Exploring the Feasibility of Cell-Free Synthesis as a Platform for Polyhydroxyalkanoate (PHA) Production: Opportunities and Challenges
Abstract
:1. Introduction
2. Overview of PHAs
3. Current Challenges in Synthesizing PHAs In Vivo
3.1. Reducing High Costs Is Difficult
3.2. Customizing PHA Properties Is Challenging
3.3. The Complexity of Cellular Regulation
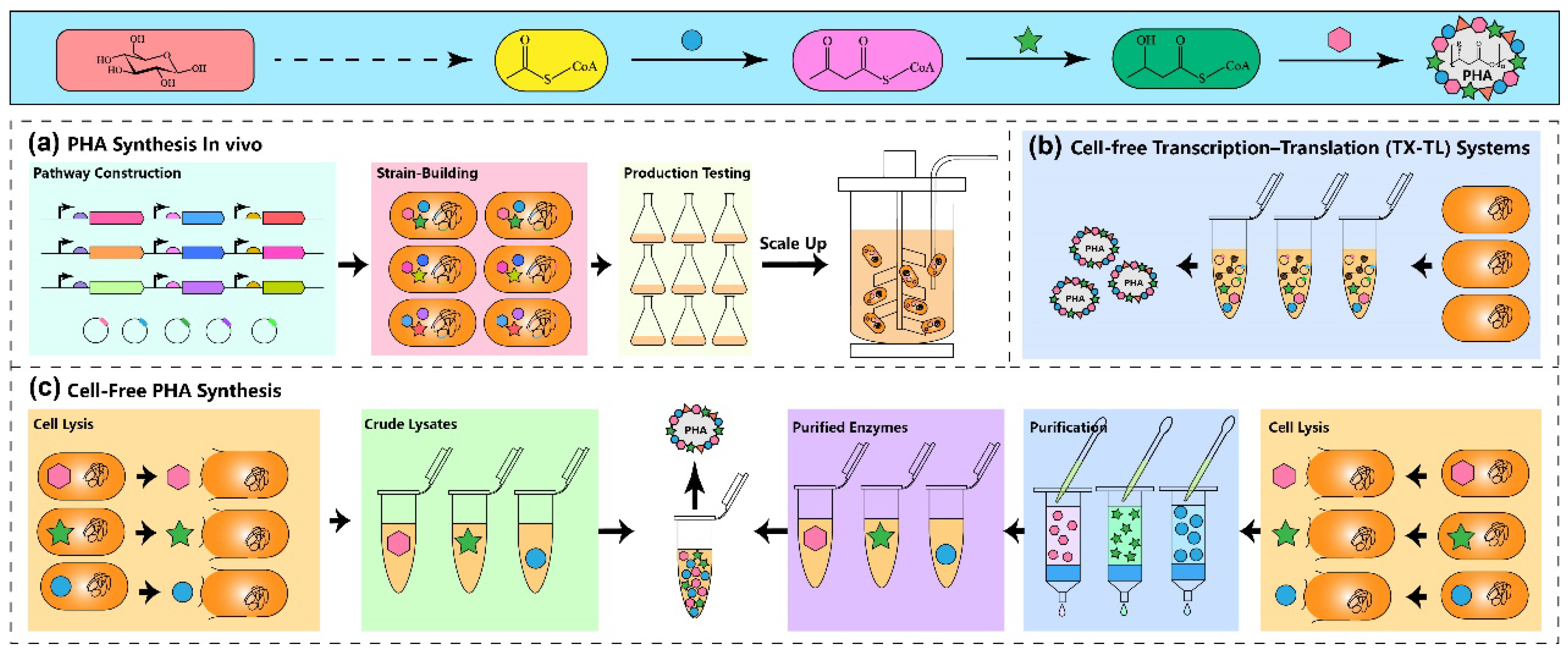
4. In Vitro PHA Synthesis
4.1. Exploration of PHA Synthetase
4.2. In Vitro Multienzyme Systems for PHA Synthesis
5. Advantages and Prospects for In Vitro PHA Synthesis
5.1. Advantages of In Vitro PHA Synthesis
5.1.1. The Limitation of Cell Volume Is Eliminated in Cell-Free Catalysis
5.1.2. Real-Time Monitoring of Product Concentration Can Be Achieved
5.1.3. Significant Reduction in Product Separation Costs Can Be Achieved
5.1.4. Compatible with New Pathways and Heterologous Enzymes
5.1.5. The Monomer Composition Is Better Controlled
5.2. Challenges with In Vitro PHA Synthesis
5.2.1. Insufficient Diversity in Monomer Composition
5.2.2. Insufficient Enzyme Activity and Stability
5.2.3. Dependence on Energy and Cofactors
5.3. Feasible Solutions (What to Work On)
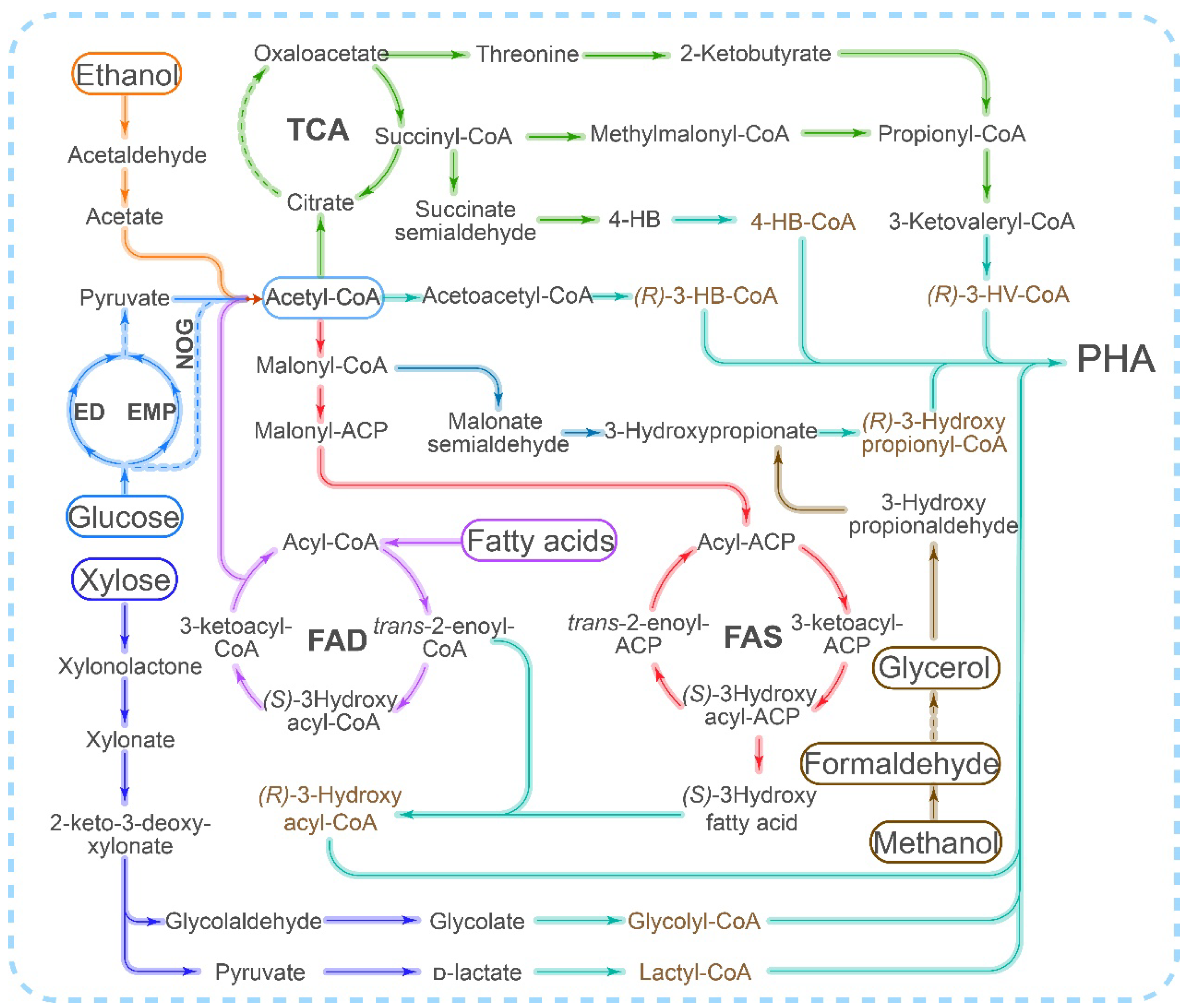
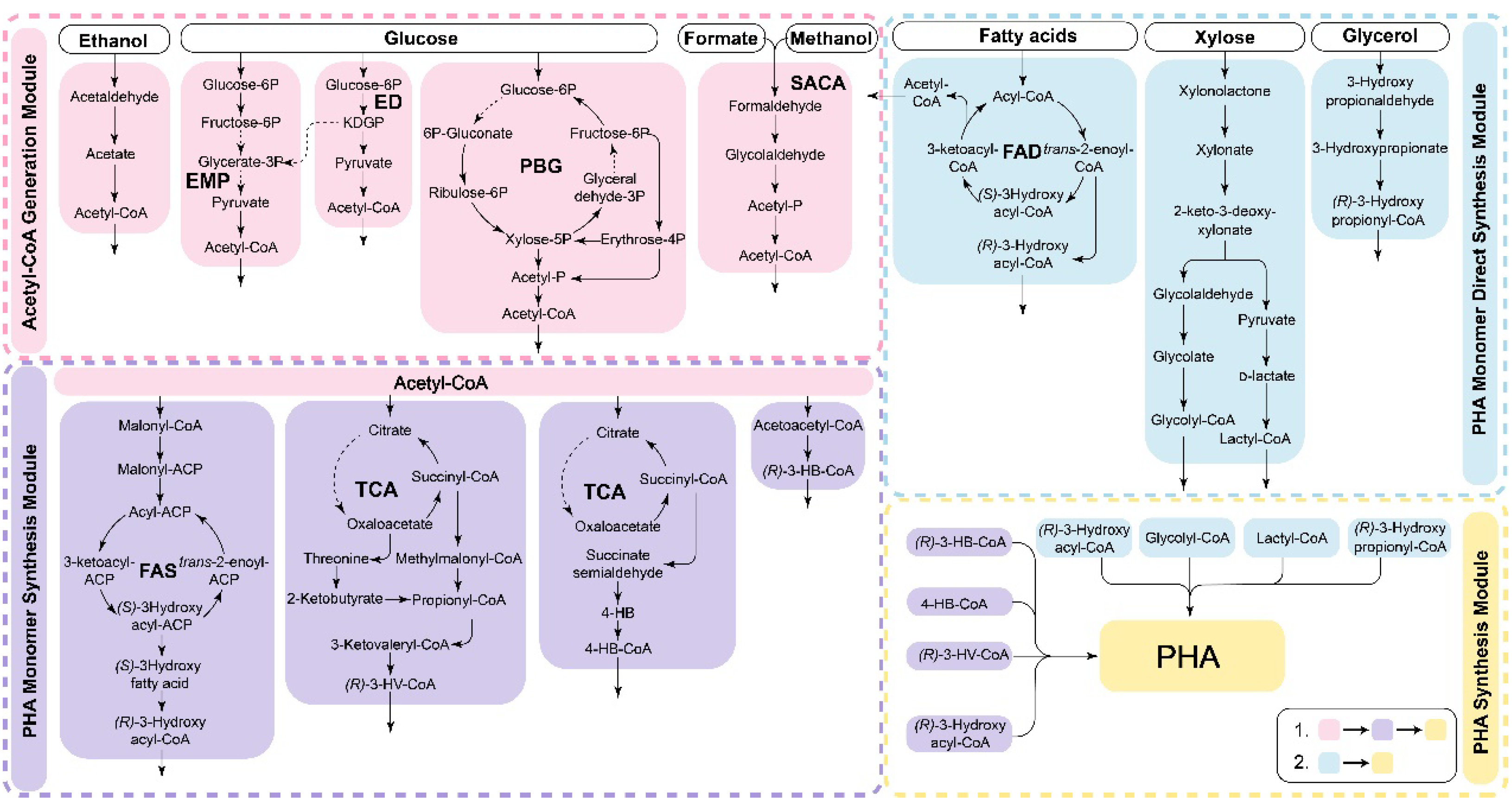
5.3.1. Utilization of Unconventional Substrates
5.3.2. Low-Cost Acquisition and Stability Modification of Enzymes
5.3.3. Cofactor Regeneration
5.3.4. Modeling In Silico Provides Quantitative Guidance
5.3.5. Exploring the Modular PHA Synthesis
6. Prospect
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bernard, M. Industrial potential of polyhydroxyalkanoate bioplastic: A brief review. USURJ Univ. Sask. Undergrad. Res. J. 2014, 1, 1–14. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, J.K.; Mallick, N.; Singh, A.K. Commercialization of bacterial cell factories for the sustainable production of polyhydroxyalkanoate thermoplastics: Progress and prospects. Recent Pat. Biotechnol. 2015, 9, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Srivastava, J.K.; Singh, A.K. Biodegradable polyhydroxyalkanoate thermoplastics substituting xenobiotic plastics: A way forward for sustainable environment. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer: Singapore, 2016; pp. 317–346. [Google Scholar]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Biological system as reactor for production of biodegradable thermoplastics, polyhydroxyalkanoates. In In Industrial Biotechnology; Apple Academic Press: Waretown, NJ, USA, 2017; pp. pp. 281–323. [Google Scholar]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364, fnx189. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, L.; Srivastava, J.K.; Mallick, N.; Ansari, M.I. Microbially originated polyhydroxyalkanoate (PHA) biopolymers: An insight into the molecular mechanism and biogenesis of PHA granules. In Sustainable Biotechnology-Enzymatic Resources of Renewable Energy; Singh, O.V., Chandel, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 355–398. [Google Scholar]
- Jiang, Y.; Yang, F.; Hassan Kazmi, S.S.U.; Zhao, Y.; Chen, M.; Wang, J. A review of microplastic pollution in seawater, sediments and organisms of the Chinese coastal and marginal seas. Chemosphere 2022, 286, 130104. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering native and synthetic pathways in Pseudomonas putida for the production of tailored polyhydroxyalkanoates. Biotechnol. J. 2021, 16, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef]
- Yuan, W.; Jia, Y.; Tian, J.; Snell, K.D.; Muh, U.; Sinskey, A.J.; Lambalot, R.H.; Walsh, C.T.; Stubbe, J. Class I and III polyhydroxyalkanoate synthases from Ralstonia eutropha and Allochromatium vinosum: Characterization and substrate specificity studies. Arch. Biochem. Biophys. 2001, 394, 87–98. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhou, X.-R.; Liu, Q.; Chen, G.-Q. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 89, 1497–1507. [Google Scholar] [CrossRef]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.-Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Afreen, R.; Tyagi, S.; Singh, G.P.; Singh, M. Challenges and perspectives of polyhydroxyalkanoate production from microalgae/cyanobacteria and bacteria as microbial factories: An assessment of hybrid biological system. Front. Bioeng. Biotechnol. 2021, 9, 624885. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Factories 2020, 19, 86. [Google Scholar] [CrossRef]
- Dudley, Q.M.; Karim, A.S.; Jewett, M.C. Cell-free metabolic engineering: Biomanufacturing beyond the cell. Biotechnol. J. 2015, 10, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Takors, R. Scale-up of microbial processes: Impacts, tools and open questions. J. Biotechnol. 2012, 160, 3–9. [Google Scholar] [CrossRef]
- Kwok, R. Five hard truths for synthetic biology. Nature 2010, 463, 288–290. [Google Scholar] [CrossRef]
- Yadav, V.G.; De Mey, M.; Giaw Lim, C.; Kumaran Ajikumar, P.; Stephanopoulos, G. The future of metabolic engineering and synthetic biology: Towards a systematic practice. Metab. Eng. 2012, 14, 233–241. [Google Scholar] [CrossRef]
- Li, D.; Lv, L.; Chen, J.-C.; Chen, G.-Q. Controlling microbial PHB synthesis via CRISPRi. Appl. Microbiol. Biotechnol. 2017, 101, 5861–5867. [Google Scholar] [CrossRef]
- Ullah, M.W.; Khattak, W.A.; Ul-Islam, M.; Khan, S.; Park, J.K. Metabolic engineering of synthetic cell-free systems: Strategies and applications. Biochem. Eng. J. 2016, 105, 391–405. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassan, M.A.; Phang, L.-Y.; Shirai, Y.; Man, H.C.; Ariffin, H. Intracellular polyhydroxyalkanoates recovery by cleaner halogen-free methods towards zero emission in the palm oil mill. J. Clean. Prod. 2012, 37, 353–360. [Google Scholar] [CrossRef]
- Bujara, M.; Panke, S. Engineering in complex systems. Curr. Opin. Biotechnol. 2010, 21, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.R. Transforming biochemical engineering with cell-free biology. AIChE J. 2012, 58, 5–13. [Google Scholar] [CrossRef]
- Jendrossek, D. Polyhydroxyalkanoate granules are complex subcellular organelles (Carbonosomes). J. Bacteriol. 2009, 191, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A.; Obruca, S.; Zinn, M. Polyhydroxyalkanoates (PHA): Microbial synthesis of natural polyesters. In Microbial Production of High-Value Products; Rehm, B.H.A., Wibowo, D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 185–236. [Google Scholar]
- Lemoigne, M. Études sur l’autolyse microbienne origine de l’acide β-oxybutyrique formé par autolyse. Ann. Inst. Pasteur 1927, 41, 148. [Google Scholar]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi. PHA-based bioplastic: A potential alternative to address microplastic pollution. Water Air Soil Pollut. 2022, 234, 21. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Mravec, F.; Obruca, S.; Krzyzanek, V.; Sedlacek, P.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Nebesarova, J. Accumulation of PHA granules in Cupriavidus necator as seen by confocal fluorescence microscopy. FEMS Microbiol. Lett. 2016, 363, fnw094. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, M.; Cai, C.; Chen, S.; Jin, M. Microbial polyhydroxyalkanoate production from lignin by Pseudomonas putida NX-1. Bioresour. Technol. 2021, 319, 124210. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.; Dong, J.; Ling, J.; Wang, W.; Wang, H.; Zhang, Z.; Yu, X. Simultaneous Improvements of pseudomonas cell growth and polyhydroxyalkanoate production from a pignin derivative for lignin-consolidated bioprocessing. Appl. Environ. Microbiol. 2018, 84, e01469-18. [Google Scholar] [CrossRef]
- Gao, J.; Vo, M.T.; Ramsay, J.A.; Ramsay, B.A. Overproduction of MCL-PHA with high 3-hydroxydecanoate Content. Biotechnol. Bioeng. 2018, 115, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chung, A.; Chen, G.-Q. Synthesis of Medium-Chain-Length polyhydroxyalkanoate homopolymers, random copolymers, and block copolymers by an engineered strain of Pseudomonas entomophila. Adv. Healthc. Mater. 2017, 6, 1601017. [Google Scholar] [CrossRef] [PubMed]
- Pederson, E.N.; McChalicher, C.W.J.; Srienc, F. Bacterial synthesis of PHA block copolymers. Biomacromolecules 2006, 7, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Wu, Q.; Wang, Y.; Chen, G.-Q. Synthetic biology and genome-editing tools for improving PHA metabolic engineering. Trends Biotechnol. 2020, 38, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.C.; Domingos, J.M.B.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates production by a mixed photosynthetic consortium of bacteria and algae. Bioresour. Technol. 2013, 132, 146–153. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef]
- Tu, W.; Zhang, D.; Wang, H. Polyhydroxyalkanoates (PHA) production from fermented thermal-hydrolyzed sludge by mixed microbial cultures: The link between phosphorus and PHA yields. Waste Manag. 2019, 96, 149–157. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, X.-R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018, 36, 856–870. [Google Scholar] [CrossRef]
- Mahler, N.; Tschirren, S.; Pflügl, S.; Herwig, C. Optimized bioreactor setup for scale-up studies of extreme halophilic cultures. Biochem. Eng. J. 2018, 130, 39–46. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Y.; Yuan, Q.; Liu, Q.; Li, Y.; Wang, Z.; Ma, H.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb. Cell Factories 2015, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.-C.; Shen, R.; Yao, H.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Engineering the diversity of polyesters. Curr. Opin. Biotechnol. 2014, 29, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Chen, G.-Q. Thirty years of metabolic engineering for biosynthesis of Polyhydroxyalkanoates. Chin. J. Biotechnol. 2021, 37, 1794–1811. [Google Scholar] [CrossRef]
- Chen, G.-Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Factories 2012, 11, 111. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Kleerebezem, R.; Cuellar, M.C.; Ramirez, A. Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: Techno-economic analysis and ex-ante environmental assessment. Bioresour. Technol. 2015, 185, 368–377. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of polyhydroxyalkanoates from single and mixed microbial cultures: A review. Front. Bioeng. Biotechnol. 2021, 9, 624021. [Google Scholar] [CrossRef]
- Jiang, X.-R.; Wang, H.; Shen, R.; Chen, G.-Q. Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab. Eng. 2015, 29, 227–237. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Chen, G.-Q. Engineering the growth pattern and cell morphology for enhanced PHB production by Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 9907–9916. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.; Roy, I.; Summers, D.; Sivaniah, E. In vitro production of polyhydroxyalkanoates: Achievements and applications. J. Chem. Technol. Biotechnol. 2010, 85, 760–767. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Song, H.M.; Joo, J.C.; Lim, S.H.; Lim, H.J.; Lee, S.; Park, S.J. Production of polyhydroxyalkanoates containing monomers conferring amorphous and elastomeric properties from renewable resources: Current status and future perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Maestro, B.; Sanz, J.M. Polyhydroxyalkanoate-associated phasins as phylogenetically heterogeneous, multipurpose proteins. Microb. Biotechnol. 2017, 10, 1323–1337. [Google Scholar] [CrossRef]
- Lee, T.-R.; Lin, J.-S.; Wang, S.-S.; Shaw, G.-C. PhaQ, A new class of poly-hydroxybutyrate (PHB)-responsive repressor, regulates phaQ and phaP (Phasin) expression in Bacillus megaterium through interaction with PHB. J. Bacteriol. 2004, 186, 3015–3021. [Google Scholar] [CrossRef]
- Pötter, M.; Müller, H.; Steinbüchel, A. Influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 2005, 151, 825–833. [Google Scholar] [CrossRef]
- Galán, B.; Dinjaski, N.; Maestro, B.; de Eugenio, L.I.; Escapa, I.F.; Sanz, J.M.; García, J.L.; Prieto, M.A. Nucleoid-associated PhaF phasin drives intracellular location and segregation of polyhydroxyalkanoate granules in Pseudomonas putida KT2442. Mol. Microbiol. 2011, 79, 402–418. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Wahl, A.; Jendrossek, D. Identification of a multifunctional protein, PhaM, that determines number, surface to volume ratio, subcellular localization and distribution to daughter cells of poly(3-hydroxybutyrate), PHB, granules in Ralstonia eutropha H16. Mol. Microbiol. 2011, 82, 936–951. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Chen, G.-Q.; Xiang, H.; Han, J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef]
- De Eugenio, L.I.; Escapa, I.F.; Morales, V.; Dinjaski, N.; Galán, B.; García, J.L.; Prieto, M.A. The turnover of medium-chain-length polyhydroxyalkanoates in Pseudomonas putida KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ. Microbiol. 2010, 12, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mitra, R.; Xiang, H.; Han, J. Deletion of the pps-like gene activates the cryptic phaC genes in Haloferax mediterranei. Appl. Microbiol. Biotechnol. 2020, 104, 9759–9771. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Qiao, G.-Q.; Shuai, B.-W.; Olavarria, K.; Yin, J.; Xiang, R.-J.; Song, K.-N.; Shen, Y.-H.; Guo, Y.; Chen, G.-Q. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA). Metab. Eng. 2018, 49, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sheng, J.; Feng, X. Mini-review: In vitro metabolic engineering for biomanufacturing of high-value products. Comput. Struct. Biotechnol. J. 2017, 15, 161–167. [Google Scholar] [CrossRef]
- Fukui, T.; Yoshimoto, A.; Matsumoto, M.; Hosokawa, S.; Saito, T.; Nishikawa, H.; Tomita, K. Enzymatic synthesis of poly-β-hydroxybutyrate inZoogloea ramigera. Arch. Microbiol. 1976, 110, 149–156. [Google Scholar] [CrossRef]
- Qi, Q.; Steinbüchel, A.; Rehm, B.H.A. In vitro synthesis of poly(3-hydroxydecanoate): Purification and enzymatic characterization of type II polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2000, 54, 37–43. [Google Scholar] [CrossRef]
- Jossek, R.; Steinbüchel, A. In vitro synthesis of poly(3-hydroxybutyric acid) by using an enzymatic coenzyme A recycling system. FEMS Microbiol. Lett. 1998, 168, 319–324. [Google Scholar] [CrossRef]
- Han, X.; Satoh, Y.; Satoh, T.; Matsumoto, K.i.; Kakuchi, T.; Taguchi, S.; Dairi, T.; Munekata, M.; Tajima, K. Chemo-enzymatic synthesis of polyhydroxyalkanoate (PHA) incorporating 2-hydroxybutyrate by wild-type class I PHA synthase from Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2011, 92, 509–517. [Google Scholar] [CrossRef]
- Han, X.; Satoh, Y.; Tajima, K.; Matsushima, T.; Munekata, M. Chemo-enzymatic synthesis of polyhydroxyalkanoate by an improved two-phase reaction system (TPRS). J. Biosci. Bioeng. 2009, 108, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Minato, M.; Kikkawa, Y.; Abe, H.; Tsuge, T. In vitro synthesis of polyhydroxyalkanoate catalyzed by class II and III PHA synthases: A useful technique for surface coatings of a hydrophobic support with PHA. J. Chem. Technol. Biotechnol. 2010, 85, 779–782. [Google Scholar] [CrossRef]
- Tajima, K.; Han, X.; Satoh, Y.; Ishii, A.; Araki, Y.; Munekata, M.; Taguchi, S. In vitro synthesis of polyhydroxyalkanoate (PHA) incorporating lactate (LA) with a block sequence by using a newly engineered thermostable PHA synthase from Pseudomonas sp. SG4502 with acquired LA-polymerizing activity. Appl. Microbiol. Biotechnol. 2012, 94, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.G.; Choi, J.; Rha, C.; Stubbe, J.; Sinskey, A.J. In vitro analysis of the chain termination reaction in the synthesis of poly-(R)-β-hydroxybutyrate by the class III Synthase from Allochromatium vinosum. Biomacromolecules 2005, 6, 2113–2119. [Google Scholar] [CrossRef]
- Tomizawa, S.; Sato, S.; Lan, J.C.-W.; Nakamura, Y.; Abe, H.; Tsuge, T. In vitro evidence of chain transfer to tetraethylene glycols in enzymatic polymerization of polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 2013, 97, 4821–4829. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Satoh, Y.; Nakazawa, K.; Tannai, H.; Erata, T.; Munekata, M.; Kamachi, M.; Lenz, R.W. Chemoenzymatic synthesis of poly(3-hydroxybutyrate) in a water-organic solvent two-phase system. Macromolecules 2004, 37, 4544–4546. [Google Scholar] [CrossRef]
- Gerngross, T.U.; Martin, D.P. Enzyme-catalyzed synthesis of poly[(R)-(-)-3-hydroxybutyrate]: Formation of macroscopic granules in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 6279–6283. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, S.; Yoshioka, M.; Ushimaru, K.; Tsuge, T. Preparative synthesis of poly[(R)-3-hydroxybutyrate] monomer for enzymatic cell-free polymerization. Polym. J. 2012, 44, 982–985. [Google Scholar] [CrossRef]
- Satoh, Y.; Tajima, K.; Tannai, H.; Munekata, M. Enzyme-catalyzed poly(3-hydroxybutyrate) synthesis from acetate with CoA recycling and NADPH regeneration in vitro. J. Biosci. Bioeng. 2003, 95, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C.; Lopez-Gallego, F. A roadmap for biocatalysis—Functional and spatial orchestration of enzyme cascades. Microb. Biotechnol. 2016, 9, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Okano, K.; Honda, K. Modules for in vitro metabolic engineering: Pathway assembly for bio-based production of value-added chemicals. Synth. Syst. Biotechnol. 2017, 2, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wilding, K.M.; Schinn, S.-M.; Long, E.A.; Bundy, B.C. The emerging impact of cell-free chemical biosynthesis. Curr. Opin. Biotechnol. 2018, 53, 115–121. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Zhang, Y.H.P. Cell-free biosystems for biomanufacturing. In Future Trends in Biotechnology; Zhong, J.-J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 89–119. [Google Scholar]
- Opgenorth, P.H.; Korman, T.P.; Bowie, J.U. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat. Chem. Biol. 2016, 12, 393–395. [Google Scholar] [CrossRef]
- Li, F.; Wei, X.; Zhang, L.; Liu, C.; You, C.; Zhu, Z. Installing a green engine to drive an enzyme cascade: A light-powered in vitro biosystem for poly(3-hydroxybutyrate) synthesis. Angew. Chem. Int. Ed. 2022, 61, e202111054. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Liu, Y.; Chu, H.; Bai, J.; Cheng, J.; Zhao, H.; Fu, S.; Liu, H.; Fu, Y.; et al. Hybrid synthesis of polyhydroxybutyrate bioplastics from carbon dioxide. Green Chem. 2023, 25, 3247–3255. [Google Scholar] [CrossRef]
- Grubbe, W.S.; Rasor, B.J.; Krüger, A.; Jewett, M.C.; Karim, A.S. Cell-free styrene biosynthesis at high titers. bioRxiv 2020. [Google Scholar] [CrossRef]
- Karim, A.S.; Dudley, Q.M.; Juminaga, A.; Yuan, Y.; Crowe, S.A.; Heggestad, J.T.; Garg, S.; Abdalla, T.; Grubbe, W.S.; Rasor, B.J.; et al. In vitro prototyping and rapid optimization of biosynthetic enzymes for cell design. Nat. Chem. Biol. 2020, 16, 912–919. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Q.; Ma, H.; Liu, Y.; Cao, G.; Zhang, X.; Zheng, P.; Sun, J.; Zhang, D.; Jiang, W.; et al. Determination of key enzymes for threonine synthesis through in vitro metabolic pathway analysis. Microb. Cell Factories 2015, 14, 86. [Google Scholar] [CrossRef]
- van den Berg, J.; Boersma, A.J.; Poolman, B. Microorganisms maintain crowding homeostasis. Nat. Rev. Microbiol. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- Tan, D.; Wu, Q.; Chen, J.-C.; Chen, G.-Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metab. Eng. 2014, 26, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, P. Polyhydroxyalkanoate production in biofermentor monitored through biosensor application. Int. J. Biosens. Bioelectron. 2018, 4, 235–240. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Q.; Zhang, J.; Zhang, M.; Wang, C.; Qu, R.; Wang, Y.; Yang, G.; Wang, J. A ratiometric fluorescent biosensor reveals dynamic regulation of long-chain fatty acyl-coa esters metabolism. Angew. Chem. Int. Ed. 2021, 60, 13996–14004. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yuan, Q.; Yang, X.; Liu, P.; Cheng, Y.; Luo, J.; Liu, H.; Yao, Y.; Sun, H.; Cai, T.; et al. Non-natural aldol reactions enable the design and construction of novel one-carbon assimilation pathways in vitro. Front. Microbiol. 2021, 12, 677596. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, I.W.; Lin, T.-S.; Liao, J.C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 2013, 502, 693–697. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA recovery from biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Zainab-L, I.; Pyary, S.; Sudesh, K. A novel biological recovery approach for PHA employing selective digestion of bacterial biomass in animals. Appl. Microbiol. Biotechnol. 2018, 102, 2117–2127. [Google Scholar] [CrossRef]
- Hodgman, C.E.; Jewett, M.C. Cell-free synthetic biology: Thinking outside the cell. Metab. Eng. 2012, 14, 261–269. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Yang, Y.; Wang, S.; Wang, Q.; Wang, X.; Yan, Z.; Cheng, J.; Liu, C.; Yang, X.; et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design. Nat. Commun. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Liu, H.; Arbing, M.A.; Bowie, J.U. Expanding the use of ethanol as a feedstock for cell-free synthetic biochemistry by implementing acetyl-CoA and ATP generating pathways. Sci. Rep. 2022, 12, 7700. [Google Scholar] [CrossRef]
- Tajima, K.; Han, X.; Hashimoto, Y.; Satoh, Y.; Satoh, T.; Taguchi, S. In vitro synthesis of polyhydroxyalkanoates using thermostable acetyl-CoA synthetase, CoA transferase, and PHA synthase from thermotorelant bacteria. J. Biosci. Bioeng. 2016, 122, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Hosoe, Y.; Guex, M.; Tomoi, M.; Tomita, H.; Zinn, M.; Matsumoto, K. Directed evolution of sequence-regulating polyhydroxyalkanoate synthase to synthesize a Medium-Chain-Length-Short-Chain-Length (MCL-SCL) block copolymer. Biomacromolecules 2022, 23, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Hori, C.; Oishi, K.; Matsumoto, K.; Taguchi, S.; Ooi, T. Site-directed saturation mutagenesis of polyhydroxylalkanoate synthase for efficient microbial production of poly[(R)-2-hydroxybutyrate]. J. Biosci. Bioeng. 2018, 125, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.C.; Tripathi, B.P. Nature inspired multienzyme immobilization: Strategies and concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef]
- Zhao, H.; van der Donk, W.A. Regeneration of cofactors for use in biocatalysis. Curr. Opin. Biotechnol. 2003, 14, 583–589. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Cai, T.; Sun, H.; Qiao, J.; Zhu, L.; Zhang, F.; Zhang, J.; Tang, Z.; Wei, X.; Yang, J.; Yuan, Q.; et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide. Science 2021, 373, 1523–1527. [Google Scholar] [CrossRef]
- Wu, R.; Li, F.; Cui, X.; Li, Z.; Ma, C.; Jiang, H.; Zhang, L.; Zhang, Y.P.J.; Zhao, T.; Zhang, Y.; et al. Enzymatic electrosynthesis of glycine from CO2 and NH3. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218387. [Google Scholar] [CrossRef]
- Welch, P.; Scopes, R.K. Studies on cell-free metabolism: Ethanol production by a yeast glycolytic system reconstituted from purified enzymes. J. Biotechnol. 1985, 2, 257–273. [Google Scholar] [CrossRef]
- Opgenorth, P.H.; Korman, T.P.; Bowie, J.U. A synthetic biochemistry molecular purge valve module that maintains redox balance. Nat. Commun. 2014, 5, 4113. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, Q.; Luo, H.; Li, F.; Mao, Y.; Zhao, X.; Du, J.; Li, P.; Ju, X.; Zheng, Y.; et al. Systematic design and in vitro validation of novel one-carbon assimilation pathways. Metab. Eng. 2019, 56, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, M.; You, C.; Li, Z.; Zhang, Y.-H.P. ATP-free biosynthesis of a high-energy phosphate metabolite fructose 1,6-diphosphate by in vitro metabolic engineering. Metab. Eng. 2017, 42, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Hollmann, F. A survey of synthetic nicotinamide cofactors in enzymatic processes. Appl. Microbiol. Biotechnol. 2016, 100, 4773–4778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tam, T.K.; Zhang, Y.H.P. Cell-free biosystems in the production of electricity and bioenergy. In Fundamentals and Application of New Bioproduction Systems; Zeng, A.-P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 125–152. [Google Scholar]
- Andexer, J.N.; Richter, M. Emerging enzymes for ATP regeneration in biocatalytic processes. ChemBioChem 2015, 16, 380–386. [Google Scholar] [CrossRef]
- Bachosz, K.; Zdarta, J.; Bilal, M.; Meyer, A.S.; Jesionowski, T. Enzymatic cofactor regeneration systems: A new perspective on efficiency assessment. Sci. Total Environ. 2023, 868, 161630. [Google Scholar] [CrossRef]
- Han, J.; Hou, J.; Zhang, F.; Ai, G.; Li, M.; Cai, S.; Liu, H.; Wang, L.; Wang, Z.; Zhang, S.; et al. Multiple propionyl coenzyme a-supplying pathways for production of the bioplastic poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferax mediterranei. Appl. Environ. Microbiol. 2013, 79, 2922–2931. [Google Scholar] [CrossRef]
- Schwander, T.; Schada von Borzyskowski, L.; Burgener, S.; Cortina, N.S.; Erb, T.J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 2016, 354, 900–904. [Google Scholar] [CrossRef]
- Elçin, A.E.; Elçin, Y.M. Polycation-coated polyanion microspheres of urease for urea hydrolysis. Artif. Cells Blood Substit. Biotechnol. 2000, 28, 95–111. [Google Scholar] [CrossRef]
- Fu, G.; Yue, J.; Li, D.; Li, Y.; Lee, S.Y.; Zhang, D. An operator-based expression toolkit for Bacillus subtilis enables fine-tuning of gene expression and biosynthetic pathway regulation. Proc. Natl. Acad. Sci. USA 2022, 119, e2119980119. [Google Scholar] [CrossRef]
- Luo, B.; Jin, M.M.; Li, X.; Makunga, N.P.; Hu, X. Yeast surface display for in vitro biosynthetic pathway reconstruction. ACS Synth. Biol. 2021, 10, 2938–2946. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, R.; Xiao, Y.; Wei, Y.; Zhang, H.; Sun, X.; Wang, S.; Cheng, Y.; Wang, X.; Tong, S.; et al. Biodegradation of highly crystallized poly(ethylene terephthalate) through cell surface codisplay of bacterial PETase and hydrophobin. Nat. Commun. 2022, 13, 7138. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zhou, S.; Wang, P. Programmable biofilm-cellulose hybrid platform for specific clustering of microbial catalysts with optimized cellular synergy. Chem. Commun. 2022, 58, 8222–8225. [Google Scholar] [CrossRef] [PubMed]
- van der Donk, W.A.; Zhao, H. Recent developments in pyridine nucleotide regeneration. Curr. Opin. Biotechnol. 2003, 14, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Nocek, B.; Kochinyan, S.; Proudfoot, M.; Brown, G.; Evdokimova, E.; Osipiuk, J.; Edwards, A.M.; Savchenko, A.; Joachimiak, A.; Yakunin, A.F. Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17730–17735. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.S.; Jewett, M.C. A cell-free framework for rapid biosynthetic pathway prototyping and enzyme discovery. Metab. Eng. 2016, 36, 116–126. [Google Scholar] [CrossRef]
- Petroll, K.; Kopp, D.; Care, A.; Bergquist, P.L.; Sunna, A. Tools and strategies for constructing cell-free enzyme pathways. Biotechnol. Adv. 2019, 37, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Novak, M.; Braunegg, G.; Horvat, P. Mathematical modelling as a tool for optimized pha production. Chem. Biochem. Eng. Q. 2015, 29, 183–220. [Google Scholar] [CrossRef]
- Rollin, J.A.; Martin del Campo, J.; Myung, S.; Sun, F.; You, C.; Bakovic, A.; Castro, R.; Chandrayan, S.K.; Wu, C.-H.; Adams, M.W.W.; et al. High-yield hydrogen production from biomass by in vitro metabolic engineering: Mixed sugars coutilization and kinetic modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 4964–4969. [Google Scholar] [CrossRef]
- Horvath, N.; Vilkhovoy, M.; Wayman, J.A.; Calhoun, K.; Swartz, J.; Varner, J.D. Toward a genome scale sequence specific dynamic model of cell-free protein synthesis in Escherichia coli. Metab. Eng. Commun. 2020, 10, e00113. [Google Scholar] [CrossRef]
- Domenzain, I.; Sánchez, B.; Anton, M.; Kerkhoven, E.J.; Millán-Oropeza, A.; Henry, C.; Siewers, V.; Morrissey, J.P.; Sonnenschein, N.; Nielsen, J. Reconstruction of a catalogue of genome-scale metabolic models with enzymatic constraints using GECKO 2.0. Nat. Commun. 2022, 13, 3766. [Google Scholar] [CrossRef]
- Yang, X.; Mao, Z.; Zhao, X.; Wang, R.; Zhang, P.; Cai, J.; Xue, C.; Ma, H. Integrating thermodynamic and enzymatic constraints into genome-scale metabolic models. Metab. Eng. 2021, 67, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Salvy, P.; Fengos, G.; Ataman, M.; Pathier, T.; Soh, K.C.; Hatzimanikatis, V. pyTFA and matTFA: A python package and a matlab toolbox for thermodynamics-based flux analysis. Bioinformatics 2019, 35, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Nogales, J.; Mueller, J.; Gudmundsson, S.; Canalejo, F.J.; Duque, E.; Monk, J.; Feist, A.M.; Ramos, J.L.; Niu, W.; Palsson, B.O. High-quality genome-scale metabolic modelling of Pseudomonas putida highlights its broad metabolic capabilities. Environ. Microbiol. 2020, 22, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Borzou, P.; Ghaisari, J.; Izadi, I.; Eshraghi, Y.; Gheisari, Y. A novel strategy for dynamic modeling of genome-scale interaction networks. Bioinformatics 2023, 39, btad079. [Google Scholar] [CrossRef]
- Goldberg, A.P.; Szigeti, B.; Chew, Y.H.; Sekar, J.A.; Roth, Y.D.; Karr, J.R. Emerging whole-cell modeling principles and methods. Curr. Opin. Biotechnol. 2018, 51, 97–102. [Google Scholar] [CrossRef]
- Zampieri, G.; Vijayakumar, S.; Yaneske, E.; Angione, C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput. Biol. 2019, 15, e1007084. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, B.S. Valorization of polyhydroxyalkanoates production process by co-synthesis of value-added products. Bioresour. Technol. 2018, 269, 544–556. [Google Scholar] [CrossRef]
- Xu, M.; Qin, J.; Rao, Z.; You, H.; Zhang, X.; Yang, T.; Wang, X.; Xu, Z. Effect of polyhydroxybutyrate (PHB) storage on l-arginine production in recombinant Corynebacterium crenatum using coenzyme regulation. Microb. Cell Factories 2016, 15, 15. [Google Scholar] [CrossRef]
| Substrates | Enzymes | Source of Enzymes | Products | Titer (mg/mL) | Conversion Rate (%) | Average M (g/mol) | Ref. |
|---|---|---|---|---|---|---|---|
| (R,S)-3HD-CoA | PhaC1, PhaC2 | P. aeruginosa | P(3HD) | 0.068 | 12 | 9.8 × 106 | [72] |
| (R)-LA and (R)-3HB, acetyl-CoA | PhaC1SG, PhaC2SG LPE, CoA-transferase, and acetyl-CoA-synthetase | Pseudomonas sp. SG4502 and Pseudomonas sp. 61-3 | P(LA-co-3HB) | 0.77 | / | 2.7 × 105 | [77] |
| D-(-)-3-hydroxybutyric acid | acetyl-CoA-synthetase, PCT, and PhaECcv | C. vinosum and C. propionicum | PHB | 2.75 | 73 | 1–2 × 106 | [73] |
| (R)-3-Hydroxyoctanoyl-CoA | PhaC1PP and PhaECAv | P. putida and Allochromatium vinosum | PHB | / | / | / | [76] |
| HB−NAC and HB−CoA | PhaECAv | A. vinosum | PHB | / | / | 5.84–7.97 × 104 | [78] |
| β-butyrolactone |
FadB1x, PhaCDa, and PhaJAc | A. caviae, D. acidovorans, and P. putida KT2440 | PHB | 1.1575 | 27 | 1.2 × 105 | [79] |
| thiophenyl (R)-3-hydroxybutyrate | PhaC | R. eutropha | PHB | 0.36 | 47 | 1.6 × 106 | [80] |
| (R)-2HB, AcETG, (R)-3HB | PHA synthase I, PHA synthase II, and PHA synthase IV PCT (propionate CoA transferase) | R. eutropha, Pseudomonas sp. SG4502, Synechocystis sp. PCC6803, Bacillus sp. INT005, and C. propionicum JCM1430 | P(2HB), PHB | 1.2 | / | 1.00 × 105 | [74,75] |
| Glucose, NADPH, acetoacetyl-CoA | PHA synthase, acetoacetyl-CoA reductase, and glucose dehydrogenase | A. eutrophus | PHB | / | / | 10 × 106 | [81] |
| crotonic anhydride | PhaJ and PhaC | A. caviae FA440 and R. eutropha H16 | PHB | / | 58 | 6.4 × 106 | [82] |
| Acetate, CoA | ACS, PhaA, PhaB, and PhaC | E. coli JM109 and R. eutropha H I6 | PHB | 1.12 | 45.5 | 6.64 × 106 | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Yang, X.; Shi, J.; Xiao, C.; Zhang, Y. Exploring the Feasibility of Cell-Free Synthesis as a Platform for Polyhydroxyalkanoate (PHA) Production: Opportunities and Challenges. Polymers 2023, 15, 2333. https://doi.org/10.3390/polym15102333
Dong H, Yang X, Shi J, Xiao C, Zhang Y. Exploring the Feasibility of Cell-Free Synthesis as a Platform for Polyhydroxyalkanoate (PHA) Production: Opportunities and Challenges. Polymers. 2023; 15(10):2333. https://doi.org/10.3390/polym15102333
Chicago/Turabian StyleDong, Huaming, Xue Yang, Jingjing Shi, Chunqiao Xiao, and Yanfei Zhang. 2023. "Exploring the Feasibility of Cell-Free Synthesis as a Platform for Polyhydroxyalkanoate (PHA) Production: Opportunities and Challenges" Polymers 15, no. 10: 2333. https://doi.org/10.3390/polym15102333
APA StyleDong, H., Yang, X., Shi, J., Xiao, C., & Zhang, Y. (2023). Exploring the Feasibility of Cell-Free Synthesis as a Platform for Polyhydroxyalkanoate (PHA) Production: Opportunities and Challenges. Polymers, 15(10), 2333. https://doi.org/10.3390/polym15102333







