How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes and Solutions
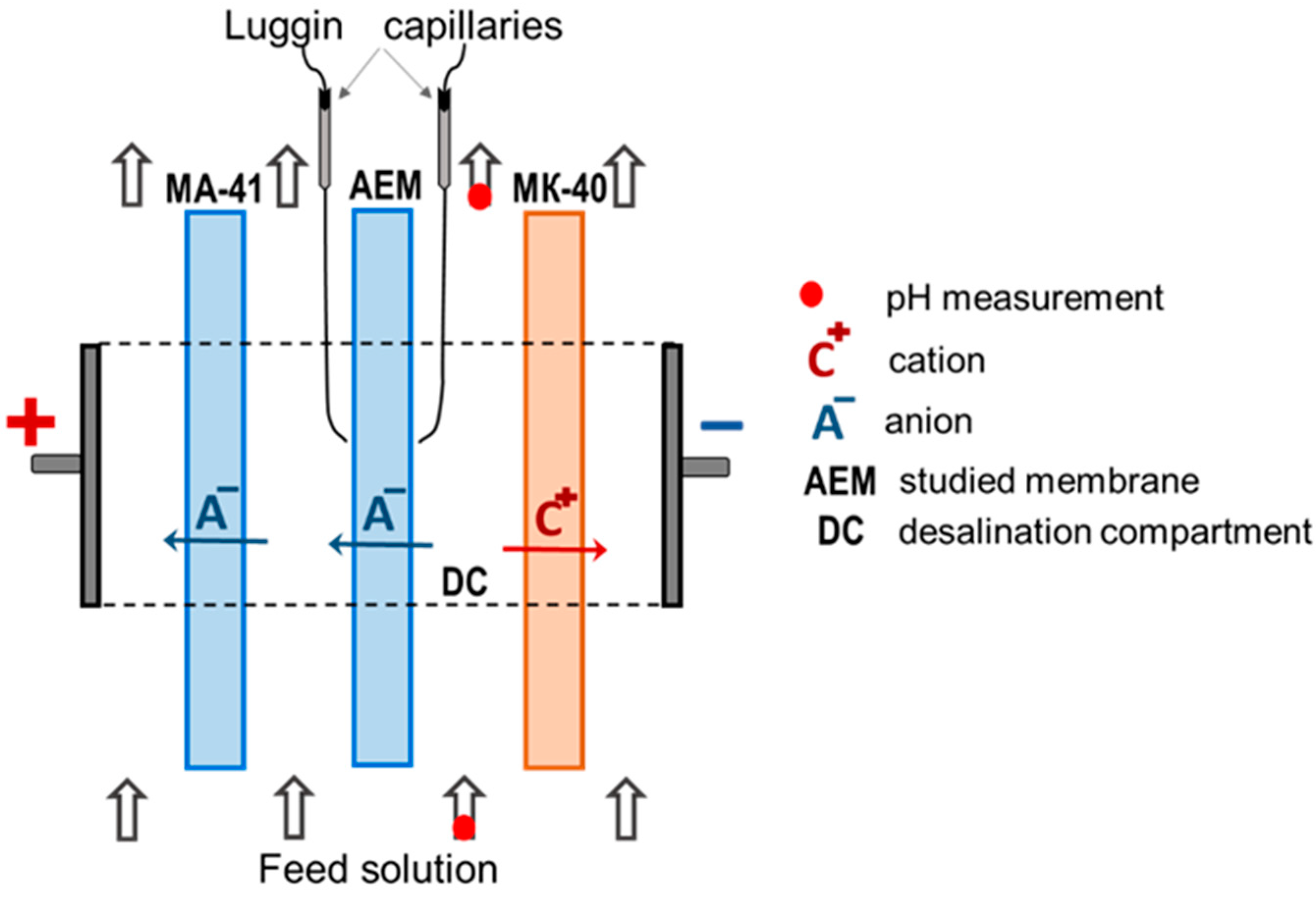
2.2. Methods
3. Results
3.1. Chemical Structure of the Membranes
3.2. Conductivity
3.3. Current-Voltage Curves
- NaCl solutions
- NaxH(3−x)PO4 solutions

3.4. Batch Electrodialysis of NaxH(3−x)PO4 Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, T.; Wu, D.; Wu, L. Poly(2,6-dimethyl-1,4-phenylene oxide) (PPO)—A versatile starting polymer for proton conductive membranes (PCMs). Prog. Polym. Sci. 2008, 33, 894–915. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Ru, Y.; Hu, M.; Din, L.; Xu, T. Anion exchange membranes (AEMs) based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and its derivatives. Polym. Chem. 2015, 6, 5809–5826. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, K.A.; Mountz, D.A.; Reuschle, D.A.; Blackwell, R.I. Self-assembled organic/inorganic hybrids as membrane materials. Electrochim. Acta 2004, 50, 565–569. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [Green Version]
- Donnan, F.G. The Theory of Membrane Equilibria. Chem. Rev. 1924, 1, 73–90. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Zhang, Z.; Wu, L.; Xu, T. Novel aromatic proton-exchange polyelectrolytes via polyacylation of pre-sulfonated monomers. J. Mater. Chem. 2012, 22, 13996. [Google Scholar] [CrossRef]
- Hori, Y.; Nakatani, T.; Mizutan, E.Y. Morphology of Ion Exchange Membranes. J. Electron Microsc. 1986, 35, 220–226. [Google Scholar] [CrossRef]
- Mizutani, Y. Structure of ion exchange membranes. J. Memb. Sci. 1990, 49, 121–144. [Google Scholar] [CrossRef]
- Nefedova, G.Z.; Klimova, Z.G.; Sapoznikova, G.S. Ion-Exchange Membranes, Granulates, Powders. Catalogue 1977. [Google Scholar]
- Sata, T.; Tsujimoto, M.; Yamaguchi, T.; Matsusaki, K. Change of anion exchange membranes in an aqueous sodium hydroxide solution at high temperature. J. Memb. Sci. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Doi, S.; Takumi, N.; Kakihana, Y.; Higa, M. Alkali Attack on Cation-Exchange Membranes with Polyvinyl Chloride Backing and Binder: Comparison with Anion-Exchange Membranes. Membranes 2020, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Yasukawa, M.; Kakihana, Y.; Higa, M. Alkali attack on anion exchange membranes with PVC backing and binder: Effect on performance and correlation between them. J. Memb. Sci. 2019, 573, 85–96. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Zhiltsova, A.V.; Chernykh, E.I.; Sirota, E.A.; Agapov, B.L. SEM diagnostics of the surface of MK-40 and MA-40 heterogeneous ion-exchange membranes in the swollen state after thermal treatment. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2013, 7, 833–840. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. A Study of Ralex Membrane Morphology by SEM. Membranes 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Astom Detailed Specification of IEMs Produced Astom Corporation. Available online: http://www.astom-corp.jp/en/product/10.html (accessed on 5 May 2023).
- Chen, G.Q.; Wei, K.; Hassanvand, A.; Freeman, B.D.; Kentish, S.E. Single and binary ion sorption equilibria of monovalent and divalent ions in commercial ion exchange membranes. Water Res. 2020, 175, 115681. [Google Scholar] [CrossRef]
- Fan, H.; Huang, Y.; Yip, N.Y. Advancing ion-exchange membranes to ion-selective membranes: Principles, status, and opportunities. Front. Environ. Sci. Eng. 2023, 17, 25. [Google Scholar] [CrossRef]
- Vatanpour, V.; Teber, O.O.; Mehrabi, M.; Koyuncu, I. Polyvinyl alcohol-based separation membranes: A comprehensive review on fabrication techniques, applications and future prospective. Mater. Today Chem. 2023, 28, 101381. [Google Scholar] [CrossRef]
- Zuo, P.; Xu, Z.; Zhu, Q.; Ran, J.; Ge, L.; Ge, X.; Wu, L.; Yang, Z.; Xu, T. Ion Exchange Membranes: Constructing and Tuning Ion Transport Channels. Adv. Funct. Mater. 2022, 32, 2207366. [Google Scholar] [CrossRef]
- Yang, B.; Cunman, Z. Progress in constructing high-performance anion exchange Membrane: Molecular design, microphase controllability and In-device property. Chem. Eng. J. 2023, 457, 141094. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Lu, F.; Su, Y.; Li, W.; An, J.; Wang, Y.; Xu, T. Electrodialytic concentration of landfill leachate effluent: Lab- and pilot-scale test, and assessment. Sep. Purif. Technol. 2021, 276, 119311. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Zhang, F.; Tan, M.; Jiang, H.; Liu, Y.; Zhang, Y. Efficient separation and recovery of cobalt(II) and lithium(I) from spent lithium ion batteries (LIBs) by polymer inclusion membrane electrodialysis (PIMED). Chem. Eng. J. 2022, 430, 132924. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Recovery of gamma-aminobutyric acid (GABA) from reaction mixtures containing salt by electrodialysis. Sep. Purif. Technol. 2016, 170, 353–359. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Xu, T. Developing Ion Exchange Membrane for Treating High Salinity Water Using Electrodialysis; Tianjin, China, 2019. [Google Scholar]
- Salinity Water Using Electrodialysis. In Proceedings of the Developing Ion Exchange Membrane for Treating High Salinity Water Using Electrodialysis, Tianjin, China, 5 May 2023.
- Sugimoto, Y.; Ujike, R.; Higa, M.; Kakihana, Y.; Higa, M. Power Generation Performance of Reverse Electrodialysis (RED) Using Various Ion Exchange Membranes and Power Output Prediction for a Large RED Stack. Membranes 2022, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhou, D.; Lai, L.; Qi, H.; Xia, L.; Depuydt, S.; Van der Bruggen, B.; Zhao, Y. A monovalent selective anion exchange membrane made by poly(2,6-dimethyl-1,4-phenyl oxide) for bromide recovery. Sep. Purif. Technol. 2023, 305, 122377. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Kozmai, A.E.; Mareev, A.A.; Gil, V.V. Theoretical and Experimental Study of Neutralization Dialysis of Phenylalanine–Mineral Salt Equimolar Mixture of Different Concentrations. Membr. Membr. Technol. 2022, 4, 306–318. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wu, L.; Wang, Y.; Xu, T. Electrodialytic concentrating lithium salt from primary resource. Desalination 2018, 425, 30–36. [Google Scholar] [CrossRef]
- Yan, H.; Xu, C.; Li, W.; Wang, Y.; Xu, T. Electrodialysis To Concentrate Waste Ionic Liquids: Optimization of Operating Parameters. Ind. Eng. Chem. Res. 2016, 55, 2144–2152. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Fu, R.; Yan, H.; Jiang, C.; Wang, Y.; Xu, T. Bipolar Membrane Electrodialysis for Cleaner Production of Gluconic Acid: Valorization of the Regenerated Base for the Upstream Enzyme Catalysis. Ind. Eng. Chem. Res. 2022, 61, 7634–7644. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Kamali, N.; Mahjouri, M. Phosphorus removal and recovery: State of the science and challenges. Environ. Sci. Pollut. Res. 2022, 29, 58561–58589. [Google Scholar] [CrossRef] [PubMed]
- Monat, L.; Zhang, W.; Jarošíková, A.; Haung, H.; Bernstein, R.; Nir, O. Circular Process for Phosphoric Acid Plant Wastewater Facilitated by Selective Electrodialysis. ACS Sustain. Chem. Eng. 2022, 10, 11567–11576. [Google Scholar] [CrossRef]
- Ye, Z.-L.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L.; Meesschaert, B. Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective-electrodialysis. Water Res. 2019, 160, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Kedwell, K.C.; Jørgensen, M.K.; Quist-Jensen, C.A.; Pham, T.D.; Van der Bruggen, B.; Christensen, M.L. Selective electrodialysis for simultaneous but separate phosphate and ammonium recovery. Environ. Technol. 2021, 42, 2177–2186. [Google Scholar] [CrossRef]
- Rotta, E.H.; Marder, L.; Pérez-Herranz, V.; Bernardes, A.M. Characterization of an anion-exchange membrane subjected to phosphate and sulfate separation by electrodialysis at overlimiting current density condition. J. Memb. Sci. 2021, 635, 119510. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.-L.; Yang, R.; Chen, S. Synchronously recovering different nutrient ions from wastewater by using selective electrodialysis. Water Sci. Technol. 2022, 86, 2627–2641. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Memb. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Ward, A.J.; Arola, K.; Thompson Brewster, E.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Wu, G.; Luo, J.; Wang, S. Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment. Chem. Eng. J. 2017, 322, 224–233. [Google Scholar] [CrossRef]
- Shi, L.; Xiao, L.; Hu, Z.; Zhan, X. Nutrient recovery from animal manure using bipolar membrane electrodialysis: Study on product purity and energy efficiency. Water Cycle 2020, 1, 54–62. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Desmidt, E.; Van Looveren, A.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Phosphate Separation and Recovery from Wastewater by Novel Electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef] [PubMed]
- Lemay, N.; Mikhaylin, S.; Mareev, S.; Pismenskaya, N.; Nikonenko, V.; Bazinet, L. How demineralization duration by electrodialysis under high frequency pulsed electric field can be the same as in continuous current condition and that for better performances? J. Memb. Sci. 2020, 603, 117878. [Google Scholar] [CrossRef]
- Schütte, T.; Niewersch, C.; Wintgens, T.; Yüce, S. Phosphorus recovery from sewage sludge by nanofiltration in diafiltration mode. J. Memb. Sci. 2015, 480, 74–82. [Google Scholar] [CrossRef]
- Sarapulova, V.; Pismenskaya, N.; Titorova, V.; Sharafan, M.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport Characteristics of CJMAEDTM Homogeneous Anion Exchange Membranes in Sodium Chloride and Sodium Sulfate Solutions. Int. J. Mol. Sci. 2021, 22, 1415. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile Drop Method: Critical Analysis and Optimization for Measuring the Contact Angle of an Ion-Exchange Membrane Surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef]
- Sarapulova, V.V.; Titorova, V.D.; Nikonenko, V.V.; Pismenskaya, N.D. Transport Characteristics of Homogeneous and Heterogeneous Ion-Exchange Membranes in Sodium Chloride, Calcium Chloride, and Sodium Sulfate Solutions. Membr. Membr. Technol. 2019, 1, 168–182. [Google Scholar] [CrossRef]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Conductivitéélectrique membranaire: Étude de l’effet de la concentration, de la nature de l’électrolyte et de la structure membranaire. Eur. Polym. J. 1999, 35, 1187–1195. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Demina, O.A.; Dvorkina, G.A.; Parshikov, S.B.; Larchet, C.; Auclair, B.; Berezina, N.P. Comparative study of methods used for the determination of electroconductivity of ion-exchange membranes. Russ. J. Electrochem. 2001, 37, 287–293. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Memb. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two mechanisms of H+/OH− ion generation in anion-exchange membrane systems with polybasic acid salt solutions. J. Memb. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Titorova, V.D.; Mareev, S.A.; Gorobchenko, A.D.; Gil, V.V.; Nikonenko, V.V.; Sabbatovskii, K.G.; Pismenskaya, N.D. Effect of current-induced coion transfer on the shape of chronopotentiograms of cation-exchange membranes. J. Memb. Sci. 2021, 624, 119036. [Google Scholar] [CrossRef]
- Iglesias, M.J.; del Río, J.C.; Laggoun-Défarge, F.; Cuesta, M.J.; Suárez-Ruiz, I. Control of the chemical structure of perhydrous coals; FTIR and Py-GC/MS investigation. J. Anal. Appl. Pyrolysis 2002, 62, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, V.; Sharma, S. Effect of various synthesis parameters on styrene–divinylbenzene copolymer properties. J. Porous Mater. 2019, 26, 1559–1571. [Google Scholar] [CrossRef]
- Danilczuk, M.; Lin, L.; Schlick, S.; Hamrock, S.J.; Schaberg, M.S. Understanding the fingerprint region in the infra-red spectra of perfluorinated ionomer membranes and corresponding model compounds: Experiments and theoretical calculations. J. Power Sources 2011, 196, 8216–8224. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Li, J.; Zhai, M. PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Vibrational spectrum of PVDF and its interpretation. Polym. Test. 2004, 23, 791–796. [Google Scholar] [CrossRef]
- Tarasevich, B.N. Infrared Spectrum of Basic Classes of Organic Compounds. IR Spectra of General Classes of Organic Compounds: Handbook; Mosk. Gos. Univ.: Moscow, Russia, 2012. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Misra, A.; Tyagi, P.; Rai, P.; Misra, D.S. FTIR Spectroscopy of Multiwalled Carbon Nanotubes: A Simple Approachto Study the Nitrogen Doping. J. Nanosci. Nanotechnol. 2007, 7, 1820–1823. [Google Scholar] [CrossRef]
- Sun, M.; Li, M.; Zhang, X.; Wu, C.; Wu, Y. Graphene oxide modified porous P84 co-polyimide membranes for boron recovery by bipolar membrane electrodialysis process. Sep. Purif. Technol. 2020, 232, 115963. [Google Scholar] [CrossRef]
- Choi, J.-H.; Moon, S.-H. Structural change of ion-exchange membrane surfaces under high electric fields and its effects on membrane properties. J. Colloid Interface Sci. 2003, 265, 93–100. [Google Scholar] [CrossRef]
- Sheorn, M.; Ahmad, H.; Kundu, S. Improved Performance of Cation Exchange Membranes Coated with Chitosan. ACS Appl. Eng. Mater. 2023, 1, 832–840. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Wang, X.; Mamlouk, M.; Scott, K. Preparation of alkaline anion exchange polymer membrane from methylated melamine grafted poly(vinylbenzyl chloride) and its fuel cell performance. J. Mater. Chem. 2011, 21, 12910. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Zhelonkina, E.A.; Kononova, T.V. Effect of chromium compounds on the properties of ion-exchange membranes. Pet. Chem. 2013, 53, 494–499. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef]
- Sistat, P.; Huguet, P.; Ruiz, B.; Pourcelly, G.; Mareev, S.A.; Nikonenko, V.V. Effect of pulsed electric field on electrodialysis of a NaCl solution in sub-limiting current regime. Electrochim. Acta 2015, 164, 267–280. [Google Scholar] [CrossRef]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of solution composition on the resistance of ion exchange membranes. J. Memb. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]
- Demina, O.A.; Kononenko, N.A.; Falina, И.V.; Demin, A.V. Theoretical Estimation of Differential Diffusion Permeability Coefficients of Ion Exchange Membranes. Colloid J. 2017, 79, 259–269. [Google Scholar] [CrossRef]
- Díaz, J.C.; Kamcev, J. Ionic conductivity of ion-exchange membranes: Measurement techniques and salt concentration dependence. J. Memb. Sci. 2021, 618, 118718. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, H.; Yip, N.Y. Influence of electrolyte on concentration-induced conductivity-permselectivity tradeoff of ion-exchange membranes. J. Memb. Sci. 2023, 668, 121184. [Google Scholar] [CrossRef]
- Sarapulova, V.; Nevakshenova, E.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Unusual concentration dependence of ion-exchange membrane conductivity in ampholyte-containing solutions: Effect of ampholyte nature. J. Memb. Sci. 2015, 479, 28–38. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Berger, L.I.; Kehiaian, H.V.; Roth, D.L.; Zwillinger, D.; Goldberg, R.N.; Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Slavinskaya, G.V.; Kurenkova, O.V. On the multifunctional character of strong basic anion-exchange resin. Sorpt. Chromatogr. Process. 2019, 19, 101–110. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L. A simple model for the response of an anion-exchange membrane to variation in concentration and pH of bathing solution. J. Memb. Sci. 2018, 567, 127–138. [Google Scholar] [CrossRef]
- Achoh, A.R.; Pribytkov, F.B.; But, A.Y.; Zabolotsky, V.I. Exchange Sorption and Electrical Conductivity of Heterogeneous Anion-Exchange Membranes in Mixed Sodium Hydroxide/Sodium Naphthenate and Sodium Sulfate/Sodium Nitrate Electrolyte Solutions. Pet. Chem. 2018, 58, 1159–1164. [Google Scholar] [CrossRef]
- Demina, O.A.; Berezina, N.P.; Sata, T.; Demin, A.V. Transport-structural parameters of domestic and foreign anion-exchange membranes. Russ. J. Electrochem. 2002, 38, 896–902. [Google Scholar] [CrossRef]
- Hernández-Pérez, L.; Martí-Calatayud, M.; Montañés, M.; Pérez-Herranz, V. Interplay between Forced Convection and Electroconvection during the Overlimiting Ion Transport through Anion-Exchange Membranes: A Fourier Transform Analysis of Membrane Voltage Drops. Membranes 2023, 13, 363. [Google Scholar] [CrossRef]
- Belloň, T.; Slouka, Z. Overlimiting behavior of surface-modified heterogeneous anion-exchange membranes. J. Memb. Sci. 2020, 610, 118291. [Google Scholar] [CrossRef]
- Nebavskaya, K.A.; Sarapulova, V.V.; Sabbatovskiy, K.G.; Sobolev, V.D.; Pismenskaya, N.D.; Sistat, P.; Cretin, M.; Nikonenko, V.V. Impact of ion exchange membrane surface charge and hydrophobicity on electroconvection at underlimiting and overlimiting currents. J. Memb. Sci. 2017, 523, 36–44. [Google Scholar] [CrossRef]
- Zabolotskiy, V.I.; But, A.Y.; Vasil’eva, V.I.; Akberova, E.M.; Melnikov, S.S. Ion transport and electrochemical stability of strongly basic anion-exchange membranes under high current electrodialysis conditions. J. Memb. Sci. 2017, 526, 60–72. [Google Scholar] [CrossRef]
- Simons, R. Electric field effects on proton transfer between ionizable groups and water in ion exchange membranes. Electrochim. Acta 1984, 29, 151–158. [Google Scholar] [CrossRef]
- Pasechnaya, E.; Tsygurina, K.; Ponomar, M.; Chuprynina, D.; Nikonenko, V.; Pismenskaya, N. Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes. Membranes 2023, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Zabolotskii, V.I.; Sharafan, M.V.; Shel’deshov, N.V. The dissociation rate of water molecules in systems with cation- and anion-exchange membranes. Russ. J. Electrochem. 2012, 48, 550–555. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Concentration polarization of interface and non-linear electrokinetic phenomena. Adv. Colloid Interface Sci. 2010, 160, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Zaltzman, B. Electro-convective versus electroosmotic instability in concentration polarization. Adv. Colloid Interface Sci. 2007, 134–135, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.; Rybalkina, O.; Moroz, I.; Mareev, S.; Nikonenko, V. Influence of Electroconvection on Chronopotentiograms of an Anion-Exchange Membrane in Solutions of Weak Polybasic Acid Salts. Int. J. Mol. Sci. 2021, 22, 13518. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Rybalkina, O.A.; Kozmai, A.E.; Tsygurina, K.A.; Melnikova, E.D.; Nikonenko, V.V. Generation of H+ and OH− ions in anion-exchange membrane/ampholyte-containing solution systems: A study using electrochemical impedance spectroscopy. J. Memb. Sci. 2020, 601, 117920. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Memb. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Mengistu, D.H.; Kooijman, E.E.; May, S. Ionization properties of mixed lipid membranes: A Gouy–Chapman model of the electrostatic–hydrogen bond switch. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.J.; Loewen, C.J. Putting the pH into phosphatidic acid signaling. BMC Biol. 2011, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Marmisollé, W.A.; Irigoyen, J.; Gregurec, D.; Moya, S.; Azzaroni, O. Supramolecular Surface Chemistry: Substrate-Independent, Phosphate-Driven Growth of Polyamine-Based Multifunctional Thin Films. Adv. Funct. Mater. 2015, 25, 4144–4152. [Google Scholar] [CrossRef]
- D’Agostino, L.; Di Luccia, A. Polyamines interact with DNA as molecular aggregates. Eur. J. Biochem. 2002, 269, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, L.P.; Kornienko, T.S.; Ovsiannikova, D.V.; Grigorova, E.V. Sorption of amino acids on phosphate cation exchangers. Proc. Vor. State Univ. Eng. Technol. 2015, 151–156. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Memb. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Ahmad, M.; Yaroshchuk, A.; Bruening, M.L. Moderate pH changes alter the fluxes, selectivities and limiting currents in ion transport through polyelectrolyte multilayers deposited on membranes. J. Memb. Sci. 2020, 616, 118570. [Google Scholar] [CrossRef]
- Dressick, W.J.; Wahl, K.J.; Bassim, N.D.; Stroud, R.M.; Petrovykh, D.Y. Divalent–Anion Salt Effects in Polyelectrolyte Multilayer Depositions. Langmuir 2012, 28, 15831–15843. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mitta, G.; Marmisollé, W.A.; Albesa, A.G.; Toimil-Molares, M.E.; Trautmann, C.; Azzaroni, O. Phosphate-Responsive Biomimetic Nanofluidic Diodes Regulated by Polyamine-Phosphate Interactions: Insights into Their Functional Behavior from Theory and Experiment. Small 2018, 14, 1702131. [Google Scholar] [CrossRef]
- Laucirica, G.; Marmisollé, W.A.; Azzaroni, O. Dangerous liaisons: Anion-induced protonation in phosphate–polyamine interactions and their implications for the charge states of biologically relevant surfaces. Phys. Chem. Chem. Phys. 2017, 19, 8612–8620. [Google Scholar] [CrossRef]
- Laucirica, G.; Pérez-Mitta, G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Amine-Phosphate Specific Interactions within Nanochannels: Binding Behavior and Nanoconfinement Effects. J. Phys. Chem. C 2019, 123, 28997–29007. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Piccinini, E.; Knoll, W.; Marmisollé, W.A.; Azzaroni, O. The Effect of Amino–Phosphate Interactions on the Biosensing Performance of Enzymatic Graphene Field-Effect Transistors. Anal. Chem. 2022, 94, 13820–13828. [Google Scholar] [CrossRef]
- Kooijman, E.E.; Tieleman, D.P.; Testerink, C.; Munnik, T.; Rijkers, D.T.S.; Burger, K.N.J.; de Kruijff, B. An Electrostatic/Hydrogen Bond Switch as the Basis for the Specific Interaction of Phosphatidic Acid with Proteins. J. Biol. Chem. 2007, 282, 11356–11364. [Google Scholar] [CrossRef] [Green Version]









| Membrane | Ion-Exchange Capacity of Wet Membrane, mmol/gwet | Water Content, gH2O/gdry, % | Thickness in 0.02 M NaCl Solution, µm | f2 in NaCl Solution | mmol/gwet |
|---|---|---|---|---|---|
| CJMA-3 | 0.57 ± 0.05 [48] | 17 ± 1 [48] | 151 ± 5 | 0.27 ± 0.02 | 0.8 ± 0.1 |
| CJMA-6 | 0.90 ± 0.05 [48] | 18 ± 1 [48] | 120 ± 3 | 0.32 ± 0.02 | 1.3 ± 0.1 |
| ASE | 1.93 ± 0.05 [28] | 20 ± 1 | 150 ± 5 | 0.06 ± 0.02 | 2.0 ± 0.1 |
| Membranes | in 0.04 M Solution, mS cm−1 | ||
|---|---|---|---|
| NaCl | NaH2PO4 | ||
| CJMA-3 | 1.1 ± 0.05 | 0.6 ± 0.05 | 1.8 ± 0.1 |
| CJMC-6 | 1.8 ± 0.05 | 0.4 ± 0.05 | 4.5 ± 0.1 |
| ASE | 3.8 ± 0.05 | 1.1 ± 0.05 | 3.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pismenskaya, N.; Rybalkina, O.; Solonchenko, K.; Pasechnaya, E.; Sarapulova, V.; Wang, Y.; Jiang, C.; Xu, T.; Nikonenko, V. How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions? Polymers 2023, 15, 2288. https://doi.org/10.3390/polym15102288
Pismenskaya N, Rybalkina O, Solonchenko K, Pasechnaya E, Sarapulova V, Wang Y, Jiang C, Xu T, Nikonenko V. How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions? Polymers. 2023; 15(10):2288. https://doi.org/10.3390/polym15102288
Chicago/Turabian StylePismenskaya, Natalia, Olesya Rybalkina, Ksenia Solonchenko, Evgeniia Pasechnaya, Veronika Sarapulova, Yaoming Wang, Chenxiao Jiang, Tongwen Xu, and Victor Nikonenko. 2023. "How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions?" Polymers 15, no. 10: 2288. https://doi.org/10.3390/polym15102288
APA StylePismenskaya, N., Rybalkina, O., Solonchenko, K., Pasechnaya, E., Sarapulova, V., Wang, Y., Jiang, C., Xu, T., & Nikonenko, V. (2023). How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions? Polymers, 15(10), 2288. https://doi.org/10.3390/polym15102288











