Bioconjugate Chem. 2018, 29, 3793–3799. [Google Scholar] [CrossRef]
- Wang, Q.F.; Schlenoff, J.B. Single- and multicompartment hollow polyelectrolyte complex microcapsules by one-step spraying. Adv. Mater. 2015, 27, 2077–2082. [Google Scholar] [CrossRef]
- Baig, M.I.; Durmaz, E.N.; Willott, J.D.; de Vos, W.M. Sustainable membrane production through polyelectrolyte complexation induced aqueous phase separation. Adv. Funct. Mater. 2020, 30, 1907344. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Baig, M.I.; Willott, J.D.; de Vos, W.M. Polyelectrolyte complex membranes via salinity change induced aqueous phase separation. ACS Appl. Polym. Mater. 2020, 2, 2612–2621. [Google Scholar] [CrossRef]
- Tang, J.D.; Caliari, S.R.; Lampe, K.J. Temperature-dependent complex coacervation of engineered elastin-like polypeptide and hyaluronic acid polyelectrolytes. Biomacromolecules 2018, 19, 3925–3935. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Yang, M.; Digby, Z.A.; Wang, Q.F. Ion content of polyelectrolyte complex coacervates and the Donnan equilibrium. Macromolecules 2019, 52, 9149–9159. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Y.; Quan, S.; Zhang, Y.; Wang, P.; Liang, H.; Ma, J.; Cheng, X.Q. Development of highly permeable polyelectrolytes (PEs)/UiO-66 nanofiltration membranes for dye removal. Chem. Eng. Res. Des. 2019, 147, 222–231. [Google Scholar] [CrossRef]
- Ye, C.-C.; An, Q.-F.; Wu, J.-K.; Zhao, F.-Y.; Zheng, P.-Y.; Wang, N.-X. Nanofiltration membranes consisting of quaternized polyelectrolyte complex nanoparticles for heavy metal removal. Chem. Eng. J. 2019, 359, 994–1005. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Goh, K.; Chong, T.H.; Wang, R. Layer-by-layer assembly based low pressure biocatalytic nanofiltration membranes for micropollutants removal. J. Membr. Sci. 2020, 615, 118514. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Zhu, G.; Zacharia, N.S. Self-healing of bulk polyelectrolyte complex material as a function of pH and salt. ACS Appl. Mater. Interfaces 2016, 8, 26258–26265. [Google Scholar] [CrossRef]
- Li, S.; Pan, H.; Wang, Y.; Sun, J. Polyelectrolyte complex-based self-healing, fatigue-resistant and anti-freezing hydrogels as highly sensitive ionic skins. J. Mater. Chem. A 2020, 8, 3667–3675. [Google Scholar] [CrossRef]
- Pershina, L.V.; Grabeklis, A.R.; Isankina, L.N.; Skorb, E.V.; Nikolaev, K.G. Determination of sodium and potassium ions in patients with SARS-Cov-2 disease by ion-selective electrodes based on polyelectrolyte complexes as a pseudo-liquid contact phase. RSC Adv. 2021, 11, 36215–36221. [Google Scholar] [CrossRef] [PubMed]
- Bucur, C.B.; Sui, Z.; Schlenoff, J.B. Ideal mixing in polyelectrolyte complexes and multilayers: Entropy driven assembly. J. Am. Chem. Soc. 2006, 128, 13690–13691. [Google Scholar] [CrossRef] [PubMed]
- Laugel, N.; Betscha, C.; Winterhalter, M.; Voegel, J.C.; Schaaf, P.; Ball, V. Relationship between the growth regime of polyelectrolyte multilayers and the polyanion/polycation complexation enthalpy. J. Phys. Chem. B 2006, 110, 19443–19449. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B.; Rmaile, A.H.; Bucur, C.B. Hydration contributions to association in polyelectrolyte multilayers and complexes: Visualizing hydrophobicity. J. Am. Chem. Soc. 2008, 130, 13589–13597. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.B.; Schlenoff, J.B. Control of dynamics in polyelectrolyte complexes by temperature and salt. Macromolecules 2019, 52, 1930–1941. [Google Scholar] [CrossRef]
- Wang, Q.F.; Schlenoff, J.B. The polyelectrolyte complex/coacervate continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Skorb, E.V.; Möhwald, H. 25th Anniversary article: Dynamic interfaces for responsive encapsulation systems. Adv. Mater. 2013, 25, 5029–5042. [Google Scholar] [CrossRef]
- Hilhorst, D.; van der Hout, R.; Mimura, M.; Ohnishi, I. A mathematical study of the one-dimensional Keller and Rubinow model for Liesegang bands. J Stat Phys. 2009, 135, 107–132. [Google Scholar] [CrossRef] [Green Version]
- Grzybowski, B.A. Chemistry in Motion: Reaction-Diffusion Systems for Micro- and Nanotechnology; Wiley: Chichester, UK, 2009. [Google Scholar]
- Duley, J.M.; Fowler, A.C.; Moyles, I.R.; O’Brien, S.B.G. On the Keller–Rubinow model for Liesegang ring formation. Proc. R. Soc. A 2017, 473, 20170128. [Google Scholar] [CrossRef]
- Overbeek, J.T.G.; Voorn, M.J. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Comp. Physiol. 1957, 49, 7–26. [Google Scholar] [CrossRef]
- Liu, Y.; Momani, B.; Winter, H.H.; Perry, S.L. Rheological characterization of liquid-to-solid transitions in bulk polyelectrolyte complexes. Soft Matter 2017, 13, 7332–7340. [Google Scholar] [CrossRef] [Green Version]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual salt and pH induced changes in polyethylenimine solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef] [Green Version]
- Shamoun, R.F.; Reisch, A.; Schlenoff, J.B. Extruded saloplastic polyelectrolyte complexes. Adv. Funct. Mater. 2012, 22, 1923–1931. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Borkovec, M.; Koper, G.J.; Piguet, C. Ion binding to polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2006, 11, 280–289. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukhtenko, E.V.; Lavrentev, F.V.; Shilovskikh, V.V.; Zyrianova, P.I.; Koltsov, S.I.; Ivanov, A.S.; Novikov, A.S.; Muravev, A.A.; Nikolaev, K.G.; Andreeva, D.V.; et al. Periodic Self-Assembly of Poly(ethyleneimine)–poly(4-styrenesulfonate) Complex Coacervate Membranes. Polymers 2023, 15, 45. https://doi.org/10.3390/polym15010045
Kukhtenko EV, Lavrentev FV, Shilovskikh VV, Zyrianova PI, Koltsov SI, Ivanov AS, Novikov AS, Muravev AA, Nikolaev KG, Andreeva DV, et al. Periodic Self-Assembly of Poly(ethyleneimine)–poly(4-styrenesulfonate) Complex Coacervate Membranes. Polymers. 2023; 15(1):45. https://doi.org/10.3390/polym15010045
Chicago/Turabian StyleKukhtenko, Ekaterina V., Filipp V. Lavrentev, Vladimir V. Shilovskikh, Polina I. Zyrianova, Semyon I. Koltsov, Artemii S. Ivanov, Alexander S. Novikov, Anton A. Muravev, Konstantin G. Nikolaev, Daria V. Andreeva, and et al. 2023. "Periodic Self-Assembly of Poly(ethyleneimine)–poly(4-styrenesulfonate) Complex Coacervate Membranes" Polymers 15, no. 1: 45. https://doi.org/10.3390/polym15010045
APA StyleKukhtenko, E. V., Lavrentev, F. V., Shilovskikh, V. V., Zyrianova, P. I., Koltsov, S. I., Ivanov, A. S., Novikov, A. S., Muravev, A. A., Nikolaev, K. G., Andreeva, D. V., & Skorb, E. V. (2023). Periodic Self-Assembly of Poly(ethyleneimine)–poly(4-styrenesulfonate) Complex Coacervate Membranes. Polymers, 15(1), 45. https://doi.org/10.3390/polym15010045






