Synthesis and Characterization of a One-Dimensional Malleable Spin-Crossover Polymer Complex Modified by Methoxy Polyethylene Glycol
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Characterizations
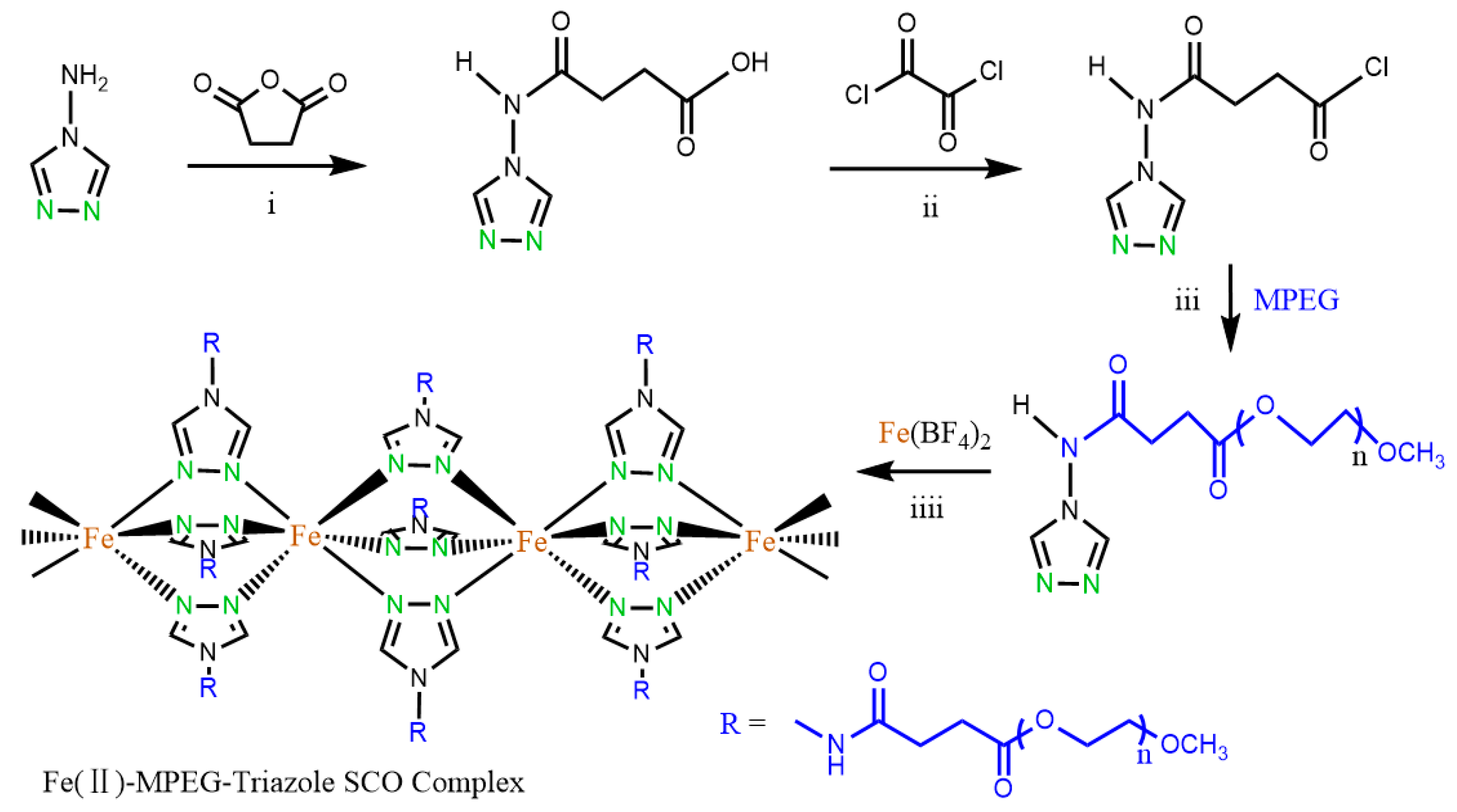
2.2. Synthesis of Carboxyl-Terminated 4-amino-1,2,4-triazole (NH2-trz-COOH)
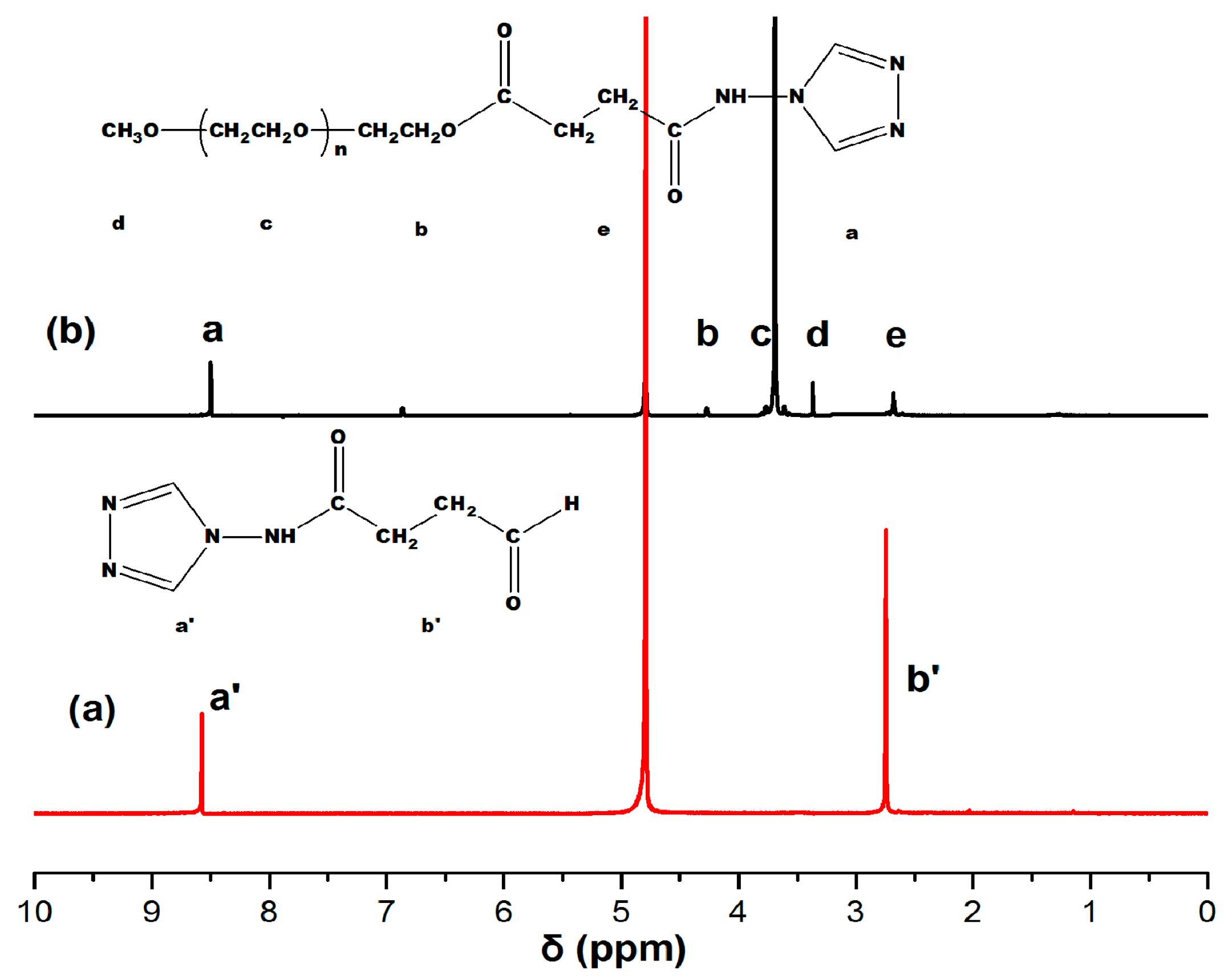
2.3. Synthesis of the Polymer-Modified Ligand MPEG-trz
2.4. Synthesis of Fe (II) Complex via MPEG-trz and Fe(BF4)2•6H2O
3. Results and Discussions
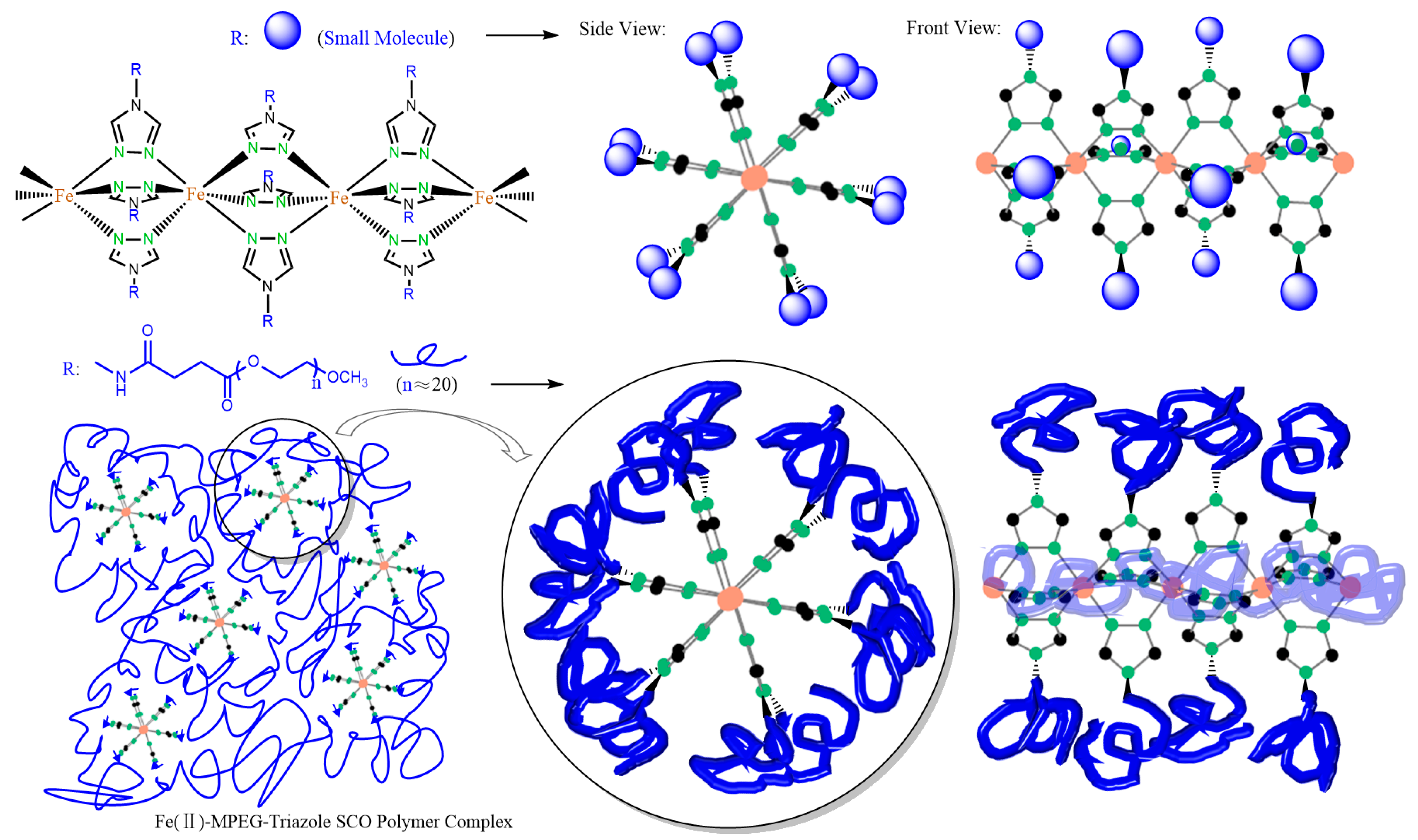
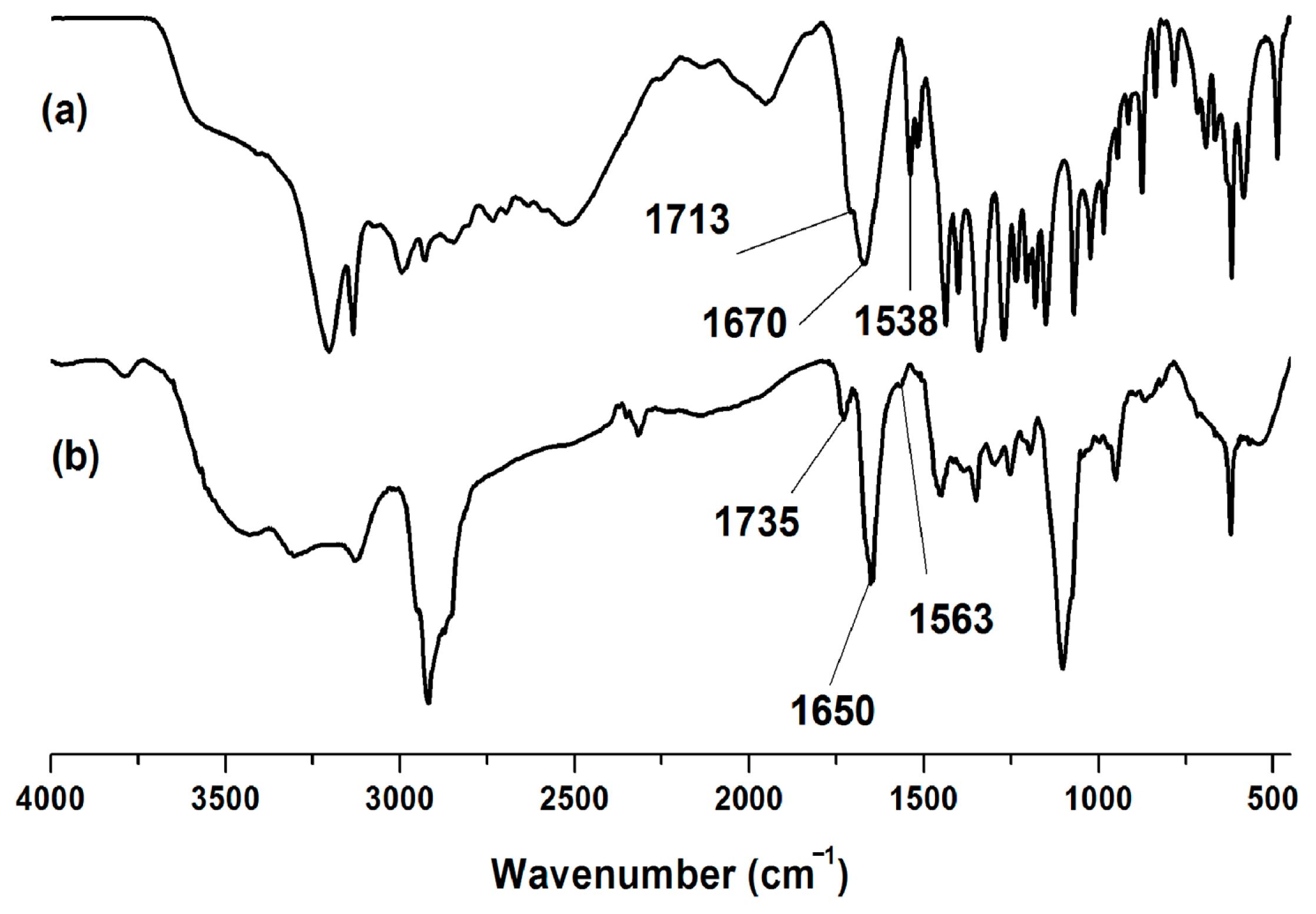
3.1. Synthesis and Characterizations of Polymer Complexes
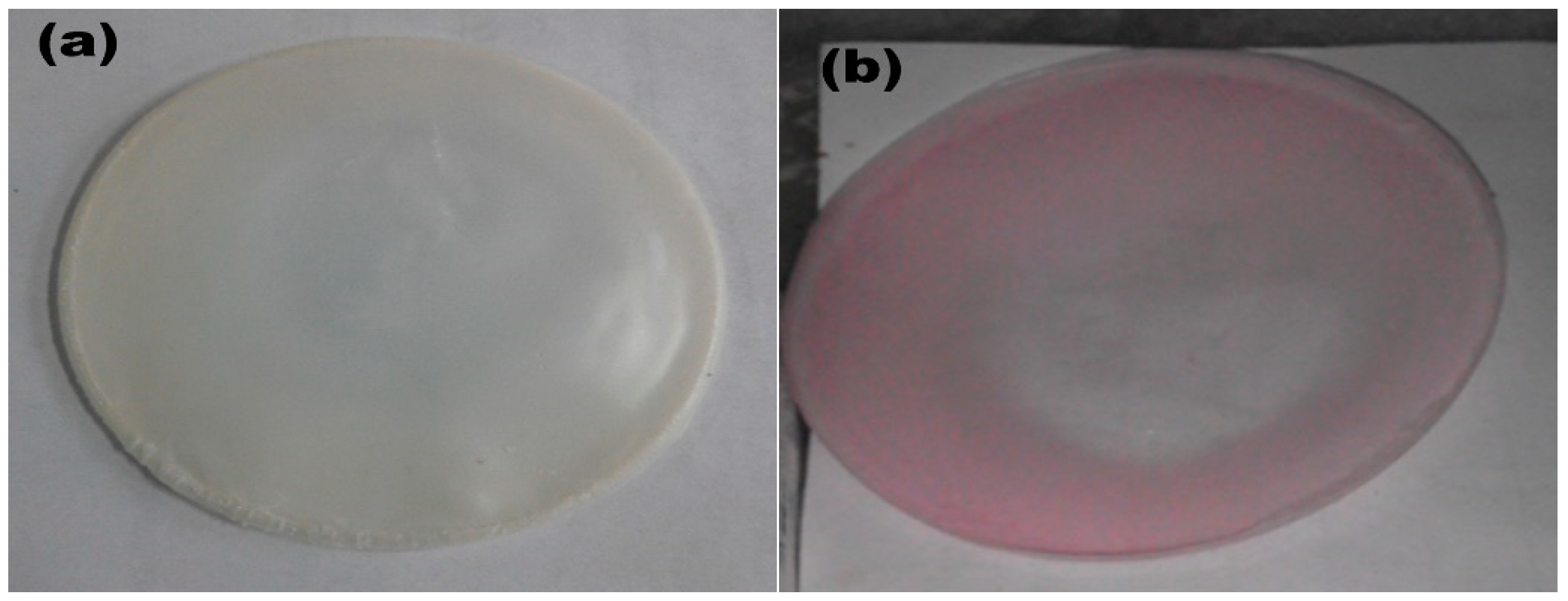
3.2. Characterizations and Description of Spin Polymer Complexes Films
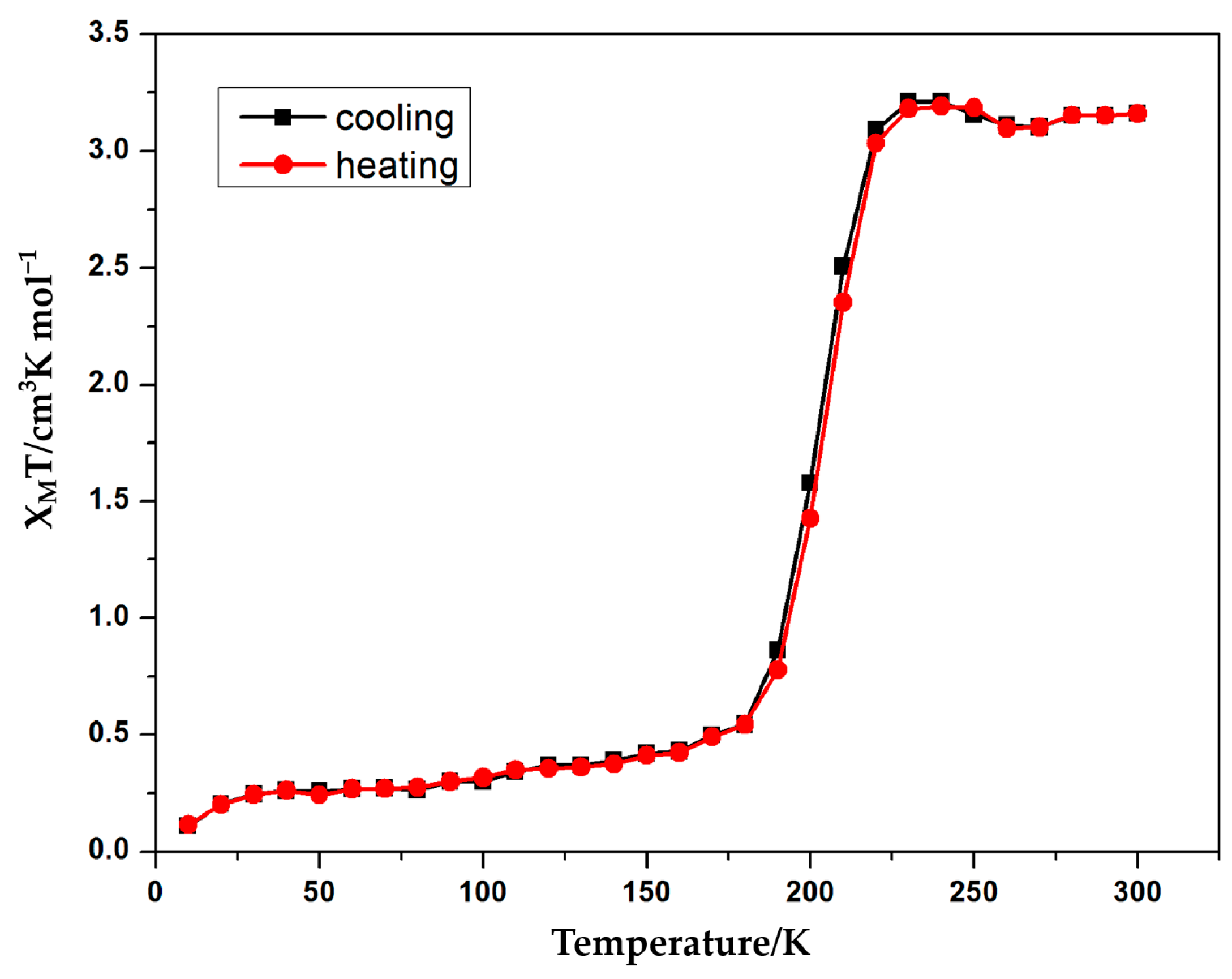
3.3. Magnetic Properties
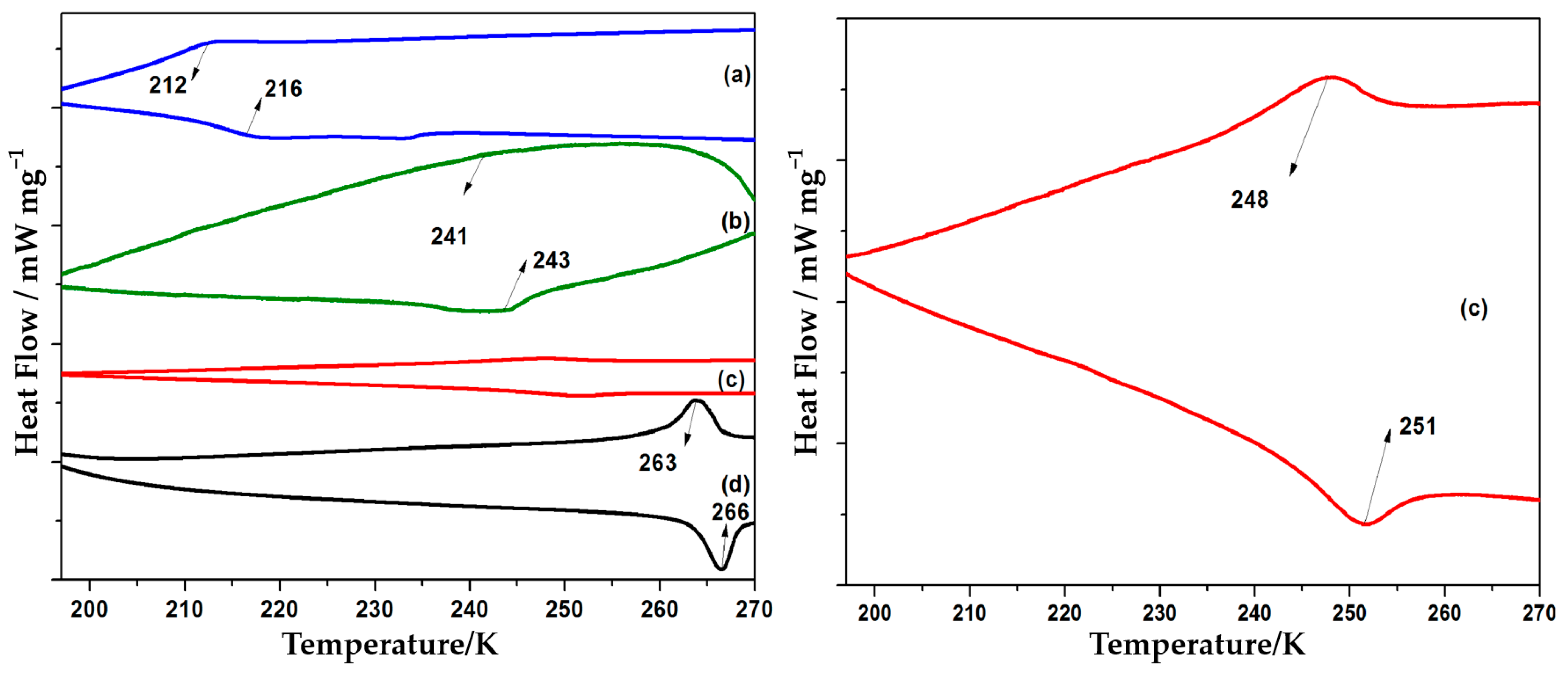
3.4. Spin Crossover Properties by Polymer Thermal Analysis
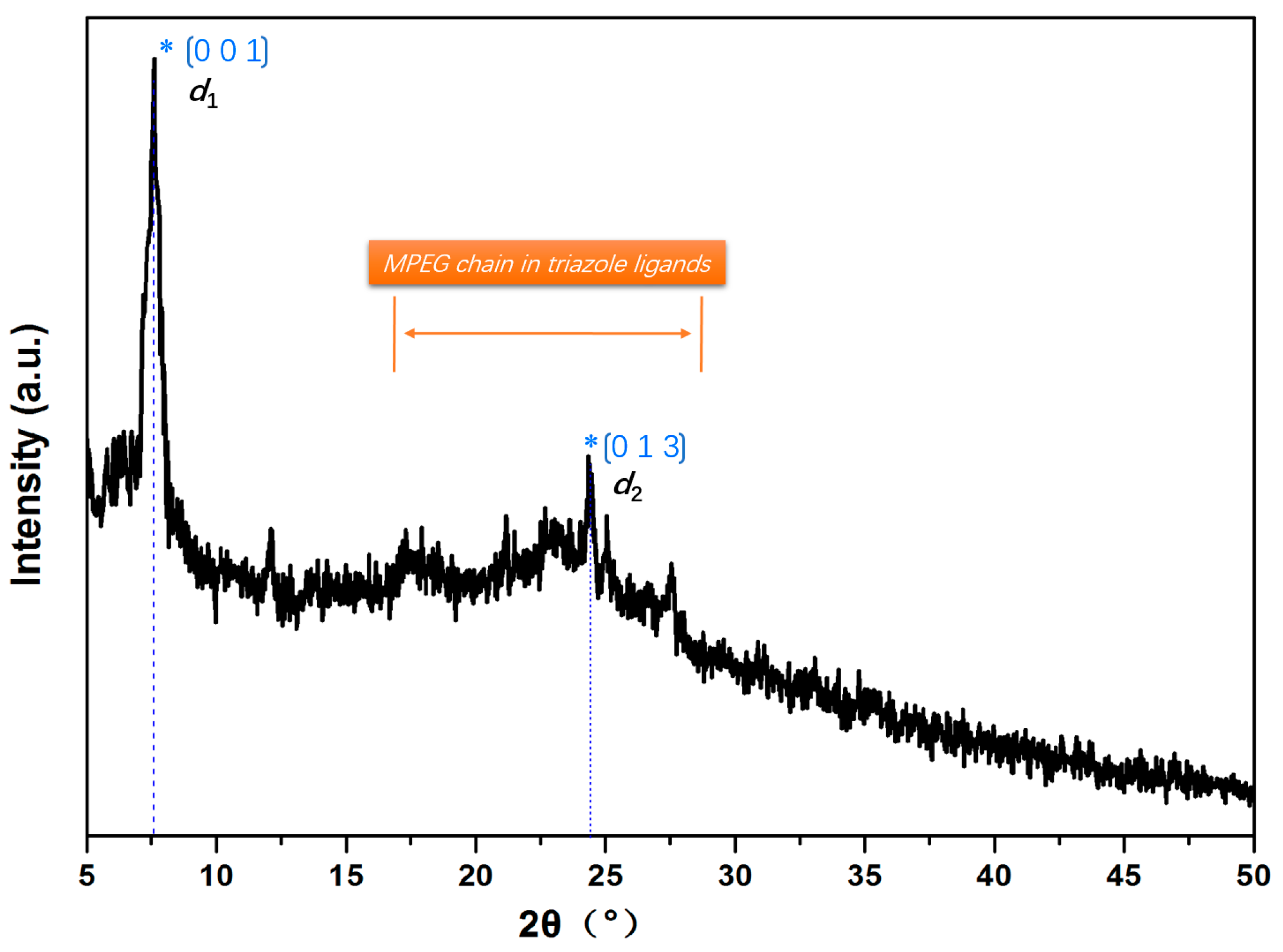
3.5. Crystal Structure Analysis
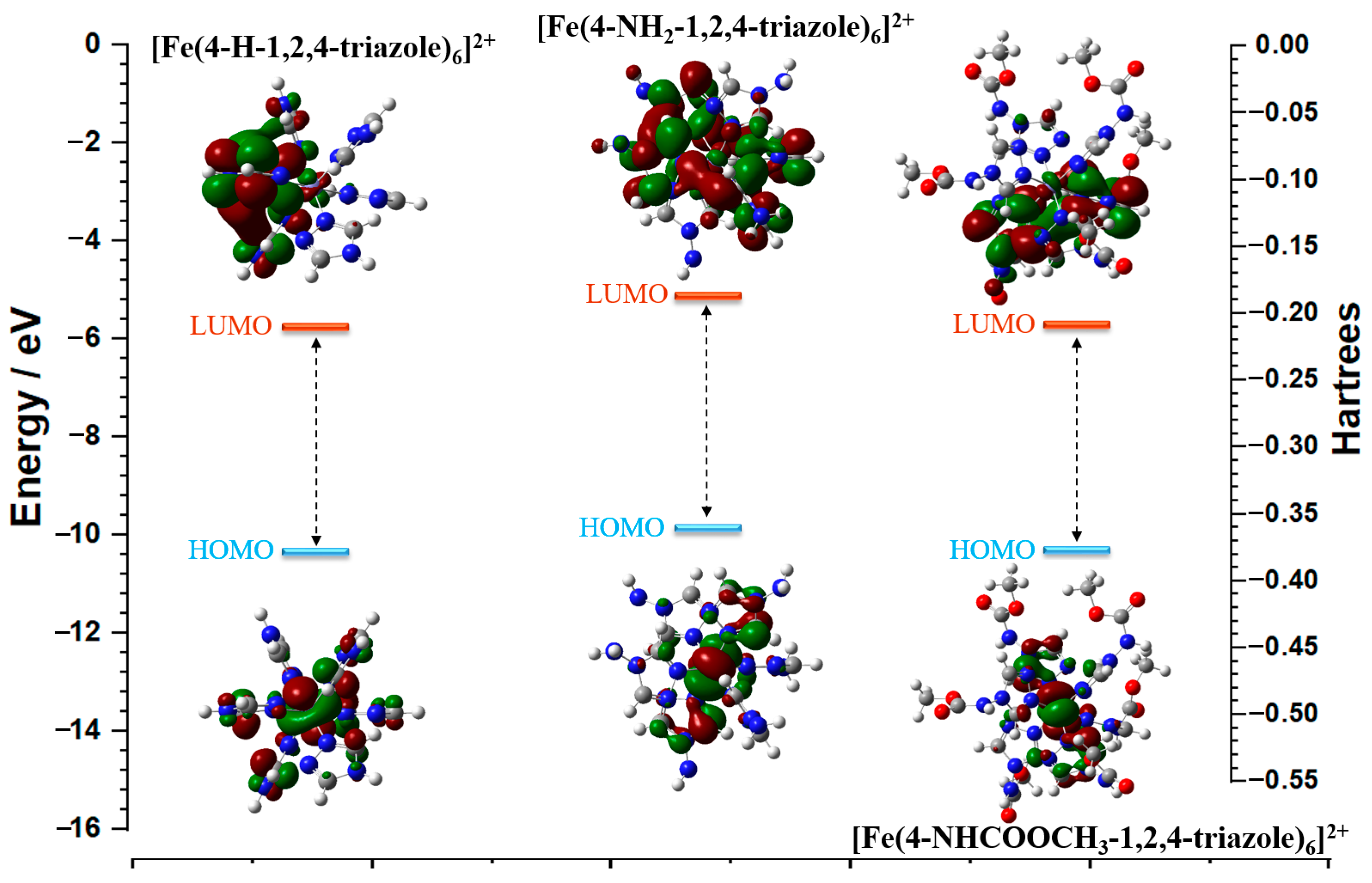
3.6. Computational Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernien, M.; Wiedemann, D.; Hermanns, C.F.; Krüger, A.; Rolf, D.; Kroener, W. Spin crossover in vacuum-deposited submonolayer of a molecular iron(II) complex. Phys. Chem. Lett. 2012, 3, 3431–3434. [Google Scholar] [CrossRef]
- Jalkanen, P.; Tuboltsev, V.; Marchand, B.; Savin, A.; Puttaswamy, M.; Vehkamäki, M.; Mizohata, K.; Kemell, M.; Hatanpää, T.; Rogozin, V.; et al. Magnetic properties of polycrystalline bismuth ferrite thin films grown by atomic layer deposition. Phys. Chem. Lett. 2014, 5, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Chen, Y.Y.; Huang, L.J.; Pan, D.C. Large-scale synthesis of well-dispersed copper nanowires in an electric pressure cooker and their application in transparent and conductive networks. Inorg. Chem. 2014, 53, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Ahoulou, S.; Vilà, N.; Pillet, S.; Schaniel, D.; Walcarius, A. Coordination polymers as template for mesoporous silica films: A novel composite material Fe(Htrz)3@SiO2 with remarkable electrochemical properties. Chem. Mater. 2019, 31, 5796–5807. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Veauthier, J.M.; Tamayo, G.F.A.; Chavez, D.E.; Lapsheva, E.; Myers, T.W.; Nelson, T.R.; Schelter, E.J. Correlating mechanical sensitivity with spin transition in the explosive spin crossover complex [Fe(Htrz)3]n[ClO4]2n. J. Am. Chem. Soc. 2020, 142, 4842–4851. [Google Scholar] [CrossRef]
- Castro, D.N.; Pineda, F.A.G.; Corcuera, A.M.; Doldan, B.P.; Guirado, F.G.; Buchholz, J.B.; Mascarós, J.R.G. Effect of mechanochemical recrystallization on the thermal hysteresis of 1D Fe II-triazole spin crossover polymers. Inorg. Chem. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Sekimoto, Y.; Ohtani, R.; Nakamura, M.; Koinuma, M.; Lindoy, L.F.; Hayami, S. Tuneable pressure effects in graphene oxide layers. Sci. Rep. 2017, 7, 12159. [Google Scholar] [CrossRef]
- Dimitrios, M.; Krzystek, J.; Eleftherios, F.; Alexander, W.; Neil, R.; Vassilis, P.; Aris, T.; Frank, N.; Panayotis, K. Investigating magnetostructural correlations in the pseudooctahedral trans-[Ni(II){(OPPh2)(EPPh2)N}2(sol)2] complexes (E = S, Se; sol = DMF, THF) by magnetometry, HFEPR, and ab initio quantum chemistry. Inorg. Chem. 2012, 51, 7218–7231. [Google Scholar]
- Cheng, L.; Zhang, W.X.; Ye, B.H.; Lin, J.B.; Chen, X.M. In situ solvothermal generation of 1,2,4-triazolates and related compounds from organonitrile and hydrazine hydrate: A mechanism study. Inorg. Chem. 2017, 46, 1135–1143. [Google Scholar] [CrossRef]
- Kahn, O.; Sommier, L.; Codjovi, E. Spin crossover behavior under pressure of Fe(PM-L)2(NCS)2 compounds with substituted 2′-pyridylmethylene 4-anilino ligands. Chem. Mater. 1997, 9, 3199–3205. [Google Scholar] [CrossRef]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory Devices. Adv. Mater. 2019, 279, 44–48. [Google Scholar] [CrossRef]
- Zhu, L.M.; Shi, W.; Zhao, R.R.; Cao, Y.L.; Ai, X.P.; Lei, A.W.; Yang, H.X. n-Dopable polythiophenes as high capacity anode materials for all-organic Li-ion batteries. J. Electroanal. Chem. 2013, 688, 118–122. [Google Scholar] [CrossRef]
- Bousseksou, A.; Salmon, L.; Varret, F.; Tuchagues, J.P. The second example of spin conversion governed by molecular vibrations: A novel ferrous complex resulting from hexacoordination of a Schiff base with an N4O2 donor set. Chem. Phys. Lett. 1998, 2, 282. [Google Scholar] [CrossRef]
- Davidson, R.J.; Ainscough, E.W.; Brodie, A.M.; Jameson, G.B.; Waterland, M.R.; Allcock, H.R.; Hindenlang, M.D.; Moubaraki, B.; Murray, K.S.; Gordon, K.C.; et al. Toward an iron(II) spin-crossover grafted phosphazene polymer. Inorg. Chem. 2012, 51, 8307–8316. [Google Scholar] [CrossRef]
- Murakami, Y.; Komatsu, T.; Kojima, N. Control of Tc and spin bistability in the spin-crossover system, [Fe(4-NH2trz)3](R-SO3)2. Synth. Met. 1999, 103, 2157–2158. [Google Scholar] [CrossRef]
- Ruan, B.F.; Lu, X.; Tang, J.F.; Wei, Y.; Wang, X.L.; Zhang, Y.B.; Wang, L.S.; Zhu, H.L. Synthesis, biological evaluation, and molecular docking studies of resveratrol derivatives possessing chalcone moiety as potential antitubulin agents. Bioorgan. Med. Chem. 2011, 19, 2688–2695. [Google Scholar] [CrossRef]
- Kavakl, C.; Kavaklı, P.A.; Güven, O. Preparation and characterization of glycidyl methacrylate grafted 4-amino-1,2,4-triazole modified nonwoven fiber adsorbent for environmental application. Phys. Chem. 2014, 94, 111–114. [Google Scholar] [CrossRef]
- Rentschler, E.; Malotki, C. Spin transition in three-dimensional bridged coordination polymers of iron(II)-urea-triazoles. Inorganica, Chimica Acta 2008, 361, 3646–3653. [Google Scholar] [CrossRef]
- Rubio, M.; López, D. Effect of solvent on the gelation properties of a metallo-organic polymer of [Fe(II) (4-octadecyl-1,2,4-triazole)3(ClO4)2]n. Eur. Polym. 2009, 45, 3339–3346. [Google Scholar] [CrossRef]
- Kitchen, J.A.; White, N.G.; Martin, C.G.; Jeffery, L.; Sally, T. Room-temperature spin crossover and Langmuir-Blodgett film formation of an iron(II) triazole complex featuring a long alkyl chain substituent: The tail that wags the dog. Chem. Commun. 2010, 46, 6464–6466. [Google Scholar] [CrossRef] [PubMed]
- Roubeau, O.; Natividad, E.; Agricole, B.; Ravaine, S. Formation, structure, and morphology of triazole-based Langmuir-Blodgett films. Langmuir 2007, 23, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Boillot, M.L.; Sour, A.; Delhaes, P.; Mingotaud, C.; Soyer, H. A photomagnetic effect for controlling spin states of Iron(II) complexes in molecular materials. Chem. Rev. 1999, 47, 192–195. [Google Scholar] [CrossRef]
- Letard, J.F.; Nguyen, O.; Soyer, H.; Mingotaud, C.; Delhaes, P.; Kahn, O. First evidence of che LIESST effect in a Langmuir-Blodgett film. Inorg. Chem. 1999, 38, 3020–3024. [Google Scholar] [CrossRef]
- Garcia, Y.; Niel, V.; Muñoz, M.C.; Real, J.A. Spin Crossover in 1D, 2D and 3D Polymeric Fe(II) Networks. Curr. Chem. 2004, 233, 229–257. [Google Scholar]
- Lee, Y.H.; Komatsu, Y.; Yamamoto, Y.; Kato, K.; Shimizu, T.; Ohta, A.; Matsui, T.; Hayami, S. Iron(II) spin crossover complexes with branched long alkyl chain. Inorg. Chem. Commun. 2011, 14, 1498–1500. [Google Scholar]
- Davidson, R.J.; Ainscough, E.W.; Brodie, A.M.; Jameson, G.B.; Waterland, M.R.; Allcock, H.R.; Hindenlang, M.D. Terpyridine and 2,6-di(1H-pyrazol-1-yl)pyridine substituted cyclotri- and polyphosphazene ruthenium(II) complexes: Chemical and physical behavior. Polyhedron 2015, 85, 429–436. [Google Scholar] [CrossRef]
- Wang, W.P.; Zhang, Z.Q.; Ji, B.B.; Zhao, H.H.; Li, G.Q.; Ma, L.X.; Zhao, H.X. Synthesis and characterization of novel coordination spin crossover poly(glycidyl methacrylate) with pendant iron(II)-4-amino-1,2,4-triazole groups. Inorg. Chem. Commun. 2015, 56, 125–128. [Google Scholar] [CrossRef]
- Feng, X.S.; Taton, D.; Borsali, R.; Chaikof, E.L.; Gnanou, Y. pH responsiveness of dendrimer-like poly(ethylene oxide)s. Chem. Soc. 2006, 128, 11551–11562. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Jemian, P.R.; Tew, G.N.; Bhatia, S.R. Micro-to nanoscale structure of biocompatible PLA-PEO-PLA hydrogels. Langmuir 2007, 23, 5039–5044. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, J.; Baumgarten, M.; Wang, D. Spirobifluorene mediating the spin−spin coupling of nitronyl nitroxide diradicals. J. Phys. Chem. A 2023, 127, 1565–1575. [Google Scholar] [CrossRef]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Galyametdinov, Y.; Verdaguer, M.; Villain, F.F.; Gütlich, P. One-dimensional iron(II) compounds exhibiting spin crossover and liquid crystalline properties in the room temperature region. Inorg. Chem. 2008, 47, 10232–10245. [Google Scholar] [CrossRef]


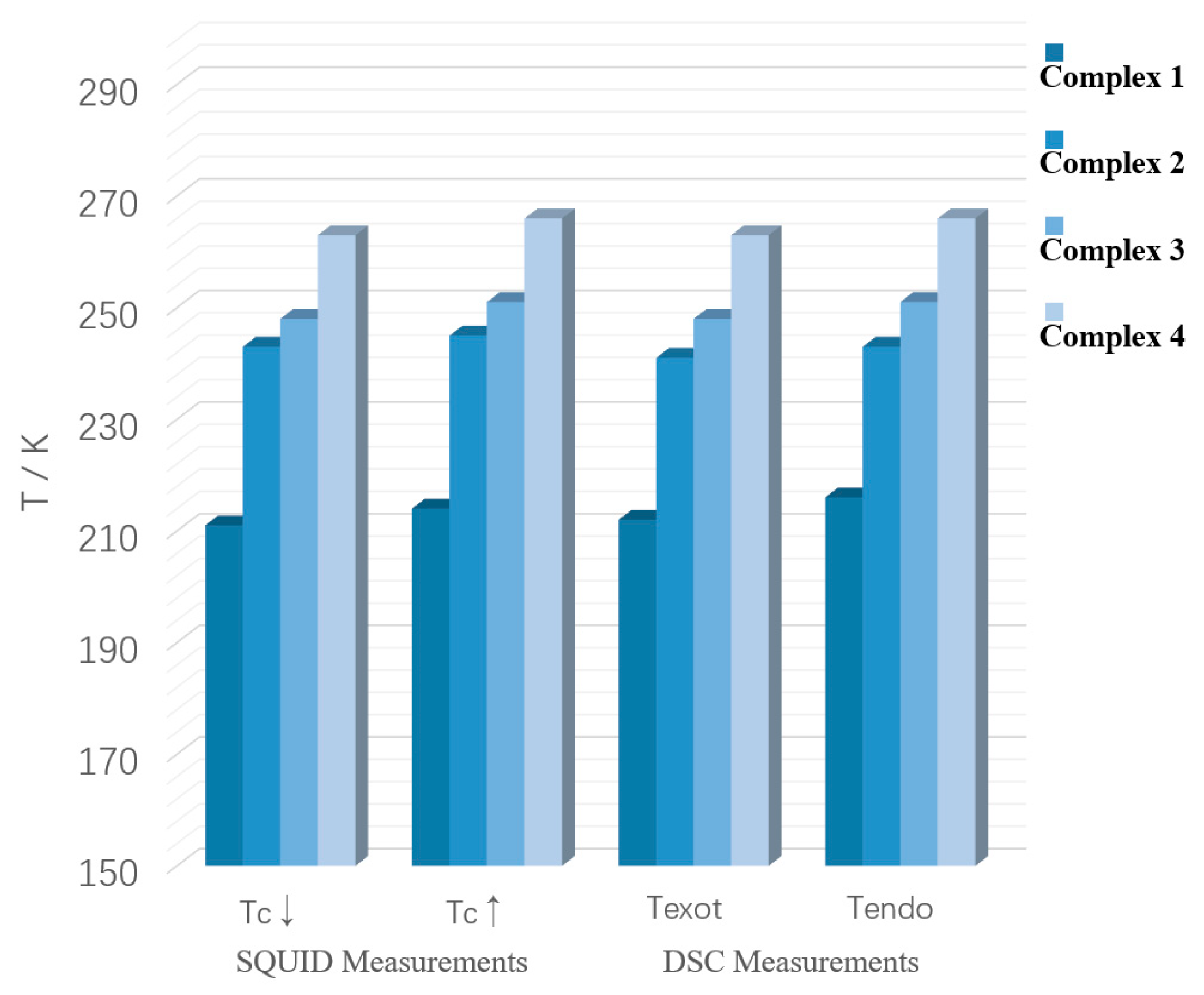


| SCO Complex | Tc↓ a (K) | Tc↑ b (K) |
|---|---|---|
| Complex 1 | 211 | 214 |
| Complex 2 | 243 | 245 |
| Complex 3 | 248 | 251 |
| Complex 4 | 263 | 266 |
| SCO Complex | Texot a (K) | Tendo b (K) |
|---|---|---|
| Complex 1 | 212 | 216 |
| Complex 2 | 241 | 243 |
| Complex 3 | 248 | 251 |
| Complex 4 | 263 | 266 |
| Fe (II) Triazoles Coordination | LUMO/Hartrees | HOMO/Hartrees | Energy Gap ΔE/Hartrees | Dipole Moment/D |
|---|---|---|---|---|
| [Fe2(4-H-1,2,4-triazole)9]4+ | −0.3757 | −0.5508 | 0.1751 | 3.131447 |
| [Fe3(4-H-1,2,4-triazole)12]6+ | −0.5310 | −0.6881 | 0.1571 | 4.758551 |
| [Fe4(4-H-1,2,4-triazole)15]8+ | −0.6675 | −0.7857 | 0.1182 | 3.924752 |
| [Fe(4-H-1,2,4-triazole)6]2+ | −0.2124 | −0.3792 | 0.1668 | 3.754252 |
| [Fe(4-NH2-1,2,4-triazole)6]2+ | −0.1883 | −0.3632 | 0.1749 | 0.207855 |
| [Fe(4-NHCOOCH3-1,2,4-triazole)6]2+ | −0.2100 | −0.3781 | 0.1681 | 2.146118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, R.; Liu, J.; Ji, B.; Wang, W.; Peng, M.; Huang, C.; Cheng, L.; Ding, Y. Synthesis and Characterization of a One-Dimensional Malleable Spin-Crossover Polymer Complex Modified by Methoxy Polyethylene Glycol. Polymers 2023, 15, 2363. https://doi.org/10.3390/polym15102363
Wang D, Zhang R, Liu J, Ji B, Wang W, Peng M, Huang C, Cheng L, Ding Y. Synthesis and Characterization of a One-Dimensional Malleable Spin-Crossover Polymer Complex Modified by Methoxy Polyethylene Glycol. Polymers. 2023; 15(10):2363. https://doi.org/10.3390/polym15102363
Chicago/Turabian StyleWang, Di, Ren Zhang, Jin Liu, Bibi Ji, Wenping Wang, Mengyuan Peng, Chen Huang, Lizhuoran Cheng, and Yi Ding. 2023. "Synthesis and Characterization of a One-Dimensional Malleable Spin-Crossover Polymer Complex Modified by Methoxy Polyethylene Glycol" Polymers 15, no. 10: 2363. https://doi.org/10.3390/polym15102363






