The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus
Abstract
:1. Introduction
2. Material and Methods
2.1. Alga
2.2. Green Synthesis of Zinc Oxide Nanoparticles
2.3. Alginate Extraction and Characterizations
2.4. Synthesis of Fu-Alg-ZnO-NCMs
2.5. Characterizations of Fu/ZnO-NPs, and Fu/Alg-ZnO-NCMs
2.5.1. Fourier Transform Infrared (FT-IR)
2.5.2. Transmission Electron Microscopy (TEM)
2.5.3. X-ray Powder Diffraction (XRD)
2.5.4. Zeta Potential Analysis
2.5.5. Energy-Dispersive Spectroscopy (EDS)
2.6. Antibacterial Activities
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Fu/ZnO-NPs and Fu-Alg-ZnO-NCMs Bio-Fabricated via F. vesiculosus
3.2. XRD of Fu/ZnO-NPs, and Fu-Alg-ZnO-NCMs
3.3. EDS Analysis of Fu/ZnO-NPs and Fu-Alg-ZnO-NCMs
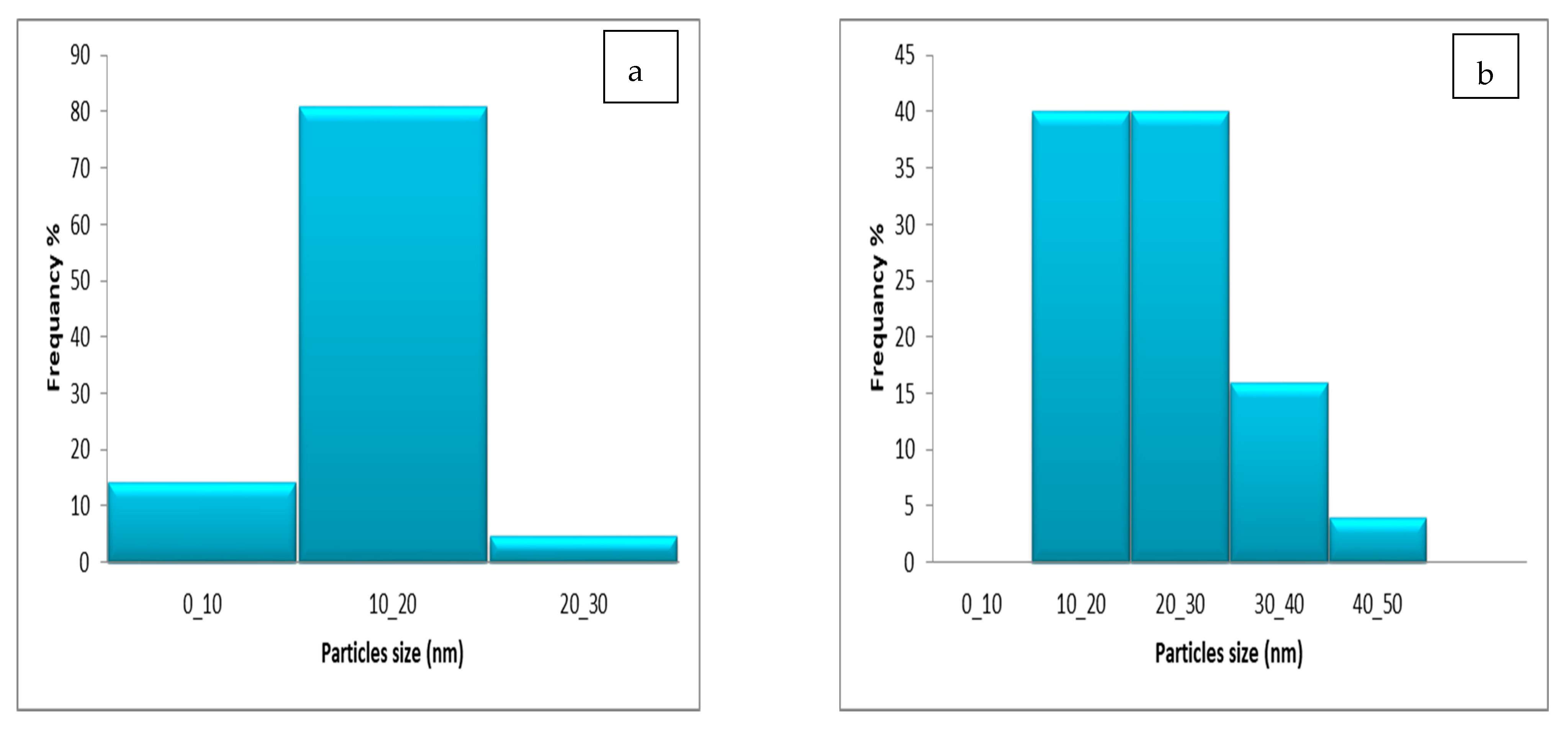
3.4. TEM Image
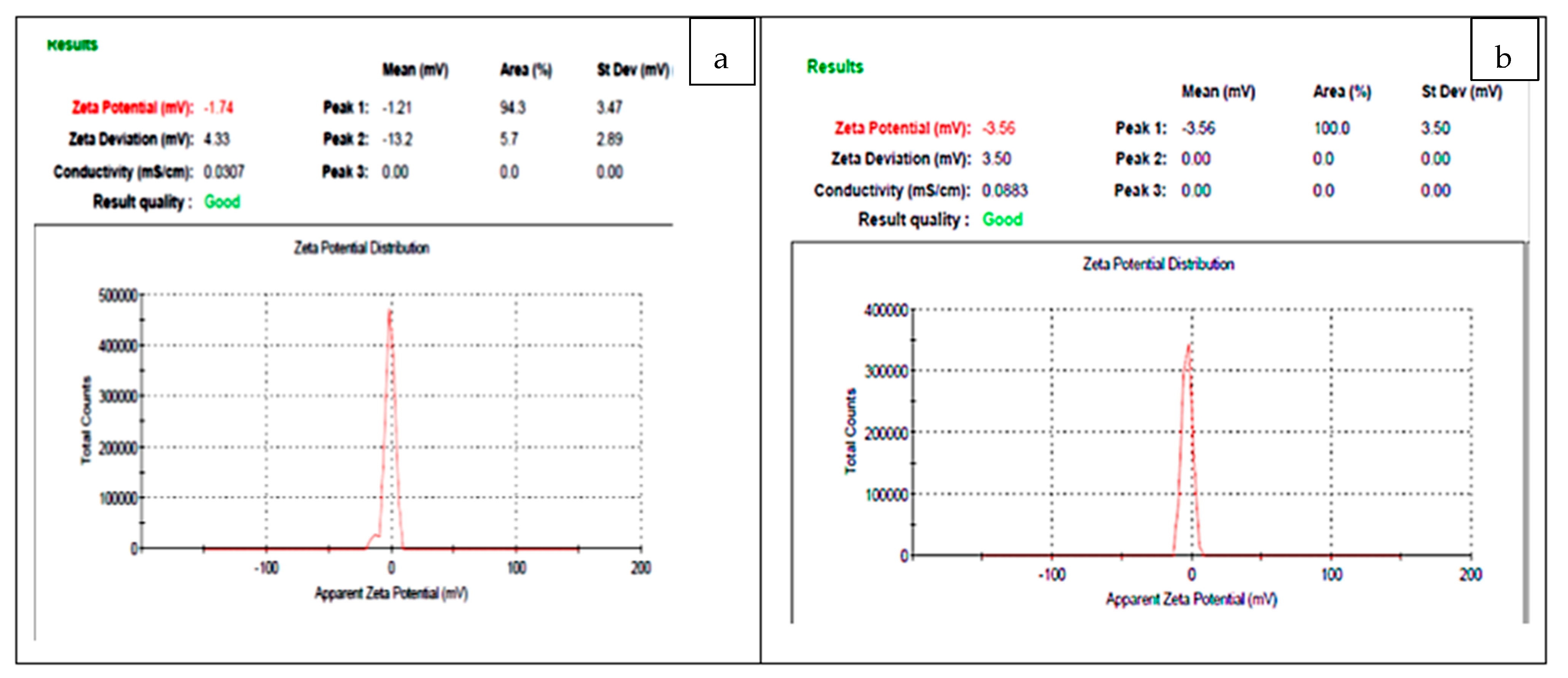
3.5. Zeta Potential Analysis
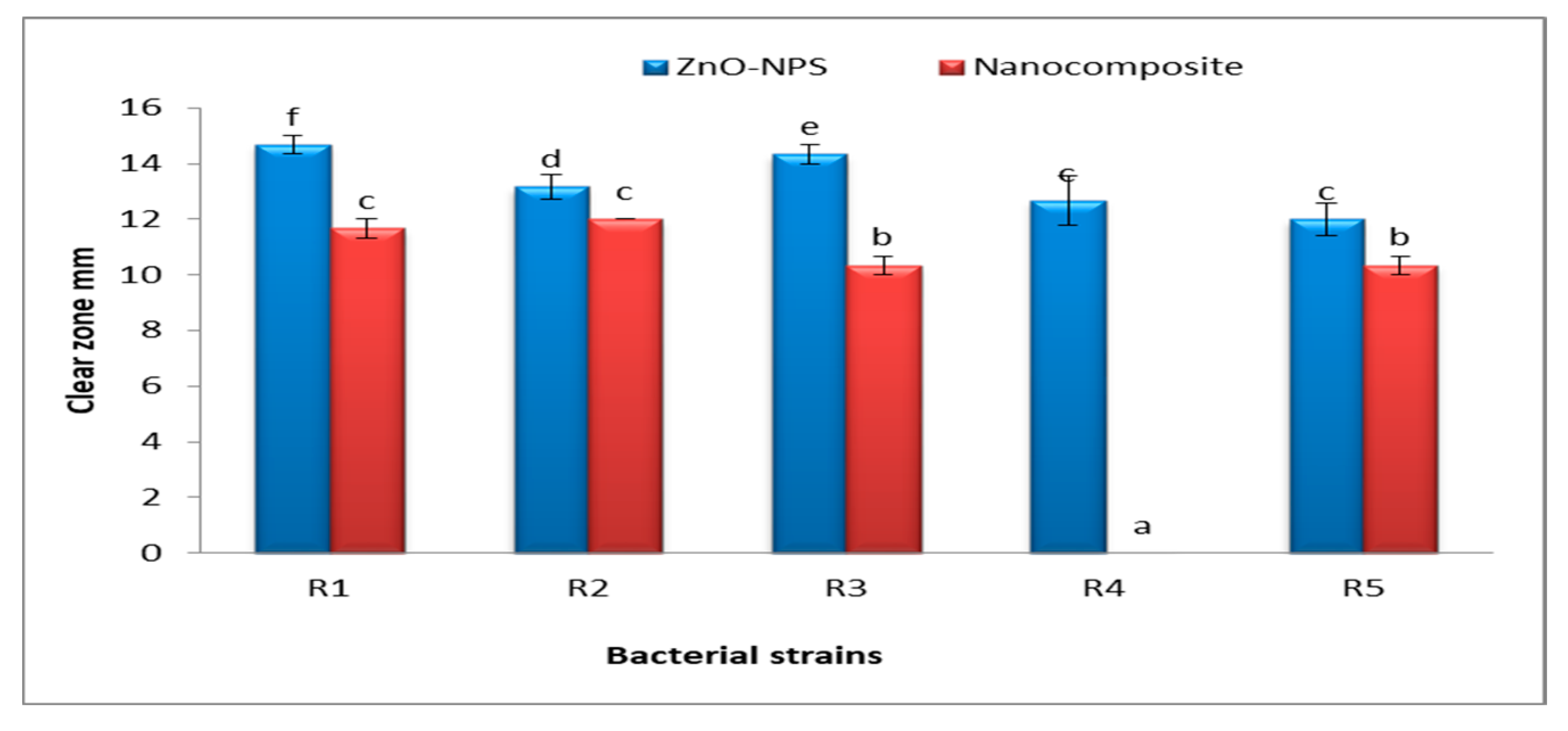
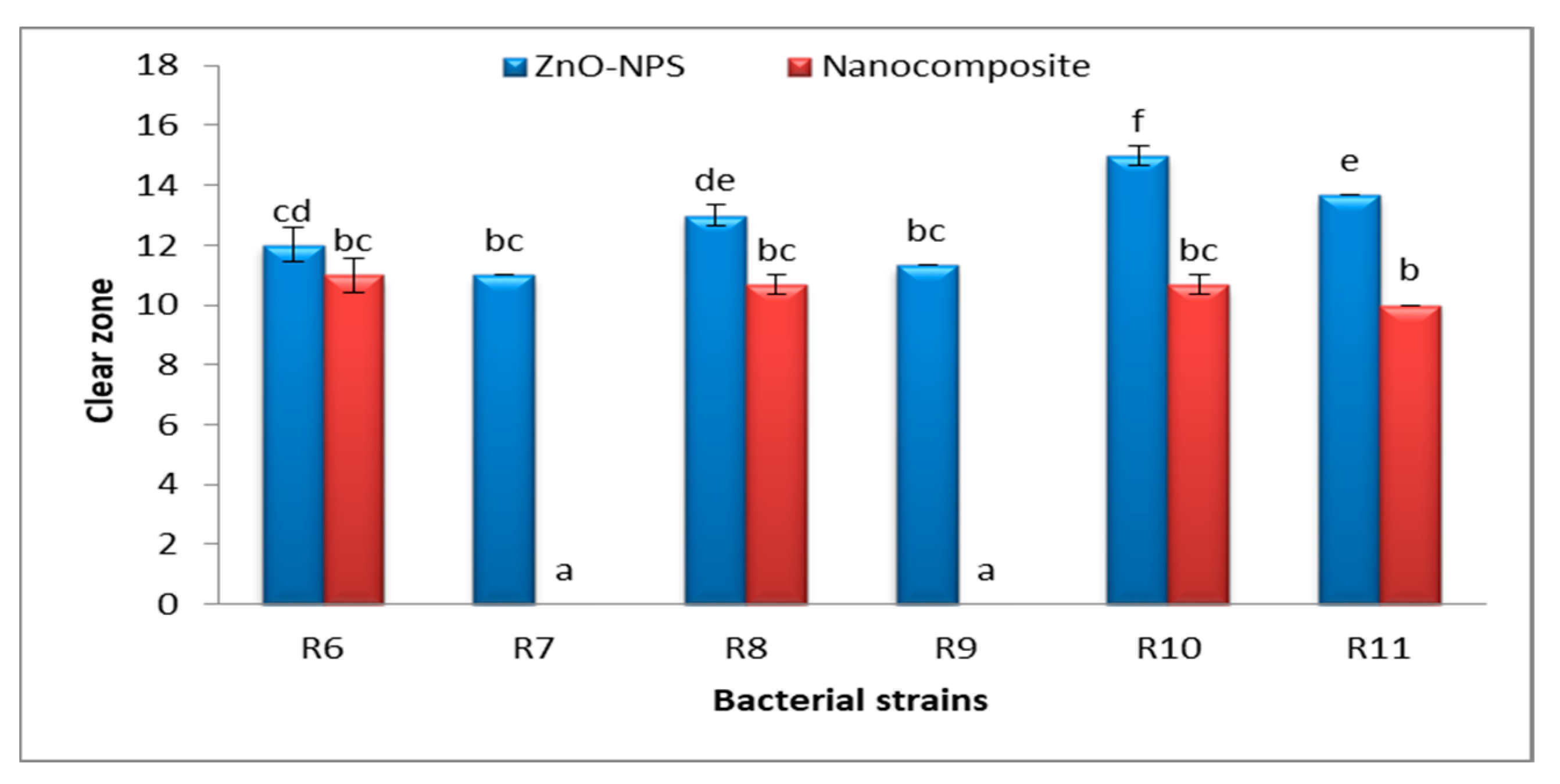
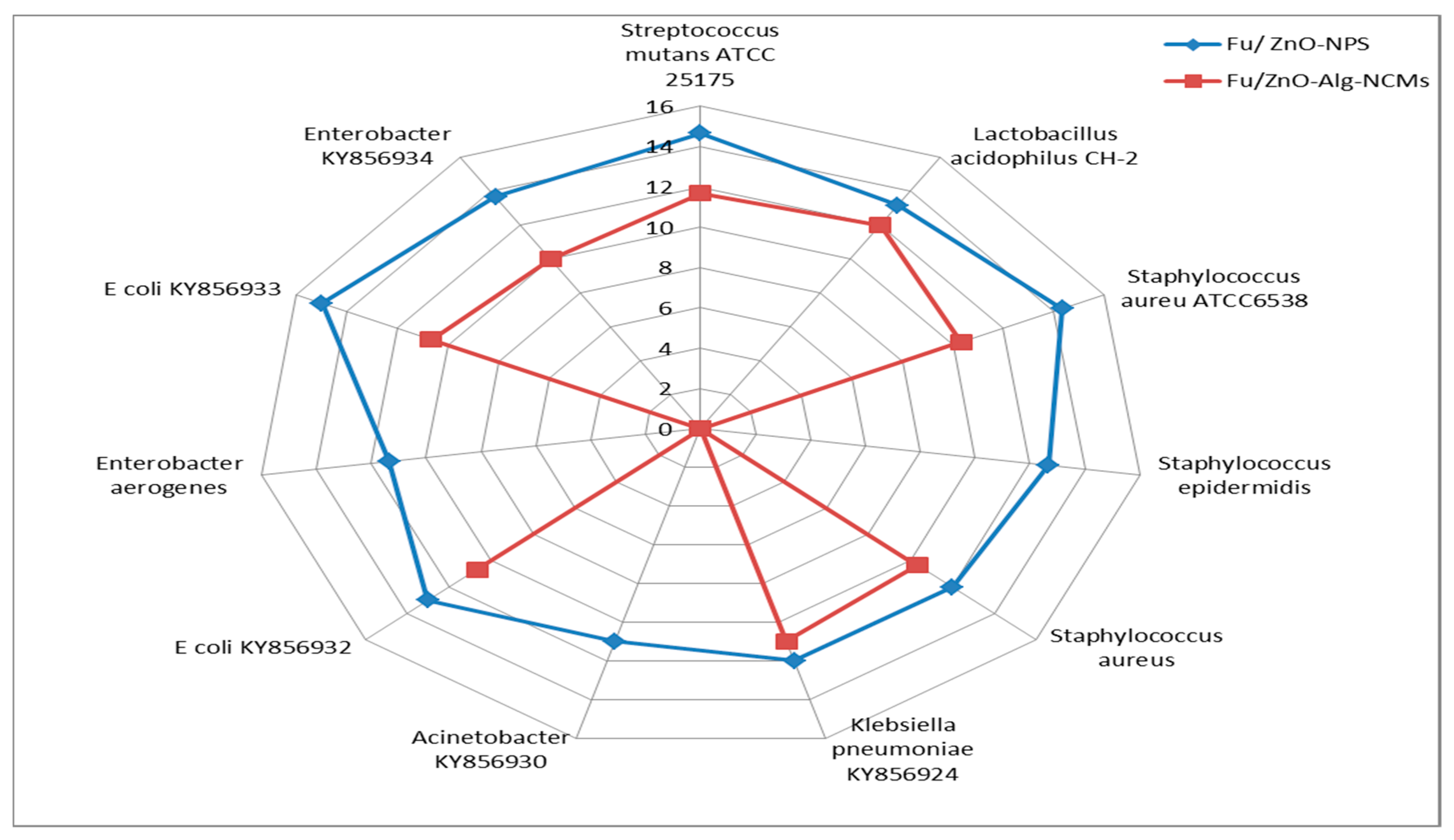
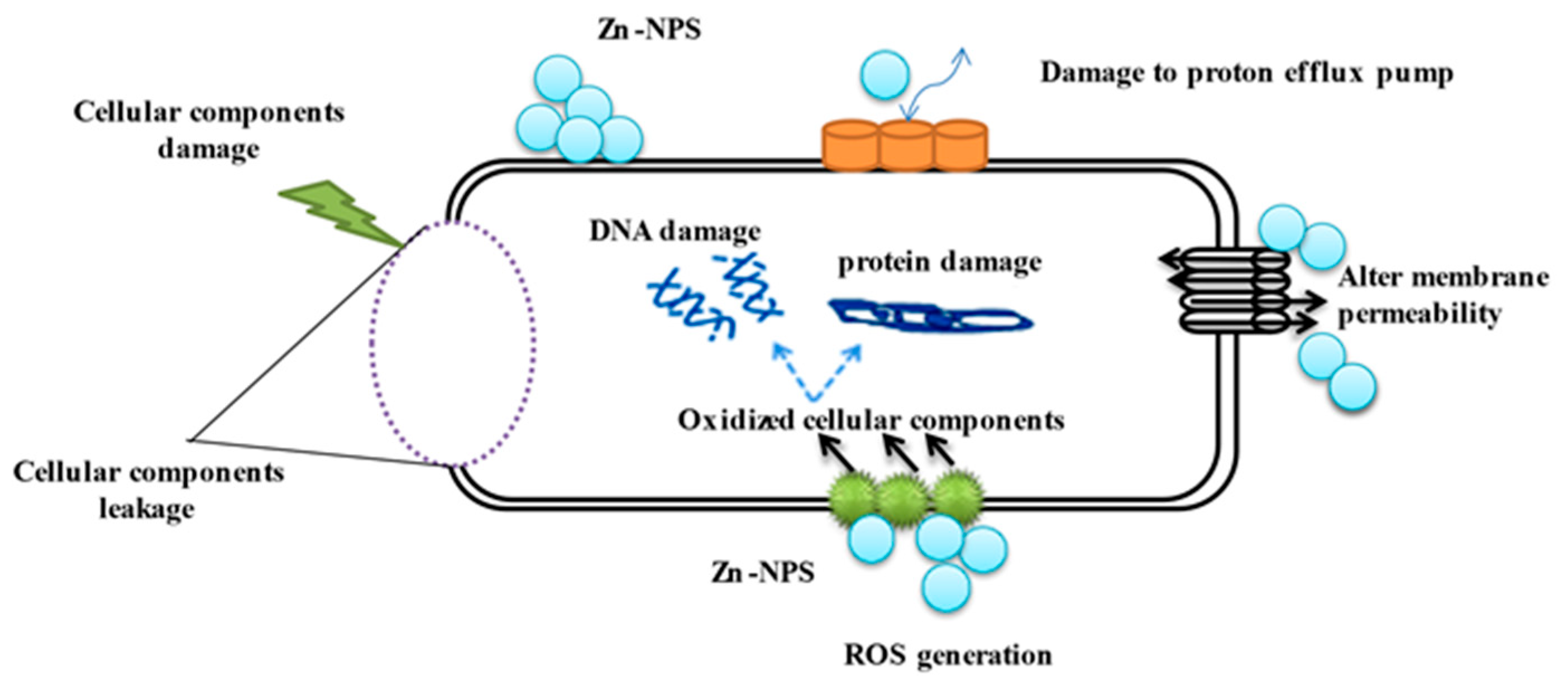
3.6. Antibacterial Activities of Fu/ZnO-NPs and Fu/ZnO-Alg-NCMs Bio-Fabricated via Brown Alga F. vesiculosus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christopher, J.F.; Jae-Young, K.; Ashley, I.B. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.-Y.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Shobha, N.; Nanda, N.; Giresha, A.S.; Manjappa, P.; Sophiya, P.; Dharmappa, K.; Nagabhushana, B. Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng. C 2018, 97, 842–850. [Google Scholar] [CrossRef]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef]
- Li, M.; Bala, H.; Lv, X.; Ma, X.; Sun, F.; Tang, L.; Wang, Z. Direct synthesis of monodispersed ZnO nanoparticles in an aqueous solution. Mater. Lett. 2007, 61, 690–693. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.; Singh, R. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig. J. Nanomater. Bios. 2010, 5, 427–432. [Google Scholar]
- Kim, S.-K.; Thomas, N.V.; Li, X. Anticancer compounds from marine macroalgae and their application as medicinal foods. Adv. Food Nutr. Res. 2011, 64, 213–224. [Google Scholar] [PubMed]
- Raja, A.; Vipin, C.; Aiyappan, A. Biological importance of marine algae—An overview. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 222–227. [Google Scholar]
- De Felício, R.; de Albuquerque, S.; Young, M.C.M.; Yokoya, N.S.; Debonsi, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Han, L. Antithrombotic effect of bromophenol, the alga-derived thrombin inhibitor. J. Biotechnol. 2008, 28, 96–98. [Google Scholar] [CrossRef]
- Na, H.-J.; Moon, P.-D.; Lee, H.-J.; Kim, H.-R.; Chae, H.-J.; Shin, T.; Seo, Y.; Hong, S.-H.; Kim, H.-M. Regulatory effect of atopic allergic reaction by Carpopeltis Affin. J. Ethnopharmacol. 2005, 101, 43–48. [Google Scholar] [CrossRef]
- Devi, G.K.; Manivannan, K.; Thirumaran, G.; Rajathi, F.A.A.; Anantharaman, P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asian Pac. J. Trop. Med. 2011, 4, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rabecca, R.; Doss, A.; Kensa, V.M.; Iswarya, S.; Mukeshbabu, N.; Pole, R.P.; Iyappan, K. Facile synthesis of zinc oxide nanoparticle using algal extract and their antibacterial potential. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Elrefaey, A.A.K.; El-Gamal, A.D.; Hamed, S.M.; El-belely, E.F. Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it’s antimicrobial and antioxidant activities. Egypt. J. Chem. 2022, 65, 231–240. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Alginate biosynthesis and biotechnological production. Alginates Biomed. Appl. 2018, 1–25. [Google Scholar] [CrossRef]
- Draget, K.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res 2009, 2, 1191–1199. [Google Scholar]
- Hampson, F.; Farndale, A.; Strugala, V.; Sykes, J.; Jolliffe, I.; Dettmar, P. Alginate rafts and their characterisation. Int. J. Pharm. 2005, 294, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of composite alginate-based electrospun membranes loaded with ZnO nanoparticles. Carbohydr. Polym. 2020, 227, 115371. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Joo, S.H.; Toborek, M. Treatment of antibiotic-resistant bacteria by encapsulation of ZnO nanoparticles in an alginate biopolymer: Insights into treatment mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Alvarez Primo, F.; Fregoso, G.; Lalitha Raveendran, N.; Noveron, J.C.; Spencer, C.T.; Joddar, B. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Reddy, C.R.K.; Thakur, M.C.; Rao, M.U. Seaweeds India: The Diversity and Distribution of Seaweeds of the Gujarat Coast; Springer: Dordrecht, The Netherlands, 2009; Volume 3, 215p. [Google Scholar]
- Hamouda, R.A.; Yousuf, W.E.; Abeer Mohammed, A.B.; Mohammed, R.S.; Darwish, D.B.; Abdeen, E.E. Comparative study between zinc oxide nanoparticles synthesis by biogenic and wet chemical methods in vivo and in vitro against Staphylococcus aureus. Microb. Pathog. 2020, 147, 104384. [Google Scholar] [CrossRef]
- Hambali, E.; Sakti, S.C.W.; Fahmi, M.Z.; Wahyudianto, F.E.; Yessi, P.; Yani, M.; Sinurat, E.; Pratama, B.S. Effect of Extraction Time and Na2CO3 Concentration on the Characteristics of Alginate Extracted from Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Salman, A.S.; Alharbi, A.A.; Alhasani, R.H.; Elshamy, M.M. Assessment of the antigenotoxic effects of alginate and zno/alginate–nanocomposites extracted from brown alga Fucus vesiculosus in Mice. Polymers 2021, 13, 3839. [Google Scholar] [CrossRef]
- Makharita, R.R.; El-Kholy, I.; Hetta, H.F.; Abdelaziz, M.H.; Hagagy, F.I.; Ahmed, A.A.; Algammal, A.M. Antibiogram and Genetic Characterization of Carbapenem-Resistant Gram-Negative Pathogens Incriminated in Healthcare-Associated Infections. Infect. Drug Resist. 2020, 13, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Safy, R.; Makharita, R. Influence of Medicinal Plant Extracts on the Growth of Oral Pathogens Streptococcus mutans and Lactobacillus acidophilus: An In-Vitro Study. Open Access Maced. J. Med. Sci. 2019, 7, 2328–2334. [Google Scholar] [CrossRef]
- Krishnan, V.; Bupesh, G.; Manikandan, E.; Thanigai, A.; Magesh, S.; Kalyanaraman, R.; Maaza, M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J. Antimicro 2016, 2, 1000123. [Google Scholar]
- Popescu, C.-M.; Popescu, M.-C.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, N.; Johnson, M. Functional groups of tree ferns (Cyathea) using FTIR: Chemotaxonomic implications. Rom. J. Biophys. 2015, 25, 131–141. [Google Scholar]
- Adochitei, A.; Drochioiu, G. Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev. Roum. Chim. 2011, 56, 783–791. [Google Scholar]
- Gokulakumar, B.; Narayanaswamy, R. Fourier transform-infrared spectra (FT-IR) analysis of root rot disease in sesame (Sesamum indicum). Rom. J. Biophys. 2008, 18, 217–223. [Google Scholar]
- Cannane, N.O.A.; Rajendran, M.; Selvaraju, R. FT-IR spectral studies on polluted soils from industrial area at Karaikal, Puducherry State, South India. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 46–54. [Google Scholar] [CrossRef]
- Ladera, R.; Finocchio, E.; Rojas, S.; Fierro, J.; Ojeda, M. Supported niobium catalysts for methanol dehydration to dimethyl ether: FTIR studies of acid properties. Catal. Today 2012, 192, 136–143. [Google Scholar] [CrossRef]
- Venkatesan, S.; Pugazhendy, K.; Sangeetha, D.; Vasantharaja, C.; Prabakaran, S.; Meenambal, M. Fourier transform infrared (FT-IR) spectoroscopic analysis of Spirulina. Int. J. Pharm. Biol. Arch. 2012, 3, 969–972. [Google Scholar]
- Wang, H.; Hollywood, K.; Jarvis, R.M.; Lloyd, J.R.; Goodacre, R. Phenotypic characterization of Shewanella oneidensis MR-1 under aerobic and anaerobic growth conditions by using Fourier transform infrared spectroscopy and high-performance liquid chromatography analyses. Appl. Environ. Microbiol. 2010, 76, 6266–6276. [Google Scholar] [CrossRef] [PubMed]
- Copikova, J.; Cerna, M.; Novotna, M.; Kaasova, J.; Synytsya, A. Application of FT-IR spectroscopy in detection of food hydrocolloids confectionery jellies and in food supplements. Czech J. Food Sci. 2001, 19, 51–56. [Google Scholar] [CrossRef]
- Mohan, P.K.; Sreelakshmi, G.; Muraleedharan, C.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Anand, B.; Meganathan, C.; Joshua, B.D. FT-IR, FT-Raman spectra and ab initio HF, DFT vibrational analysis of 2, 3-difluoro phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Šarić, A.; Musić, S.; Nomura, K.; Popović, S. Microstructural properties of Fe–oxide powders obtained by precipitation from FeCl3 solutions. Mater. Sci. Eng. B 1998, 56, 43–52. [Google Scholar] [CrossRef]
- Anariba, F.; Viswanathan, U.; Bocian, D.F.; McCreery, R.L. Determination of the structure and orientation of organic molecules tethered to flat graphitic carbon by ATR-FT-IR and Raman spectroscopy. Anal. Chem. 2006, 78, 3104–3112. [Google Scholar] [CrossRef]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Fourier transform infrared and ultraviolet-visible spectroscopic characterization of ammonium acetate and ammonium chloride: An impact of biofield treatment. Mod. Chem. Appl. 2015, 3, 1–10. [Google Scholar]
- Sajan, D.; Bena Jothy, V.; Kuruvilla, T.; Hubert, J.I. NIR-FT Raman, FT-IR and surface-enhanced Raman scattering and DFT based theoretical studies on the adsorption behaviour of (S)-phenylsuccinic acid on silver nanoparticles. J. Chem. Sci. 2010, 122, 511–519. [Google Scholar] [CrossRef]
- Rangaswamy, V.; Renuka, S.; Venda, I. Synthesis, spectral characterization and antibacterial activity of transition metal (II) complexes of tetradentate Schiff base ligand. Mater. Today Proc. 2022, 51, 1810–1816. [Google Scholar] [CrossRef]
- El-Nahass, M.; Kamel, M.; El-Deeb, A.; Atta, A.; Huthaily, S. Ab initio HF, DFT and experimental (FT-IR) investigation of vibrational spectroscopy of PN, N-dimethylaminobenzylidenemalononitrile (DBM). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, P.; Gunasekaran, S. Experimental and theoretical studies of (FT-IR, FT-Raman, UV–Visible and DFT) 4-(6-methoxynaphthalen-2-yl) butan-2-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghareebawi, A.; Al-Okaily, B.; Ibrahim, O. Characterization of Zinc Oxide Nanoparticles Synthesized by Olea Europaea Leaves Extract (Part L). Iraqi J. Agric. Sci. 2021, 52, 580–588. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Aslam, N.; Mumtaz, S.; Jaafar, H.Z.; Wahab, P.E.M.; Qayum, M.; Ormenisan, A.N. Impact of Zn nanoparticles synthesized via green and chemical approach on okra (Abelmoschus esculentus L.) growth under salt stress. Sustainability 2021, 13, 3694. [Google Scholar]
- El-Belely, E.F.; Farag, M.M.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (Class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hezma, A.; Shaltout, W.A.; Kabary, H.A.; El-Bahy, G.S.; Abdelrazzak, A.B. Fabrication, characterization and adsorption investigation of Nano zinc oxide–sodium alginate beads for effective removal of chromium (VI) from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2023, 1–9. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar]
- Keshavarz, M.; Alizadeh, P. On the role of alginate coating on the mechanical and biological properties of 58S bioactive glass scaffolds. Int. J. Biol. Macromol. 2021, 167, 947–961. [Google Scholar] [CrossRef]
- Singh, R.P.P.; Hudiara, I.S.; Rana, S.B. Effect of calcination temperature on the structural, optical and magnetic properties of pure and Fe-doped ZnO nanoparticles. Mater. Sci. 2016, 34, 451–459. [Google Scholar]
- Govindan, N.; Vairaprakasam, K.; Chinnasamy, C.; Sivalingam, T.; Mohammed, M.K. Green synthesis of Zn-doped Catharanthus Roseus nanoparticles for enhanced anti-diabetic activity. Mater. Adv. 2020, 1, 3460–3465. [Google Scholar] [CrossRef]
- El-Sonbaty, S.; Kandil, E.I.; Haroun, R.A.-H. Assessment of the antitumor activity of green biosynthesized zinc nanoparticles as therapeutic agent against renal cancer in rats. Biol. Trace Elem. Res. 2023, 201, 272–281. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Zheng, Y. Biosynthesis of polyphenols functionalized ZnO nanoparticles: Characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 2018, 183, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Khan, A.U.; Hoque, M.; Suhail, M.; Khan, M.I.; Zughaibi, T.A. Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer. Nanotechnol. Rev. 2022, 11, 2714–2725. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdelkareem, A.; Said, H.A.; El-Belely, E.F.; Alkhalifah, D.H.M.; Alshallash, K.S.; Hassan, S.E.-D. Phyco-synthesized zinc oxide nanoparticles using marine macroalgae, Ulva fasciata Delile, characterization, antibacterial activity, photocatalysis, and tanning wastewater treatment. Catalysts 2022, 12, 756. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and antibacterial properties of ZnO–alginate nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, P.; Yao, Y.; Liu, Y.; Zhang, Y.; Wang, X.; Wang, Y.; Wu, J. Gliadin-mediated green preparation of hybrid zinc oxide nanospheres with antibacterial activity and low toxicity. Sci. Rep. 2021, 11, 10373. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar]
- Haider, J.; Mehdi, M.M.S. Effect of Experimental Parameters on the Fabrication of Silver Nanoparticles by Laser Ablation. Eng. Technol. J. 2014, 32, 704–709. [Google Scholar]
- Farhadi, S.; Ajerloo, B.; Mohammadi, A. Green biosynthesis of spherical silver nanoparticles by using date palm (Phoenix dactylifera) fruit extract and study of their antibacterial and catalytic activities. Acta Chim. Slov. 2017, 64, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium alginate-zinc oxide nanocomposite film for antibacterial wound healing applications. Biointerface Res. Appl. Chem. 2020, 10, 6289–6296. [Google Scholar]
- Salama, A.; Diab, M.A.; Abou-Zeid, R.E.; Aljohani, H.A.; Shoueir, K.R. Crosslinked alginate/silica/zinc oxide nanocomposite: A sustainable material with antibacterial properties. Compos. Commun. 2018, 7, 7–11. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Gong, C.-P.; Luo, Y.; Pan, Y.-Y. Novel synthesized zinc oxide nanoparticles loaded alginate-chitosan biofilm to enhanced wound site activity and anti-septic abilities for the management of complicated abdominal wound dehiscence. J. Photochem. Photobiol. B Biol. 2019, 192, 124–130. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Seo, J. Nano zinc oxide–sodium alginate antibacterial cellulose fibres. Carbohydr. Polym. 2016, 135, 349–355. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]

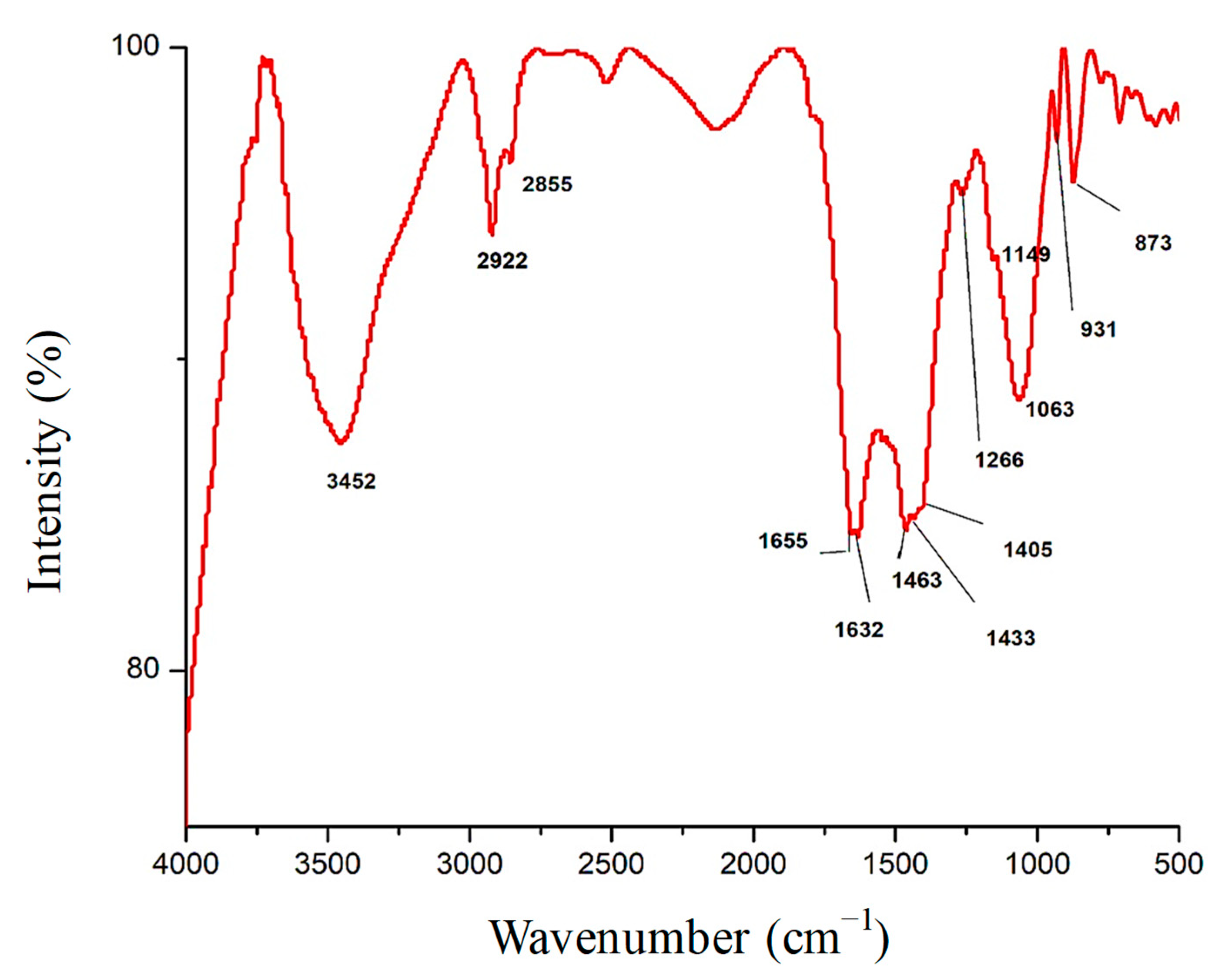
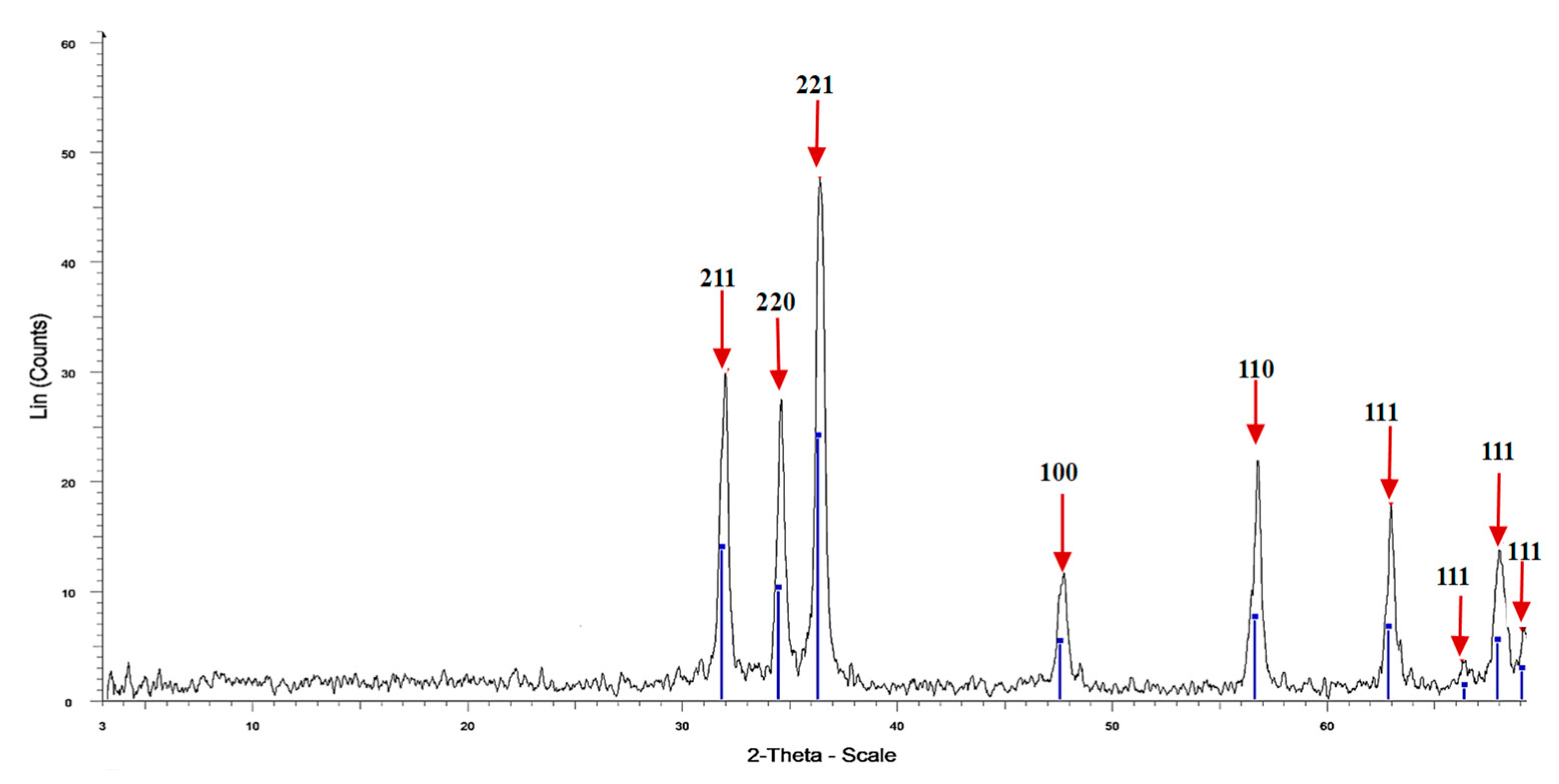
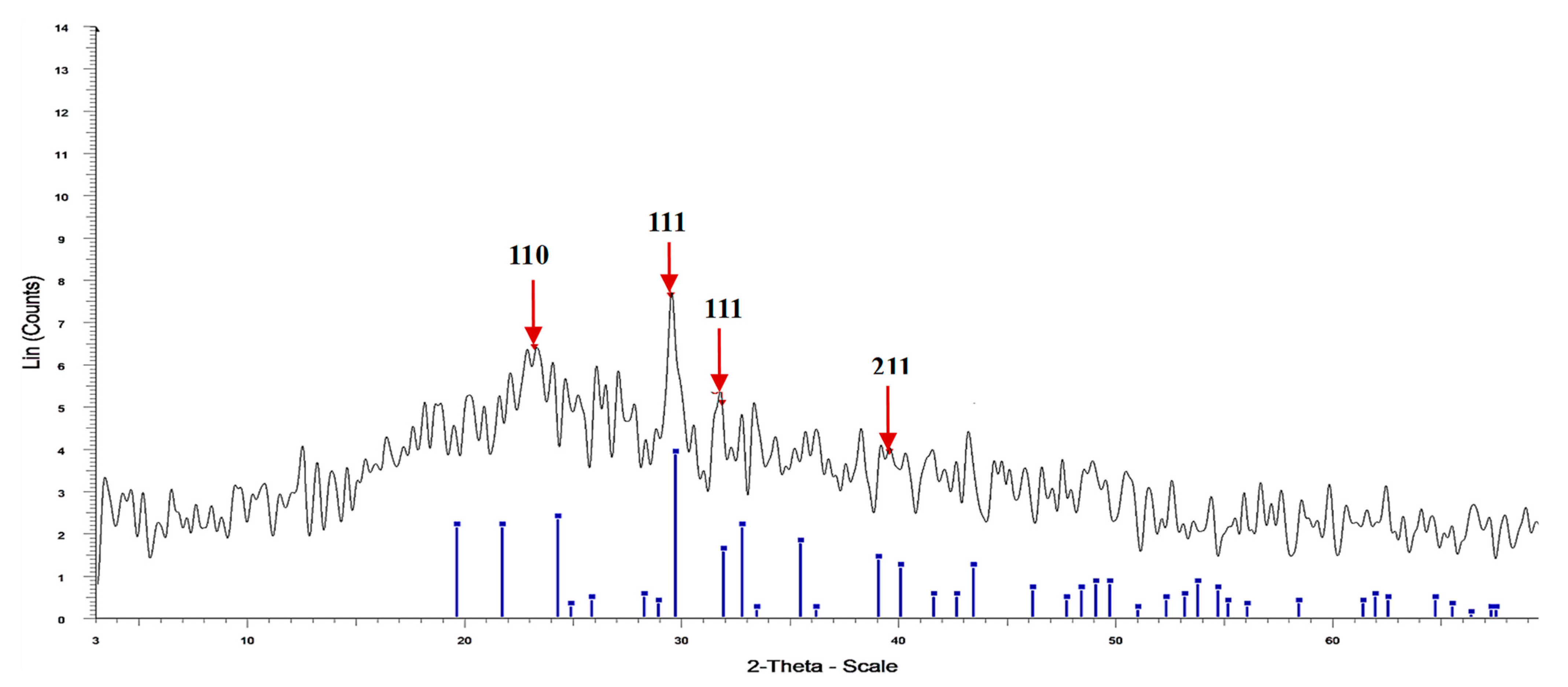
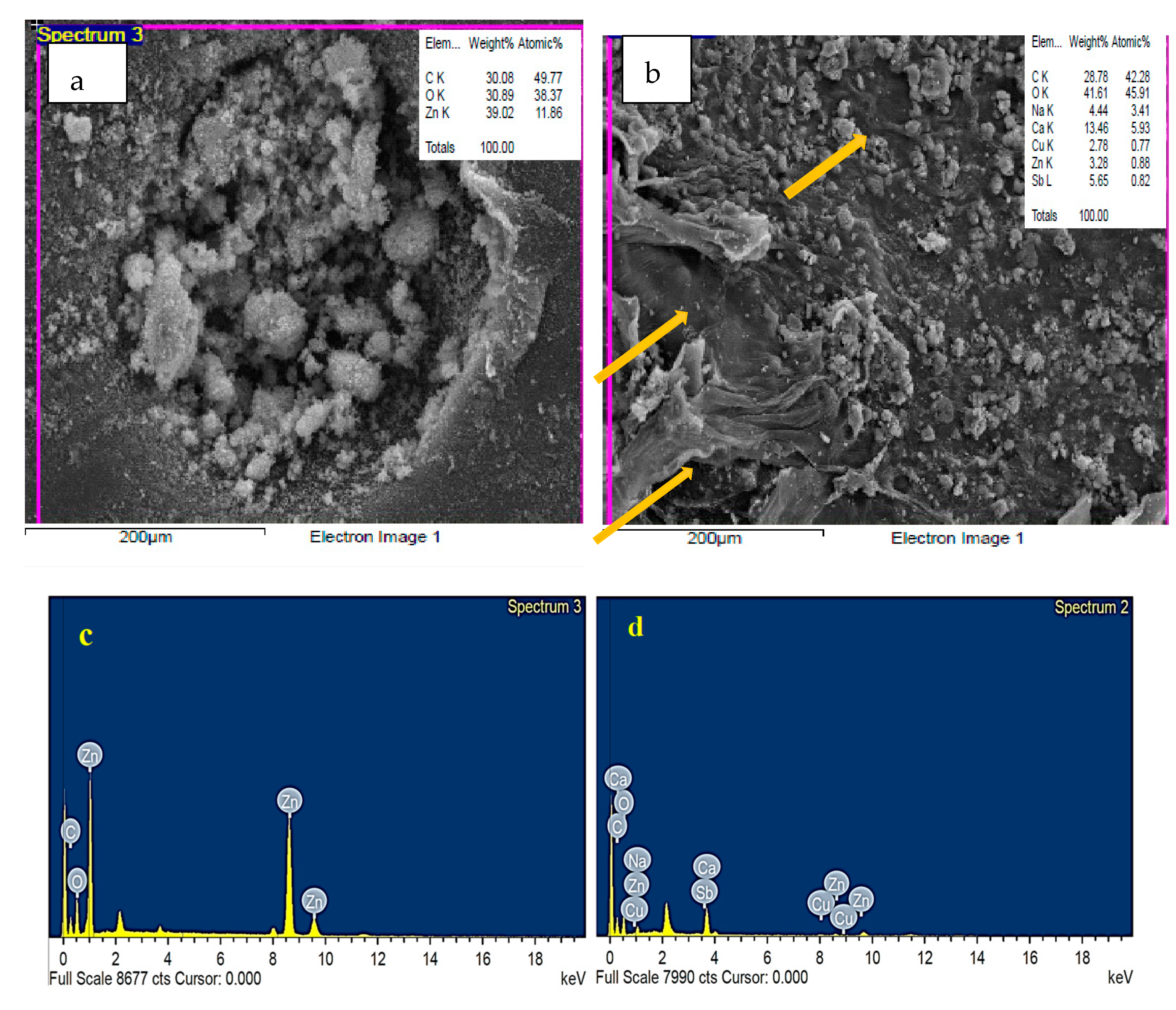

| Wavenumber cm−1 | Fu/ZnO-NPS | Fu/Alg-ZnO-NCMs | Assignment | References |
|---|---|---|---|---|
| 3421 | + | +31 | OH symmetric stretching | [35] |
| 2924 | + | −2 | OH stretching | [36] |
| 2854 | + | +1 | C–H stretching | [37] |
| 1655 | + | + | amide I-III band | [38] |
| 1634 | + | −2 | amide I-III band | [38] |
| 1549 | + | _ | N–H deformation | [39] |
| 1463 | _ | + | O–H bending | [40] |
| 1439 | + | +6 | C single bond H deformation | [41] |
| 1405 | _ | + | CH2 bending vibration | [42] |
| 1266 | _ | + | Amide III | [43] |
| 1149 | _ | + | Glycosidic bonds | [44] |
| 1082 | −19 | C single bond H | [45] | |
| 931 | _ | + | C single bond H out-of-plane | [46] |
| 875 | + | −2 | C–H stretching vibrations | [47] |
| 843 | + | _ | –OH groups | [48] |
| 775 | _ | + | C-H | [49] |
| 710 | _ | + | N-Cl stretching bond | [50] |
| 606 | + | _ | ring deformation of phenyl | [51] |
| 582 | _ | + | M-N stretching vibrations of the complexes. | [52] |
| 565 | + | −33 | C–C–C deformation of the phenyl ring | [53] |
| 472 | _ | + | C–C torsion vibrations | [54] |
| 433 | + | _ | Metal oxygen | [55] |
| 2-Theta ° | Area | Cry Size D (nm) | Intensity% | d Value (Angstrom) |
|---|---|---|---|---|
| 31.97 | 17.09386 | 26.8 | 62.4 | 2.79713 |
| 34.579 | 13.82135 | 31.5 | 58.3 | 2.59188 |
| 36.388 | 28.63312 | 29.9 | 100 | 2.46708 |
| 47.745 | 7.448441 | 25.3 | 24.6 | 1.90339 |
| 56.814 | 11.46893 | 34.5 | 46.6 | 1.61918 |
| 63.009 | 9.757796 | 33.7 | 37.2 | 1.47407 |
| 66.33 | 1.604286 | 26.7 | 7.1 | 1.4081 |
| 68.072 | 10.56759 | 25.2 | 29 | 1.37624 |
| 69.174 | 3.084169 | 50.6 | 6.12 | 1.35699 |
| 2-Theta° | Area | Cry Size (D) (nm) | Intensity % | d Value (Angstrom) |
|---|---|---|---|---|
| 23.159 | 67.99954 | 0.7 | 83.8 | 3.83746 |
| 29.457 | 2.635086 | 21.7 | 100 | 3.0298 |
| 31.844 | 9.625473 | 1.3 | 66.2 | 2.80792 |
| 39.564 | 21.01589 | 0.7 | 50.9 | 2.27601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, R.A.; Alharbi, A.A.; Al-Tuwaijri, M.M.; Makharita, R.R. The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus. Polymers 2023, 15, 2335. https://doi.org/10.3390/polym15102335
Hamouda RA, Alharbi AA, Al-Tuwaijri MM, Makharita RR. The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus. Polymers. 2023; 15(10):2335. https://doi.org/10.3390/polym15102335
Chicago/Turabian StyleHamouda, Ragaa A., Asrar A. Alharbi, Majdah M. Al-Tuwaijri, and Rabab R. Makharita. 2023. "The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus" Polymers 15, no. 10: 2335. https://doi.org/10.3390/polym15102335





