Synthesis and Characterization of a New Molecularly Imprinted Polymer for Selective Extraction of Mandelic Acid Metabolite from Human Urine as a Biomarker of Environmental and Occupational Exposures to Styrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Pre-Treatment of Urine
2.3. Analytical Instruments
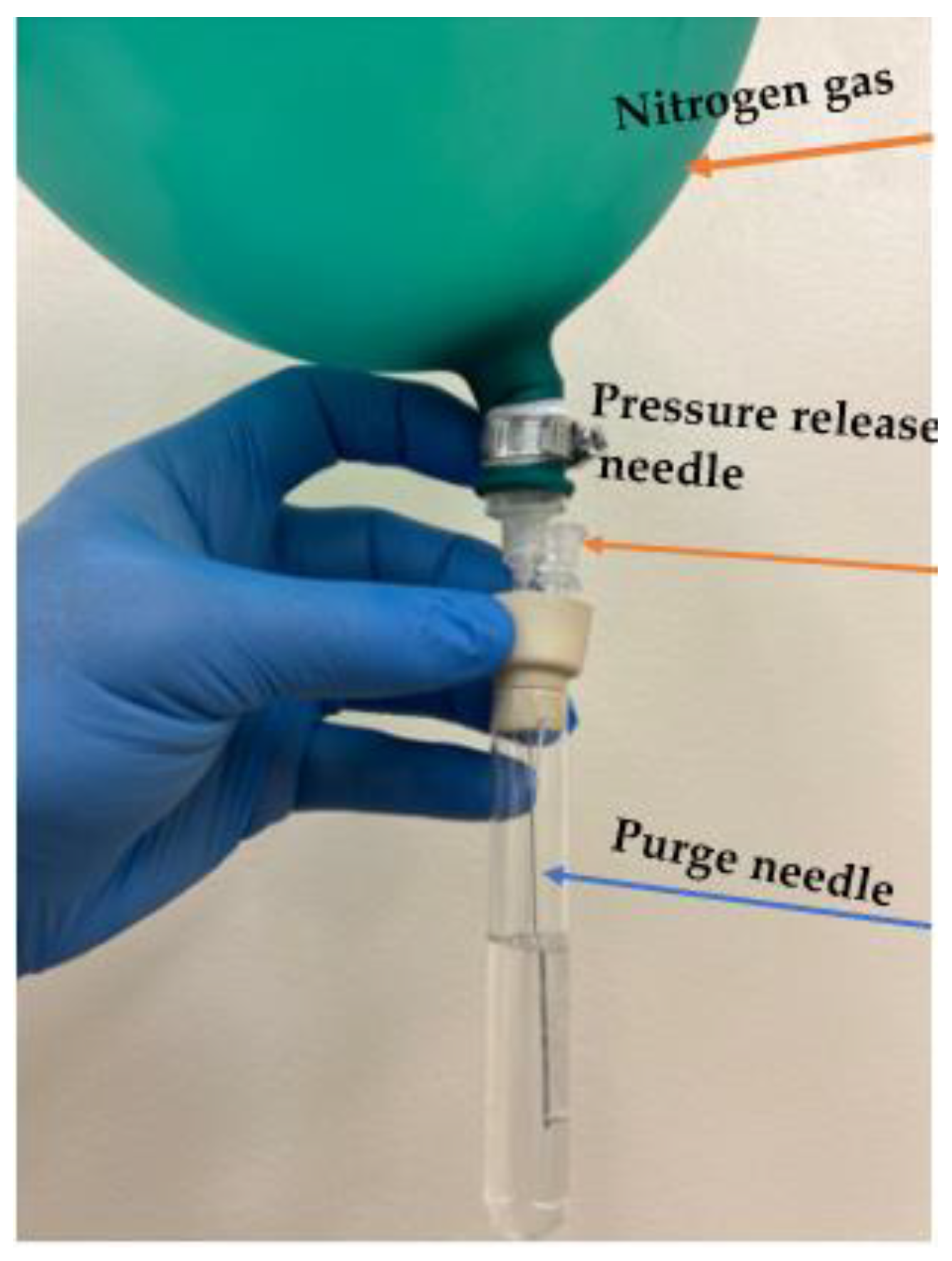
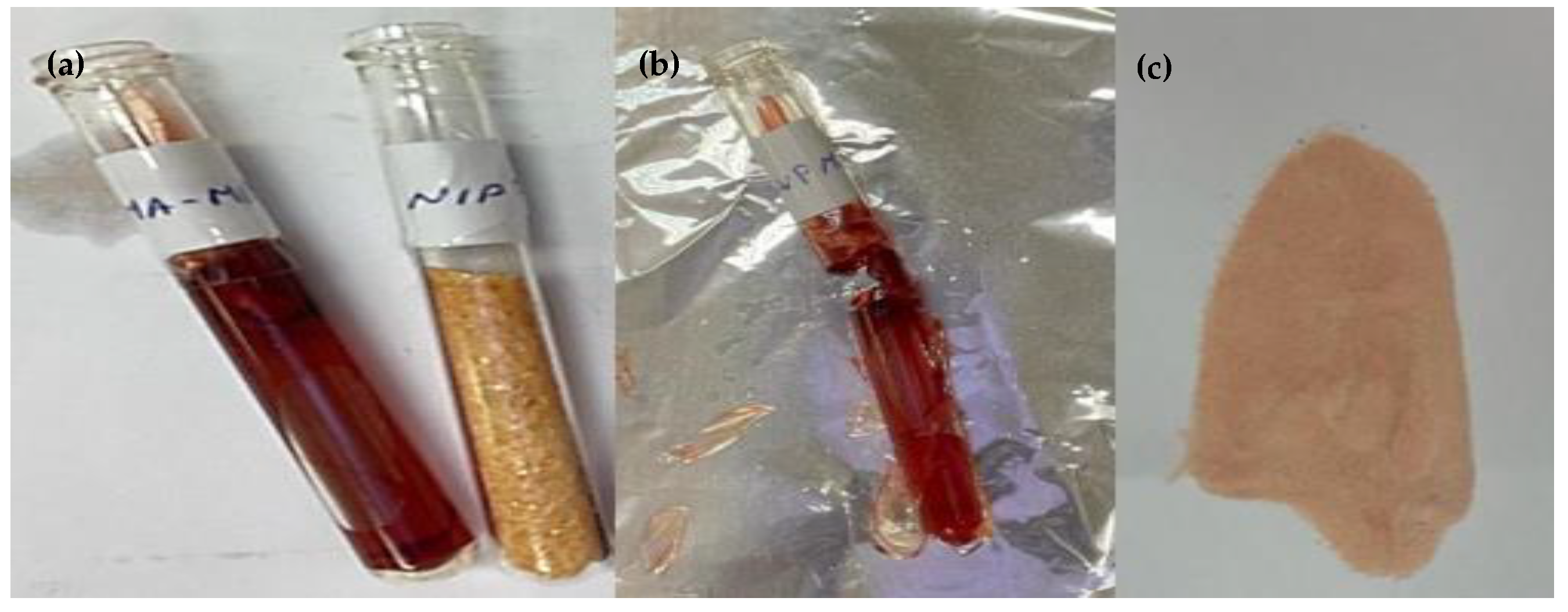
2.4. Synthesis of MA-MIP and NIP
2.5. MA Elution
2.6. Adsorption Experiments
2.7. Adsorptive Selectivity
2.8. Solid-phase Extraction Experiments
2.9. Adsorption Kinetics
2.10. Adsorption Isotherm
3. Results and Discussion
3.1. Characterization of Physical Properties of 4-VPMIP and NIP
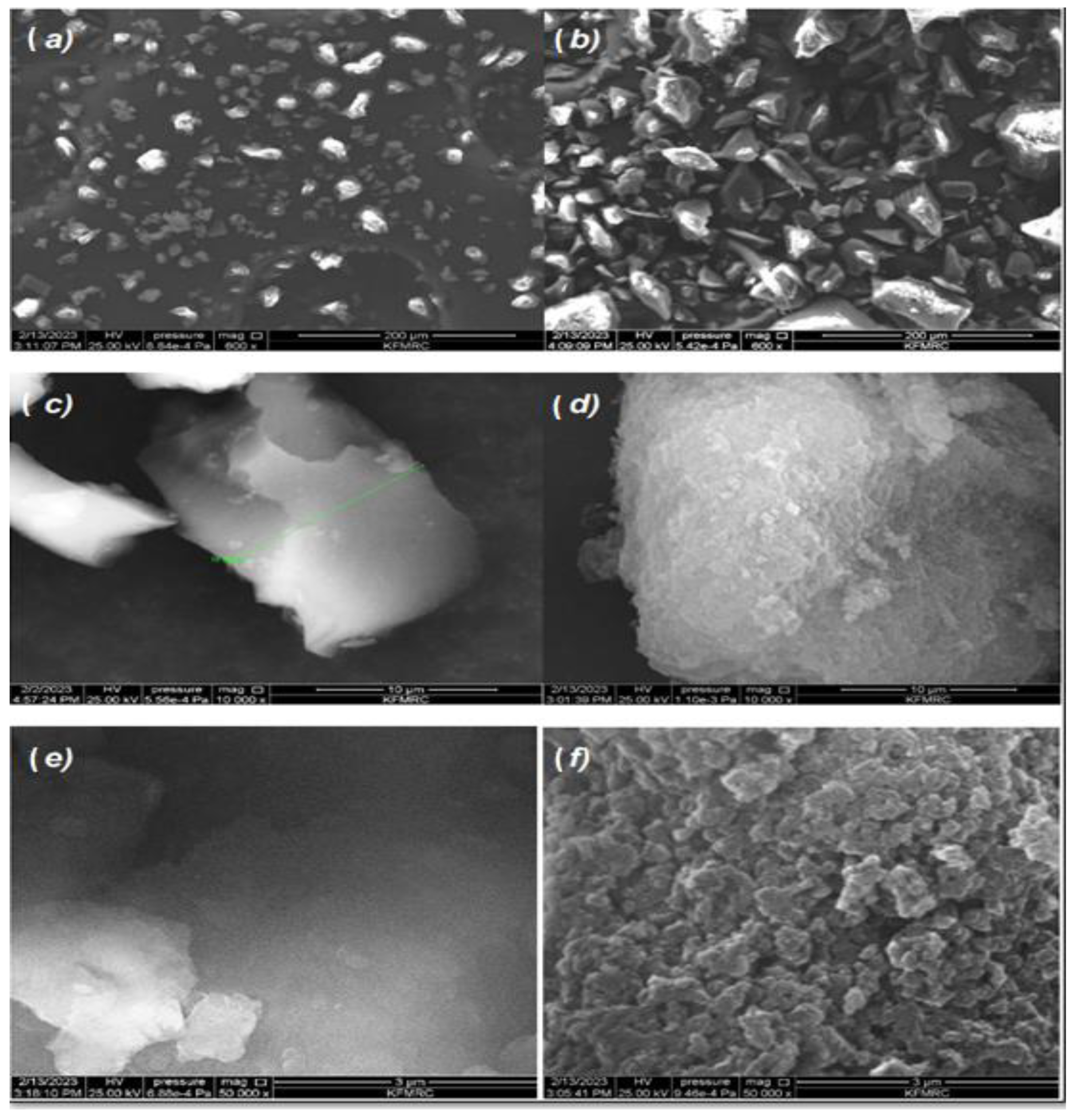
3.1.1. Scanning Electron Microscopy (SEM)
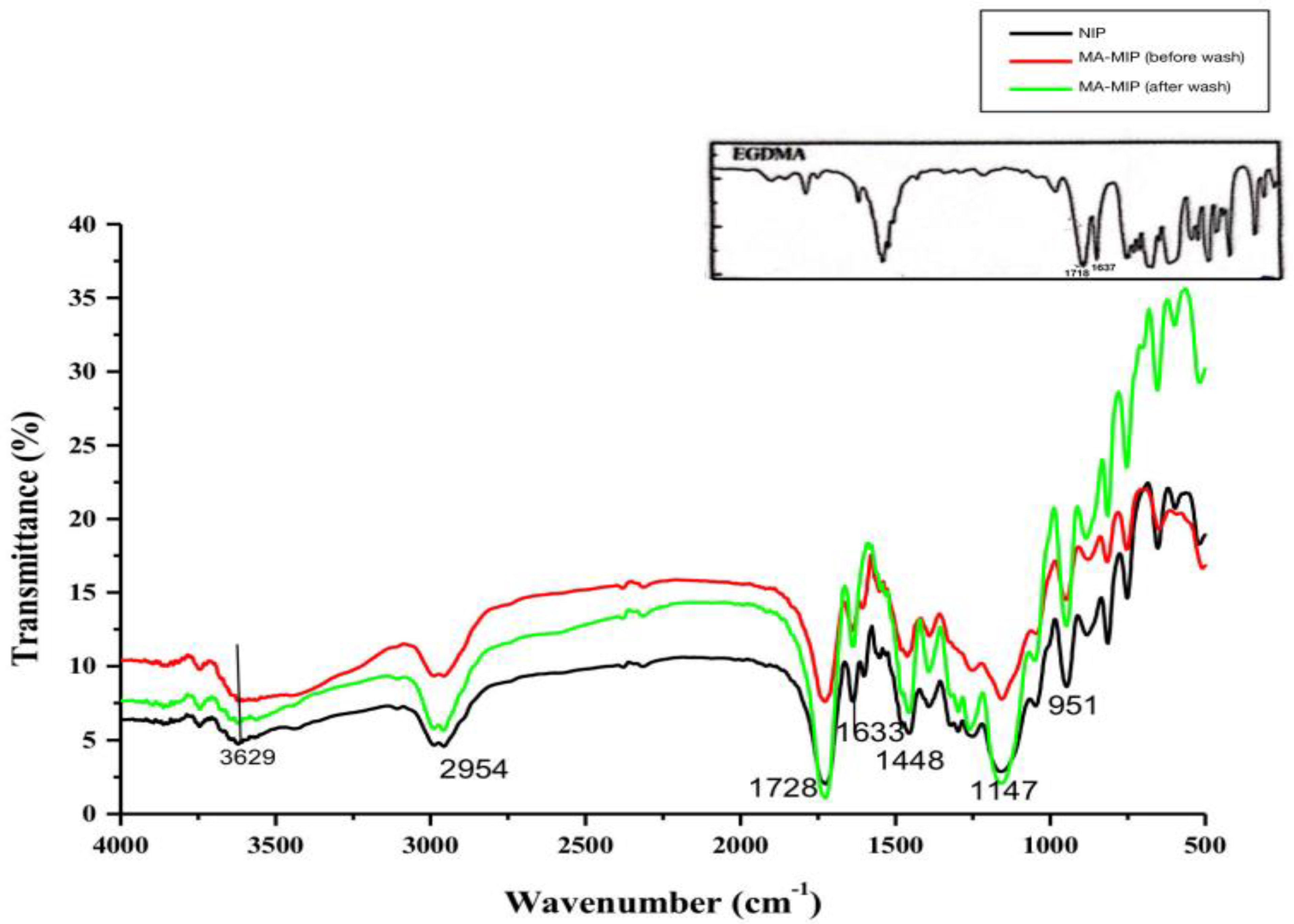
3.1.2. FT-IR Spectroscopy
3.2. Effect of Initial Concentration on Adsorption Capacity
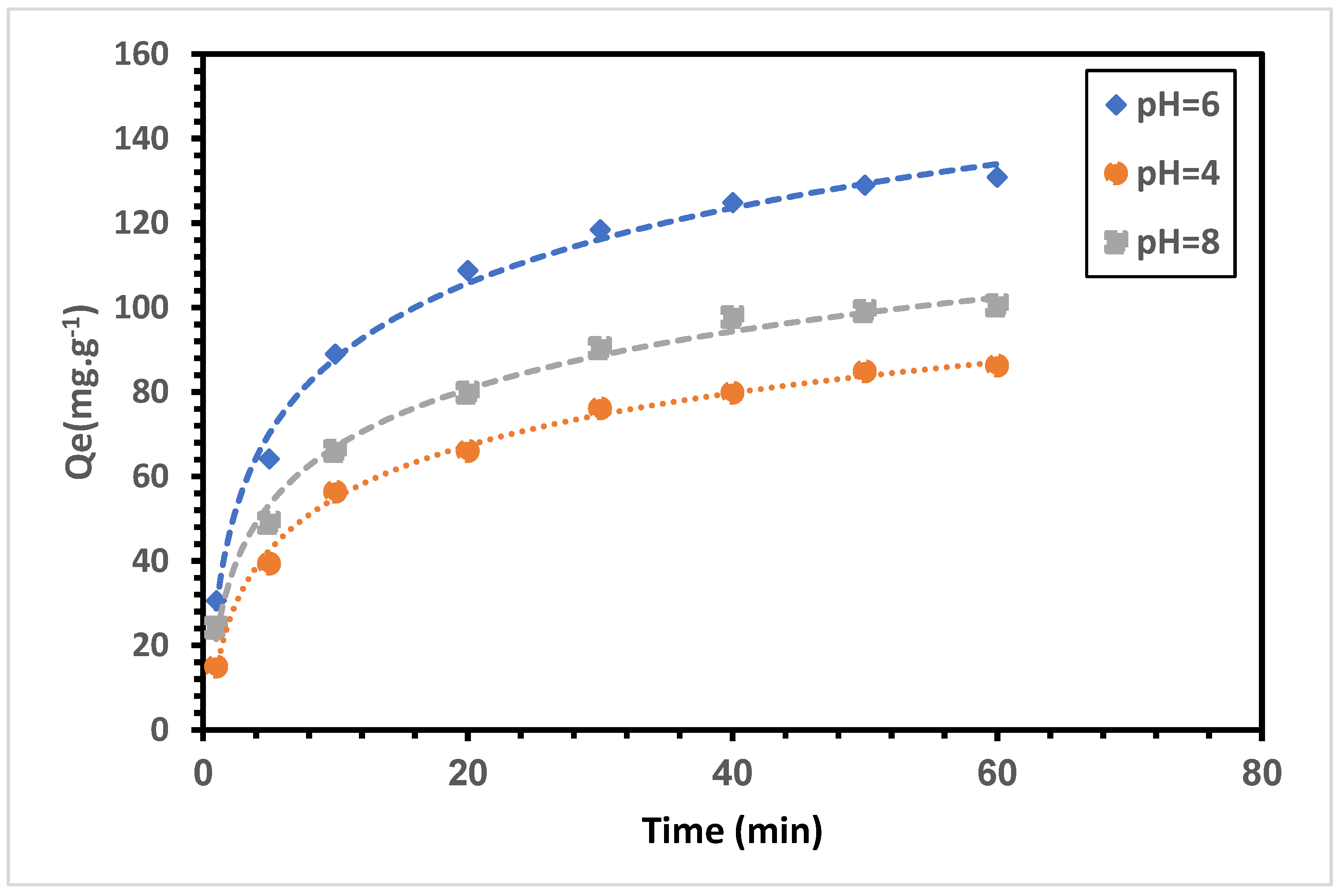
3.3. Effect of pH on Adsorption Capacity
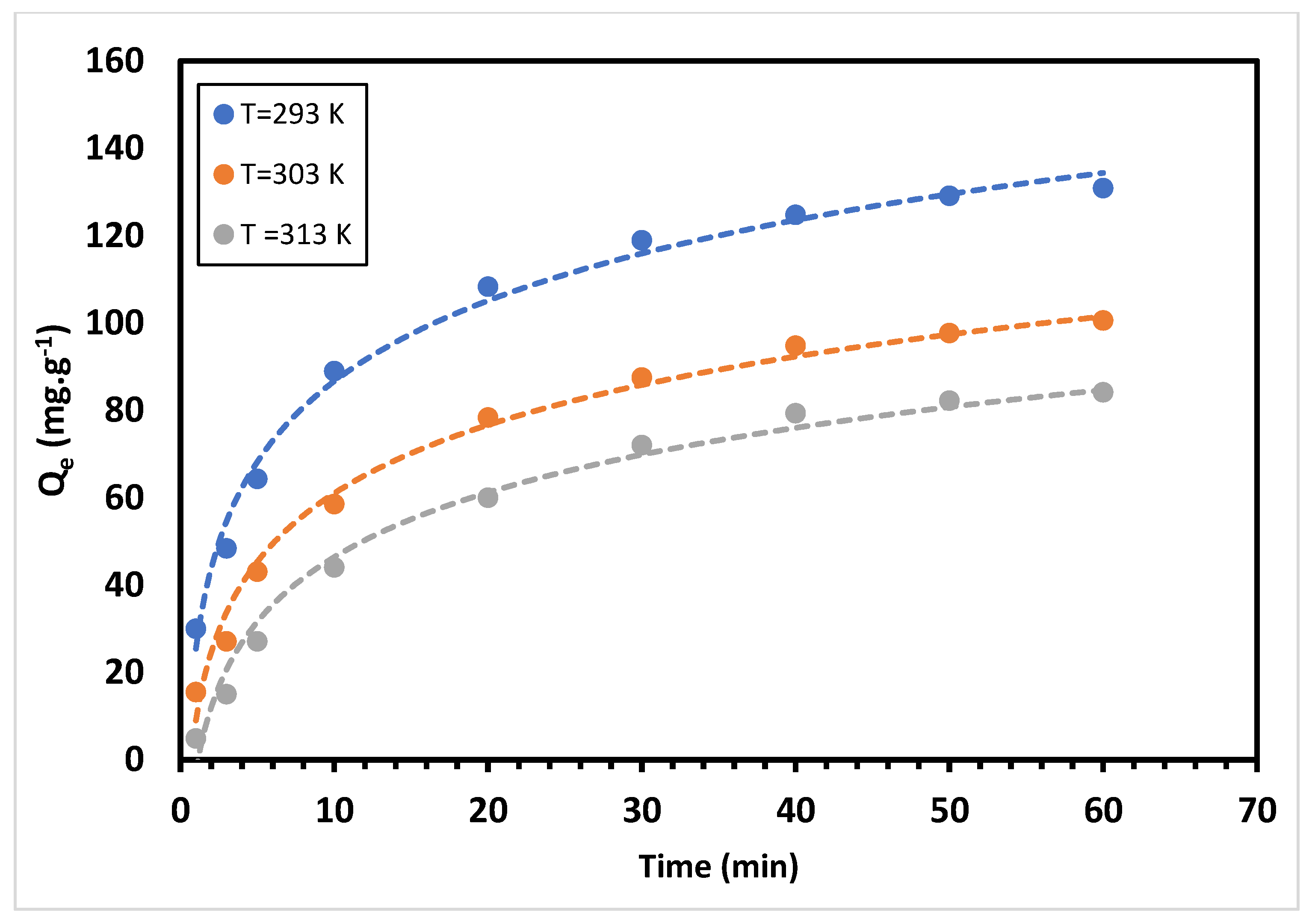
3.4. Effect of Temperature on Adsorption Capacity
3.5. Adsorptive Selectivity
3.6. Adsorption Kinetics and Isotherms
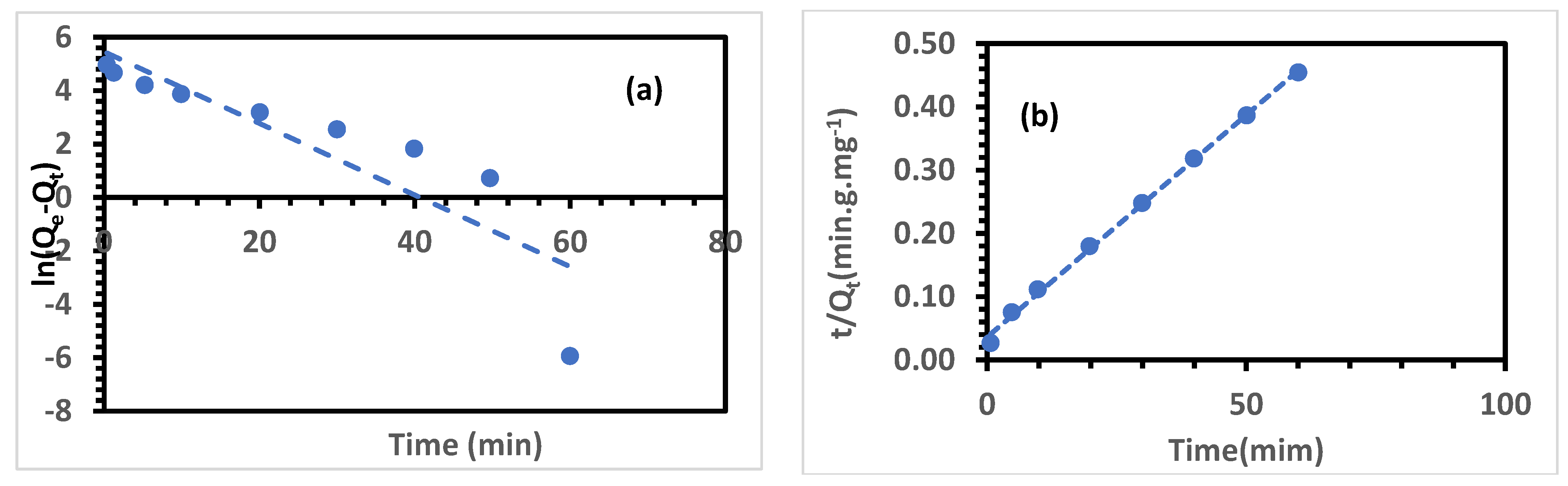
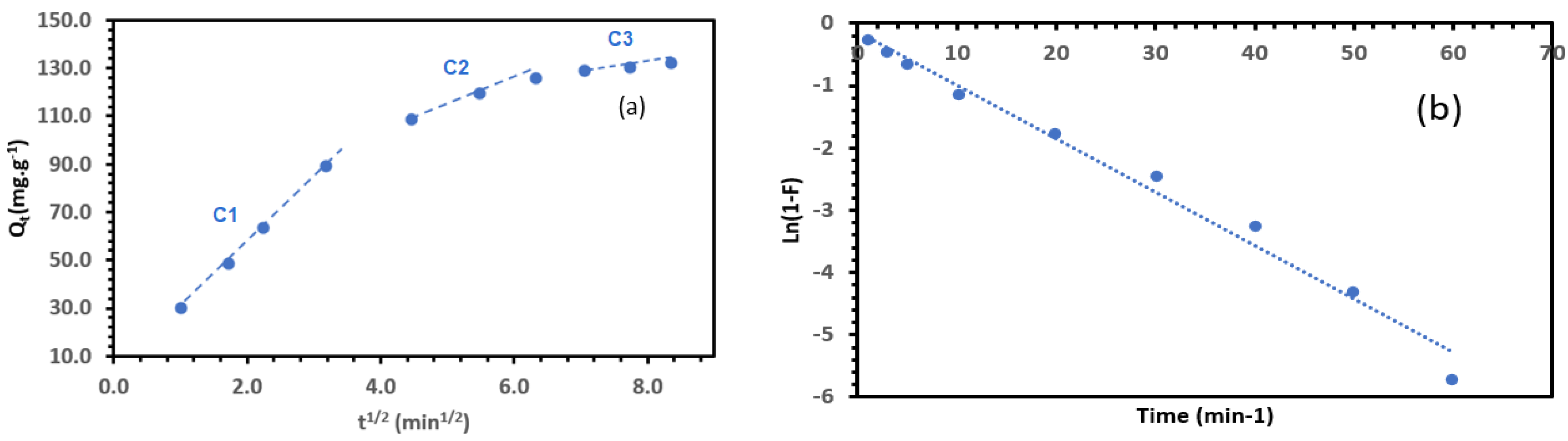
3.6.1. Adsorption Kinetics
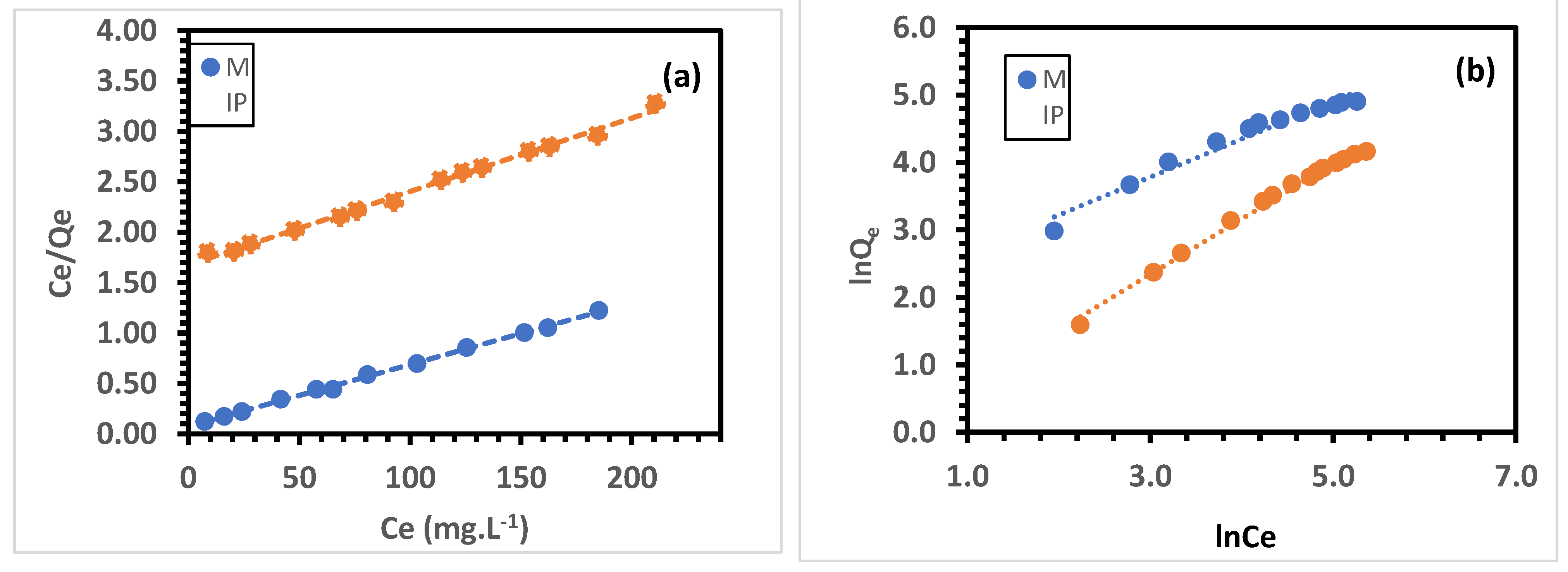
3.6.2. Adsorption Isotherm
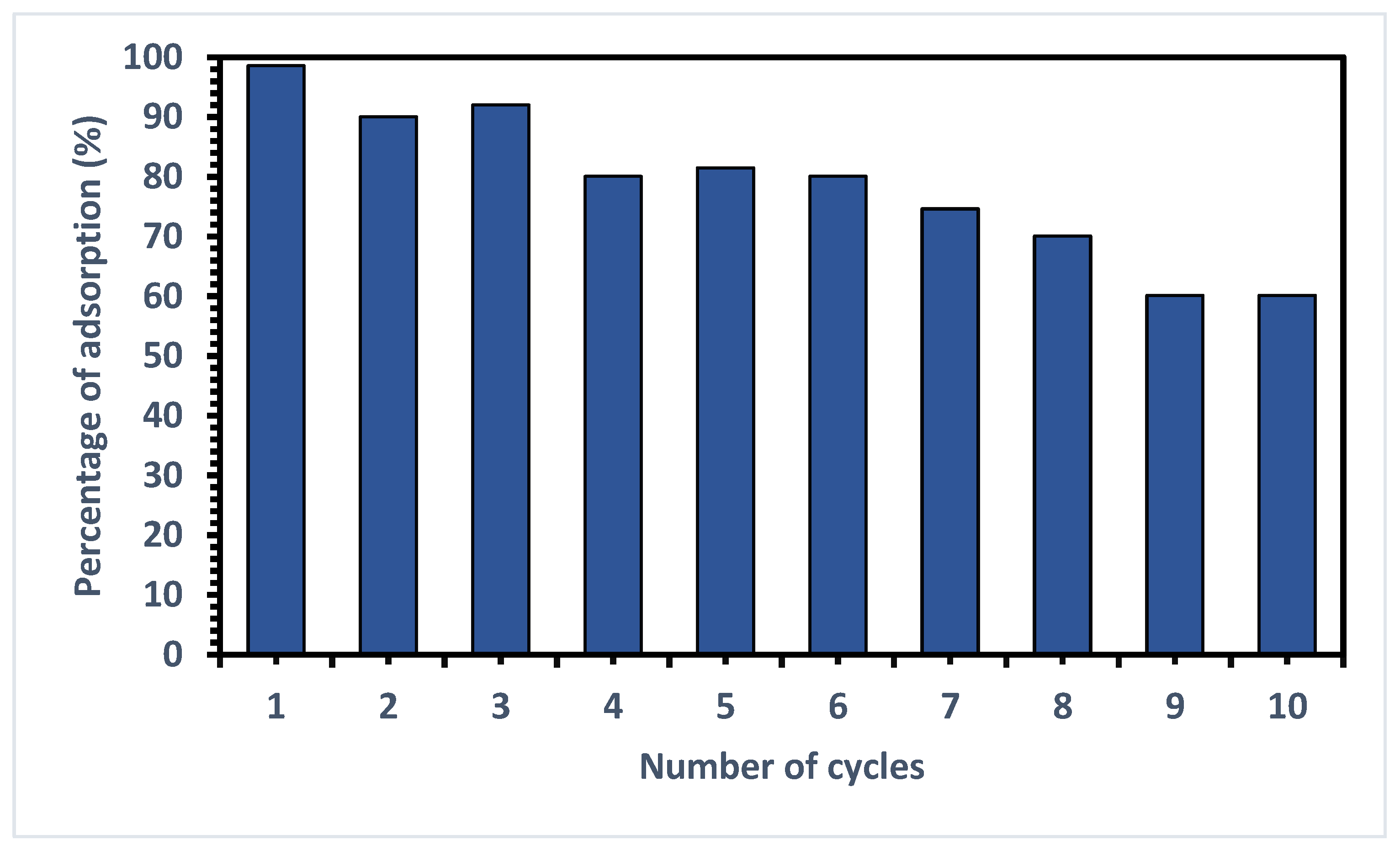
3.7. Reusability of SPE-4-VPMIP
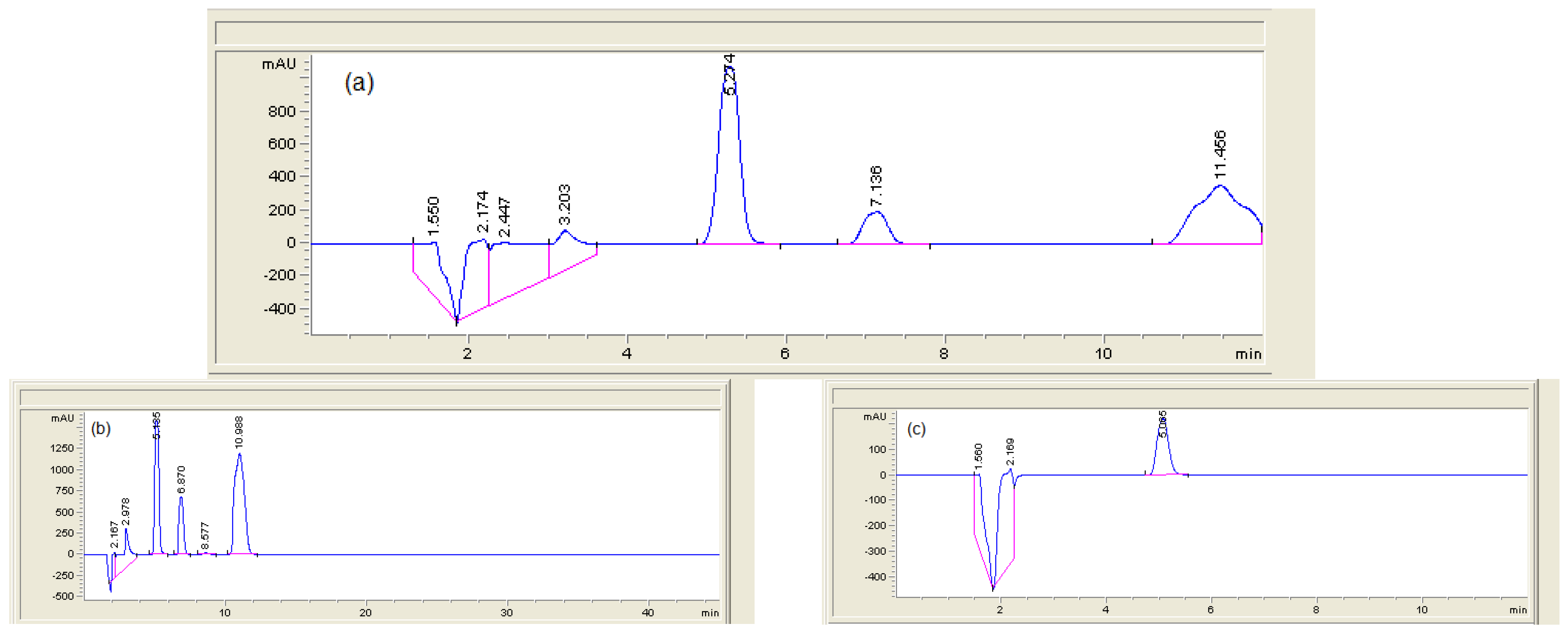
3.8. Application of the Proposed SPE4-VPMIP to the Extraction of MA from Human Urine with HPLC-DAD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahtaheri, S.; Abdollahi, M.; Golbabaei, F.; Rahimi, F.A.; Ghamari, F. Monitoring of Mandelic Acid as a Biomarker of Environmental and Occupational Exposures to Styrene. Int. J. Environ. 2008, 2, 169–176. [Google Scholar]
- Soleimani, E.; Bahrami, A.; Afkhami, A.; Shahna, F.G. Selective determination of mandelic acid in urine using molecularly imprinted polymer in microextraction by packed sorbent. Arch. Toxicol. J. 2018, 92, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Habibagahi, A.; Alderman, N.; Kubwabo, C. A review of the analysis of biomarkers of exposure to tobacco and vaping products. Anal. Methods 2020, 12, 4276–4302. [Google Scholar] [CrossRef] [PubMed]
- Capella, K.M.; Roland, K.; Geldner, N.; deCastro, B.R.; De Jesús, V.R.; van Bemmel, D.; Blount, B.C. Ethylbenzene and styrene exposure in the United States based on urinary mandelic acid and phenylglyoxylic acid: NHANES 2005–2006 and 2011–2012. Environ. Res. 2019, 171, 101–110. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Teiri, H.; Nazmara, S.; Parseh, I. Environmental and biological monitoring of exposures to VOCs in a petrochemical complex in Iran. Environ. Sci. Pollut. Res. 2018, 25, 6656–6667. [Google Scholar] [CrossRef]
- Mohamadyan, M.; Moosazadeh, M.; Borji, A.; Khanjani, N.; Moghadam, S.R. Occupational exposure to styrene and its relation with urine mandelic acid, in plastic injection workers. Environ. Monit. Assess J. 2019, 191, 62. [Google Scholar] [CrossRef]
- Bahrami, A.; Ghamari, F.; Yamini, Y.; Ghorbani Shahna, F.; Koolivand, A. Ion-pair-based hollow-fiber liquid-phase microextraction combined with high-performance liquid chromatography for the simultaneous determination of urinary benzene, toluene, and styrene metabolites. J. Sep. Sci. 2017, 41, 501–508. [Google Scholar] [CrossRef]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation, and biomonitoring of volatile organic compounds. Toxicol. Environ. Chem. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Takeuchi, A.; Namera, A.; Sakui, N.; Yamamoto, S.; Yamamuro, K.; Nishinoiri, O.; Endo, Y.; Endo, G. Direct methyl esterification with 2,2-dimethoxypropane for the simultaneous determination of urinary metabolites of toluene, xylene, styrene, and ethylbenzene by gas chromatography-mass spectrometry. J. Occup. Health 2019, 61, 82–90. [Google Scholar] [CrossRef]
- Arabi, M.; Ghaedi, M.; Ostovan, A.; Tashkhourian, J.; Asadallahzadeh, H. Synthesis and application of molecularly imprinted nanoparticles combined ultrasonic assisted for highly selective solid phase extraction trace amount of celecoxib from human plasma samples using design expert (DXB) software. Ultrason. Sonochem. 2016, 33, 67–76. [Google Scholar] [CrossRef]
- Organization, W.H. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; World Health Organization: Geneva, Switzerland, 1996; Volume 1. [Google Scholar]
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Shahna, F.G.; Farhadian, M. Facile and sensitive determination of urinary mandelic acid by combination of metal organic frameworks with microextraction by packed sorbents. J. Chromatogr. B 2019, 1114, 45–54. [Google Scholar] [CrossRef]
- Pratiwi, R.; Megantara, S.; Rahayu, D.; Pitaloka, I.; Hasanah, A.N. Comparison of Bulk and Precipitation Polymerization Method of Synthesis Molecular Imprinted Solid Phase Extraction for Atenolol using Methacrylic Acid. J. Young Pharm. 2019, 11, 12–16. [Google Scholar] [CrossRef]
- Javanbakht, M.; Namjumanesh, M.H.; Akbari-Adergani, B. Molecularly imprinted solid-phase extraction for the selective determination of bromhexine in human serum and urine with high performance liquid chromatography. Talanta 2009, 80, 133–138. [Google Scholar] [CrossRef]
- Ren, J.-W.; Luo, X.-Y.; Zhao, X.; Wang, X.; Zhu, J.; Bie, M.-J.; Liu, J.-S.; Cong, X.; Zou, X.-L. Determination of Aromatic Compounds Metabolites in Human Urine by Solid Phase Extraction-liquid Chromatography-Tandem Mass Spectrometry. J. Sichuan Univ. 2020, 51, 695–701. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Mulder, H.A.; Halquist, M.S. Growing Trends in the Efficient and Selective Extraction of Compounds in Complex Matrices Using Molecularly Imprinted Polymers and Their Relevance to Toxicological Analysis. J. Anal. Toxicol. 2021, 45, 312–321. [Google Scholar] [CrossRef]
- Wan, Y.; Ma, H.; Lu, B. MIPs in aqueous environments. Mol. Impr. Polym. Biotechnol. 2015, 150, 131–166. [Google Scholar] [CrossRef]
- Vieira, A.C.; Zampieri, R.A.; de Siqueira, M.E.P.B.; Martins, I.; Figueiredo, E.C. Molecularly imprinted solid-phase extraction and high-performance liquid chromatography with ultraviolet detection for the determination of urinary trans, trans-muconic acid: A comparison with ionic exchange extraction. Analyst 2012, 137, 2462–2469. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef]
- Pichon, V.; Delaunay, N.; Combès, A. Sample Preparation Using Molecularly Imprinted Polymers. Anal. Chem. 2019, 92, 16–33. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Marcé, R.M.; Cormack, P.A.G. Molecularly-imprinted polymers: Useful sorbents for selective extractions. Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Fauzi, D.; Witka, B.Z.; Rahayu, D.; Pratiwi, R. Molecular Imprinted Polymer for Ethylmorphine with Methacrylic Acid and Acrylamide as Functional Monomer in Butanol Using Two Polymerization Method. Mediterr. J. Chem. 2020, 10, 277–288. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Du, F.; Zheng, W.; Liu, Z.; Xu, Z.; Hu, X.; Liu, H. Dummy-template molecularly imprinted micro-solid-phase extraction coupled with high-performance liquid chromatography for bisphenol A determination in environmental water samples. Microchem 2019, 145, 337–344. [Google Scholar] [CrossRef]
- Pawliszyn, J. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists: Theory of Extraction Techniques, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 2, pp. 1–25. [Google Scholar]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Pratama, K.F.; Manik, M.E.R.; Rahayu, D.; Hasanah, A.N. Effect of the Molecularly Imprinted Polymer Component Ratio on Analytical Performance. Chem. Pharm. Bull. 2020, 68, 1013–1024. [Google Scholar] [CrossRef]
- Qin, S.; Deng, S.; Su, L.; Wang, P. Simultaneous determination of five sulfonamides in wastewater using group-selective molecularly imprinted solid-phase extraction coupled with HPLC-DAD. Anal. Methods 2012, 4, 4278–4283. [Google Scholar] [CrossRef]
- Vasconcelos, I.; da Silva, P.H.R.; Dias, D.R.D.; de Freitas Marques, M.B.; da Nova Mussel, W.; Pedrosa, T.A.; e Silva, M.E.S.R.; de Souza Freitas, R.F.; de Sousa, R.G.; Fernandes, C. Synthesis and characterization of a molecularly imprinted polymer (MIP) for solid-phase extraction of the antidiabetic gliclazide from human plasma. Mater. Sci. Eng. C 2020, 116, 111191. [Google Scholar] [CrossRef]
- Martín-Esteban, A. Molecularly Imprinted Polymers: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Tarley, C.R.; Segatelli, M.G.; César, I.C.; Lima, J.L.; Borges, D.L. Development of a solid-phase extraction method for preconcentration and determination of uric acid in human urine samples by UV-visible spectrophotometry. J. Braz. Chem. Soc. 2011, 22, 2140–2147. [Google Scholar]
- Mao, S.; Li, Y.; Li, X.; Sun, Y.; Wang, L.; Wu, Y. Chromatographic Resolution and Isotherm Determination of (R, S)-Mandelic Acid on Chiralcel-OD Column. J. Sep. Sci. 2012, 35, 2273–2281. [Google Scholar] [CrossRef]
- Jamoussi, B.; Chakroun, R.; Jablaoui, C.; Rhazi, L. Efficiency of Acacia Gummifera powder as biosorbent for simultaneous decontamination of water polluted with metals. Arab. J. Chem. 2020, 13, 7459–7481. [Google Scholar] [CrossRef]
- Hajri, A.K.; Jamoussi, B.; Albalawi, A.E.; Alhawiti, O.H.; Alsharif, A.A. Designing of modified ion-imprinted chitosan particles for selective removal of mercury (II) ions. Carbohydr. Polym. 2022, 286, 119207. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Hu, Y.; Yang, X.; Yao, S. Synthesis of a novel composite imprinted material based on multiwalled carbon nanotubes as a selective melamine absorbent. J. Agric. Food Chem. 2011, 59, 1063–1071. [Google Scholar] [CrossRef]
- Zakaria, N.D.; Yusof, N.A.; Haron, J.; Abdullah, A.H. Synthesis and Evaluation of a Molecularly Imprinted Polymer for 2,4-Dinitrophenol. Int. J. Mol. Sci. 2009, 10, 354–365. [Google Scholar] [CrossRef]
- Roland, R.M.; Bhawani, S.A. Synthesis and Characterization of Molecular Imprinting Polymer Microspheres of Piperine: Extraction of Piperine from Spiked Urine. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Liu, M.; Han, X.; Peng, Y.; Tian, X.; Liu, J.; Zhang, S. Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation. Appl. Sci. 2020, 10, 2868. [Google Scholar] [CrossRef]
- Martins, G.V.; Marques, A.C.; Fortunato, E.; Sales, M.G.F. Paper-based (bio)sensor for label-free detection of 3-nitrotyrosine in human urine samples using molecular imprinted polymer. Sens. Bio-Sens. Res. 2020, 28, 100333. [Google Scholar] [CrossRef]
- Kamran Bashir, K.; Guo, P.; Chen, G.; Li, Y.; Ge, Y.; Shu, H.; Fu, Q. Synthesis, characterization, and application of griseofulvin surface molecularly imprinted polymers as the selective solid phase extraction sorbent in rat plasma samples. Arab. J. Chem. 2020, 13, 4082–4091. [Google Scholar] [CrossRef]
- Roushani, M.; Nezhadali, A.; Jalilian, Z.; Azadbakht, A. Development of novel electrochemical sensor on the base of molecular imprinted polymer decorated on SiC nanoparticles modified glassy carbon electrode for selective determination of loratadine. Mater. Sci. Eng. C 2017, 71, 1106–1114. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, J. Selective detection of chloramphenicol based on molecularly imprinted solid-phase extraction in seawater from Jiaozhou Bay, China. Mar. Pollut. Bull. 2018, 133, 750–755. [Google Scholar] [CrossRef]
- Zhao, X.; Pei, W.; Guo, R.; Li, X. Selective adsorption and purification of the acteoside in cistanche tubulosa by molecularly imprinted polymers. Front. Chem. 2020, 7, 903. [Google Scholar] [CrossRef]






| Code | 4-VPMIP | NIP |
|---|---|---|
| Template (mmole) | MA (1) | -------- |
| Functional Monomer (mmole) | 4-VP (4) | 4-VP (4) |
| Cross-linker (mmole) | EGDMA (20) | EGDMA (20) |
| Porogen (mL) | ACN (3 mL) | ACN (3 mL) |
| Equation * | Definition | Equation Number |
|---|---|---|
| Pseudo-first-order model (PFO) | (7) | |
| Pseudo-second-order model (PSO) | (8) |
| Target and Metabolites Solution | Polymer | Kd (mL/g) | IF | αT/M | |
|---|---|---|---|---|---|
| αMA/PGA | αMA/Hip | ||||
| MA | MIP | 654.18 | 2.31 | 2.91 | 4.21 |
| NIP | 283.60 | ||||
| PGA | MIP | 219.25 | 0.79 | ||
| NIP | 276.75 | ||||
| Hip | MIP | 154.25 | 0.54 | ||
| NIP | 281.75 | ||||
| Qexp (mg.g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| Q1,cal | k1 (min−1) | Q2,cal | k2 (g.mg−1.min−1) | |||
| 130.84 | 234.042 | 0.7754 | 0.134 | 142.857 | 0.998 | 1.357.10–3 |
| Intraparticle Diffusion Model | Liquid Film Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|
| Kd1 | Kd2 | Kd3 | c1 | c2 | c3 | kf | Intercept |
| 27.353 | 9.255 | 2.296 | 2.149 | 67.899 | 112.8 | 0.086 | 0.138 |
| Polymer | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|
| Qm (mg.g−1) | k (L.mg−1) | R2 | 1/n | kf [(mg.g−1)1/n] | R2 | |
| MIP | 163.93 | 0.0821 | 0.9983 | 0.5627 | 8.1327 | 0.9631 |
| NIP | 136.98 | 0.0043 | 0.9943 | 0.9926 | 0.8814 | 0.9926 |
| Spiked Urine (mg.L−1) | Recovery (%) | RSD (%) |
|---|---|---|
| 50 | 96 | 4.8 |
| 100 | 98 | 5.1 |
| 150 | 98 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qronfla, M.M.; Jamoussi, B.; Chakroun, R. Synthesis and Characterization of a New Molecularly Imprinted Polymer for Selective Extraction of Mandelic Acid Metabolite from Human Urine as a Biomarker of Environmental and Occupational Exposures to Styrene. Polymers 2023, 15, 2398. https://doi.org/10.3390/polym15102398
Qronfla MM, Jamoussi B, Chakroun R. Synthesis and Characterization of a New Molecularly Imprinted Polymer for Selective Extraction of Mandelic Acid Metabolite from Human Urine as a Biomarker of Environmental and Occupational Exposures to Styrene. Polymers. 2023; 15(10):2398. https://doi.org/10.3390/polym15102398
Chicago/Turabian StyleQronfla, Murad. M., Bassem Jamoussi, and Radhouane Chakroun. 2023. "Synthesis and Characterization of a New Molecularly Imprinted Polymer for Selective Extraction of Mandelic Acid Metabolite from Human Urine as a Biomarker of Environmental and Occupational Exposures to Styrene" Polymers 15, no. 10: 2398. https://doi.org/10.3390/polym15102398
APA StyleQronfla, M. M., Jamoussi, B., & Chakroun, R. (2023). Synthesis and Characterization of a New Molecularly Imprinted Polymer for Selective Extraction of Mandelic Acid Metabolite from Human Urine as a Biomarker of Environmental and Occupational Exposures to Styrene. Polymers, 15(10), 2398. https://doi.org/10.3390/polym15102398







