Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships
Abstract
1. Introduction
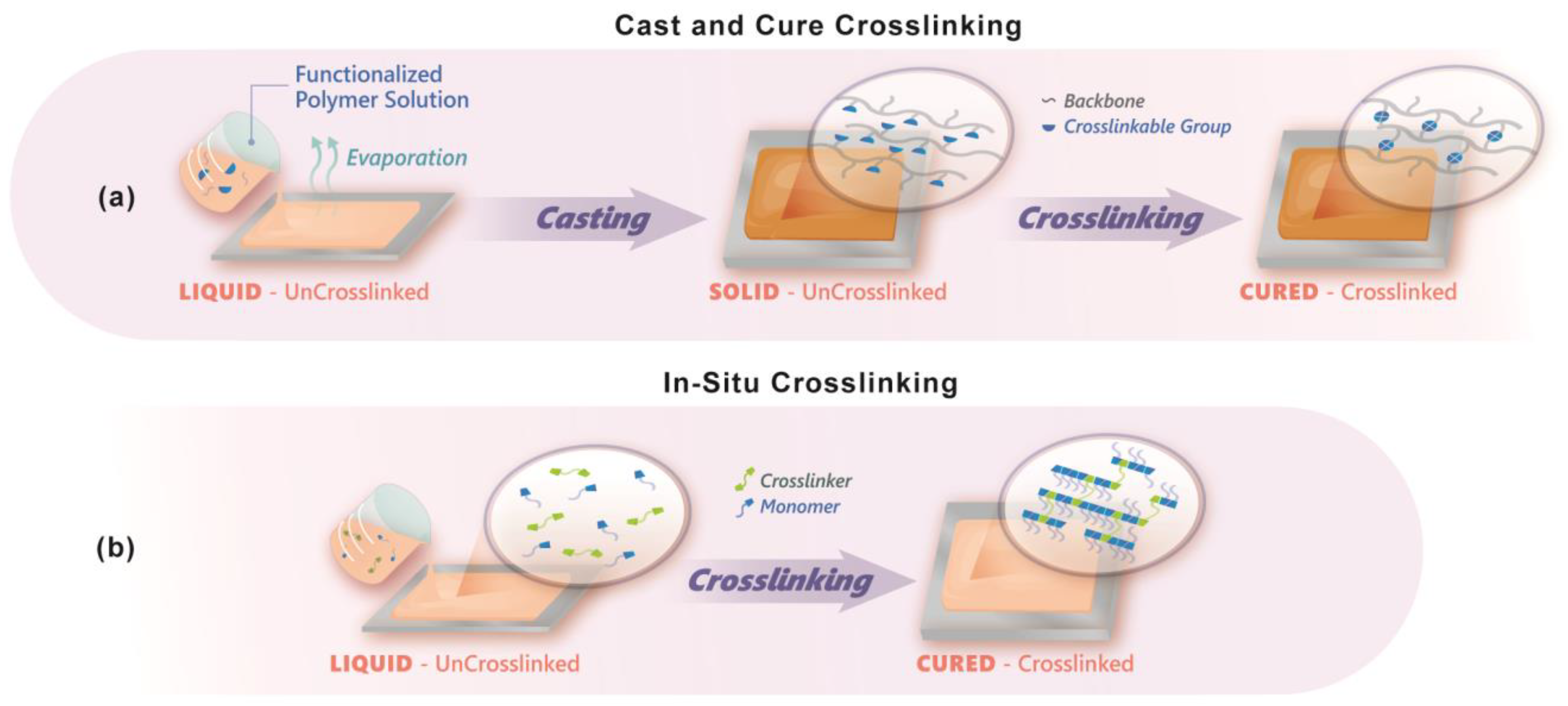
2. Crosslinked Ion Exchange Membranes
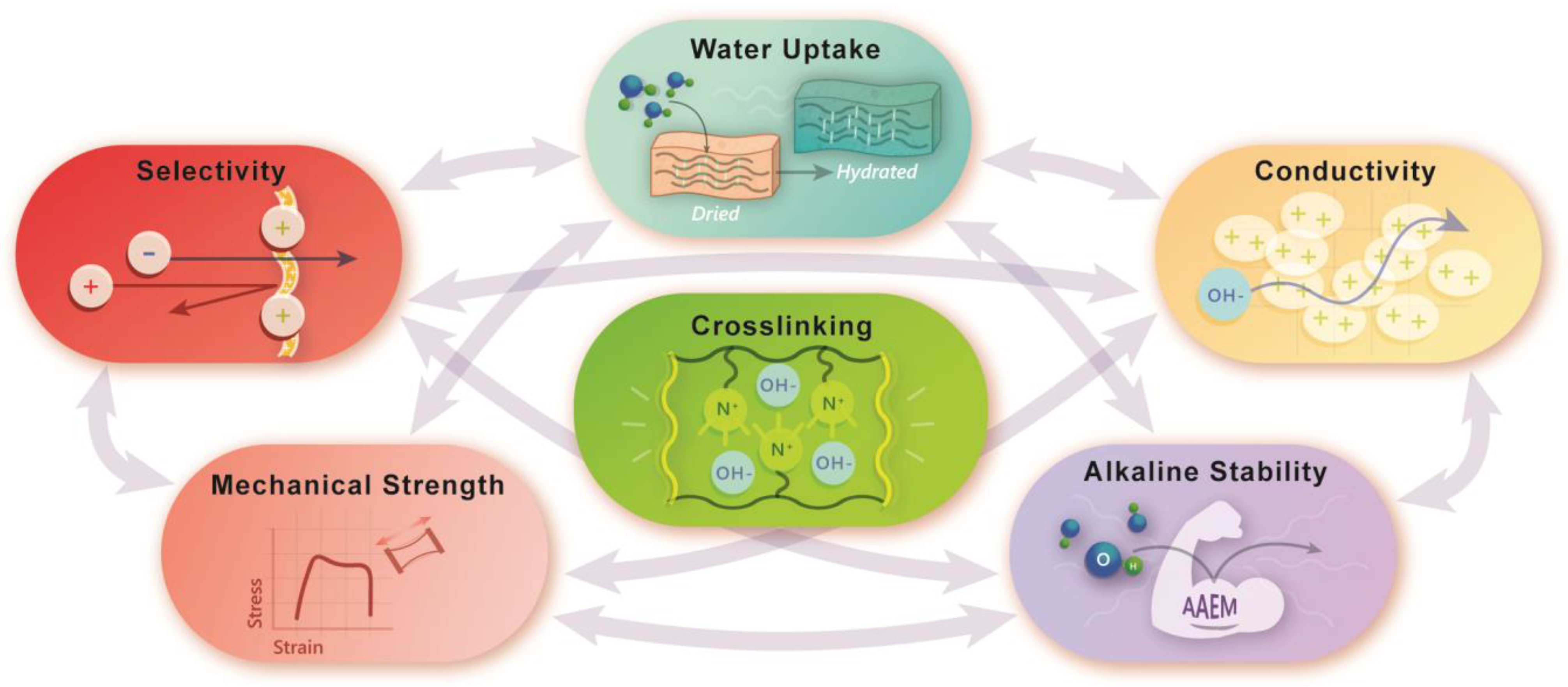
3. Effect of Crosslinking on the Material Properties of AAEMs
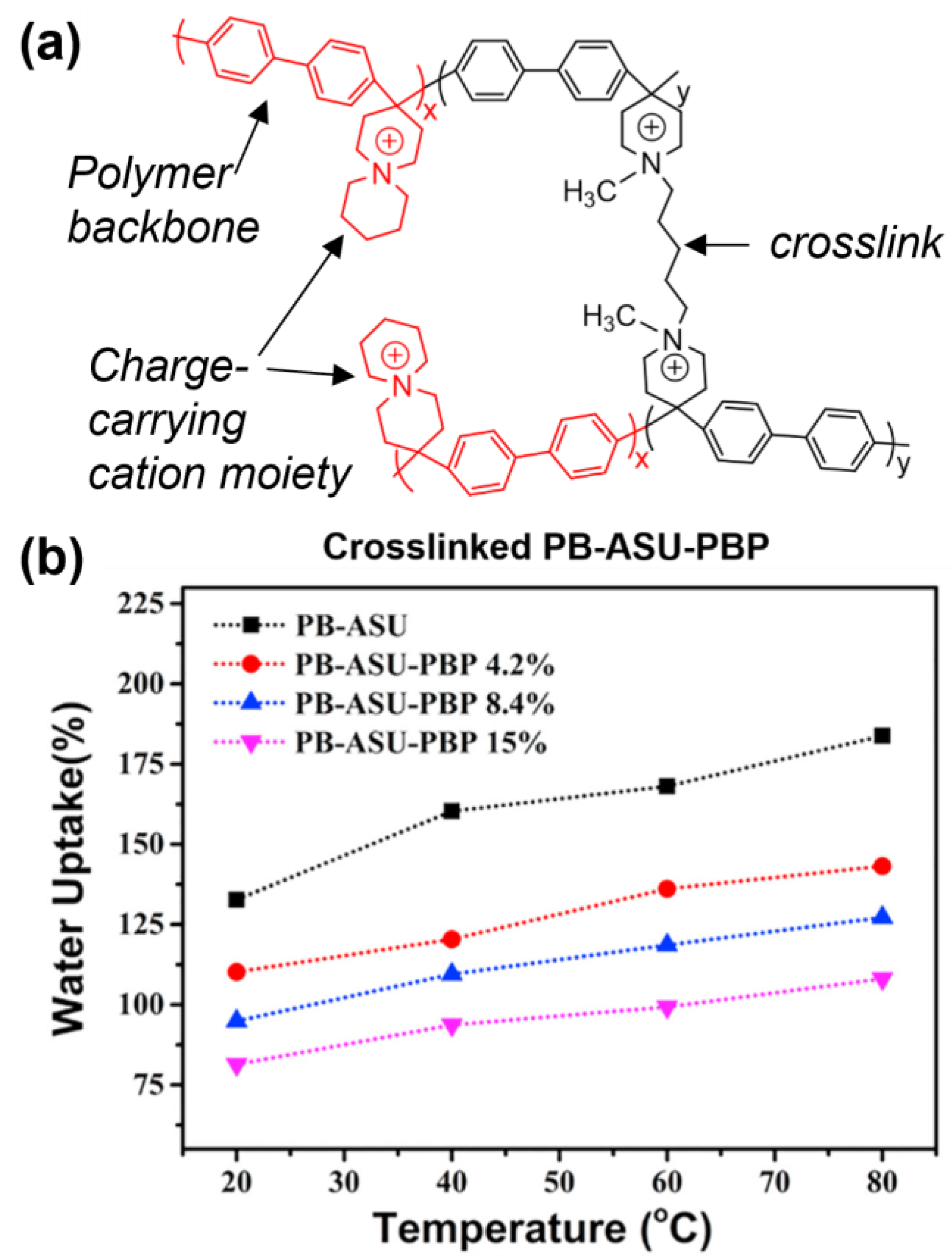
3.1. Crosslinking and Mechanical Properties
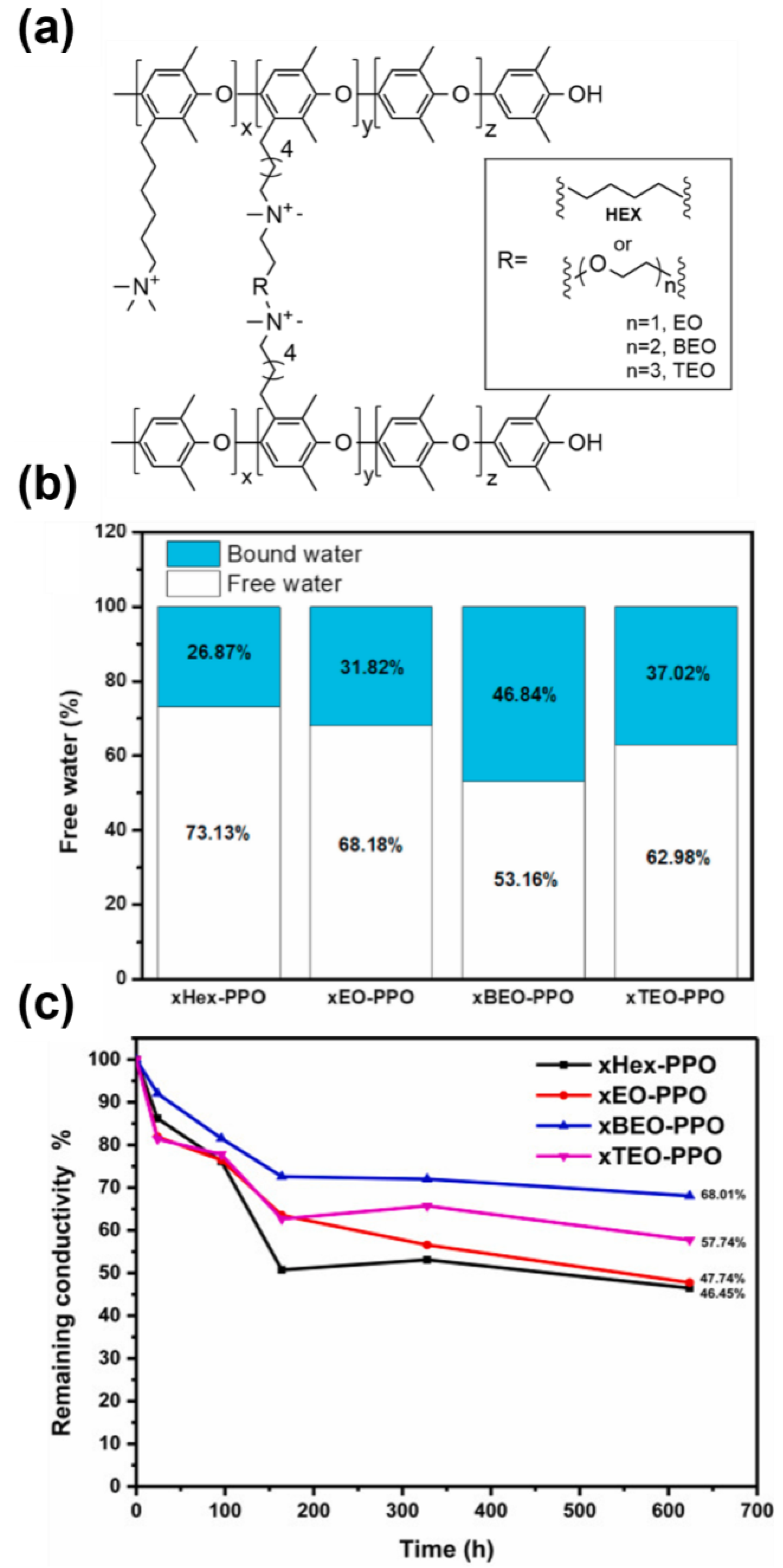
3.2. Crosslinking and (Ionic) Conductivity

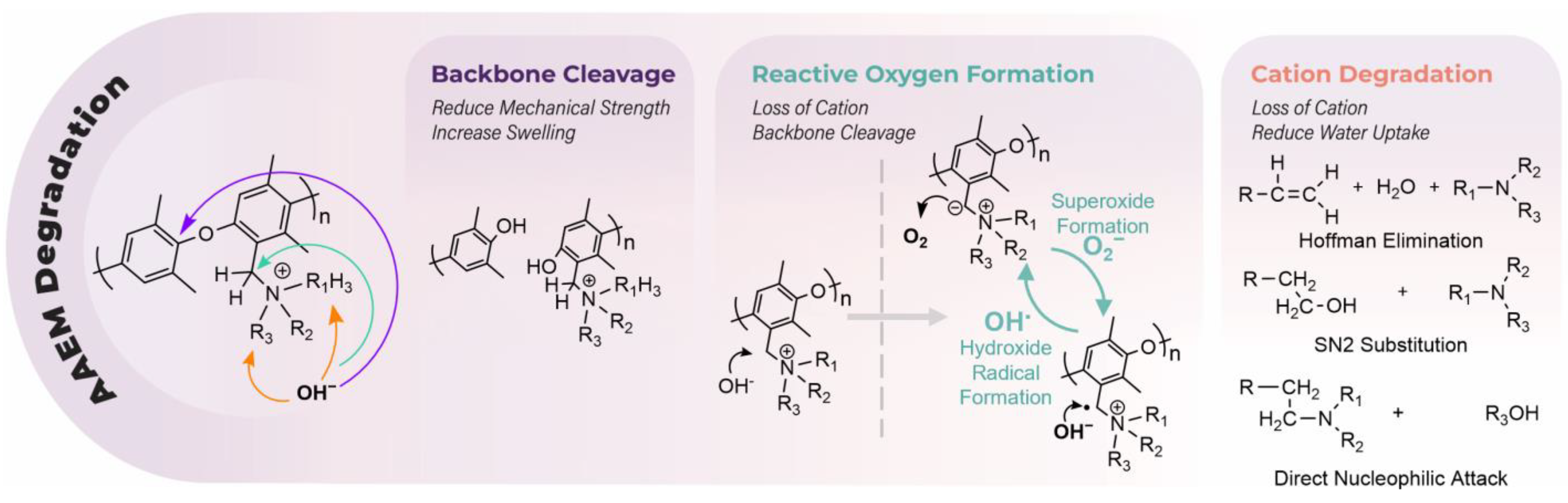
3.3. Crosslinking and Alkaline Stability
3.4. Crosslinking and Selectivity
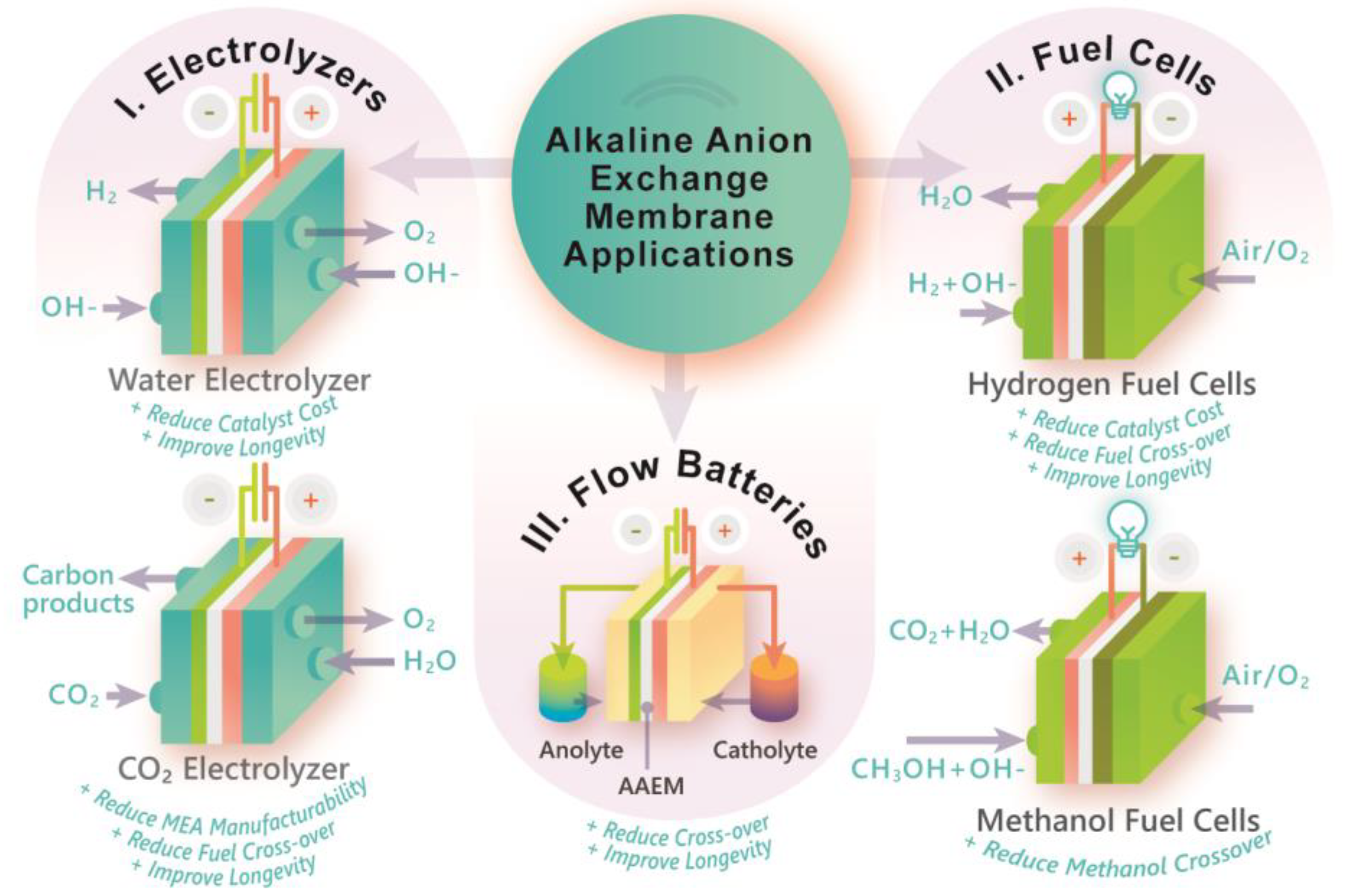
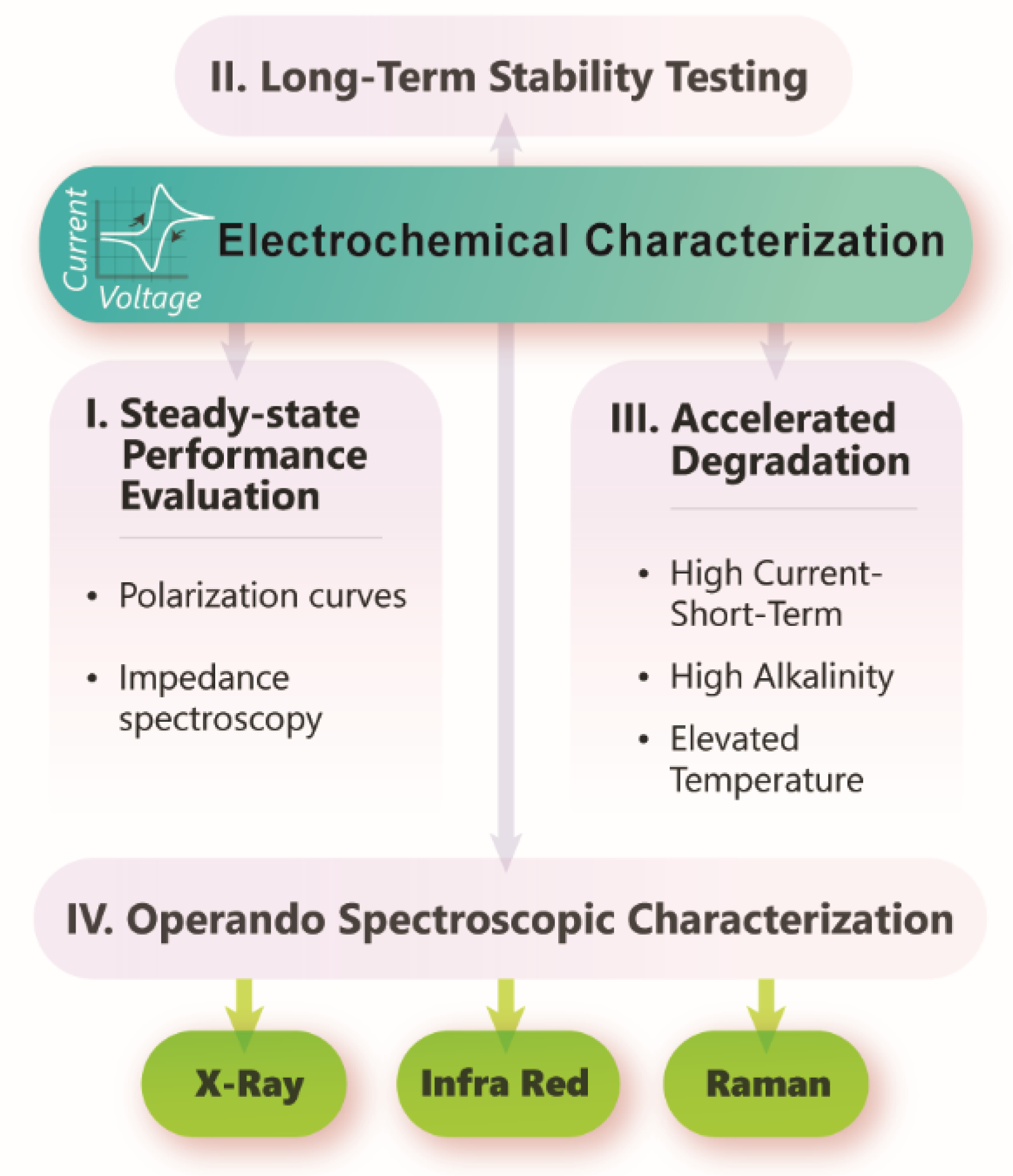
4. Application in Electrochemical Reactors
5. AAEM Design and Crosslinking Strategies
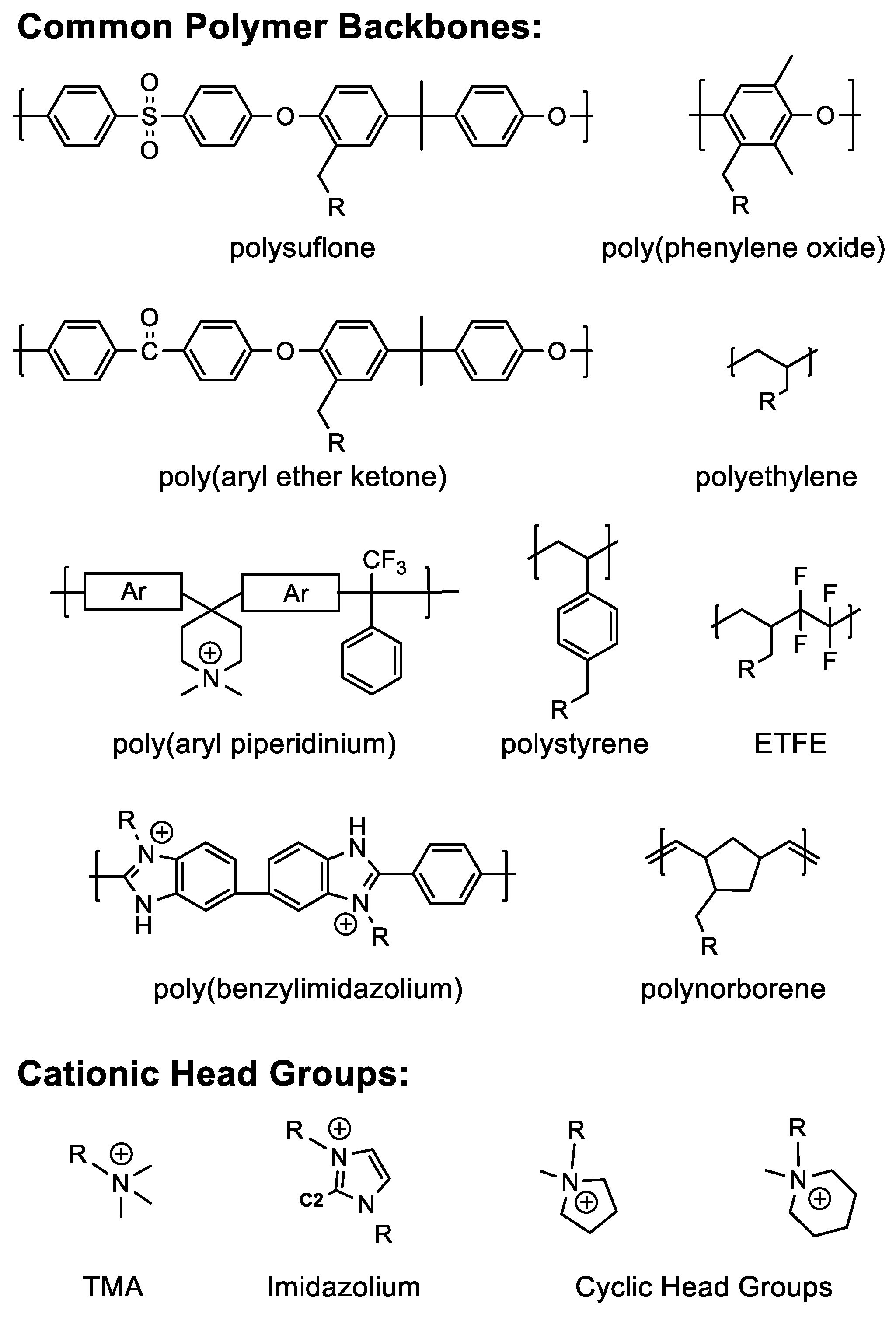
5.1. Polymer Design
5.2. Crosslink Chemistry
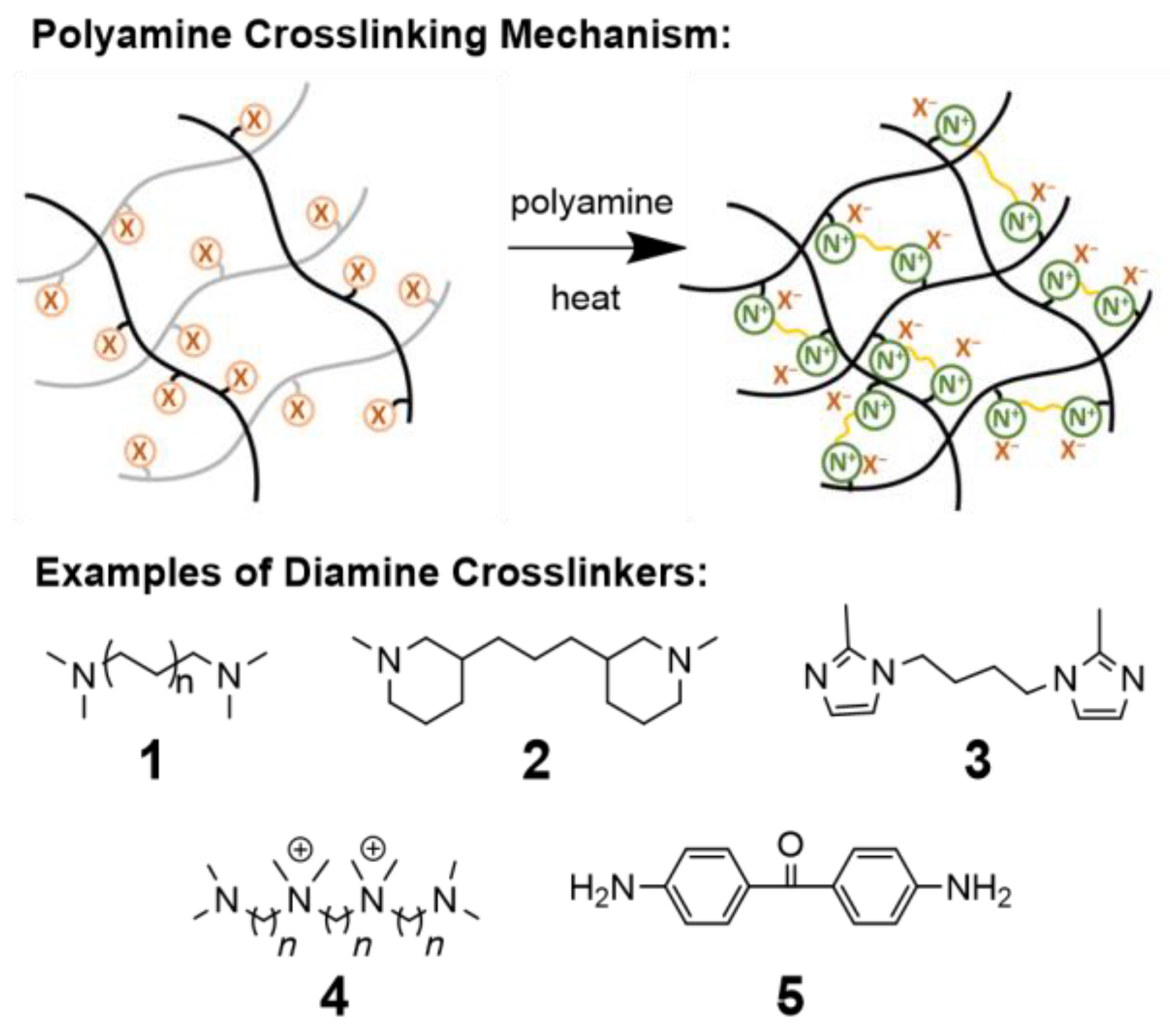
5.2.1. Poly-Functional Amines
5.2.2. Radical Polymerization
5.2.3. Olefin Metathesis
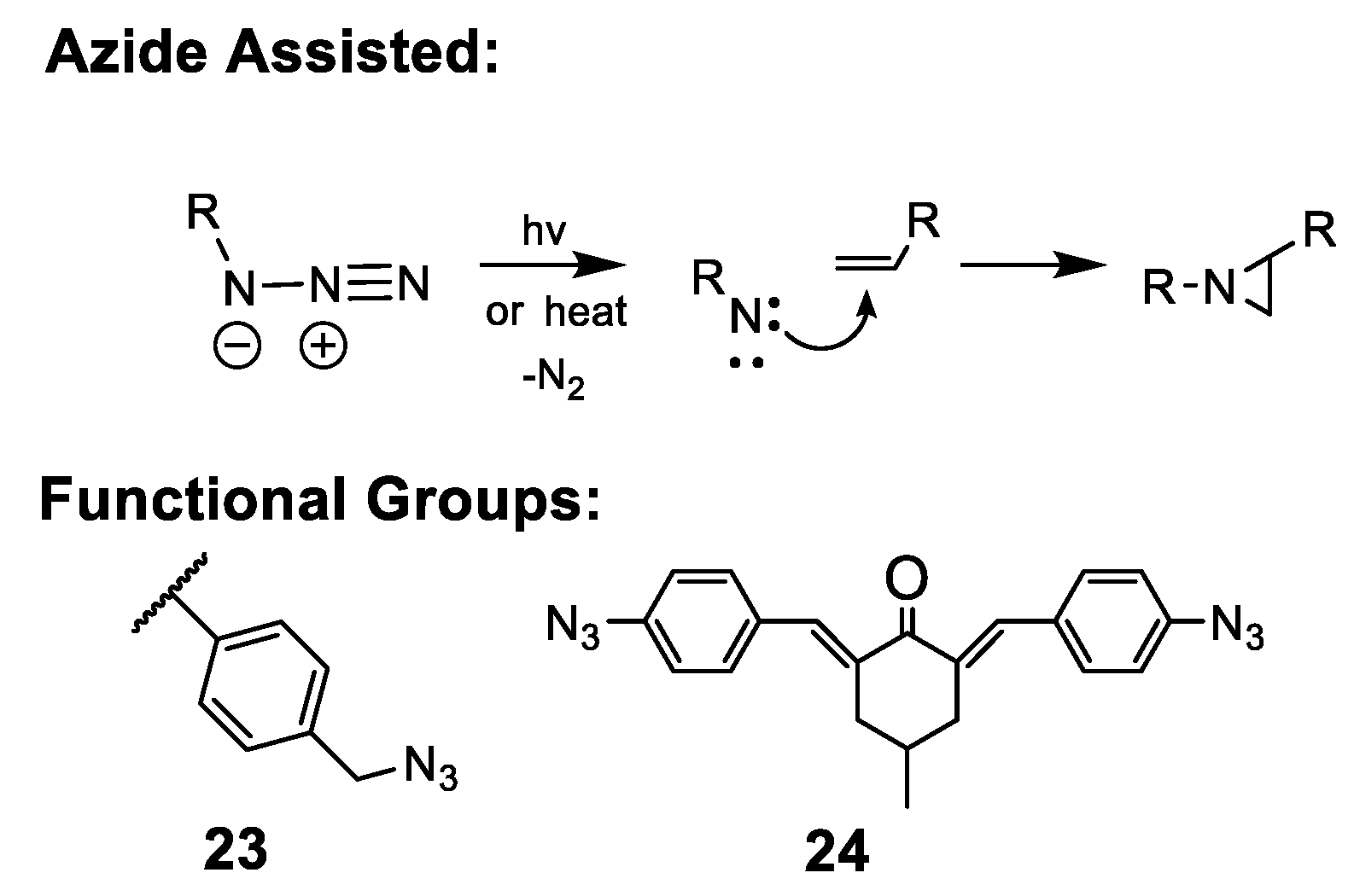
5.2.4. Organic Azide-Mediated Crosslinking
5.2.5. Thiol-Ene Click Crosslinking
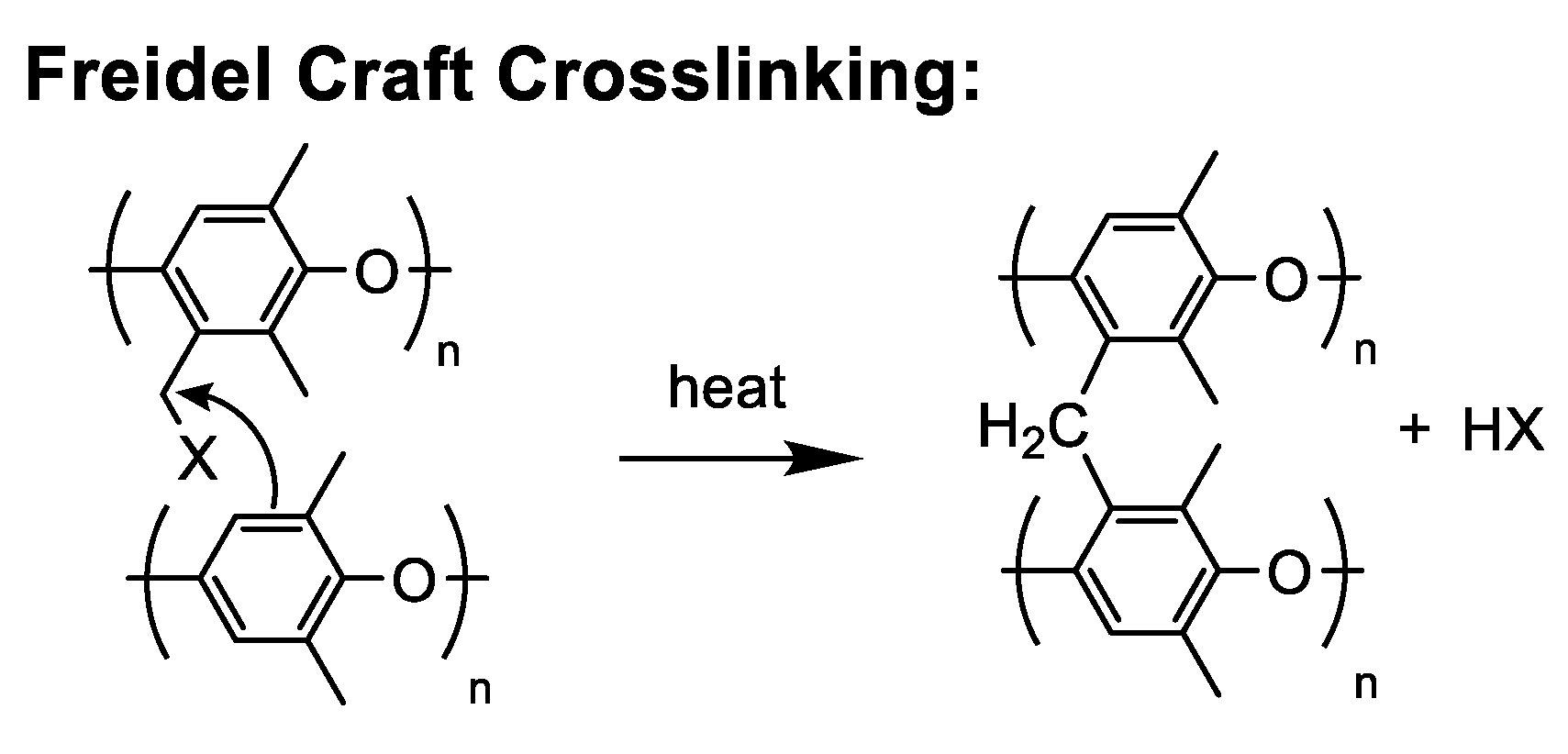
5.2.6. Thermal Friedel–Crafts Electrophilic Substitution
5.2.7. Epoxide Crosslinking
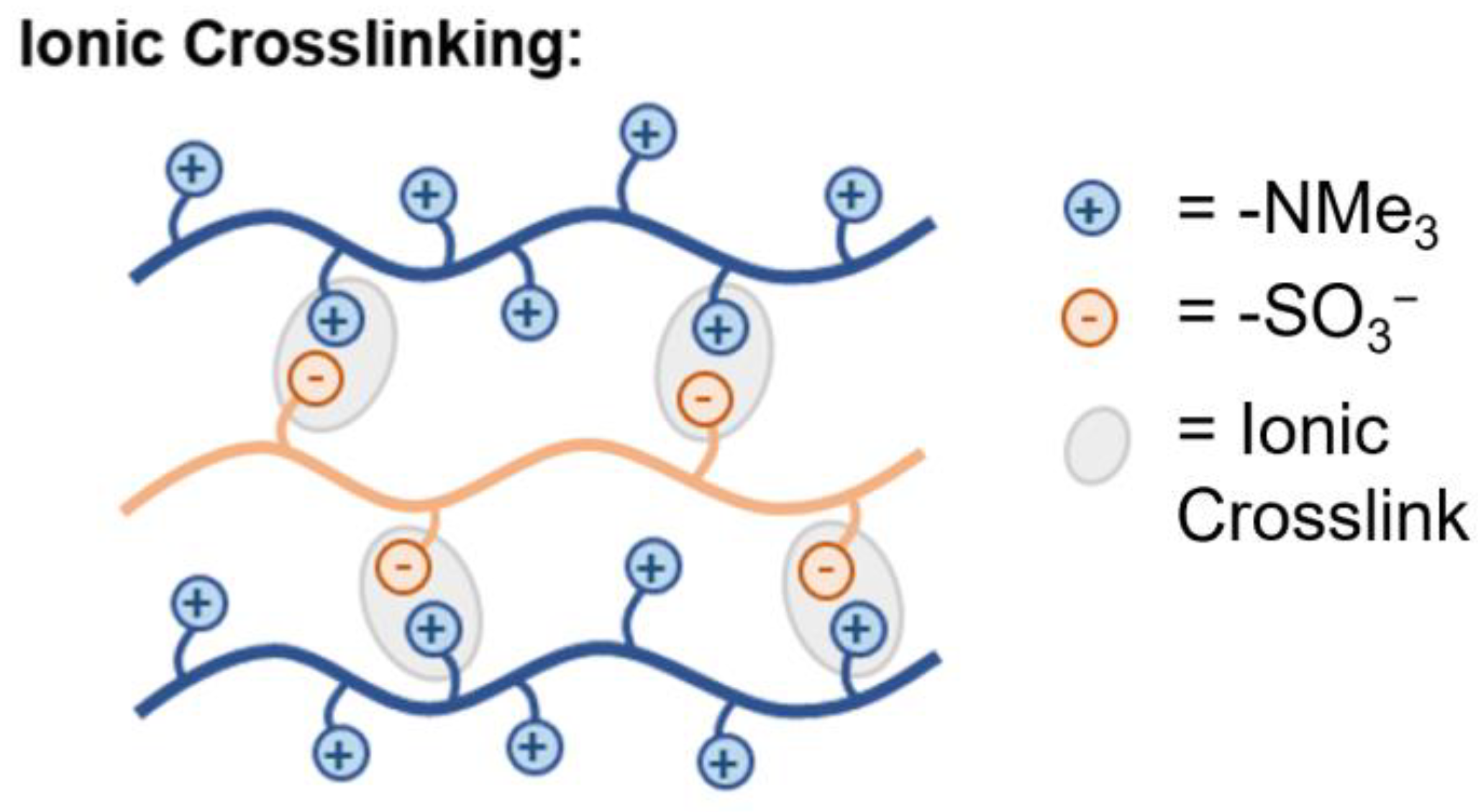
5.2.8. Ionic Crosslinking
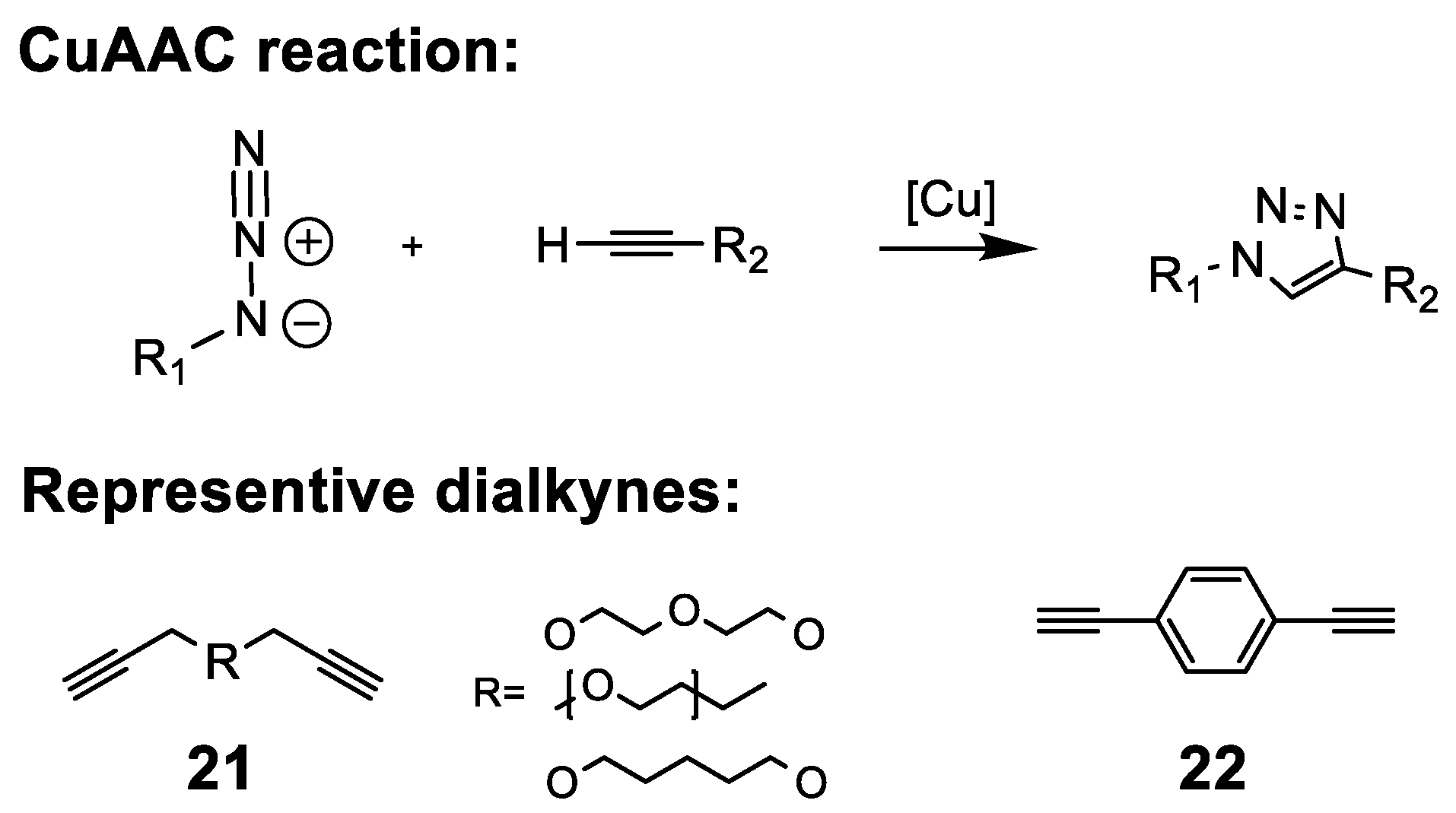
5.2.9. Cycloadditions
5.2.10. Radiation-Activated Crosslinking
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olabi, A.G. Renewable Energy and Energy Storage Systems. Energy 2017, 136, 1–6. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging Electrochemical Energy Conversion and Storage Technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A Review of the Synthesis and Characterization of Anion Exchange Membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of Cell Performance in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion Exchange Membrane Fuel Cells: Current Status and Remaining Challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Lin, B. Progress of Alkaline Anion Exchange Membranes for Fuel Cells: The Effects of Micro-Phase Separation. Front. Mater. 2020, 7, 4. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric Materials as Anion-Exchange Membranes for Alkaline Fuel Cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef]
- Kuppusamy, H.G.; Dhanasekaran, P.; Nagaraju, N.; Neeshma, M.; Dass, B.M.; Dhavale, V.M.; Unni, S.M.; Bhat, S.D. Anion Exchange Membranes for Alkaline Polymer Electrolyte Fuel Cells—A Concise Review. Materials 2022, 15, 5601. [Google Scholar] [CrossRef]
- Fathabadi, H. Combining a Proton Exchange Membrane Fuel Cell (PEMFC) Stack with a Li-Ion Battery to Supply the Power Needs of a Hybrid Electric Vehicle. Renew. Energy 2019, 130, 714–724. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, Q.; Das, P.K.; Mao, B.; Scott, K. Membrane Electrode Assemblies for PEM Fuel Cells: A Review of Functional Graded Design and Optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Pavel, C.C.; Cecconi, F.; Emiliani, C.; Santiccioli, S.; Scaffidi, A.; Catanorchi, S.; Comotti, M. Highly Efficient Platinum Group Metal Free Based Membrane-Electrode Assembly for Anion Exchange Membrane Water Electrolysis. Angew. Chem. Int. Ed. 2014, 53, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Pivovar, B.; Seung Kim, Y. 2019 Anion Exchange Membrane Workshop Summary Report; Technical Report; U.S. Department of Energy: Washington, DC, USA, 2019. [Google Scholar]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Cho, M.K.; Lim, A.; Lee, S.Y.; Kim, H.-J.; Yoo, S.J.; Sung, Y.-E.; Park, H.S.; Jang, J.H. A Review on Membranes and Catalysts for Anion Exchange Membrane Water Electrolysis Single Cells. J. Electrochem. Sci. Technol. 2017, 8, 183–196. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in Alkaline Water Electrolyzers: A Review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Zhang, J.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Anion Exchange Membrane Water Electrolyzer: Electrode Design, Lab-Scaled Testing System and Performance Evaluation. EnergyChem 2022, 4, 100087. [Google Scholar] [CrossRef]
- Wang, F.; Fu, B. Anion-Exchange Membranes for Direct Methanol Alkaline Fuel Cells. In Direct Methanol Fuel Cell Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–106. ISBN 978-0-12-819158-3. [Google Scholar]
- Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon Dioxide and Water Electrolysis Using New Alkaline Stable Anion Membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and Challenges in Electrochemical CO 2 Reduction Processes: An Engineering and Design Perspective Looking beyond New Catalyst Materials. J. Mater. Chem. A 2020, 8, 1511–1544. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Gabardo, C.M.; Reyes, A.; O’Brien, C.P.; Holdcroft, S.; Pintauro, P.; Bahar, B.; Hickner, M.; Bae, C.; Sinton, D.; et al. Designing Anion Exchange Membranes for CO2 Electrolysers. Nat. Energy 2021, 6, 339–348. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, Q.-F.; Zheng, M.-S.; Tian, Z.-W. The Preparation of a Novel Anion-Exchange Membrane and Its Application in All-Vanadium Redox Batteries. J. Membr. Sci. 2012, 421–422, 232–237. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Wu, M.C. Anion Exchange Membranes for Aqueous Acid-Based Redox Flow Batteries: Current Status and Challenges. Appl. Energy 2019, 233–234, 622–643. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A Review on Recent Developments of Anion Exchange Membranes for Fuel Cells and Redox Flow Batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Weitzner, S.E.; Akhade, S.A.; Varley, J.B.; Wood, B.C.; Otani, M.; Baker, S.E.; Duoss, E.B. Toward Engineering of Solution Microenvironments for the CO 2 Reduction Reaction: Unraveling PH and Voltage Effects from a Combined Density-Functional–Continuum Theory. J. Phys. Chem. Lett. 2020, 11, 4113–4118. [Google Scholar] [CrossRef]
- Zhang, Z.; Melo, L.; Jansonius, R.P.; Habibzadeh, F.; Grant, E.R.; Berlinguette, C.P. PH Matters When Reducing CO 2 in an Electrochemical Flow Cell. ACS Energy Lett. 2020, 5, 3101–3107. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Yang, J.H.; Kim, H.-T. A Review of Vanadium Electrolytes for Vanadium Redox Flow Batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Dekel, D.R. Water—A Key Parameter in the Stability of Anion Exchange Membrane Fuel Cells. Curr. Opin. Electrochem. 2018, 9, 173–178. [Google Scholar] [CrossRef]
- Zelovich, T.; Simari, C.; Nicotera, I.; Dekel, D.R.; Tuckerman, M.E. The Impact of Carbonation on Hydroxide Diffusion in Nano-Confined Anion Exchange Membranes. J. Mater. Chem. A 2022, 10, 11137–11149. [Google Scholar] [CrossRef]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion Exchange Membranes: Current Status and Moving Forward. J. Polym. Sci. B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Yang, B.; Cunman, Z. Progress in Constructing High-Performance Anion Exchange Membrane: Molecular Design, Microphase Controllability and In-Device Property. Chem. Eng. J. 2023, 457, 141094. [Google Scholar] [CrossRef]
- Gutru, R.; Turtayeva, Z.; Xu, F.; Maranzana, G.; Vigolo, B.; Desforges, A. A Comprehensive Review on Water Management Strategies and Developments in Anion Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 19642–19663. [Google Scholar] [CrossRef]
- Zhang, B.; Hua, Y.; Gao, Z. Strategies to Optimize Water Management in Anion Exchange Membrane Fuel Cells. J. Power Sources 2022, 525, 231141. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A Mini-Review on Anion Exchange Membranes for Fuel Cell Applications: Stability Issue and Addressing Strategies. Int. J. Hydrog. Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability Challenges of Anion Exchange Membrane Fuel Cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-Stable Anion Exchange Membranes: A Review of Synthetic Approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Ouma, C.N.M.; Obodo, K.O.; Bessarabov, D. Computational Approaches to Alkaline Anion-Exchange Membranes for Fuel Cell Applications. Membranes 2022, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Karibayev, M.; Kalybekkyzy, S.; Wang, Y.; Mentbayeva, A. Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications. Molecules 2022, 27, 3574. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Liu, X.; Huang, T.; Jiang, H.; Yin, Y.; Qin, Y.; Guiver, M.D. Toward Alkaline-Stable Anion Exchange Membranes in Fuel Cells: Cycloaliphatic Quaternary Ammonium-Based Anion Conductors. Electrochem. Energy Rev. 2022, 5, 348–400. [Google Scholar] [CrossRef]
- Masel, R.I.; Sajjad, S.D.; Chen, Q.; Liu, Z. Ion-Conducting Membranes. Patent No US20200030787A1, 30 January 2020. [Google Scholar]
- Xergy™ Membranes. Fuel Cell Store Website. Xergy Xion Composite Pention Anion Exchange Membranes are commercially sold with varying degrees of crosslinking from 5 or 15%. Available online: https://www.fuelcellstore.com/fuel-cell-components/membranes/xergy-ion-exchange-membranes (accessed on 17 March 2023).
- Wright, A.; Holdcroft, S.; Weissbach, T.; Peckham, T.J.; Britton, B. Crosslinking of Hydroxide Stable, Polybenzimidazoliums and Polyimidazoliums Membranes and Ionomers. Patent No WO2018026743A1, 8 February 2018. [Google Scholar]
- Nielsen, L.E. Cross-Linking–Effect on Physical Properties of Polymers. J. Macromol. Sci. Part C 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Tsusaka, K.; Yamamoto, T.; Asano, T.; Muto, A.; Hasegawa, N.; Kawasumi, M. Imide Crosslinked Perfluorinated Proton Exchange Membranes by Gas Treatment. J. Electrochem. Soc. 2016, 163, F372–F376. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, J.; Wang, C.; Chu, D. Fuel Cell Durability Enhancement by Crosslinking Alkaline Anion Exchange Membrane Electrolyte. Electrochem. Commun. 2012, 16, 65–68. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Du, F.; Warsinger, D.M.; Urmi, T.I.; Thiel, G.P.; Kumar, A.; Lienhard V, J.H. Sodium Hydroxide Production from Seawater Desalination Brine: Process Design and Energy Efficiency. Environ. Sci. Technol. 2018, 52, 5949–5958. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Liu, Y.; You, K.; Song, N.; Liu, B.; Guan, S. Pendant-Group Cross-Linked Highly Sulfonated Co-Polyimides for Proton Exchange Membranes. J. Membr. Sci. 2015, 480, 83–92. [Google Scholar] [CrossRef]
- Yao, H.; Song, N.; Shi, K.; Feng, S.; Zhu, S.; Zhang, Y.; Guan, S. Highly Sulfonated Co-Polyimides Containing Hydrophobic Cross-Linked Networks as Proton Exchange Membranes. Polym. Chem. 2016, 7, 4728–4735. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Yang, K.; Zhang, H.; Wang, Z. Synthesis and Properties of Novel Cross-Linked Composite Sulfonated Poly (Aryl Ether Ketone Sulfone) Containing Multiple Sulfonic Side Chains for High-Performance Proton Exchange Membranes. Renew. Energy 2019, 138, 1104–1113. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Ma, W.; Zhang, N.; Zhang, Y.; Zhang, G.; Liu, Z.; Na, H. Silane-Cross-Linked Polybenzimidazole with Improved Conductivity for High Temperature Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Wang, D.; Ni, H.; Wang, Z. Benzimidazole Grafted Polybenzimidazole Cross-Linked Membranes with Excellent PA Stability for High-Temperature Proton Exchange Membrane Applications. Appl. Surf. Sci. 2019, 465, 332–339. [Google Scholar] [CrossRef]
- Shen, C.-H.; Hsu, S.L. Synthesis of Novel Cross-Linked Polybenzimidazole Membranes for High Temperature Proton Exchange Membrane Fuel Cells. J. Membr. Sci. 2013, 443, 138–143. [Google Scholar] [CrossRef]
- Luo, H.; Vaivars, G.; Mathe, M. Covalent-Ionically Cross-Linked Polyetheretherketone Proton Exchange Membrane for Direct Methanol Fuel Cell. J. Power Sources 2010, 195, 5197–5200. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Na, H. Novel Cross-Linked Sulfonated Poly (Arylene Ether Ketone) Membranes for Direct Methanol Fuel Cell. Int. J. Hydrog. Energy 2010, 35, 2176–2182. [Google Scholar] [CrossRef]
- Chen, C.; Tse, Y.-L.S.; Lindberg, G.E.; Knight, C.; Voth, G.A. Hydroxide Solvation and Transport in Anion Exchange Membranes. J. Am. Chem. Soc. 2016, 138, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.G.; Melchior, J.P.; Wohlfarth, A.; Kreuer, K.D. Hydroxide, Halide and Water Transport in a Model Anion Exchange Membrane. J. Membr. Sci. 2014, 464, 61–71. [Google Scholar] [CrossRef]
- Marx, D.; Chandra, A.; Tuckerman, M.E. Aqueous Basic Solutions: Hydroxide Solvation, Structural Diffusion, and Comparison to the Hydrated Proton. Chem. Rev. 2010, 110, 2174–2216. [Google Scholar] [CrossRef]
- Grew, K.N.; Chiu, W.K.S. A Dusty Fluid Model for Predicting Hydroxyl Anion Conductivity in Alkaline Anion Exchange Membranes. J. Electrochem. Soc. 2010, 157, B327. [Google Scholar] [CrossRef]
- Ramani, D.; Khattra, N.S.; Singh, Y.; Orfino, F.P.; Dutta, M.; Kjeang, E. Mitigation of Mechanical Membrane Degradation in Fuel Cells—Part 2: Bonded Membrane Electrode Assembly. J. Power Sources 2021, 512, 230431. [Google Scholar] [CrossRef]
- Zheng, Y.; Ash, U.; Pandey, R.P.; Ozioko, A.G.; Ponce-González, J.; Handl, M.; Weissbach, T.; Varcoe, J.R.; Holdcroft, S.; Liberatore, M.W.; et al. Water Uptake Study of Anion Exchange Membranes. Macromolecules 2018, 51, 3264–3278. [Google Scholar] [CrossRef]
- Zhegur, A.; Gjineci, N.; Willdorf-Cohen, S.; Mondal, A.N.; Diesendruck, C.E.; Gavish, N.; Dekel, D.R. Changes of Anion Exchange Membrane Properties During Chemical Degradation. ACS Appl. Polym. Mater. 2020, 2, 360–367. [Google Scholar] [CrossRef]
- Barnett, A.; Karnes, J.J.; Clemens, A.L.; Oakdale, J.S.; Molinero, V. Post-Hydration Crosslinking of Ion Exchange Membranes to Control Water Content. J. Phys. Chem. C 2023. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, D.H.; Kim, Y.M.; Moon, S.J.; Seong, J.G.; Shin, D.W.; Sohn, J.-Y.; Kim, J.F.; Lee, Y.M. Highly Conductive and Durable Poly(Arylene Ether Sulfone) Anion Exchange Membrane with End-Group Cross-Linking. Energy Environ. Sci. 2017, 10, 275–285. [Google Scholar] [CrossRef]
- Dong, J.; Li, H.; Ren, X.; Che, X.; Yang, J.; Aili, D. Anion Exchange Membranes of Bis-Imidazolium Cation Crosslinked Poly(2,6-Dimethyl-1,4- Phenylene Oxide) with Enhanced Alkaline Stability. Int. J. Hydrog. Energy 2019, 44, 22137–22145. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, N.; Zhang, D.; Yang, J.; He, R. Ionic Crosslinking of Imidazolium Functionalized Poly(Aryl Ether Ketone) by Sulfonated Poly(Ether Ether Ketone) for Anion Exchange Membranes. J. Colloid Interface Sci. 2017, 497, 333–342. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Chu, F.; Ren, Y.; Ding, J.; Yan, F. Bis-Imidazolium Based Poly(Phenylene Oxide) Anion Exchange Membranes for Fuel Cells: The Effect of Cross-Linking. J. Mater. Chem. A 2019, 7, 13275–13283. [Google Scholar] [CrossRef]
- Hao, J.; Gao, X.; Jiang, Y.; Zhang, H.; Luo, J.; Shao, Z.; Yi, B. Crosslinked High-Performance Anion Exchange Membranes Based on Poly(Styrene-b-(Ethylene-Co-Butylene)-b-Styrene). J. Membr. Sci. 2018, 551, 66–75. [Google Scholar] [CrossRef]
- He, S.; Liu, L.; Wang, X.; Zhang, S.; Guiver, M.D.; Li, N. Azide-Assisted Self-Crosslinking of Highly Ion Conductive Anion Exchange Membranes. J. Membr. Sci. 2016, 509, 48–56. [Google Scholar] [CrossRef]
- Lin, C.X.; Zhuo, Y.Z.; Hu, E.N.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Crosslinked Side-Chain-Type Anion Exchange Membranes with Enhanced Conductivity and Dimensional Stability. J. Membr. Sci. 2017, 539, 24–33. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Tuning Poly(Arylene Piperidinium) Anion-Exchange Membranes by Copolymerization, Partial Quaternization and Crosslinking. J. Membr. Sci. 2019, 578, 183–195. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Ge, Q.; Chen, Y.; Xu, T. High Performance Anion Exchange Membranes Obtained through Graft Architecture and Rational Cross-Linking. J. Membr. Sci. 2014, 470, 229–236. [Google Scholar] [CrossRef]
- Kwon, S.; Rao, A.H.N.; Kim, T.-H. Anion Exchange Membranes Based on Terminally Crosslinked Methyl Morpholinium-Functionalized Poly(Arylene Ether Sulfone)s. J. Power Sources 2018, 375, 421–432. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q.; Wang, Y.; Zheng, C.; Wu, L.; Xu, T. In-Situ Crosslinking of Anion Exchange Membrane Bearing Unsaturated Moieties for Electrodialysis. Sep. Purif. Technol. 2015, 156, 226–233. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Chen, N.; Lu, C.; Long, C.; Li, Z.; Liu, Q. Controllable Physical-Crosslinking Poly(Arylene 6-Azaspiro[5.5] Undecanium) for Long-Lifetime Anion Exchange Membrane Applications. J. Membr. Sci. 2019, 590, 117307. [Google Scholar] [CrossRef]
- Hu, C.; Deng, X.; Dong, X.; Hong, Y.; Zhang, Q.; Liu, Q. Rigid Crosslinkers towards Constructing Highly-Efficient Ion Transport Channels in Anion Exchange Membranes. J. Membr. Sci. 2021, 619, 118806. [Google Scholar] [CrossRef]
- Wang, C.; He, Z.; Xie, X.; Mai, X.; Li, Y.; Li, T.; Zhao, M.; Yan, C.; Liu, H.; Wujcik, E.K.; et al. Controllable Cross-Linking Anion Exchange Membranes with Excellent Mechanical and Thermal Properties. Macromol. Mater. Eng. 2018, 303, 1700462. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Shao, Q.; Pan, D.; Wujick, E.; Guo, J.; Xie, X.; Xie, X.; Guo, Z. Crosslinked Norbornene Copolymer Anion Exchange Membrane for Fuel Cells. J. Membr. Sci. 2018, 556, 118–125. [Google Scholar] [CrossRef]
- Ertem, S.P.; Tsai, T.-H.; Donahue, M.M.; Zhang, W.; Sarode, H.; Liu, Y.; Seifert, S.; Herring, A.M.; Coughlin, E.B. Photo-Cross-Linked Anion Exchange Membranes with Improved Water Management and Conductivity. Macromolecules 2016, 49, 153–161. [Google Scholar] [CrossRef]
- Sung, S.; Mayadevi, T.S.; Min, K.; Lee, J.; Chae, J.E.; Kim, T.-H. Crosslinked PPO-Based Anion Exchange Membranes: The Effect of Crystallinity versus Hydrophilicity by Oxygen-Containing Crosslinker Chain Length. J. Membr. Sci. 2020, 619, 118774. [Google Scholar] [CrossRef]
- Pandey, A.K.; Goswami, A.; Sen, D.; Mazumder, S.; Childs, R.F. Formation and Characterization of Highly Crosslinked Anion-Exchange Membranes. J. Membr. Sci. 2003, 217, 117–130. [Google Scholar] [CrossRef]
- Huang, T.; He, G.; Xue, J.; Otoo, O.; He, X.; Jiang, H.; Zhang, J.; Yin, Y.; Jiang, Z.; Douglin, J.C.; et al. Self-Crosslinked Blend Alkaline Anion Exchange Membranes with Bi-Continuous Phase Separated Morphology to Enhance Ion Conductivity. J. Membr. Sci. 2020, 597, 117769. [Google Scholar] [CrossRef]
- Xue, J.; Liu, L.; Liao, J.; Shen, Y.; Li, N. UV-Crosslinking of Polystyrene Anion Exchange Membranes by Azidated Macromolecular Crosslinker for Alkaline Fuel Cells. J. Membr. Sci. 2017, 535, 322–330. [Google Scholar] [CrossRef]
- Hao, L.; Liao, J.; Liu, Y.; Ruan, H.; Sotto, A.; der Bruggen, B.V.; Shen, J. Highly Conductive Anion Exchange Membranes with Low Water Uptake and Performance Evaluation in Electrodialysis. Sep. Purif. Technol. 2019, 211, 481–490. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Pan, J.; Zhu, Y.; Ge, X.; Yang, Z.; Ran, J.; Xu, T. A Mechanically Robust Anion Exchange Membrane with High Hydroxide Conductivity. J. Membr. Sci. 2016, 504, 47–54. [Google Scholar] [CrossRef]
- Xia, Z.; Patchan, M.; Maranchi, J.; Elisseeff, J.; Trexler, M. Determination of Crosslinking Density of Hydrogels Prepared from Microcrystalline Cellulose. J. Appl. Polym. Sci. 2013, 127, 4537–4541. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Highly Conductive Anion-Exchange Membranes Based on Cross-Linked Poly(Norbornene): Vinyl Addition Polymerization. ACS Appl. Energy Mater. 2019, 2, 2447–2457. [Google Scholar] [CrossRef]
- Park, A.M.; Turley, F.E.; Wycisk, R.J.; Pintauro, P.N. Electrospun and Cross-Linked Nanofiber Composite Anion Exchange Membranes. Macromolecules 2014, 47, 227–235. [Google Scholar] [CrossRef]
- Wang, J.J.; Gao, W.T.; Choo, Y.S.L.; Cai, Z.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Highly Conductive Branched Poly(Aryl Piperidinium) Anion Exchange Membranes with Robust Chemical Stability. J. Colloid Interface Sci. 2023, 629, 377–387. [Google Scholar] [CrossRef]
- Abdi, Z.G.; Chiu, T.-H.; Pan, Y.-Z.; Chen, J.-C. Anion Exchange Membranes Based on Ionic Polybenzimidazoles Crosslinked by Thiol-Ene Reaction. React. Funct. Polym. 2020, 156, 104719. [Google Scholar] [CrossRef]
- Wang, F.; Cui, Y.; Sang, J.; Zhang, H.; Zhu, H. Cross-linked of Poly(Biphenyl Pyridine) and Poly(Styrene-b-(Ethylene-co-butylene)-b-styrene) Grafted with Double Cations for Anion Exchange Membrane. Electrochim. Acta 2022, 405, 139770. [Google Scholar] [CrossRef]
- Zhao, T.; Long, C.; Wang, Z.; Zhu, H. Multication Cross-Linked Poly( p -Terphenyl Isatin) Anion Exchange Membranes for Fuel Cells: Effect of Cross-Linker Length on Membrane Performance. ACS Appl. Energy Mater. 2021, 4, 14476–14487. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Yuan, Y.; Wang, Z. Constructing Micro-Phase Separation Structure to Improve the Performance of Anion-Exchange Membrane Based on Poly(Aryl Piperidinium) Cross-Linked Membranes. J. Power Sources 2021, 487, 229429. [Google Scholar] [CrossRef]
- Kumari, M.; Douglin, J.C.; Dekel, D.R. Crosslinked Quaternary Phosphonium-Functionalized Poly(Ether Ether Ketone) Polymer-Based Anion-Exchange Membranes. J. Membr. Sci. 2021, 626, 119167. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, J.; Wu, Z.; Zhou, J.; Li, Z.; Gao, S.; Shen, C. Crosslinked Anion Exchange Membranes Based on Styrene/Acrylonitrile/Vinylimidazole Copolymer and PPO. J. Electrochem. Soc. 2021, 168, 094506. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Liu, W.; Xu, J.; Chen, Z.; Wang, C. Imidazolium-Functionalized Poly (Arylene Ether Ketone) Cross-Linked Anion Exchange Membranes. J. Membr. Sci. 2018, 566, 205–212. [Google Scholar] [CrossRef]
- Li, X.; Tao, J.; Nie, G.; Wang, L.; Li, L.; Liao, S. Cross-Linked Multiblock Copoly(Arylene Ether Sulfone) Ionomer/Nano-ZrO2 Composite Anion Exchange Membranes for Alkaline Fuel Cells. RSC Adv. 2014, 4, 41398–41410. [Google Scholar] [CrossRef]
- Han, J.; Zhu, L.; Pan, J.; Zimudzi, T.J.; Wang, Y.; Peng, Y.; Hickner, M.A.; Zhuang, L. Elastic Long-Chain Multication Cross-Linked Anion Exchange Membranes. Macromolecules 2017, 50, 3323–3332. [Google Scholar] [CrossRef]
- Lee, S.-B.; Min, C.-M.; Jang, J.; Lee, J.-S. Enhanced Conductivity and Stability of Anion Exchange Membranes Depending on Chain Lengths with Crosslinking Based on Poly(Phenylene Oxide). Polymer 2020, 192, 122331. [Google Scholar] [CrossRef]
- Hu, B.; Miao, L.; Bai, Y.; Lü, C. Facile Construction of Crosslinked Anion Exchange Membranes Based on Fluorenyl-Containing Polysulfone via Click Chemistry. Polym. Chem. 2017, 8, 4403–4413. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y.; Kang, F.; Liu, C.; Song, W.; Zheng, X.; Liu, X.; Wang, M.; Zhou, X.; Ren, Z.; et al. Poly(Phenylene Oxide)-Based Anion Exchange Membranes Having Linear Cross-Linkers or Star Cross-Linkers. ACS Appl. Energy Mater. 2022, 5, 11613–11623. [Google Scholar] [CrossRef]
- Ren, J.; Xu, J.; Ju, M.; Chen, X.; Zhao, P.; Meng, L.; Lei, J.; Wang, Z. Long-Term Durable Anion Exchange Membranes Based on Imidazole-Functionalized Poly(Ether Ether Ketone) Incorporating Cationic Metal−organic Framework. Adv. Powder Mater. 2022, 1, 100017. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Sang, J.; Li, J. Poly(Terphenylene) and Bromohexylated Polystyrene- b -Poly(Ethylene- Co -Butene)- b -Polystyrene Cross-Linked Anion Exchange Membrane with Excellent Comprehensive Performance. Energy Fuels 2022, 36, 7795–7805. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Investigation of Cation Degradation in Anion Exchange Membranes Using Multi-Dimensional NMR Spectroscopy. J. Electrochem. Soc. 2013, 160, F1006–F1021. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-Dimensional NMR Spectroscopy Reveals Cation-Triggered Backbone Degradation in Polysulfone-Based Anion Exchange Membranes. Proc. Natl. Acad. Sci. 2013, 110, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Parrondo, J.; Wang, Z.; Jung, M.-S.J.; Ramani, V. Reactive Oxygen Species Accelerate Degradation of Anion Exchange Membranes Based on Polyphenylene Oxide in Alkaline Environments. Phys. Chem. Chem. Phys. 2016, 18, 19705–19712. [Google Scholar] [CrossRef]
- Chempath, S.; Einsla, B.R.; Pratt, L.R.; Macomber, C.S.; Boncella, J.M.; Rau, J.A.; Pivovar, B.S. Mechanism of Tetraalkylammonium Headgroup Degradation in Alkaline Fuel Cell Membranes. J. Phys. Chem. C 2008, 112, 3179–3182. [Google Scholar] [CrossRef]
- Yang, K.; Chu, X.; Zhang, X.; Li, X.; Zheng, J.; Li, S.; Li, N.; Sherazi, T.A.; Zhang, S. The Effect of Polymer Backbones and Cation Functional Groups on Properties of Anion Exchange Membranes for Fuel Cells. J. Membr. Sci. 2020, 603, 118025. [Google Scholar] [CrossRef]
- You, W.; Hugar, K.M.; Selhorst, R.C.; Treichel, M.; Peltier, C.R.; Noonan, K.J.T.; Coates, G.W. Degradation of Organic Cations under Alkaline Conditions. J. Org. Chem. 2021, 86, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.; Tan, J.L.; Lim, L.H.; Arco, S. Density Functional Theory Study on the Degradation of Fuel Cell Anion Exchange Membranes via Removal of Vinylbenzyl Quaternary Ammonium Head Group. J. Phys. Org. Chem. 2020, 33, e4049. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, N.; Gellrich, F.; Reumert, A.K.; Kraglund, M.R.; Dong, J.; Aili, D.; Yang, J. Diamine Crosslinked Anion Exchange Membranes Based on Poly(Vinyl Benzyl Methylpyrrolidinium) for Alkaline Water Electrolysis. J. Membr. Sci. 2021, 633, 119418. [Google Scholar] [CrossRef]
- Dekel, D.R.; Amar, M.; Willdorf, S.; Kosa, M.; Dhara, S.; Diesendruck, C.E. Effect of Water on the Stability of Quaternary Ammonium Groups for Anion Exchange Membrane Fuel Cell Applications. Chem. Mater. 2017, 29, 4425–4431. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Mondal, A.N.; Dekel, D.R.; Diesendruck, C.E. Chemical Stability of Poly(Phenylene Oxide)-Based Ionomers in an Anion Exchange-Membrane Fuel Cell Environment. J. Mater. Chem. A 2018, 6, 22234–22239. [Google Scholar] [CrossRef]
- Kwasny, M.T.; Zhu, L.; Hickner, M.A.; Tew, G.N. Thermodynamics of Counterion Release Is Critical for Anion Exchange Membrane Conductivity. J. Am. Chem. Soc. 2018, 140, 7961–7969. [Google Scholar] [CrossRef]
- Pusara, S.; Srebnik, S.; Dekel, D.R. Molecular Simulation of Quaternary Ammonium Solutions at Low Hydration Levels. J. Phys. Chem. C 2018, 122, 11204–11213. [Google Scholar] [CrossRef]
- Sung, S.; Chae, J.E.; Min, K.; Kim, H.-J.; Nam, S.Y.; Kim, T.-H. Preparation of Crosslinker-Free Anion Exchange Membranes with Excellent Physicochemical and Electrochemical Properties Based on Crosslinked PPO-SEBS. J. Mater. Chem. A 2021, 9, 1062–1079. [Google Scholar] [CrossRef]
- Yun, D.; Yim, T.; Kwon, O.J.; Kim, T.-H. Click Chemistry-Induced Terminally Crosslinked Poly(Ether Sulfone) as a Highly Conductive Anion Exchange Membrane Under Humidity Condition. Macromol. Res. 2019, 27, 1050–1059. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.; Yang, Y.; Wu, C.; Fang, H.; Yang, S.; Wei, H.; Ding, Y. Dimensionally and Oxidatively Stable Anion Exchange Membranes Based on Bication Cross-Linked Poly(Meta-Terphenylene Alkylene)s. Polymer 2021, 216, 123433. [Google Scholar] [CrossRef]
- Long, H.; Kim, K.; Pivovar, B.S. Hydroxide Degradation Pathways for Substituted Trimethylammonium Cations: A DFT Study. J. Phys. Chem. C 2012, 116, 9419–9426. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Jiang, Z.; Yin, Y.; Zhang, B.; He, G.; Tong, Z.; Wu, H.; Jiao, K. Enhancing Hydroxide Conductivity and Stability of Anion Exchange Membrane by Blending Quaternary Ammonium Functionalized Polymers. Electrochim. Acta 2017, 240, 486–494. [Google Scholar] [CrossRef]
- Komkova, E.; Stamatialis, D.; Strathmann, H.; Wessling, M. Anion-Exchange Membranes Containing Diamines: Preparation and Stability in Alkaline Solution. J. Membr. Sci. 2004, 244, 25–34. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Singh, R.K.; Dekel, D.R.; Kruczała, K. Operando EPR Study of Radical Formation in Anion-Exchange Membrane Fuel Cells. ACS Catal. 2023, 13, 2744–2750. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Kostuch, A.; Dekel, D.R.; Kruczała, K. Are Radicals Formed During Anion-Exchange Membrane Fuel Cell Operation? J. Phys. Chem. Lett. 2020, 11, 7630–7636. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, W.; Xu, K.; Li, M.; Fan, M.; Wang, K. Preparation of Anion Exchange Membrane with Branch Polyethyleneimine as Main Skeleton Component. Mater. Des. 2018, 160, 698–707. [Google Scholar] [CrossRef]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-Assisted Crosslinked Quaternized Polysulfone with Reduced Graphene Oxide for Highly Stable Anion Exchange Membranes. J. Membr. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Chen, N.; Lu, C.; Wang, F.; Zhu, H. Crosslinked Poly (2,6-Dimethyl-1,4-Phenylene Oxide) Polyelectrolyte Enhanced with Poly (Styrene-b-(Ethylene-Co-Butylene)-b-Styrene) for Anion Exchange Membrane Applications. J. Membr. Sci. 2018, 564, 492–500. [Google Scholar] [CrossRef]
- Noyé, P.; Li, Q.; Pan, C.; Bjerrum, N.J. Cross-Linked Polybenzimidazole Membranes for High Temperature Proton Exchange Membrane Fuel Cells with Dichloromethyl Phosphinic Acid as a Cross-Linker. Polym. Adv. Technol. 2008, 19, 1270–1275. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Neelakandan, S.; Wang, L.; Chen, Y. Cross-Linked Polybenzimidazoles Containing Hyperbranched Cross-Linkers and Quaternary Ammoniums as High-Temperature Proton Exchange Membranes: Enhanced Stability and Conductivity. J. Membr. Sci. 2020, 593, 117435. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Liu, Z.; Wang, S.; Li, M.; Zhu, J.; Li, H.; Zhang, Y.; Lew, C.M.; Na, H. Cross-Linked Polybenzimidazole with Enhanced Stability for High Temperature Proton Exchange Membrane Fuel Cells. J. Mater. Chem. 2011, 21, 2187–2193. [Google Scholar] [CrossRef]
- Weissbach, T.; Peckham, T.J.; Holdcroft, S. CeO2, ZrO2 and YSZ as Mitigating Additives against Degradation of Proton Exchange Membranes by Free Radicals. J. Membr. Sci. 2016, 498, 94–104. [Google Scholar] [CrossRef]
- Yu, T.H.; Sha, Y.; Liu, W.-G.; Merinov, B.V.; Shirvanian, P.; Goddard, W.A. Mechanism for Degradation of Nafion in PEM Fuel Cells from Quantum Mechanics Calculations. J. Am. Chem. Soc. 2011, 133, 19857–19863. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Aricò, A.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of Direct Methanol Fuel Cell Membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef]
- Hwang, G. Crosslinking of Anion Exchange Membrane by Accelerated Electron Radiation as a Separator for the All-Vanadium Redox Flow Battery. J. Membr. Sci. 1997, 132, 55–61. [Google Scholar] [CrossRef]
- Krödel, M.; Carter, B.M.; Rall, D.; Lohaus, J.; Wessling, M.; Miller, D.J. Rational Design of Ion Exchange Membrane Material Properties Limits the Crossover of CO2 Reduction Products in Artificial Photosynthesis Devices. ACS Appl. Mater. Interfaces 2020, 12, 12030–12042. [Google Scholar] [CrossRef]
- Geise, G.M.; Hickner, M.A.; Logan, B.E. Ionic Resistance and Permselectivity Tradeoffs in Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef]
- Kamcev, J. Reformulating the permselectivity-conductivity Tradeoff Relation in ion-exchange Membranes. J. Polym. Sci. 2021, 59, 2510–2520. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Van der Bruggen, B.; Yaroslavtsev, A.B. Novel Anion Exchange Membrane with Low Ionic Resistance Based on Chloromethylated/Quaternized-grafted Polystyrene for Energy Efficient Electromembrane Processes. J. Appl. Polym. Sci. 2020, 137, 48656. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Liu, Q.L.; Broadwell, I.; Zhu, A.M.; Xiong, Y.; Tu, X.P. Anion Exchange Membranes Based on Quaternized Polystyrene-Block-Poly(Ethylene-Ran-Butylene)-Block-Polystyrene for Direct Methanol Alkaline Fuel Cells. J. Membr. Sci. 2010, 349, 237–243. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.-H.; Xu, R.-W.; Zhang, S.; Wu, Y.-X. Cross-Linked Quaternized Poly(Styrene- b -(Ethylene- Co -Butylene)- b -Styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. ACS Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef]
- Mo, Z.-H.; Yang, R.; Hong, S.; Wu, Y.-X. In-Situ Preparation of Cross-Linked Hybrid Anion Exchange Membrane of Quaternized Poly (Styrene-b-Isobutylene-b-Styrene) Covalently Bonded with Graphene. Int. J. Hydrog. Energy 2018, 43, 1790–1804. [Google Scholar] [CrossRef]
- Bance-Soualhi, R.; Choolaei, M.; Franklin, S.A.; Willson, T.R.; Lee, J.; Whelligan, D.K.; Crean, C.; Varcoe, J.R. Radiation-Grafted Anion-Exchange Membranes for Reverse Electrodialysis: A Comparison of N, N, N′, N′-Tetramethylhexane-1,6-Diamine Crosslinking (Amination Stage) and Divinylbenzene Crosslinking (Grafting Stage). J. Mater. Chem. A 2021, 9, 22025–22038. [Google Scholar] [CrossRef]
- Yang, S.; Kim, W.-S.; Choi, J.; Choi, Y.-W.; Jeong, N.; Kim, H.; Nam, J.-Y.; Jeong, H.; Kim, Y.H. Fabrication of Photocured Anion-Exchange Membranes Using Water-Soluble Siloxane Resins as Cross-Linking Agents and Their Application in Reverse Electrodialysis. J. Membr. Sci. 2019, 573, 544–553. [Google Scholar] [CrossRef]
- Toshikatsu, S.; Kazuyoshi, T.; Takanori, Y. Permselectivity between Two Anions in Anion Exchange Membranes Crosslinked with Various Diamines in Electrodialysis. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1475–1482. [Google Scholar]
- Li, C.; Wang, G.; Yu, D.; Sheng, F.; Shehzad, M.A.; He, T.; Xu, T.; Ren, X.; Cao, M.; Wu, B.; et al. Cross-Linked Anion Exchange Membranes with Hydrophobic Side-Chains for Anion Separation. J. Membr. Sci. 2019, 581, 150–157. [Google Scholar] [CrossRef]
- Zeng, L.; He, Q.; Liao, Y.; Kuang, S.; Wang, J.; Ding, W.; Liao, Q.; Wei, Z. Anion Exchange Membrane Based on Interpenetrating Polymer Network with Ultrahigh Ion Conductivity and Excellent Stability for Alkaline Fuel Cell. Research 2020, 2020, 4794706. [Google Scholar] [CrossRef]
- Min, K.; Chae, J.E.; Lee, Y.; Kim, H.-J.; Kim, T.-H. Crosslinked Poly(m-Terphenyl N-Methyl Piperidinium)-SEBS Membranes with Aryl-Ether Free and Kinked Backbones as Highly Stable and Conductive Anion Exchange Membranes. J. Membr. Sci. 2022, 653, 120487. [Google Scholar] [CrossRef]
- Han, J.; Lin, B.; Peng, H.; Zhu, Y.; Ren, Z.; Xiao, L.; Zhuang, L. Aggregated and Ionic Cross-Linked Anion Exchange Membrane with Enhanced Hydroxide Conductivity and Stability. J. Power Sources 2020, 459, 227838. [Google Scholar] [CrossRef]
- Zhang, F.; He, X.; Cheng, C.; Huang, S.; Duan, Y.; Zhu, C.; Guo, Y.; Wang, K.; Chen, D. Bis-Imidazolium Functionalized Self-Crosslinking Block Polynorbornene Anion Exchange Membrane. Int. J. Hydrog. Energy 2020, 45, 13090–13100. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Wu, H.; Deng, X.; Yang, Q.; Liu, P.; Hong, Y.; Zhu, A.; Liu, Q. Dual Hydrophobic Modifications toward Anion Exchange Membranes with Both High Ion Conductivity and Excellent Dimensional Stability. J. Membr. Sci. 2020, 595, 117521. [Google Scholar] [CrossRef]
- Xu, F.; Qiu, K.; Lin, B.; Ren, Y.; Li, J.; Ding, J.; Hickner, M.A. Enhanced Performance of Poly(Olefin)-Based Anion Exchange Membranes Cross-Linked by Triallylmethyl Ammonium Iodine and Divinylbenzene. J. Membr. Sci. 2021, 637, 119629. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; Wang, J.; He, G. Ether Spaced N-Spirocyclic Quaternary Ammonium Functionalized Crosslinked Polysulfone for High Alkaline Stable Anion Exchange Membranes. J. Membr. Sci. 2020, 598, 117650. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S. High-Performance Alkaline Ionomer for Alkaline Exchange Membrane Fuel Cells. Electrochem. Commun. 2013, 34, 278–281. [Google Scholar] [CrossRef]
- Qaisrani, N.A.; Ma, L.; Hussain, M.; Liu, J.; Li, L.; Zhou, R.; Jia, Y.; Zhang, F.; He, G. Hydrophilic Flexible Ether Containing, Cross-Linked Anion-Exchange Membrane Quaternized with DABCO. ACS Appl. Mater. Interfaces 2020, 12, 3510–3521. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, Y.; France-Lanord, A.; Wang, Y.; Wu, Y.-C.M.; Lin, S.; Li, Y.; Grossman, J.C.; Swager, T.M. Ionic Highways from Covalent Assembly in Highly Conducting and Stable Anion Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2019, 141, 18152–18159. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Lin, C.; Lin, Z.; Li, L.; Soyekwo, F.; Zhu, A.; Liu, Q. Multi-Cation Crosslinked Anion Exchange Membranes from Microporous Tröger’s Base Copolymers. J. Mater. Chem. A 2018, 6, 13302–13311. [Google Scholar] [CrossRef]
- Wakerley, D.; Lamaison, S.; Wicks, J.; Clemens, A.; Feaster, J.; Corral, D.; Jaffer, S.A.; Sarkar, A.; Fontecave, M.; Duoss, E.B.; et al. Gas Diffusion Electrodes, Reactor Designs and Key Metrics of Low-Temperature CO2 Electrolysers. Nat. Energy 2022, 7, 130–143. [Google Scholar] [CrossRef]
- Ashdot, A.; Kattan, M.; Kitayev, A.; Tal-Gutelmacher, E.; Amel, A.; Page, M. Design Strategies for Alkaline Exchange Membrane–Electrode Assemblies: Optimization for Fuel Cells and Electrolyzers. Membranes 2021, 11, 686. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; Seifitokaldani, A.; Gabardo, C.M.; García de Arquer, F.P.; Kiani, A.; Edwards, J.P.; De Luna, P.; Bushuyev, O.S.; et al. CO2 Electroreduction to Ethylene via Hydroxide-Mediated Copper Catalysis at an Abrupt Interface. Science 2018, 360, 783–787. [Google Scholar] [CrossRef]
- Cha, M.S.; Jeong, H.Y.; Shin, H.Y.; Hong, S.H.; Kim, T.-H.; Oh, S.-G.; Lee, J.Y.; Hong, Y.T. Crosslinked Anion Exchange Membranes with Primary Diamine-Based Crosslinkers for Vanadium Redox Flow Battery Application. J. Power Sources 2017, 363, 78–86. [Google Scholar] [CrossRef]
- Seo, S.-J.; Kim, B.-C.; Sung, K.-W.; Shim, J.; Jeon, J.-D.; Shin, K.-H.; Shin, S.-H.; Yun, S.-H.; Lee, J.-Y.; Moon, S.-H. Electrochemical Properties of Pore-Filled Anion Exchange Membranes and Their Ionic Transport Phenomena for Vanadium Redox Flow Battery Applications. J. Membr. Sci. 2013, 428, 17–23. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, P.; Singh, K.; Shahi, V.K. Improved Performance of Vanadium Redox Flow Battery with Tuneable Alkyl Spacer Based Cross-Linked Anion Exchange Membranes. J. Power Sources 2022, 520, 230856. [Google Scholar] [CrossRef]
- Cha, M.S.; Lee, J.Y.; Kim, T.-H.; Jeong, H.Y.; Shin, H.Y.; Oh, S.-G.; Hong, Y.T. Preparation and Characterization of Crosslinked Anion Exchange Membrane (AEM) Materials with Poly(Phenylene Ether)-Based Short Hydrophilic Block for Use in Electrochemical Applications. J. Membr. Sci. 2017, 530, 73–83. [Google Scholar] [CrossRef]
- Yao, D.; Jao, T.-C.; Zhang, W.; Xu, L.; Xing, L.; Ma, Q.; Xu, Q.; Li, H.; Pasupathi, S.; Su, H. In-Situ Diagnosis on Performance Degradation of High Temperature Polymer Electrolyte Membrane Fuel Cell by Examining Its Electrochemical Properties under Operation. Int. J. Hydrog. Energy 2018, 43, 21006–21016. [Google Scholar] [CrossRef]
- Hyun, J.; Yang, S.H.; Doo, G.; Choi, S.; Lee, D.-H.; Lee, D.W.; Kwen, J.; Jo, W.; Shin, S.-H.; Lee, J.Y.; et al. Degradation Study for the Membrane Electrode Assembly of Anion Exchange Membrane Fuel Cells at a Single-Cell Level. J. Mater. Chem. A 2021, 9, 18546–18556. [Google Scholar] [CrossRef]
- Lee, S.H.; Lin, J.C.; Farmand, M.; Landers, A.T.; Feaster, J.T.; Avilés Acosta, J.E.; Beeman, J.W.; Ye, Y.; Yano, J.; Mehta, A.; et al. Oxidation State and Surface Reconstruction of Cu under CO 2 Reduction Conditions from In Situ X-Ray Characterization. J. Am. Chem. Soc. 2021, 143, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, S. An Investigation of Active Sites for Electrochemical CO2 Reduction Reactions: From In Situ Characterization to Rational Design. Adv. Sci. 2021, 8, 2003579. [Google Scholar] [CrossRef]
- Yang, K.; Li, X.; Guo, J.; Zheng, J.; Li, S.; Zhang, S.; Cao, X.; Sherazi, T.A.; Liu, X. Preparation and Properties of Anion Exchange Membranes with Exceptional Alkaline Stable Polymer Backbone and Cation Groups. J. Membr. Sci. 2020, 596, 117720. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic Alkaline Stability Study of Polymer Backbones for Anion Exchange Membrane Applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Yang, Z.; Ran, J.; Wu, B.; Wu, L.; Xu, T. Stability Challenge in Anion Exchange Membrane for Fuel Cells. Curr. Opin. Chem. Eng. 2016, 12, 22–30. [Google Scholar] [CrossRef]
- Nuñez, S.A.; Hickner, M.A. Quantitative 1 H NMR Analysis of Chemical Stabilities in Anion-Exchange Membranes. ACS Macro Lett. 2013, 2, 49–52. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly Quaternized Polystyrene Ionomers for High Performance Anion Exchange Membrane Water Electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Xie, X.; Zhao, C.X.; Zhang, L.; Shao, Q.; Guo, X.; Wujcik, E.K.; Guo, Z. Hydroxide Ions Transportation in Polynorbornene Anion Exchange Membrane. Polymer 2018, 138, 363–368. [Google Scholar] [CrossRef]
- Espiritu, R.; Mamlouk, M.; Scott, K. Study on the Effect of the Degree of Grafting on the Performance of Polyethylene-Based Anion Exchange Membrane for Fuel Cell Application. Int. J. Hydrog. Energy 2016, 41, 1120–1133. [Google Scholar] [CrossRef]
- Biancolli, A.L.G.; Bsoul-Haj, S.; Douglin, J.C.; Barbosa, A.S.; de Sousa, R.R.; Rodrigues, O.; Lanfredi, A.J.C.; Dekel, D.R.; Santiago, E.I. High-Performance Radiation Grafted Anion-Exchange Membranes for Fuel Cell Applications: Effects of Irradiation Conditions on ETFE-Based Membranes Properties. J. Membr. Sci. 2022, 641, 119879. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(Aryl Piperidinium) Membranes and Ionomers for Hydroxide Exchange Membrane Fuel Cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Liu, M.; Hu, X.; Hu, B.; Liu, L.; Li, N. Soluble Poly(Aryl Piperidinium) with Extended Aromatic Segments as Anion Exchange Membranes for Alkaline Fuel Cells and Water Electrolysis. J. Membr. Sci. 2022, 642, 119966. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Liu, D.; Sun, F. A Highly Durable Long Side-Chain Polybenzimidazole Anion Exchange Membrane for AEMFC. J. Membr. Sci. 2018, 546, 15–21. [Google Scholar] [CrossRef]
- Disabb-Miller, M.L.; Zha, Y.; DeCarlo, A.J.; Pawar, M.; Tew, G.N.; Hickner, M.A. Water Uptake and Ion Mobility in Cross-Linked Bis(Terpyridine)Ruthenium-Based Anion Exchange Membranes. Macromolecules 2013, 46, 9279–9287. [Google Scholar] [CrossRef]
- Gu, S.; Cai, R.; Yan, Y. Self-Crosslinking for Dimensionally Stable and Solvent-Resistant Quaternary Phosphonium Based Hydroxide Exchange Membranes. Chem. Commun. 2011, 47, 2856. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.d.A.; Jang, H.; Sutradhar, S.C.; Ha, J.; Yoo, J.; Lee, C.; Lee, S.; Kim, W. Novel Hydroxide Conducting Sulfonium-Based Anion Exchange Membrane for Alkaline Fuel Cell Applications. Int. J. Hydrog. Energy 2016, 41, 10458–10465. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Dai, J.; Wang, H.; Jin, B.; Bai, R. A Facile Strategy for the Synthesis of Guanidinium-Functionalized Polymer as Alkaline Anion Exchange Membrane with Improved Alkaline Stability. J. Membr. Sci. 2014, 453, 52–60. [Google Scholar] [CrossRef]
- Salma, U.; Shalahin, N. A Mini-Review on Alkaline Stability of Imidazolium Cations and Imidazolium-Based Anion Exchange Membranes. Results Mater. 2023, 17, 100366. [Google Scholar] [CrossRef]
- Long, H.; Pivovar, B. Hydroxide Degradation Pathways for Imidazolium Cations: A DFT Study. J. Phys. Chem. C 2014, 118, 9880–9888. [Google Scholar] [CrossRef]
- Price, S.C.; Williams, K.S.; Beyer, F.L. Relationships between Structure and Alkaline Stability of Imidazolium Cations for Fuel Cell Membrane Applications. ACS Macro Lett. 2014, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, S.-H.; Yim, S.-D.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Performance of Solid Alkaline Fuel Cells Employing Anion-Exchange Membranes. J. Power Sources 2008, 178, 620–626. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, Q.; Gao, X.L.; Zhu, Z.Y.; Sun, Q.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Novel Crosslinked Aliphatic Anion Exchange Membranes with Pendant Pentafluorophenyl Groups. Electrochim. Acta 2019, 321, 134634. [Google Scholar] [CrossRef]
- Hossain, M.d.M.; Wu, L.; Liang, X.; Yang, Z.; Hou, J.; Xu, T. Anion Exchange Membrane Crosslinked in the Easiest Way Stands out for Fuel Cells. J. Power Sources 2018, 390, 234–241. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Ertem, S.P.; Maes, A.M.; Seifert, S.; Herring, A.M.; Coughlin, E.B. Thermally Cross-Linked Anion Exchange Membranes from Solvent Processable Isoprene Containing Ionomers. Macromolecules 2015, 48, 655–662. [Google Scholar] [CrossRef]
- Wu, L.; Pan, Q.; Varcoe, J.R.; Zhou, D.; Ran, J.; Yang, Z.; Xu, T. Thermal Crosslinking of an Alkaline Anion Exchange Membrane Bearing Unsaturated Side Chains. J. Membr. Sci. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Qiu, L.; Yan, F. Alkaline Imidazolium- and Quaternary Ammonium-Functionalized Anion Exchange Membranes for Alkaline Fuel Cell Applications. J. Mater. Chem. 2012, 22, 1040–1045. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-Imidazolium-Based Anion-Exchange Membranes for Alkaline Fuel Cells. J. Power Sources 2012, 217, 329–335. [Google Scholar] [CrossRef]
- Lin, B.; Qiu, L.; Lu, J.; Yan, F. Cross-Linked Alkaline Ionic Liquid-Based Polymer Electrolytes for Alkaline Fuel Cell Applications. Chem. Mater. 2010, 22, 6718–6725. [Google Scholar] [CrossRef]
- Seo, J.; Kushner, D.I.; Hickner, M.A. 3D Printing of Micropatterned Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2016, 8, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts †. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Clark, T.J.; Robertson, N.J.; Kostalik IV, H.A.; Lobkovsky, E.B.; Mutolo, P.F.; Abruña, H.D.; Coates, G.W. A Ring-Opening Metathesis Polymerization Route to Alkaline Anion Exchange Membranes: Development of Hydroxide-Conducting Thin Films from an Ammonium-Functionalized Monomer. J. Am. Chem. Soc. 2009, 131, 12888–12889. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, L.; Gao, J.; Zhao, Y.; Wang, S.; Ramani, V.; Zhang, Z.; Xie, X. Study on Tunable Crosslinking Anion Exchange Membranes Fabrication and Degradation Mechanism. Int. J. Hydrog. Energy 2016, 41, 16264–16274. [Google Scholar] [CrossRef]
- Robertson, N.J.; Kostalik, H.A.; Clark, T.J.; Mutolo, P.F.; Abruña, H.D.; Coates, G.W. Tunable High Performance Cross-Linked Alkaline Anion Exchange Membranes for Fuel Cell Applications. J. Am. Chem. Soc. 2010, 132, 3400–3404. [Google Scholar] [CrossRef]
- You, W.; Hugar, K.M.; Coates, G.W. Synthesis of Alkaline Anion Exchange Membranes with Chemically Stable Imidazolium Cations: Unexpected Cross-Linked Macrocycles from Ring-Fused ROMP Monomers. Macromolecules 2018, 51, 3212–3218. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Hickner, M. Cross-Linked Comb-Shaped Anion Exchange Membranes with High Base Stability. Chem. Commun. 2014, 50, 4092. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Y.; Zhang, M.; Wang, Y.; Tang, H.; Li, N. Olefin Metathesis-Crosslinked, Bulky Imidazolium-Based Anion Exchange Membranes with Excellent Base Stability and Mechanical Properties. J. Membr. Sci. 2020, 598, 117793. [Google Scholar] [CrossRef]
- Wang, L.; Hickner, M.A. Low-Temperature Crosslinking of Anion Exchange Membranes. Polym. Chem. 2014, 5, 2928. [Google Scholar] [CrossRef]
- You, W.; Padgett, E.; MacMillan, S.N.; Muller, D.A.; Coates, G.W. Highly Conductive and Chemically Stable Alkaline Anion Exchange Membranes via ROMP of Trans -Cyclooctene Derivatives. Proc. Natl. Acad. Sci. USA 2019, 116, 9729–9734. [Google Scholar] [CrossRef]
- Price, S.C.; Ren, X.; Savage, A.M.; Beyer, F.L. Synthesis and Characterization of Anion-Exchange Membranes Based on Hydrogenated Poly(Norbornene). Polym. Chem. 2017, 8, 5708–5717. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Felkin, H.; Morris, G.E. Cationic Iridium Diolefin Complexes as Alkene Hydrogenation Catalysts and the Isolation of Some Related Hydrido Complexes. J. Organomet. Chem. 1977, 141, 205–215. [Google Scholar] [CrossRef]
- Ge, Q.; Ran, J.; Miao, J.; Yang, Z.; Xu, T. Click Chemistry Finds Its Way in Constructing an Ionic Highway in Anion-Exchange Membrane. ACS Appl. Mater. Interfaces 2015, 7, 28545–28553. [Google Scholar] [CrossRef]
- Liu, L.; He, S.; Zhang, S.; Zhang, M.; Guiver, M.D.; Li, N. 1,2,3-Triazolium-Based Poly(2,6-Dimethyl Phenylene Oxide) Copolymers as Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2016, 8, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mondal, R.; Guha, S.; Chatterjee, U.; Jewrajka, S.K. Crosslinked Terpolymer Anion Exchange Membranes for Selective Ion Separation and Acid Recovery. J. Membr. Sci. 2020, 612, 118459. [Google Scholar] [CrossRef]
- Dhean, W.; Bakulev, V.A. Chemistry of 1,2,3-Triazolium Salts. In Chemistry of 1,2,3-Triazoles; Springer: Berlin/Heidelberg, Germany, 2015; Volume 40, pp. 167–210. [Google Scholar]
- Kinumoto, T.; Inaba, M.; Nakayama, Y.; Ogata, K.; Umebayashi, R.; Tasaka, A.; Iriyama, Y.; Abe, T.; Ogumi, Z. Durability of Perfluorinated Ionomer Membrane against Hydrogen Peroxide. J. Power Sources 2006, 158, 1222–1228. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC “Click Chemistry” Goes Heterogeneous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
- Xue, J.; Liu, L.; Liao, J.; Shen, Y.; Li, N. Semi-Interpenetrating Polymer Networks by Azide–Alkyne Cycloaddition as Novel Anion Exchange Membranes. J. Mater. Chem. A 2018, 6, 11317–11326. [Google Scholar] [CrossRef]
- Jia, J.; Baker, G.L. Cross-linking of Poly[1-(Trimethylsilyl)-1-propyne] Membranes Using Bis(Aryl Azides). J. Polym. Sci. Part B: Polym. Phys. 1998, 36, 959–968. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Liao, J.; Wang, L.; Li, N. Self-Crosslinking of Comb-Shaped Polystyrene Anion Exchange Membranes for Alkaline Fuel Cell Application. J. Membr. Sci. 2017, 536, 133–140. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Li, N.; Pan, J.; Lin, B.; Hickner, M.A. Crosslinking of Comb-Shaped Polymer Anion Exchange Membranes via Thiol–Ene Click Chemistry. Polym. Chem. 2016, 7, 2464–2475. [Google Scholar] [CrossRef]
- Tibbits, A.C.; Mumper, L.E.; Kloxin, C.J.; Yan, Y.S. A Single-Step Monomeric Photo-Polymerization and Crosslinking via Thiol-Ene Reaction for Hydroxide Exchange Membrane Fabrication. J. Electrochem. Soc. 2015, 162, F1206–F1211. [Google Scholar] [CrossRef]
- Tibbits, A.C.; Yan, Y.S.; Kloxin, C.J. Covalent Incorporation of Ionic Liquid into Ion-Conductive Networks via Thiol-Ene Photopolymerization. Macromol. Rapid Commun. 2017, 38, 1700113. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, C.; Zhang, G.; Zhang, Y.; Wang, J.; Ma, W.; Liu, Z.; Na, H. Novel Self-Crosslinked Poly(Aryl Ether Sulfone) for High Alkaline Stable and Fuel Resistant Alkaline Anion Exchange Membranes. Chem. Commun. 2011, 47, 8943–8945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ünlü, M.; Anestis-Richard, I.; Kohl, P.A. Crosslinked, Epoxy-Based Anion Conductive Membranes for Alkaline Membrane Fuel Cells. J. Membr. Sci. 2010, 350, 286–292. [Google Scholar] [CrossRef]
- Merle, G.; Hosseiny, S.S.; Wessling, M.; Nijmeijer, K. New Cross-Linked PVA Based Polymer Electrolyte Membranes for Alkaline Fuel Cells. J. Membr. Sci. 2012, 409–410, 191–199. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Tian, H.; Wang, S.; Zhang, B.; Cao, Y.; He, G.; Li, Z.; Wu, H. Preparing Alkaline Anion Exchange Membrane with Enhanced Hydroxide Conductivity via Blending Imidazolium-Functionalized and Sulfonated Poly(Ether Ether Ketone). J. Power Sources 2015, 288, 384–392. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, S.; Wan, R.; Yang, Y.; He, R. Functionalized Graphene Oxide Cross-Linked Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Anion Exchange Membranes with Superior Ionic Conductivity. J. Power Sources 2022, 517, 230720. [Google Scholar] [CrossRef]
- Arunkumar, I.; Kim, A.R.; Lee, S.H.; Yoo, D.J. Enhanced Fumion Nanocomposite Membranes Embedded with Graphene Oxide as a Promising Anion Exchange Membrane for Fuel Cell Application. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Ponce-González, J.; Ouachan, I.; Varcoe, J.R.; Whelligan, D.K. Radiation-Induced Grafting of a Butyl-Spacer Styrenic Monomer onto ETFE: The Synthesis of the Most Alkali Stable Radiation-Grafted Anion-Exchange Membrane to Date. J. Mater. Chem. A 2018, 6, 823–827. [Google Scholar] [CrossRef]
- Fang, J.; Yang, Y.; Lu, X.; Ye, M.; Li, W.; Zhang, Y. Cross-Linked, ETFE-Derived and Radiation Grafted Membranes for Anion Exchange Membrane Fuel Cell Applications. Int. J. Hydrog. Energy 2012, 37, 594–602. [Google Scholar] [CrossRef]
- Espiritu, R.; Golding, B.T.; Scott, K.; Mamlouk, M. Degradation of Radiation Grafted Hydroxide Anion Exchange Membrane Immersed in Neutral PH: Removal of Vinylbenzyl Trimethylammonium Hydroxide Due to Oxidation. J. Mater. Chem. A 2017, 5, 1248–1267. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemens, A.L.; Jayathilake, B.S.; Karnes, J.J.; Schwartz, J.J.; Baker, S.E.; Duoss, E.B.; Oakdale, J.S. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers 2023, 15, 1534. https://doi.org/10.3390/polym15061534
Clemens AL, Jayathilake BS, Karnes JJ, Schwartz JJ, Baker SE, Duoss EB, Oakdale JS. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers. 2023; 15(6):1534. https://doi.org/10.3390/polym15061534
Chicago/Turabian StyleClemens, Auston L., Buddhinie S. Jayathilake, John J. Karnes, Johanna J. Schwartz, Sarah E. Baker, Eric B. Duoss, and James S. Oakdale. 2023. "Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships" Polymers 15, no. 6: 1534. https://doi.org/10.3390/polym15061534
APA StyleClemens, A. L., Jayathilake, B. S., Karnes, J. J., Schwartz, J. J., Baker, S. E., Duoss, E. B., & Oakdale, J. S. (2023). Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers, 15(6), 1534. https://doi.org/10.3390/polym15061534





