Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Nanofibers and Nanoparticles
2.3. Characterization
2.4. Experimental Design
3. Results
3.1. Precursors Selection Rationale
- Creating in situ a reducing environment because it is completely disintegrated under an inert environment to zero-valent nickel (Ni°), as reported by many researchers; upon heating, the acetate anion breaks down to produce reducing gases (CO and H2) [48].
- Having a polycondensation tendency (as illustrated in Equation (2)), which helps preserve the formed nanofibrous structure throughout the calcination process [49].

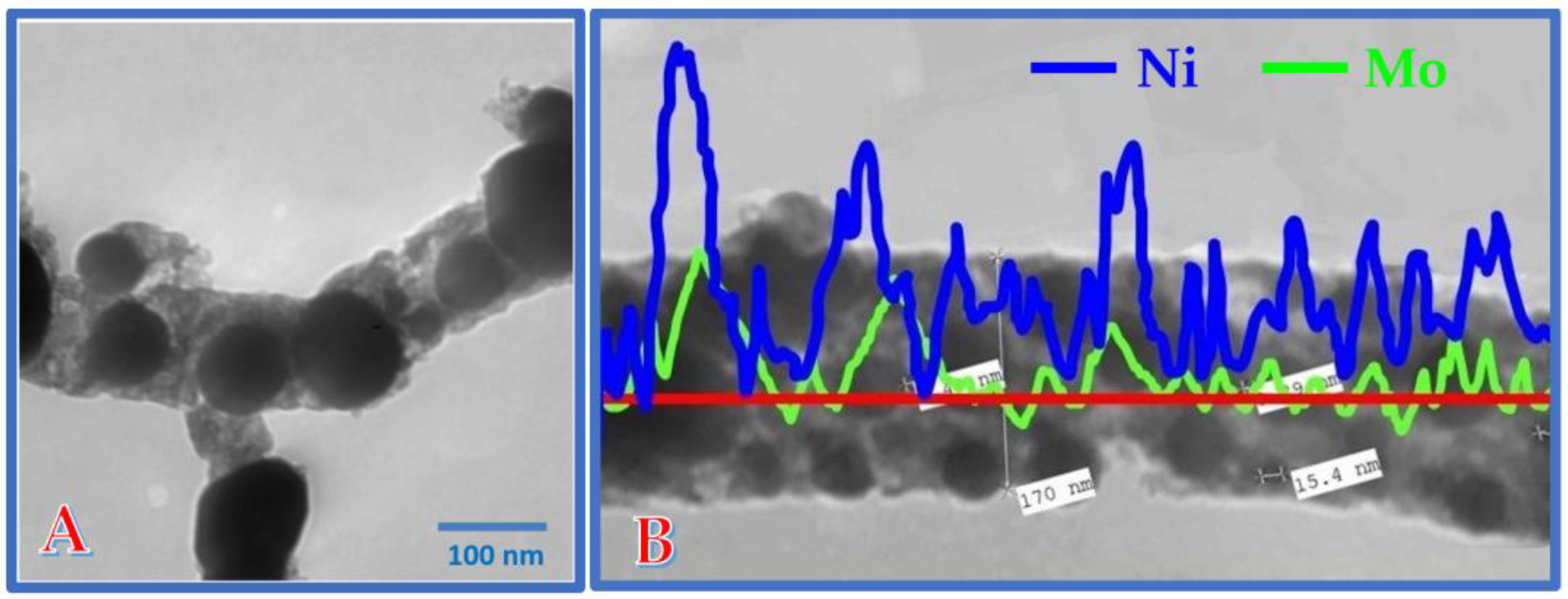
3.2. Characterization of the Prepared Composites
3.3. Electrochemical Performance
3.3.1. Electroactive Surface Area (ESA)
3.3.2. Influence of CNFs Matrix and Mo2C Incorporation
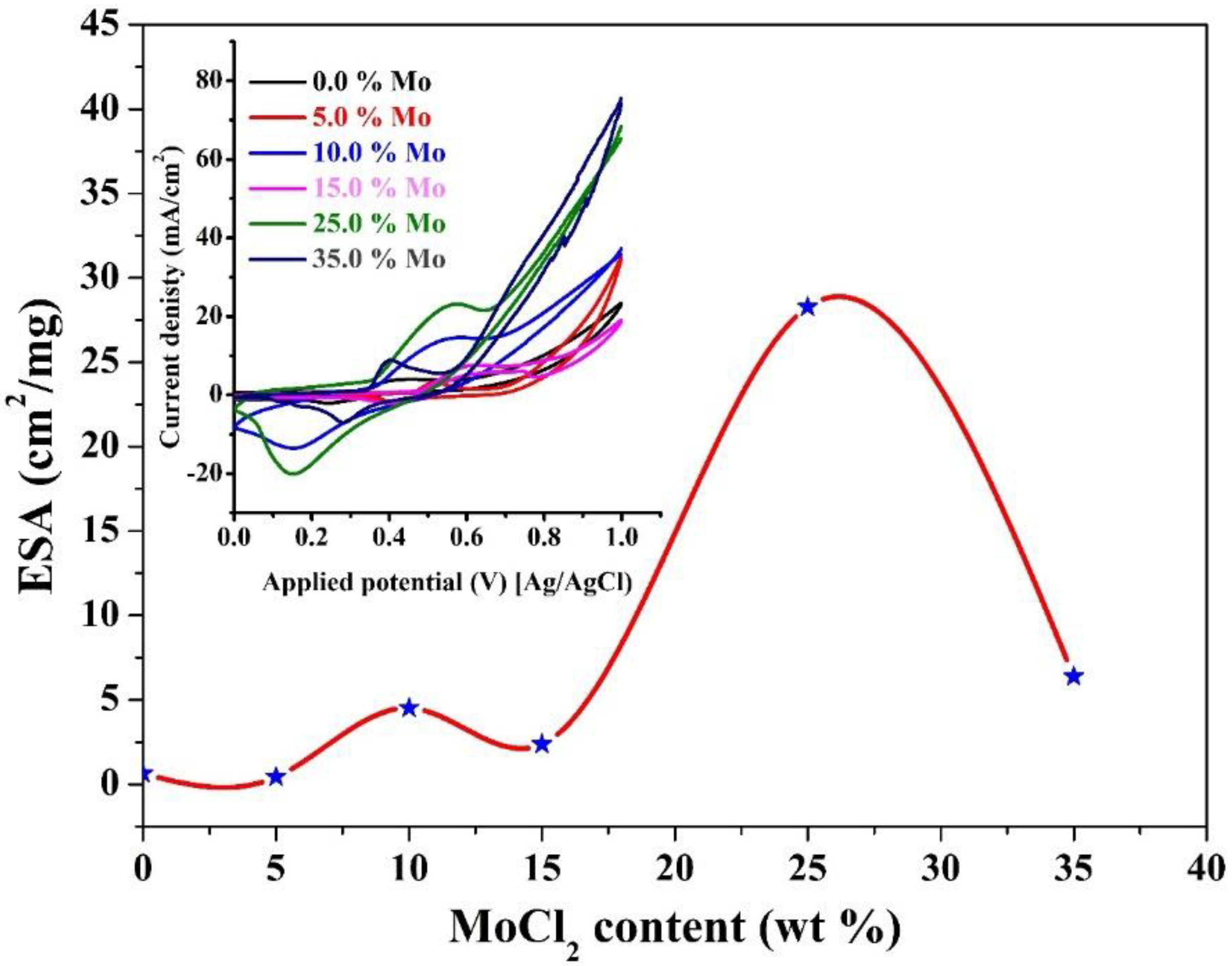
3.3.3. Influence Mo2C Content and Methanol Concentration
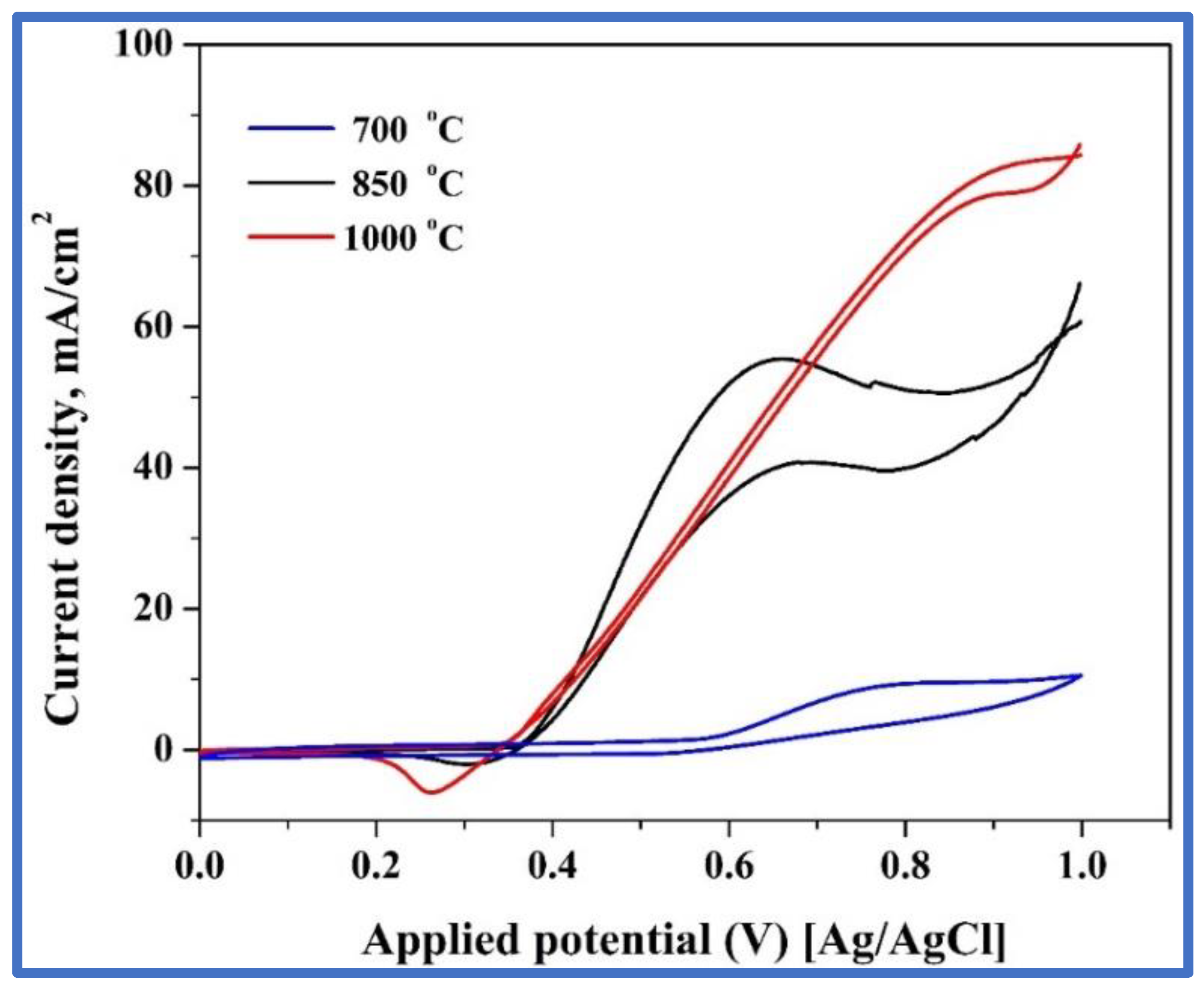
3.3.4. Effect of Calcination Temperature
3.3.5. Effect of Nanostructure Morphology
3.3.6. Electrooxidation Mechanism
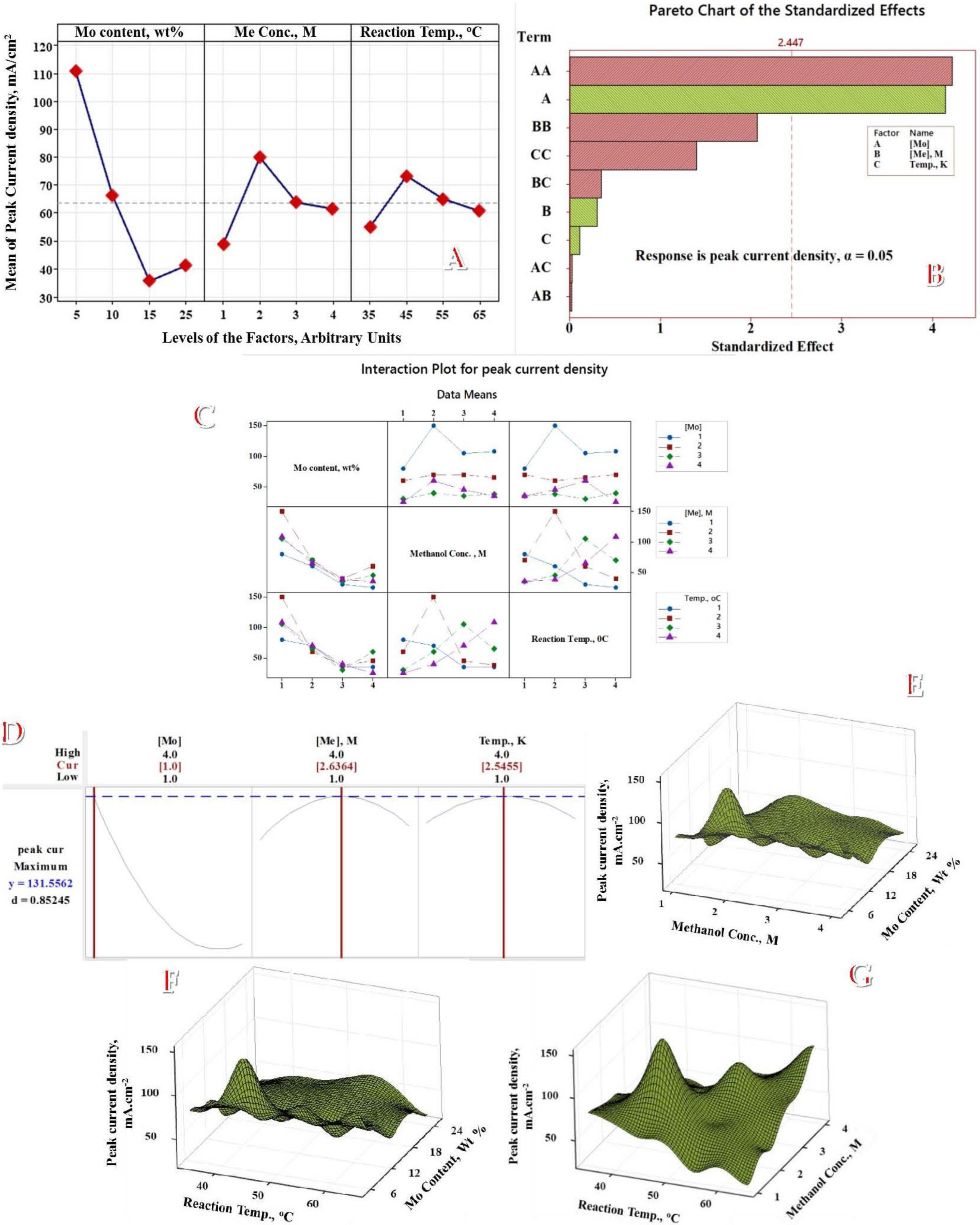
3.4. Taguchi Optimization of Methanol Electrooxidation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, N.; Ullah, S.; Khan, S.; Guziejewski, D.; Mirceski, V. A review: Metal-organic framework based electrocatalysts for methanol electro-oxidation reaction. Int. J. Hydrog. Energy 2022, 48, 3340–3354. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Qin, H.; Chen, X.; Li, J.; Jiao, L. Integrating energy-saving hydrogen production with methanol electrooxidation over Mo modified Co 4 N nanoarrays. J. Mater. Chem. A 2021, 9, 21094–21100. [Google Scholar] [CrossRef]
- Sanchez, C.; Espinos, F.J.; Barjola, A.; Escorihuela, J.; Compañ, V. Hydrogen Production from Methanol–Water Solution and Pure Water Electrolysis Using Nanocomposite Perfluorinated Sulfocationic Membranes Modified by Polyaniline. Polymers 2022, 14, 4500. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, E.; Caravaca, A.; Vernoux, P.; Dorado, F.; de Lucas-Consuegra, A. Over-faradaic hydrogen production in methanol electrolysis cells. Chem. Eng. J. 2020, 396, 125217. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Kamarudin, N.H.N. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrog. Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.; Daud, W.; Yaakob, Z.; Kadhum, A. Novel anode catalyst for direct methanol fuel cells. Sci. World J. 2014, 2014, 547604. [Google Scholar] [CrossRef]
- Sarkar, R.; Farghaly, A.A.; Arachchige, I.U. Oxidative Self-Assembly of Au/Ag/Pt Alloy Nanoparticles into High-Surface Area, Mesoporous, and Conductive Aerogels for Methanol Electro-oxidation. Chem. Mater. 2022, 34, 5874–5887. [Google Scholar] [CrossRef]
- Wang, L.; Tian, X.L.; Xu, Y.; Zaman, S.; Qi, K.; Liu, H.; Xia, B.Y. Engineering one-dimensional and hierarchical PtFe alloy assemblies towards durable methanol electrooxidation. J. Mater. Chem. A 2019, 7, 13090–13095. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.; Ismail, A.; Rahman, M.; Yusof, N.; Aziz, F. One-pot synthesis of efficient reduced graphene oxide supported binary Pt-Pd alloy nanoparticles as superior electro-catalyst and its electro-catalytic performance toward methanol electro-oxidation reaction in direct methanol fuel cell. J. Alloys Compd. 2019, 793, 232–246. [Google Scholar] [CrossRef]
- Ravichandran, S.; Bhuvanendran, N.; Xu, Q.; Maiyalagan, T.; Su, H. Improved methanol electrooxidation catalyzed by ordered mesoporous Pt-Ru-Ir alloy nanostructures with trace Ir content. Electrochim. Acta 2021, 394, 139148. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, F.; Gan, M.; Ma, L.; Zhang, Y.; Li, X.; Wang, L.; Hua, X. Highly graphitized carbon-wrapped PtFeCo alloy with enhanced durability and activity toward methanol electro-oxidation. Mater. Today Chem. 2022, 24, 100788. [Google Scholar] [CrossRef]
- Barakat, N.A.; Yassin, M.A.; Al-Mubaddel, F.S.; Amen, M.T. New electrooxidation characteristic for Ni-based electrodes for wide application in methanol fuel cells. Appl. Catal. A Gen. 2018, 555, 148–154. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. In-situ synthesis of Ni/N-doped CNFs-supported graphite disk as effective immobilized catalyst for methanol electrooxidation. Int. J. Hydrog. Energy 2015, 40, 14845–14856. [Google Scholar] [CrossRef]
- Yang, J.-H.; Song, X.; Zhao, X.; Wang, Y.; Yang, Y.; Gao, L. Nickel phosphate materials regulated by doping cobalt for urea and methanol electro-oxidation. Int. J. Hydrog. Energy 2019, 44, 16305–16314. [Google Scholar] [CrossRef]
- Ye, N.; Zhao, P.; Qi, X.; Zhang, R.; Yan, B.; Sheng, W.; Jiang, Z.; Fang, T. Probing the activity origin of the enhanced methanol electrooxidation on Ni-induced PdNix (OH) y-TaN/C catalyst with nitrogen vacancies. Appl. Catal. B Environ. 2023, 322, 122142. [Google Scholar] [CrossRef]
- Moazzami, N.; Khadempir, S.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Enhanced methanol electrooxidation by electroactivated Pd/Ni (OH) 2/N-rGO catalyst. Int. J. Hydrog. Energy 2022, 48, 6680–6690. [Google Scholar] [CrossRef]
- Li, J.; Xing, C.; Zhang, Y.; Zhang, T.; Spadaro, M.C.; Wu, Q.; Yi, Y.; He, S.; Llorca, J.; Arbiol, J. Nickel iron diselenide for highly efficient and selective electrocatalytic conversion of methanol to formate. Small 2021, 17, 2006623. [Google Scholar] [CrossRef]
- Oloye, F.; McCue, A.; Anderson, J. n-Heptane hydroconversion over sulfated-zirconia-supported molybdenum carbide catalysts. Appl. Petrochem. Res. 2016, 6, 341–352. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Salley, S.O.; Ng, K.S. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Deeva, E.B.; Kurlov, A.; Abdala, P.M.; Lebedev, D.; Kim, S.M.; Gordon, C.P.; Tsoukalou, A.; Fedorov, A.; Müller, C.R. In situ XANES/XRD study of the structural stability of two-dimensional molybdenum carbide Mo2CT x: Implications for the catalytic activity in the water–gas shift reaction. Chem. Mater. 2019, 31, 4505–4513. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Lødeng, R.; Bergem, H.; Stöcker, M.; Hannevold, L.; Blekkan, E.A. Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal. Today 2014, 223, 44–53. [Google Scholar] [CrossRef]
- Cheng, H.; Ding, L.X.; Chen, G.F.; Zhang, L.; Xue, J.; Wang, H. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions. Adv. Mater. 2018, 30, 1803694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Smith, K.J. Understanding selectivity changes during hydrodesulfurization of dibenzothiophene on Mo2C/carbon catalysts. J. Catal. 2019, 369, 427–439. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhu, Y.; Tian, D.; Wang, C.; Lu, X. Mo/Mo2C encapsulated in nitrogen-doped carbon nanofibers as efficiently integrated heterojunction electrocatalysts for hydrogen evolution reaction in wide pH range. Appl. Surf. Sci. 2019, 496, 143672. [Google Scholar] [CrossRef]
- Wu, Q.; Christensen, J.M.; Chiarello, G.L.; Duchstein, L.D.; Wagner, J.B.; Temel, B.; Grunwaldt, J.-D.; Jensen, A.D. Supported molybdenum carbide for higher alcohol synthesis from syngas. Catal. Today 2013, 215, 162–168. [Google Scholar] [CrossRef]
- Kawamura, G.; Okamoto, H.; Ishikawa, A.; Kudo, T. Tungsten molybdenum carbide for electrocatalytic oxidation of methanol. J. Electrochem. Soc. 1987, 134, 1653. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Zhang, M.; Jiang, Z.; Jiang, T.; Xie, J. Pt supported on Mo2C particles with synergistic effect and strong interaction force for methanol electro-oxidation. Electrochim. Acta 2013, 95, 218–224. [Google Scholar] [CrossRef]
- Duraisamy, M.; Mari, E.; Chinnuswamy, V.; Senthilkumar, S.; Lin, Y.-C.; Kumar Ponnusamy, V. Novel palladium-decorated molybdenum carbide/polyaniline nanohybrid material as superior electrocatalyst for fuel cell application. Int. J. Hydrog. Energy 2022, 47, 37599–37608. [Google Scholar] [CrossRef]
- Barakat, N.A.; Motlak, M.; Ghouri, Z.K.; Yasin, A.S.; El-Newehy, M.H.; Al-Deyab, S.S. Nickel nanoparticles-decorated graphene as highly effective and stable electrocatalyst for urea electrooxidation. J. Mol. Catal. A Chem. 2016, 421, 83–91. [Google Scholar] [CrossRef]
- Bian, L.; Du, Q.; Luo, M.; Qu, L.; Li, M. Monodisperse nickel nanoparticles supported on multi-walls carbon nanotubes as an effective catalyst for the electro-oxidation of urea. Int. J. Hydrog. Energy 2017, 42, 25244–25250. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Abdelkareem, M.A.; Kim, H.Y.; Barakat, N.A. Cobalt-incorporated, nitrogen-doped carbon nanofibers as effective non-precious catalyst for methanol electrooxidation in alkaline medium. Appl. Catal. A Gen. 2015, 498, 230–240. [Google Scholar] [CrossRef]
- Barakat, N.A.; Ali, M.A. Molybdenum carbide/Ni nanoparticles-incorporated carbon nanofibers as effective non-precious catalyst for urea electrooxidation reaction. Sci. Rep. 2022, 12, 22574. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2–10. [Google Scholar] [CrossRef]
- Liu, X.; He, J.-H.; Sakthivel, R.; Chung, R.-J. Rare earth erbium molybdate nanoflakes decorated functionalized carbon nanofibers: An affordable and potential catalytic platform for the electrooxidation of phenothiazine. Electrochim. Acta 2020, 358, 136885. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Amiri, S.; Masoumi, H.R.F.; Moghri, M. Optimization of Direct Yellow 12 dye removal by nanoscale zero-valent iron using response surface methodology. J. Ind. Eng. Chem. 2014, 20, 2535–2542. [Google Scholar] [CrossRef]
- Gomaa, H.E.; Alotaibi, A.A.; Gomaa, F.A.; Bajuayfir, E.; Ahmad, A.; Alotaibi, K.M. Integrated ion exchange-based system for nitrate and sulfate removal from water of different matrices: Analysis and optimization using response surface methodology and Taguchi experimental design techniques. Process Saf. Environ. Prot. 2021, 153, 500–517. [Google Scholar] [CrossRef]
- Nordstokke, D.; Colp, S.M. Factorial design. Encycl. Qual. Life Well Being Res. 2014, 1, 2144–2145. [Google Scholar]
- Mughal, M.A.; Newell, M.J.; Vangilder, J.; Thapa, S.; Wood, K.; Engelken, R.; Carroll, B.R.; Johnson, J.B. Optimization of the electrodeposition parameters to improve the stoichiometry of In2S3 films for solar applications using the taguchi method. J. Nanomater. 2014, 2014, 4. [Google Scholar] [CrossRef]
- Berger, P.D.; Maurer, R.E.; Celli, G.B. Introduction to Taguchi methods. In Experimental Design: With Application in Management, Engineering, and the Sciences; Springer: Cham, Switzerland, 2018; pp. 449–480. [Google Scholar]
- Do Kim, K.; Choi, D.W.; Choa, Y.-H.; Kim, H.T. Optimization of parameters for the synthesis of zinc oxide nanoparticles by Taguchi robust design method. Colloids Surf. A 2007, 311, 170–173. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Eng. Sci. Technol. Int. J. 2022, 33, 101070. [Google Scholar] [CrossRef]
- Francis, D.V.; Aiswarya, T.; Gokhale, T. Optimization of the incubation parameters for biogenic synthesis of WO3 nanoparticles using Taguchi method. Heliyon 2022, 8, e10640. [Google Scholar] [CrossRef] [PubMed]
- Mashentseva, A.; Zdorovets, M. Determination of optimal synthesis conditions of the Cu@ PET composites using Taguchi robust experiment design. In Proceedings of the 10th International Conference on Nanomaterials and Advanced Energy Storage Systems, Astana, Kazakhstan, 4–6 August 2022. [Google Scholar]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A.M. Ni-Cd carbon nanofibers as an effective catalyst for urea fuel cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Fatema, U.K.; Uddin, A.J.; Uemura, K.; Gotoh, Y. Fabrication of carbon fibers from electrospun poly (vinyl alcohol) nanofibers. Text. Res. J. 2011, 81, 659–672. [Google Scholar] [CrossRef]
- Barakat, N.A.; Khalil, K.A.; Mahmoud, I.H.; Kanjwal, M.A.; Sheikh, F.A.; Kim, H.Y. CoNi bimetallic nanofibers by electrospinning: Nickel-based soft magnetic material with improved magnetic properties. J. Phys. Chem. C 2010, 114, 15589–15593. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Unnithan, A.R.; Al-Deyab, S.S.; Kim, H.Y. Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: Effective visible light photocatalyst for waste water treatment. J. Lumin. 2012, 132, 1668–1677. [Google Scholar] [CrossRef]
- De Jesus, J.C.; González, I.; Quevedo, A.; Puerta, T. Thermal decomposition of nickel acetate tetrahydrate: An integrated study by TGA, QMS and XPS techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Barakat, N.A.; Khalil, K.A.; Kim, H.Y. Toward facile synthesizing of diamond nanostructures via nanotechnological approach: Lonsdaleite carbon nanofibers by electrospinning. Mater. Res. Bull. 2012, 47, 2140–2147. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Britovsek, G.J.; Gibson, V.C.; White, A.J. Electronic effects in oxo transfer reactions catalysed by salan molybdenum (VI) cis-dioxo complexes. Dalton Trans. 2009, 13, 2337–2344. [Google Scholar] [CrossRef]
- Barakat, N.A.; Ahmed, E.; Amen, M.T.; Abdelkareem, M.A.; Farghali, A. N-doped Ni/C/TiO2 nanocomposite as effective photocatalyst for water splitting. Mater. Lett. 2018, 210, 317–320. [Google Scholar] [CrossRef]
- Barakat, N.A.; Abdelkareem, M.A.; Abdelghani, E.A.M. Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation. Catalysts 2019, 9, 330. [Google Scholar] [CrossRef]
- Wang, W.; Chai, D.; Zhang, J.; Xue, S.; Wang, Y.; Lei, Z. Ni5Sm-P/C ternary alloyed catalyst as highly efficient electrocatalyst for urea electrooxidation. J. Taiwan Inst. Chem. Eng. 2017, 80, 326–332. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Cyclic voltammetry measurements of electroactive surface area of porous nickel: Peak current and peak charge methods and diffusion layer effect. Mater. Chem. Phys. 2019, 233, 60–67. [Google Scholar] [CrossRef]
- Watzele, S.; Hauenstein, P.; Liang, Y.; Xue, S.; Fichtner, J.; Garlyyev, B.; Scieszka, D.; Claudel, F.; Maillard, F.; Bandarenka, A.S. Determination of electroactive surface area of Ni-, Co-, Fe-, and Ir-based oxide electrocatalysts. ACS Catal. 2019, 9, 9222–9230. [Google Scholar] [CrossRef]
- Brown, I.; Sotiropoulos, S. Preparation and characterization of microporous Ni coatings as hydrogen evolving cathodes. J. Appl. Electrochem. 2000, 30, 107–111. [Google Scholar] [CrossRef]
- Machado, S.A.; Avaca, L.A. The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 1994, 39, 1385–1391. [Google Scholar] [CrossRef]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B Environ. 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Hamadanian, M.; Reisi-Vanani, A.; Majedi, A. Preparation and characterization of S-doped TiO2 nanoparticles, effect of calcination temperature and evaluation of photocatalytic activity. Mater. Chem. Phys. 2009, 116, 376–382. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Effect of calcination temperature on electrocatalytic activities of Ti/IrO2 electrodes in methanol aqueous solutions. Electrochim. Acta 2006, 51, 6258–6267. [Google Scholar] [CrossRef]
- Xu, F.; Le, Y.; Cheng, B.; Jiang, C. Effect of calcination temperature on formaldehyde oxidation performance of Pt/TiO2 nanofiber composite at room temperature. Appl. Surf. Sci. 2017, 426, 333–341. [Google Scholar] [CrossRef]
- Barakat, N.A.; Al-Mubaddel, F.S.; Karim, M.R.; Alrashed, M.; Kim, H.Y. Influence of Sn content on the electrocatalytic activity of NiSn alloy nanoparticles-incorporated carbon nanofibers toward methanol oxidation. Int. J. Hydrogen Energy 2018, 43, 21333–21344. [Google Scholar] [CrossRef]
- Geng, D.; Zhu, S.; Chai, M.; Zhang, Z.; Fan, J.; Xu, Q.; Min, Y. Pd x Fe y alloy nanoparticles decorated on carbon nanofibers with improved electrocatalytic activity for ethanol electrooxidation in alkaline media. New J. Chem. 2020, 44, 5023–5032. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Gan, M.; Li, X.; Zhang, Y.; Hua, X.; Wang, L. Engineering intermetallic-metal oxide interface with low platinum loading for efficient methanol electrooxidation. J. Colloid Interface Sci. 2021, 604, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Perrot, H.; Sel, O.; Debiemme-Chouvy, C.; Lafdi, K.; El Rhazi, M. Electrosynthesis of hierarchical Cu2O–Cu(OH)2 nanodendrites supported on carbon nanofibers/poly (para-phenylenediamine) nanocomposite as high-efficiency catalysts for methanol electrooxidation. Int. J. Hydrog. Energy 2021, 46, 19926–19938. [Google Scholar]
- Salcı, A.; Şahin, E.A.; Solmaz, R. Methanol electrooxidation at nickel-modified rhodanine self assembled monolayer films: A new class of multilayer electrocatalyst. Int. J. Hydrog. Energy 2019, 44, 14228–14234. [Google Scholar] [CrossRef]
- Elbasri, M.; Perrot, H.; Sel, O.; Lafdi, K.; El Rhazi, M. Synthesis of carbon nanofibers/poly (para-phenylenediamine)/nickel particles nanocomposite for enhanced methanol electrooxidation. Int. J. Hydrog. Energy 2019, 44, 24534–24545. [Google Scholar]
- Shi, Y.; Li, H.; Ao, D.; Chang, Y.; Xu, A.; Jia, M.; Jia, J. 3D nickel diselenide architecture on nitrogen-doped carbon as a highly efficient electrode for the electrooxidation of methanol and urea. J. Alloys Compd. 2021, 885, 160919. [Google Scholar] [CrossRef]
- Chen, F.-K.; Ho, Y.-H.; Chang, H.-W.; Tsai, Y.-C. Nanocomposite integrating tube-like NiCo2S4 and carbon nanotubes for electrooxidation of methanol. Electrochem. Commun. 2020, 117, 106783. [Google Scholar] [CrossRef]
- Sarkar, C.; Nath, J.; Bhuyan, S.; Dolui, S.K. Multifunctional ternary nanocomposites of Ni/Polypyrrole/Reduced graphene oxide as supercapacitor and electrocatalyst in methanol oxidation. ChemistrySelect 2019, 4, 2529–2537. [Google Scholar] [CrossRef]
- Murugesan, B.; Pandiyan, N.; Sonamuthu, J.; Samayanan, S.; Mahalingam, S. Ternary nanocomposite designed by MWCNT backbone PPy/Pd for efficient catalytic approach toward reduction and oxidation reactions. Adv. Powder Technol. 2018, 29, 3173–3182. [Google Scholar] [CrossRef]
- Nazal, M.K.; Olakunle, O.S.; Al-Ahmed, A.; Merzougui, B.; Abualkibash, A.; Sultan, A.; Yousaf, A.B.; Zaidi, S.J. Precious metal free Ni/Cu/Mo trimetallic nanocomposite supported on multi-walled carbon nanotubes as highly efficient and durable anode-catalyst for alkaline direct methanol fuel cells. J. Electroanal. Chem. 2018, 823, 98–105. [Google Scholar] [CrossRef]
- Haldorai, Y.; Arreaga-Salas, D.; Rak, C.S.; Huh, Y.S.; Han, Y.-K.; Voit, W. Platinized titanium nitride/graphene ternary hybrids for direct methanol fuel cells and titanium nitride/graphene composites for high performance supercapacitors. Electrochim. Acta 2016, 220, 465–474. [Google Scholar] [CrossRef]
- Pattanayak, P.; Pramanik, N.; Kumar, P.; Kundu, P.P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC. Int. J. Hydrog. Energy 2018, 43, 11505–11519. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, J.; Sun, Q.; Yan, Z.; Chen, M.; Jing, J. Enhanced electrocatalytic activity of high Pt-loadings on surface functionalized graphene nanosheets for methanol oxidation. Int. J. Hydrog. Energy 2013, 38, 16402–16409. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Rezaei-Moghadam, B.; Asghari, E. Electrosynthesis of polypyrrole–nanodiamond composite film under ultrasound irradiation: Promotion for methanol electrooxidation by gold and Cu2O nanostructures. J. Taiwan Inst. Chem. Eng. 2017, 75, 263–270. [Google Scholar] [CrossRef]
- Roy, S.; Payra, S.; Challagulla, S.; Arora, R.; Roy, S.; Chakraborty, C. Enhanced photoinduced electrocatalytic oxidation of methanol using Pt nanoparticle-decorated TiO2–polyaniline ternary nanofibers. ACS Omega 2018, 3, 17778–17788. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Sulfonated polypyrrole matrix induced enhanced efficiency of Ni nanocatalyst for application as an anode material for DMFCs. Mater. Chem. Phys. 2016, 176, 143–151. [Google Scholar]
- Li, P.; Du, C.; Gao, X.; Zhuang, Z.; Xiang, D.; Zhang, C.; Chen, W. Insights into the morphology and composition effects of one-dimensional CuPt nanostructures on the electrocatalytic activities and methanol oxidation mechanism by in situ FTIR. Nanoscale 2020, 12, 13688–13696. [Google Scholar]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The kinetics and mechanism of the oxidation of amines and alcohols at oxide-covered nickel, silver, copper, and cobalt electrodes. J. Chem. Soc. Perkin Trans. 2 1972, 10, 1396–1403. [Google Scholar] [CrossRef]
- El-Shafei, A.A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J. Electroanal. Chem. 1999, 471, 89–95. [Google Scholar] [CrossRef]
- Abdel Rahim, M.A.; Abdel Hameed, R.M.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Hosseini, S.R. An electrochemical investigation of methanol oxidation on nickel hydroxide nanoparticles. S. Afr. J. Chem. 2013, 66, 47–53. [Google Scholar]
- Ferrin, P.; Mavrikakis, M. Structure sensitivity of methanol electrooxidation on transition metals. J. Am. Chem. Soc. 2009, 131, 14381–14389. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Shojaei, S.; Band, S.S.; Farizhandi, A.A.K.; Ghoroqi, M.; Mosavi, A. Application of Taguchi method and response surface methodology into the removal of malachite green and auramine-O by NaX nanozeolites. Sci. Rep. 2021, 11, 16054. [Google Scholar] [CrossRef] [PubMed]






| Variable | Symbol | Unit | Level | |||
|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | |||
| Mo/Ni content (Mo wt.%) | A | % | 5% | 10% | 15% | 25% |
| Methanol Concentration | B | M | 1 | 2 | 3 | 4 |
| Reaction temperature | C | °C | 35 | 45 | 55 | 65 |
| No | Material | Current Density mA/Cm2 | Ref. |
|---|---|---|---|
| 1 | PtFe(1:2)@a-FeOx/NC-C (7. Wt% Pt) | 2.34 | [65] |
| 2 | CNF/PpPD/CuNDs | 50 | [66] |
| 3 | Cu/Rh-SAM-Ni | 28 | [67] |
| 4 | Cu/Rh-SAM | 24 | [67] |
| 5 | Cu bare | 22 | [67] |
| 6 | CPE/CNF/PpPD/NiPs | 38.11 | [68] |
| 7 | NiSe2/NC-450 | 164.68 | [69] |
| 8 | NiCo2S4/CNT | 160 | [70] |
| 9 | Ni/PPy/rGO | 32.39 | [70] |
| 10 | Ni/rGO | 23.45 | [70] |
| 11 | PPy/rGO | 20 | [71] |
| 12 | MWCNT/PPy/Pd | 45.31 | [72] |
| 13 | Ni/Cu/Mo@MWCNTs | 45 | [73] |
| 14 | Pt@TiN/rGO | 3.09 | [73] |
| 15 | Pt/rGO | 2.01 | [73] |
| 16 | Pt/Vulcan | 1.61 | [74] |
| 17 | Cu2O/PPy-Go | 0.3 | [75] |
| 18 | Pt/PDDA-G | 40 | [76] |
| 19 | PPy(ND)/Au/Cu2O | 62 | [77] |
| 20 | Pt/TiO2/PTP | 0.84 | [78] |
| 21 | Pt-Ru/C (Commercial) | 0.28 | [79] |
| 22 | Pt/C | 2.65 | [80] |
| 23 | Mo2C/Ni-CNFs (5%) | 107 | Current study |
| Exp. No | (Mo) wt.% | (Me), M | Temp., °C | EOP, mA/cm2 | S/N Ratio |
|---|---|---|---|---|---|
| 1 | 5 | 1 | 35 | 80.0 | 37.7725 |
| 2 | 5 | 2 | 45 | 150.0 | 43.2119 |
| 3 | 5 | 3 | 55 | 105.0 | 40.5050 |
| 4 | 5 | 4 | 65 | 108.0 | 40.7474 |
| 5 | 10 | 1 | 45 | 60.0 | 35.1687 |
| 6 | 10 | 2 | 35 | 70.0 | 37.1913 |
| 7 | 10 | 3 | 65 | 70.0 | 37.1913 |
| 8 | 10 | 4 | 55 | 65.0 | 36.1204 |
| 9 | 15 | 1 | 55 | 30.0 | 29.2325 |
| 10 | 15 | 2 | 65 | 39.6 | 31.6490 |
| 11 | 15 | 3 | 35 | 35.0 | 30.6183 |
| 12 | 15 | 4 | 45 | 38.0 | 31.8127 |
| 13 | 25 | 1 | 65 | 25.0 | 28.6788 |
| 14 | 25 | 2 | 55 | 60.0 | 35.8967 |
| 15 | 25 | 3 | 45 | 45.0 | 32.5227 |
| 16 | 25 | 4 | 35 | 35.0 | 31.2238 |
| F | 75.9 | 11.89 | 1.9 | 80.0 | |
| P | 0.00 | 0.006 | 0.23 | ||
| R2 | 97.80 | ||||
| Rank | 1 | 2 | 3 | ||
| SS | 235.36 | 36.88 | 5.903 | ||
| Contribution, % | 82.77 | 12.97 | 2.08 | ||
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Mo% | 3 | 235.359 | 82.77% | 235.359 | 78.453 | 75.90 | 0.000 |
| (Me) | 3 | 36.880 | 12.97% | 36.880 | 12.293 | 11.89 | 0.006 |
| T | 3 | 5.903 | 2.08% | 5.903 | 1.968 | 1.90 | 0.230 |
| Error | 6 | 6.202 | 2.18% | 6.202 | 1.034 | ||
| Total | 15 | 284.343 | 100.00% |
| No. | Experimental | Predicted by Taguchi | % Diff. | ||||
|---|---|---|---|---|---|---|---|
| Mo% | Methanol, M | Temp. °C | Peak CD | S/N Ratio | Peak CD | ||
| 1 | 10 | 1 | 35 | 55 | 33.4 | 43 | 21.82 |
| 2 | 10 | 3 | 35 | 70 | 35.93 | 58.05 | 17.07 |
| 3 | 15 | 1 | 35 | 30 | 27.98 | 12.5 | 58.33 |
| 4 | 15 | 2 | 35 | 35 | 32.19 | 43.6 | −24.57 |
| 5 | 5 | 3 | 55 | 105 | 41.46 | 112.6 | −7.24 |
| 6 | 5 | 2.5 | 50 | 128 | 42.8 | 136 | −6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Aty, M.M.; Gomaa, H.E.; Abdu, H.M.; Almasri, R.A.; Irfan, O.M.; Barakat, N.A.M. Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation. Polymers 2023, 15, 2430. https://doi.org/10.3390/polym15112430
Abdel-Aty MM, Gomaa HE, Abdu HM, Almasri RA, Irfan OM, Barakat NAM. Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation. Polymers. 2023; 15(11):2430. https://doi.org/10.3390/polym15112430
Chicago/Turabian StyleAbdel-Aty, Marwa M., Hassan E. Gomaa, Hany Mohamed Abdu, Radwan A. Almasri, Osama M. Irfan, and Nasser A. M. Barakat. 2023. "Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation" Polymers 15, no. 11: 2430. https://doi.org/10.3390/polym15112430






