Renewable Poly(Lactic Acid)Lignocellulose Biocomposites for the Enhancement of the Water Retention Capacity of the Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLA
2.3. Preparation of the PLA/Lignocellulose Composite Material
2.4. Characterization
2.4.1. Structural Properties
2.4.2. Thermal Properties
2.4.3. Swelling Ratio and Water Retention in Soil
2.4.4. Degradation Studies
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
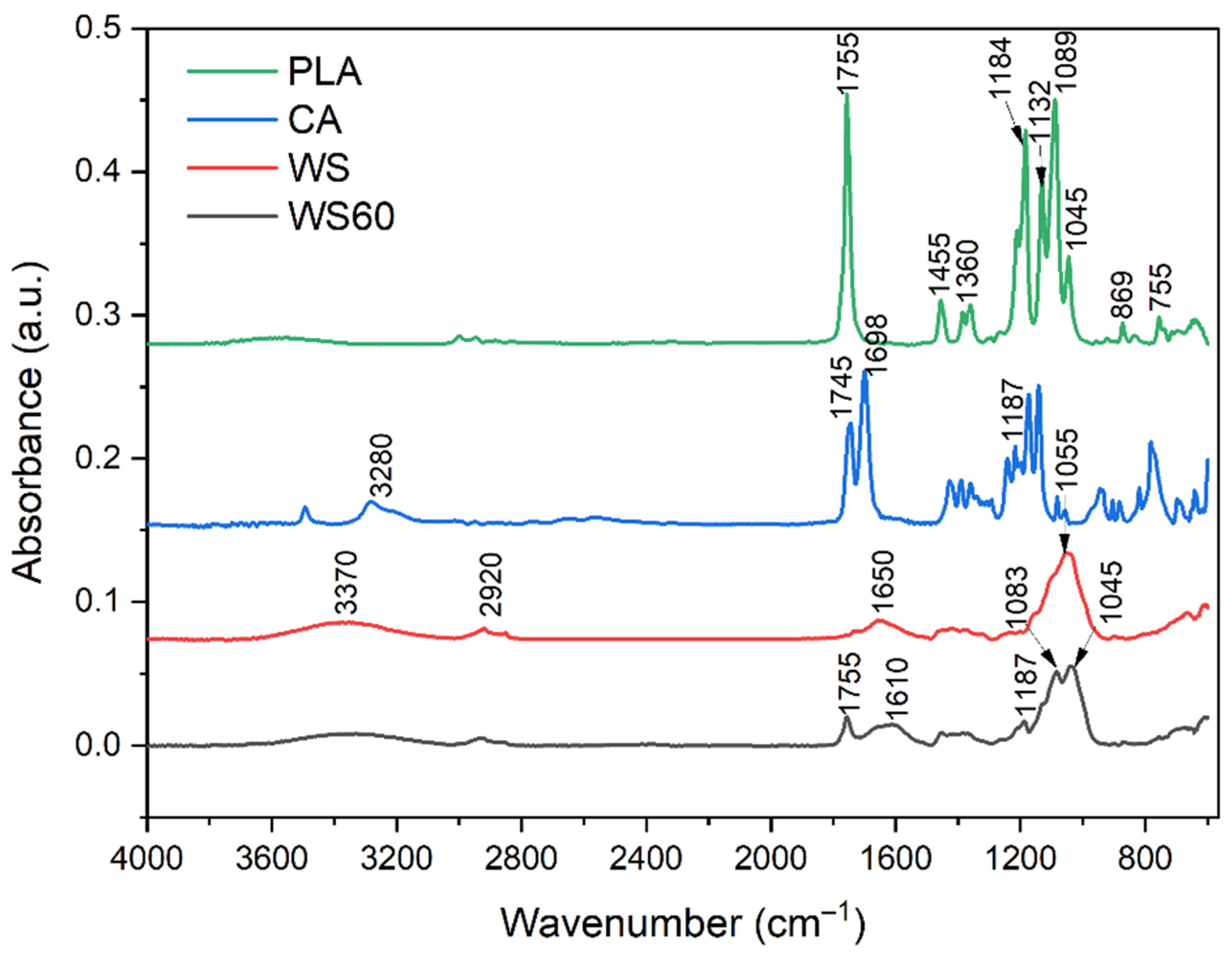
3.2. Structural Analysis
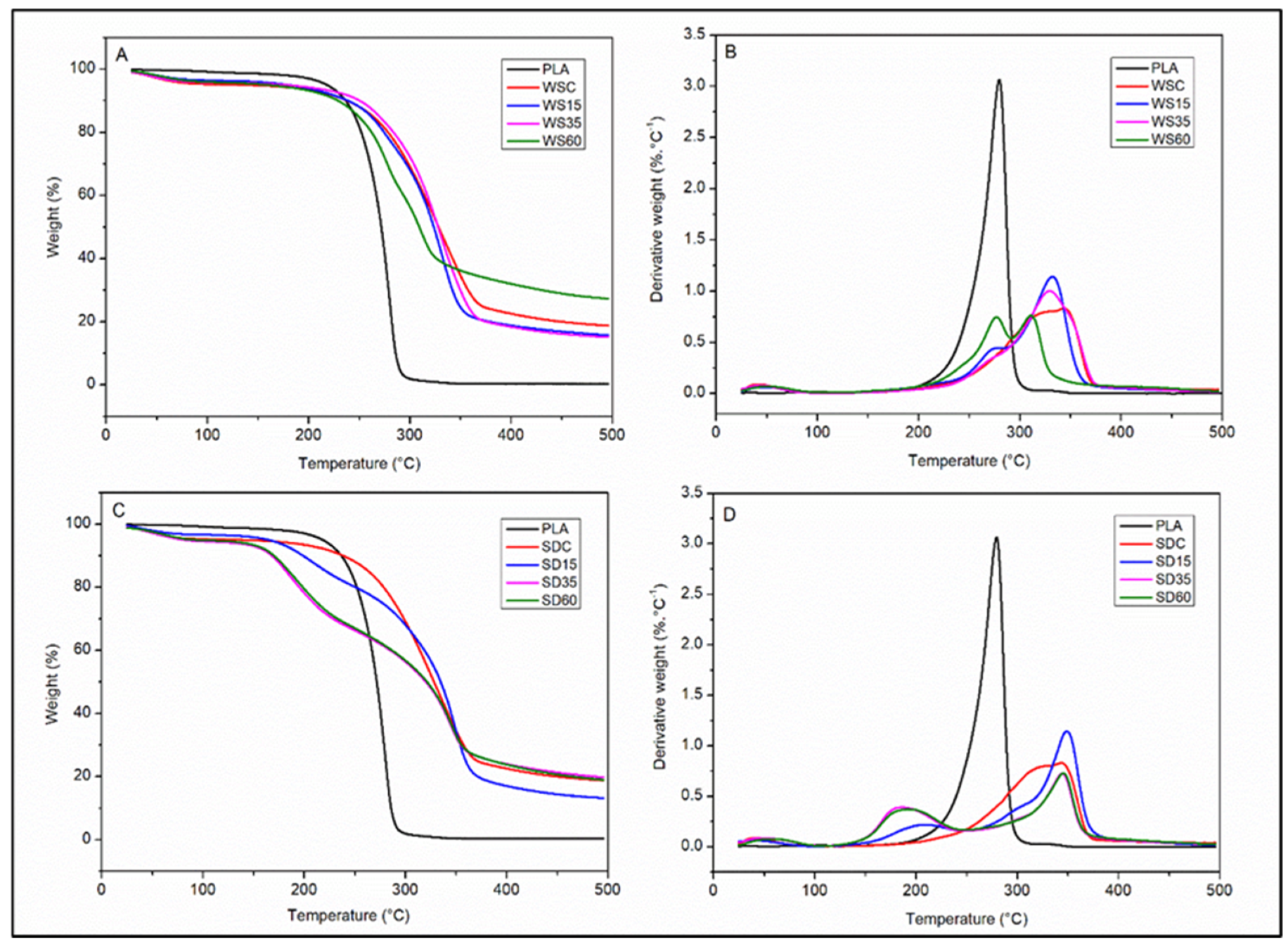
3.3. Thermal Properties
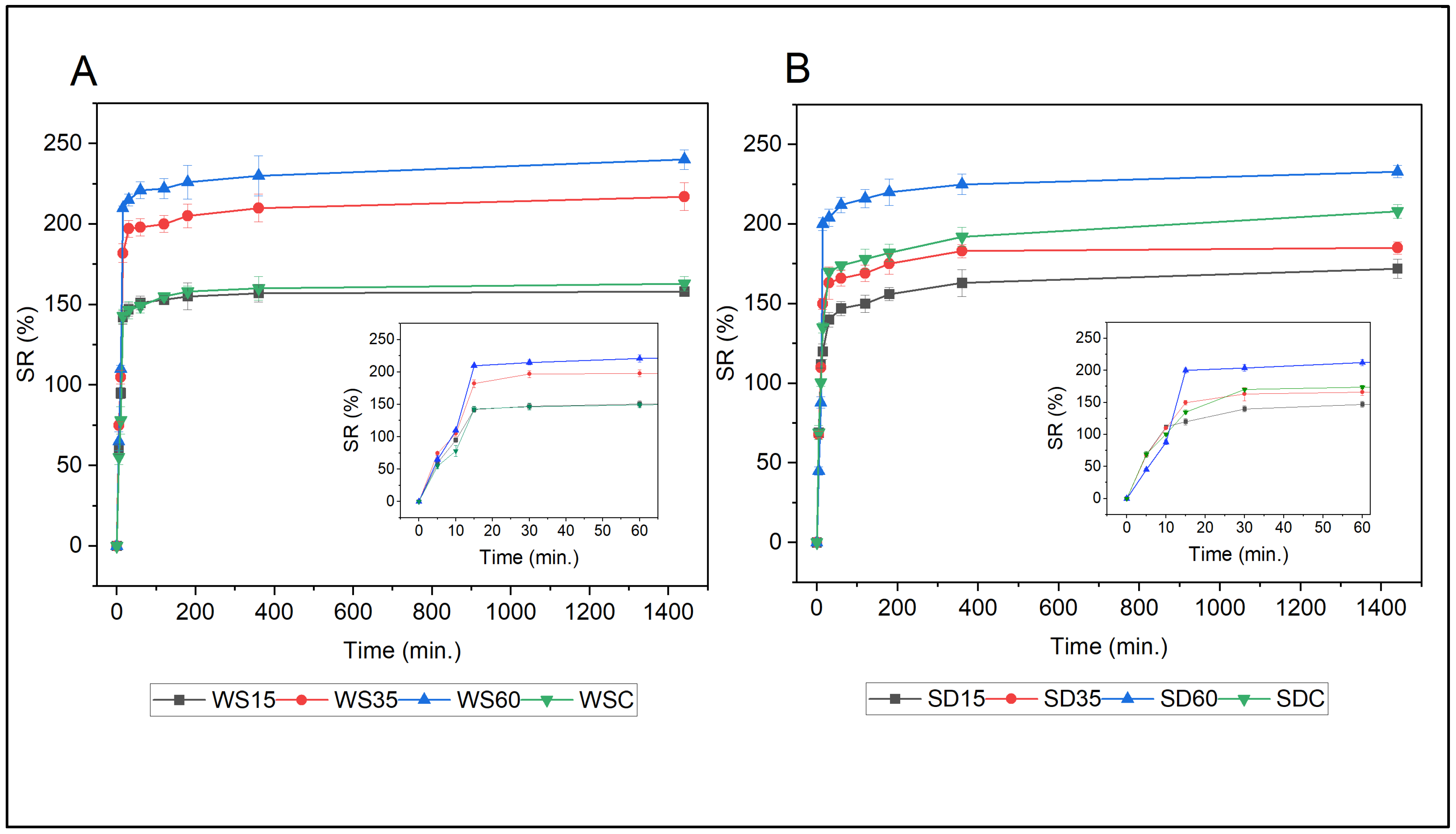
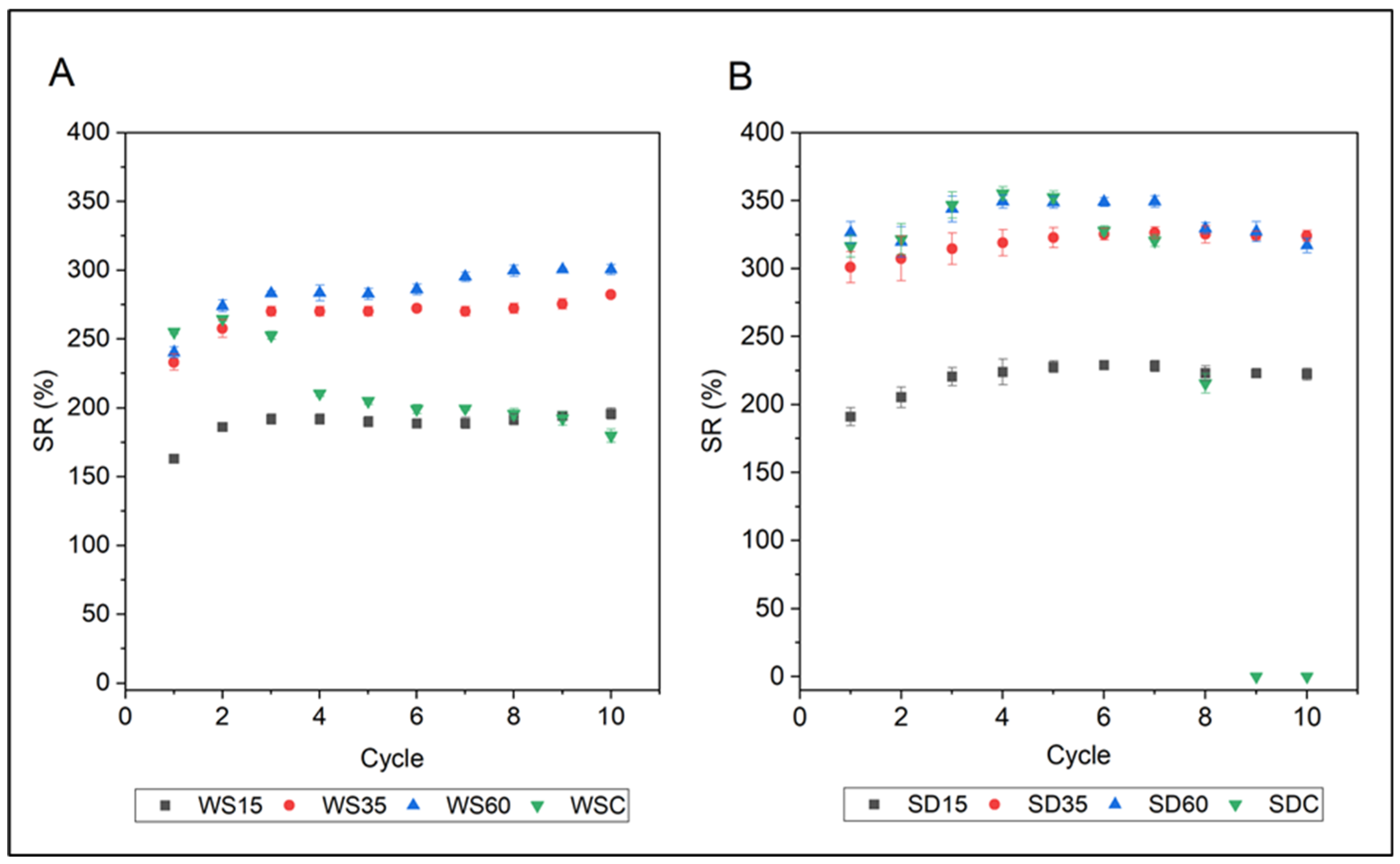
3.4. Swelling Properties
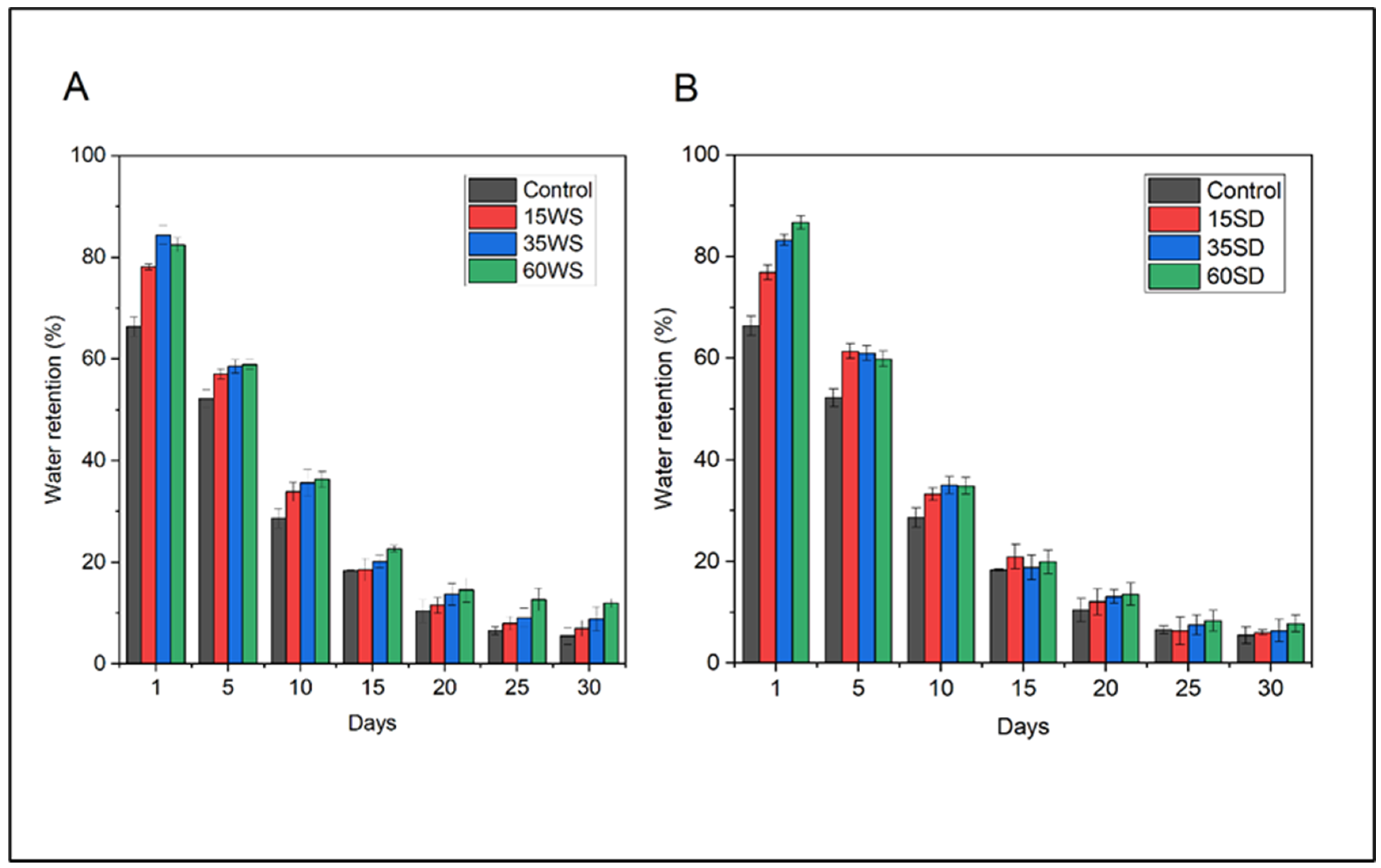
3.5. Water Retention in Soil
3.6. Biodegradability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. Open Access 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Tomadoni, B.; Casalongué, C.; Alvarez, V.A. Biopolymer-Based Hydrogels for Agriculture Applications: Swelling Behavior and Slow Release of Agrochemicals BT. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 99–125. ISBN 978-3-030-19416-1. [Google Scholar]
- Heise, K.; Kirsten, M.; Schneider, Y.; Jaros, D.; Keller, H.; Rohm, H.; Kalbitz, K.; Fischer, S. From Agricultural Byproducts to Value-Added Materials: Wheat Straw-Based Hydrogels as Soil Conditioners? ACS Sustain. Chem. Eng. 2019, 7, 8604–8612. [Google Scholar] [CrossRef]
- Zandi, A.; Zanganeh, A.; Hemmati, F.; Mohammadi-Roshandeh, J. Thermal and biodegradation properties of poly(lactic acid)/rice straw composites: Effects of modified pulping products. Iran. Polym. J. 2019, 28, 403–415. [Google Scholar] [CrossRef]
- Mallik, A.K.; Shahruzzaman, M.; Sakib, M.N.; Zaman, A.; Rahman, M.S.; Islam, M.M.; Islam, M.S.; Haque, P.; Rahman, M.M. Benefits of Renewable Hydrogels over Acrylate- and Acrylamide-Based Hydrogels BT. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–47. ISBN 978-3-319-76573-0. [Google Scholar]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, e1900670. [Google Scholar] [CrossRef]
- Kalinoski, R.M.; Shi, J. Hydrogels derived from lignocellulosic compounds: Evaluation of the compositional, structural, mechanical and antimicrobial properties. Ind. Crops Prod. 2019, 128, 323–330. [Google Scholar] [CrossRef]
- Ferede, E. Evaluation of mechanical and water absorption properties of alkaline-treated sawdust-reinforced polypropylene composite. J. Eng. 2020, 2020, 3706176. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yang, Y.; Kim, M.S.; Lee, C.H.; Zhang, R.; Xu, T.; Choi, S.E.; Si, C. Hemicellulose-based hydrogels for advanced applications. Front. Bioeng. Biotechnol. 2023, 10, 1–18. [Google Scholar] [CrossRef]
- Idrus, M.A.M.M.; Hamdan, S.; Rahman, M.R.; Islam, M.S. Treated Tropical Wood Sawdust-Polypropylene Polymer Composite: Mechanical and Morphological Study. J. Biomater. Nanobiotechnol. 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Maraveas, C. Production of sustainable and biodegradable polymers from agricultural waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Lu, P.; Zhang, M. Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers. Green Process. Synth. 2020, 9, 139–152. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xu, X.; Su, Y.; Yue, Q.; Gao, B. Characterization, swelling and slow-release properties of a new controlled release fertilizer based on wheat straw cellulose hydrogel. J. Taiwan Inst. Chem. Eng. 2016, 60, 564–572. [Google Scholar] [CrossRef]
- Malinconico, M.; Vink, E.T.H.; Cain, A. Applications of Poly(lactic Acid) in Commodities and Specialties BT. In Industrial Applications of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 35–50. ISBN 978-3-319-75459-8. [Google Scholar]
- Calcagnile, P.; Sibillano, T.; Giannini, C.; Sannino, A.; Demitri, C. Biodegradable poly(lactic acid)/cellulose-based superabsorbent hydrogel composite material as water and fertilizer reservoir in agricultural applications. J. Appl. Polym. Sci. 2019, 136, 47546. [Google Scholar] [CrossRef]
- Shao, H.; Sun, H.; Yang, B.; Zhang, H.; Hu, Y. Facile and green preparation of hemicellulose-based film with elevated hydrophobicity: Via cross-linking with citric acid. RSC Adv. 2019, 9, 2395–2401. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Poljansek, I.; Sedlarik, V.; Kasparkova, V.; Salakova, A.; Drbohlav, J.; Cvelbar, U.; Saha, P. Functionalization of polylactic acid through direct melt polycondensation in the presence of tricarboxylic acid. J. Appl. Polym. Sci. 2011, 122, 1275–1285. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fiţigău, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterisation of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Chougan, M.; Ghaffar, S.H.; Al-Kheetan, M.J.; Gecevicius, M. Wheat straw pre-treatments using eco-friendly strategies for enhancing the tensile properties of bio-based polylactic acid composites. Ind. Crops Prod. 2020, 155, 112836. [Google Scholar] [CrossRef]
- Sedlarik, V.; Kucharczyk, P.; Kasparkova, V.; Drbohlav, J.; Salakova, A.; Saha, P. Optimization of the reaction conditions and characterization of L-lactic acid direct polycondensation products catalyzed by a non-metal-based compound. J. Appl. Polym. Sci. 2010, 116, 1597–1602. [Google Scholar] [CrossRef]
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef]
- Stloukal, P.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Koutny, M. The influence of a hydrolysis-inhibiting additive on the degradation and biodegradation of PLA and its nanocomposites. Polym. Test. 2015, 41, 124–132. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, Y.; Yang, G.; Zhang, A. Water- and Fertilizer-Integrated Hydrogel Derived from the Polymerization of Acrylic Acid and Urea as a Slow-Release N Fertilizer and Water Retention in Agriculture. J. Agric. Food Chem. 2018, 66, 5762–5769. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, H.; Huang, Y. Structure and morphology of cellulose in wheat straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Jiang, D.; An, P.; Cui, S.; Sun, S.; Zhang, J.; Tuo, T. Effect of Modification Methods of Wheat Straw Fibers on Water Absorbency and Mechanical Properties of Wheat Straw Fiber Cement-Based Composites. Adv. Mater. Sci. Eng. 2020, 2020, 5031025. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; De Brito, E.S.; Rodrigues, T.H.S.; Rosa, M.F.; Azeredo, H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Properties of natural rubber biocomposities filled with alkaline modified oat straw. J. Renew. Mater. 2018, 6, 746–754. [Google Scholar] [CrossRef]
- Cai, Z.; Ji, B.; Yan, K.; Zhu, Q. Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy. Polymers 2019, 11, 2071. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohydr. Polym. 2011, 85, 664–673. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improvement of tensile and thermal properties of poly(lactic acid) composites with admicellar-treated rice straw fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Petinakis, E.; Yu, L.; Simon, G. Natural Fibre Bio-Composites Incorporating Poly(Lactic Acid). In Fiber Reinforced Polymers—The Technology Applied for Concrete Repair; Masuelli, M., Ed.; IntechOpen: London, UK, 2013; p. 242. ISBN 978-953-51-0938-9. [Google Scholar]
- Meng, Y.; Liu, X.; Li, C.; Liu, H.; Cheng, Y.; Lu, J.; Zhang, K.; Wang, H. Super-swelling lignin-based biopolymer hydrogels for soil water retention from paper industry waste. Int. J. Biol. Macromol. 2019, 135, 815–820. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Gao, B. A biodegradable biomass-based polymeric composite for slow release and water retention. J. Environ. Manag. 2019, 230, 190–198. [Google Scholar] [CrossRef]
- Yaacob, N.D.; Ismail, H.; Ting, S.S. Soil burial of polylactic acid/paddy straw powder biocomposite. BioResources 2016, 11, 1255–1269. [Google Scholar] [CrossRef]
- Wilfred, O.; Tai, H.; Marriott, R.; Liu, Q.; Tverezovskiy, V.; Curling, S.; Tai, H.; Fan, Z.; Wang, W. Biodegradation of Polylactic Acid and Starch Composites in Compost and Soil. Int. J. Nano Res. 2018, 1, 1–11. [Google Scholar]
- Azeredo, H.M.C.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.F.; Waldron, K.W. Wheat straw hemicellulose films as affected by citric acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef]






| Sample Designation | PLA Solution (% w/v) | Cross-Linking Agent (%) * | Residual Biomass (%) ** | |
|---|---|---|---|---|
| PLA *** | - | - | - | |
| Wheat straw (WS) | WS15 | 10 | 10 | 15 |
| WS35 | 10 | 35 | ||
| WS60 | 10 | 60 | ||
| WSC | 0 | 60 | ||
| Sawdust (SD) | SD15 | 10 | 15 | |
| SD35 | 10 | 35 | ||
| SD60 | 10 | 60 | ||
| SDC | 0 | 60 |
| Formulation | Tm (°C) | Tg (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|---|
| PLA | 135 | 50 | 102 | 2 |
| WS15 | 145 | 53 | 106 | 5 |
| WS35 | 148 | 55 | 117 | 13 |
| WS60 | 149 | 55 | 121 | 20 |
| WSC | 143 | 52 | 120 | 21 |
| SD15 | 138 | 47 | 117 | 13 |
| SD35 | 142 | 49 | 123 | 20 |
| SD60 | 143 | 49 | 125 | 22 |
| SDC | 135 | 48 | 119 | 24 |
| Formulation | Tmax (°C) | Total Loss in Mass at 500 °C (%) |
|---|---|---|
| PLA | 279 | 99 |
| WS15 | 332 | 84 |
| WS35 | 329 | 84 |
| WS60 | 311 | 72 |
| WSC | 326 | 82 |
| SD15 | 349 | 86 |
| SD35 | 344 | 79 |
| SD60 | 345 | 80 |
| SDC | 341 | 81 |
| Days | 0 | 15 | 30 | 45 | ||||
|---|---|---|---|---|---|---|---|---|
| Sample | Mw (g.mol−1) | Mw/Mn (Ð) | Mw (g.mol−1) | Mw/Mn (Ð) | Mw (g.mol−1) | Mw/Mn (Ð) | Mw (g.mol−1) | Mw/Mn (Ð) |
| PLA | 7700 | 1.64 | 7000 | 1.84 | 6500 | 2.23 | 6300 | 2.41 |
| WS15 | 10,000 | 1.59 | 9400 | 1.57 | 8000 | 2.36 | 5000 | 3.30 |
| WS35 | 9900 | 1.53 | 8300 | 1.44 | 7300 | 3.04 | 5200 | 3.67 |
| WS60 | 10,600 | 1.59 | 8200 | 1.38 | 6600 | 3.74 | 5100 | 4.10 |
| WSC | 10,900 | 1.55 | 8200 | 1.75 | 6300 | 2.87 | 5600 | 3.99 |
| SD15 | 9900 | 1.59 | 8400 | 1.57 | 7000 | 2.90 | 5200 | 3.61 |
| SD35 | 9700 | 1.57 | 8900 | 1.50 | 7500 | 3.22 | 6000 | 3.48 |
| SD60 | 10,900 | 1.51 | 9000 | 1.36 | 8300 | 3.36 | 5100 | 3.87 |
| SDC | 10,800 | 1.55 | 9700 | 1.45 | 7900 | 3.14 | 6000 | 3.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Fabian, D.R.; Durpekova, S.; Dusankova, M.; Cisar, J.; Drohsler, P.; Elich, O.; Borkova, M.; Cechmankova, J.; Sedlarik, V. Renewable Poly(Lactic Acid)Lignocellulose Biocomposites for the Enhancement of the Water Retention Capacity of the Soil. Polymers 2023, 15, 2243. https://doi.org/10.3390/polym15102243
Cruz Fabian DR, Durpekova S, Dusankova M, Cisar J, Drohsler P, Elich O, Borkova M, Cechmankova J, Sedlarik V. Renewable Poly(Lactic Acid)Lignocellulose Biocomposites for the Enhancement of the Water Retention Capacity of the Soil. Polymers. 2023; 15(10):2243. https://doi.org/10.3390/polym15102243
Chicago/Turabian StyleCruz Fabian, Dalila Rubicela, Silvie Durpekova, Miroslava Dusankova, Jaroslav Cisar, Petra Drohsler, Ondrej Elich, Marketa Borkova, Jarmila Cechmankova, and Vladimir Sedlarik. 2023. "Renewable Poly(Lactic Acid)Lignocellulose Biocomposites for the Enhancement of the Water Retention Capacity of the Soil" Polymers 15, no. 10: 2243. https://doi.org/10.3390/polym15102243






