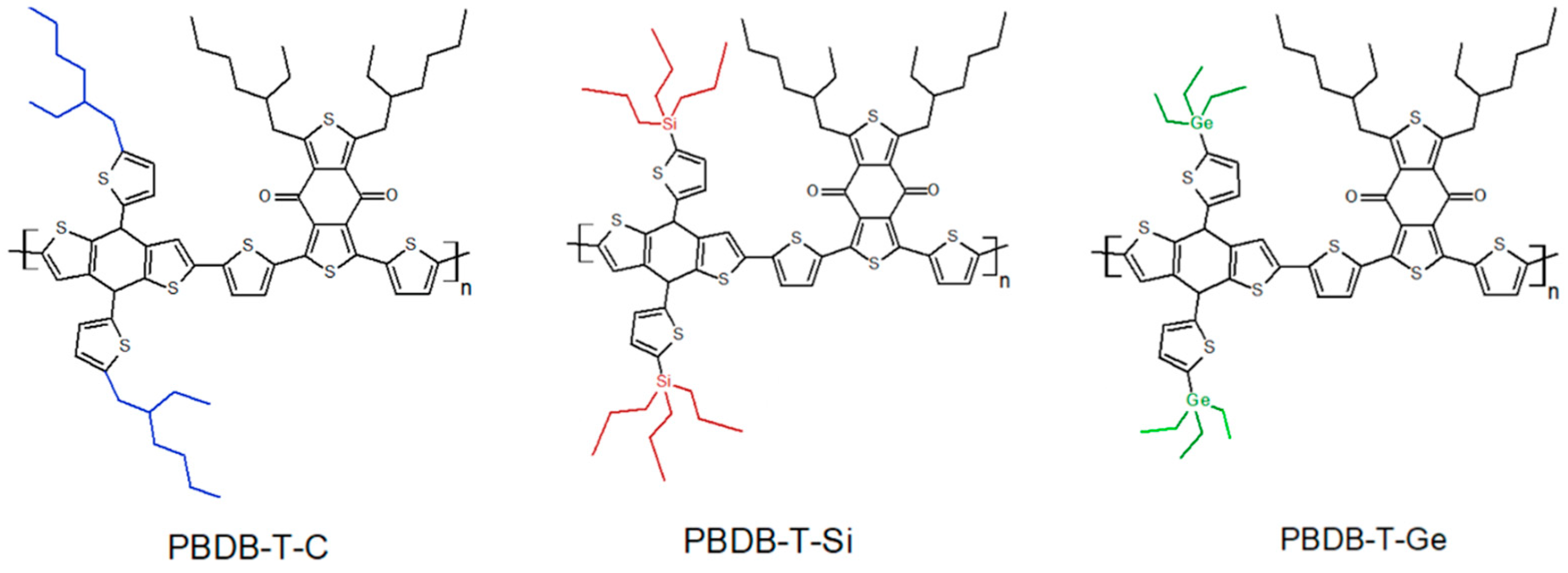
Synthesis and Characterization of the Donor-Acceptor Conjugated Polymer PBDB-T Implementing Group IV Element Germanium
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Synthesis of PBDB-T-Ge
2.2.1. Synthesis of Triethyl (Thiophen-2-yl) Germane (2)
2.2.2. Synthesis of 4,8-Bis(5-triethyl germanium)thiophene-2-yl)benzo[1,2-b:4,5-b′]dithiophene (3)
2.2.3. Synthesis of [4,8-Bis[5-triethyl germanium) thiophene-2-yl]-2-trimethylstannyl thieno[2,3-f][1]benzothiol-6-yl]-trimethylstannane (4)
2.2.4. Synthesis of PBDB-T-Ge (6)
2.3. Characterization
3. Results and Discussion
3.1. Synthesis of PBDB-T-Ge
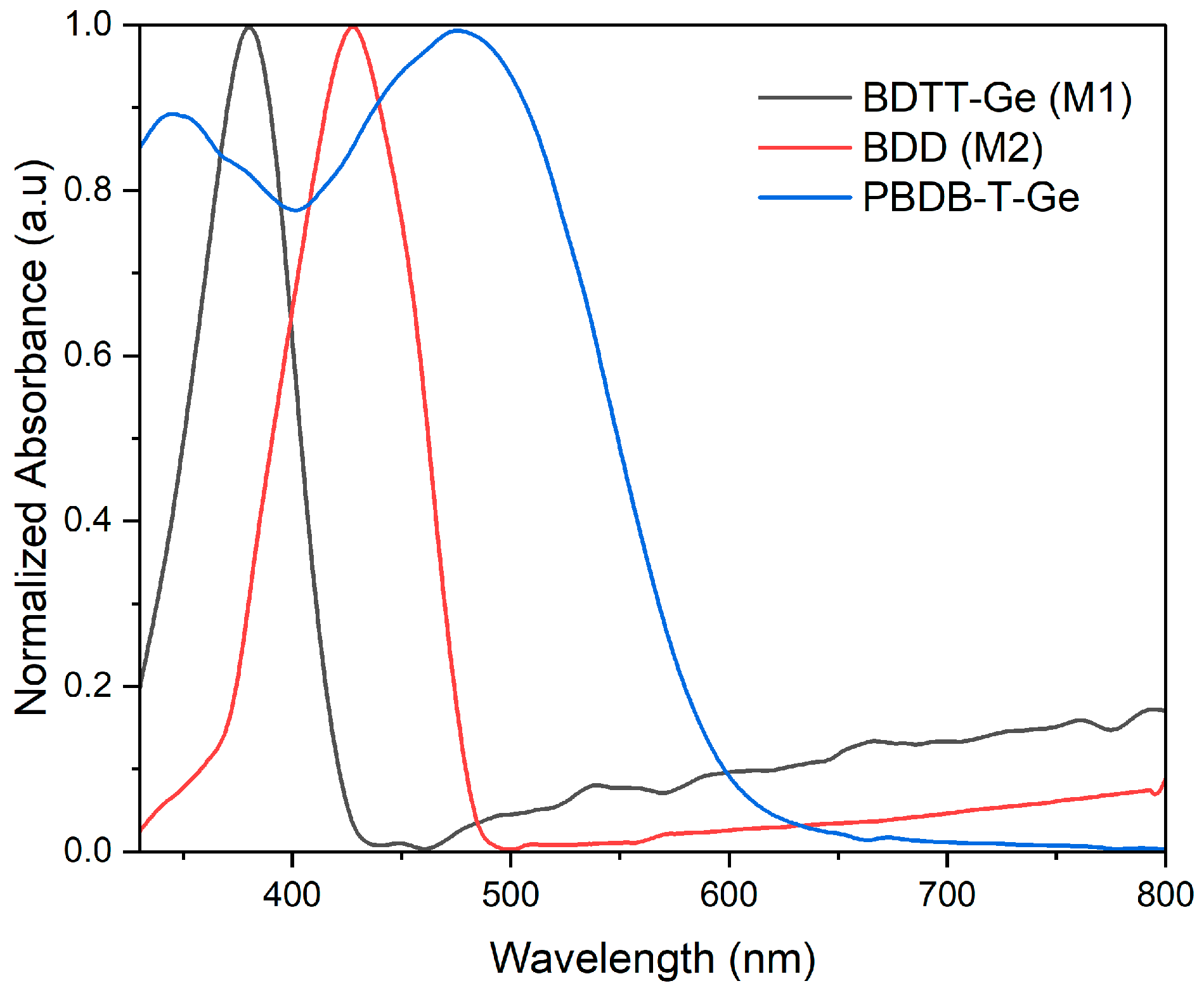
3.2. UV-Vis Absorption of PBDB-T-Ge
3.3. Photoluminescence of PBDB-T-Ge
3.4. Electrochemical Characteristics of PBDB-T-Ge
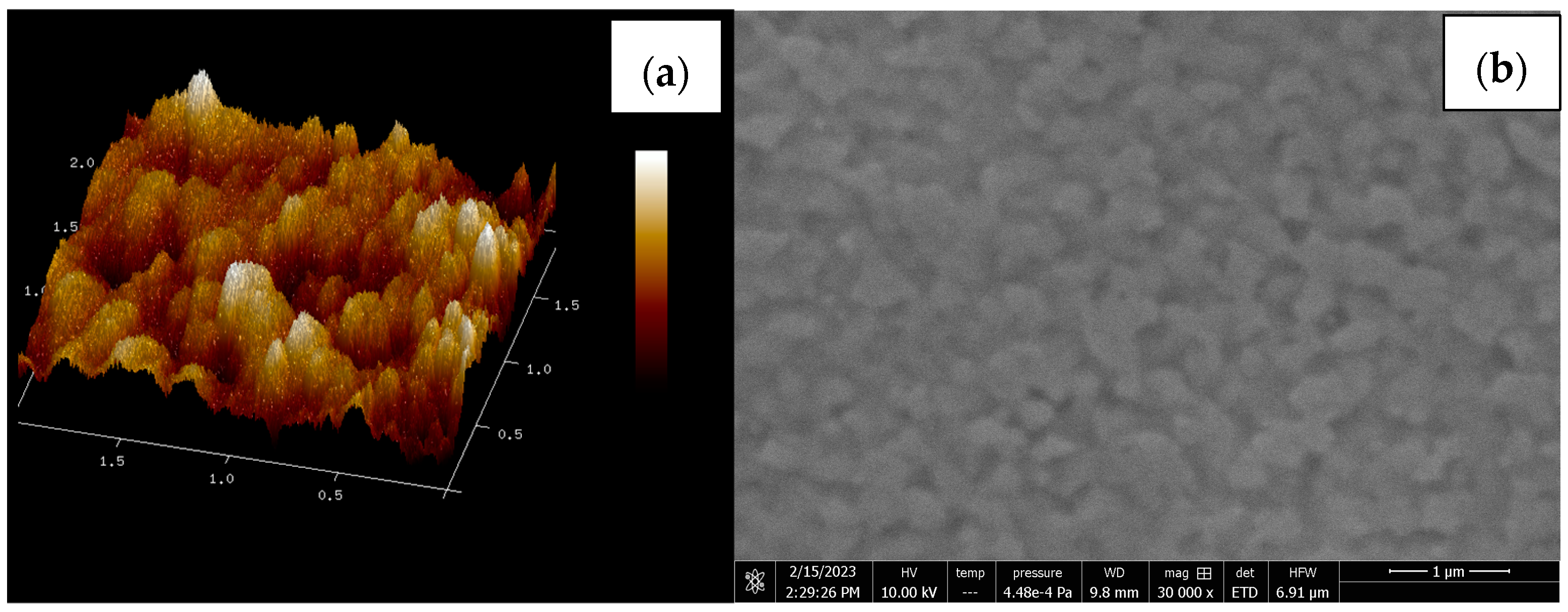
3.5. Morphology Study
3.6. Structural Optimization and Density Functional Theory (DFT) Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Ji, Q.; Yin, L.; Zhang, N.; Liu, T.; Li, N.; He, X.; Wen, G.; Zhang, W.; Yu, L.; et al. Synergistic engineering of substituents and backbones on donor polymers: Toward terpolymer design of high-performance polymer solar cells. ACS Appl. Mater. Interfaces 2021, 13, 23993–24004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, Y.; Xu, Z.; Hou, J.; Zhang, M.; Min, J.; Chen, H.Y.; Ye, M.; Hong, Z.; Yang, Y.; et al. Effect of carbon chain length in the substituent of PCBM-like molecules on their photovoltaic properties. Adv. Funct. Mater. 2010, 20, 1480–1487. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Wei, W.; Jiang, Z. Fluorinated benzothiadiazole-based conjugated polymers for high-performance polymer solar cells without any processing additives or post-treatments. J. Am. Chem. Soc. 2013, 135, 17060–17068. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, Z.; Yang, C.; Yu, J.; Feng, J.; Adil, M.A.; Deng, D.; Zou, W.; Zhang, J.; Lu, K.; et al. All-small-molecule organic solar cells with over 14% efficiency by optimizing hierarchical morphologies. Nat. Commun. 2019, 10, 5393. [Google Scholar] [CrossRef]
- Lin, Y.; Firdaus, Y.; Nugraha, M.I.; Liu, F.; Karuthedath, S.; Emwas, A.H.; Zhang, W.; Seitkhan, A.; Neophytou, M.; Faber, H.; et al. 17.1% efficient single-junction organic solar cells enabled by n-type doping of the bulk-heterojunction. Adv. Sci. 2020, 7, 1903419. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Fan, B.; Zhong, W.; Ying, L.; Zhang, D.; Li, M.; Lin, Y.; Xia, R.; Liu, F.; Yip, H.L.; Li, N.; et al. Surpassing the 10% efficiency milestone for 1-cm2 all-polymer solar cells. Nat. Commun. 2019, 10, 4100. [Google Scholar] [CrossRef]
- Nagarjuna, P.; Bagui, A.; Hou, J.; Singh, S.P. New electron acceptor derived from fluorene: Synthesis and its photovoltaic properties. J. Phys. Chem. C 2016, 120, 13390–13397. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, C.P.; Sharma, G.D.; Kurchania, R.; Roy, M.S. Synthesis of a modified PC70BM and its application as an electron acceptor with poly(3-hexylthiophene) as an electron donor for efficient bulk heterojunction solar cells. Adv. Funct. Mater. 2012, 22, 4087–4095. [Google Scholar] [CrossRef]
- Kuhlmann, J.C.; de Bruyn, P.; Bouwer, R.K.M.; Meetsma, A.; Blom, P.W.M.; Hummelen, J.C. Improving the compatibility of fullerene acceptors with fluorene-containing donor-polymers in organic photovoltaic devices. Chem. Commun. 2010, 46, 7232–7234. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; et al. Fused nonacyclic electron acceptors for efficient polymer solar cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, B.; Peng, R.; Fan, L.; Cai, L.; Fan, B.; Chu, S. Photovoltaic performance of PCBM analogs with different ester groups as acceptor in the polymer solar cells. Synth. Met. 2016, 212, 44–50. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Courtright, B.A.E.; Ferreira, A.S.; Tolbert, S.H.; Jenekhe, S.A. 7.7% Efficient all-polymer solar cells. Adv. Mater. 2015, 27, 4578–4584. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Trinh, M.T.; Chen, R.; Purdum, G.E.; Khlyabich, P.P.; Sezen, M.; Oh, S.; Zhu, H.; Fowler, B.; Zhang, B.; et al. Molecular helices as electron acceptors in high-performance bulk heterojunction solar cells. Nat. Commun. 2015, 6, 8242. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.H.; Kang, T.E.; Lee, C.; Kang, H.; Shin, M.; Wang, C.; Ma, B.; Jeong, U.; Kim, T.S.; et al. Flexible, highly efficient all-polymer solar cells. Nat. Commun. 2015, 6, 8547. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, G.; Li, Y.; Zhang, Z.; Li, T.; Zuo, X.; Lu, X.; Lin, Y. An electron acceptor analogue for lowering trap density in organic solar cells. Adv. Mater. 2021, 33, 2008134. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wu, F.P.; Zhou, Y.; Jiang, Z.Q.; Song, B.; Xia, Y.; Zhang, Z.G.; Gao, F.; Inganäs, O.; et al. Non-fullerene acceptor with low energy loss and high external quantum efficiency: Towards high performance polymer solar cells. J. Mater. Chem. A 2016, 4, 5890–5897. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; He, Q.; Huo, L.; Wu, Y.; Parker, T.C.; Ma, W.; Sun, Y.; Wang, C.; Zhu, D.; et al. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, X.; Li, Y.; Li, Z.; Peng, Q. Chalcogen-atom-annulated perylene diimide trimers for highly efficient nonfullerene polymer solar cells. Macromol. Rapid Commun. 2017, 38, 1700405. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Awartani, O.; Zhao, J.; Han, H.; Ade, H.; Zhao, D.; Yan, H. A Vinylene-bridged perylenediimide-based polymeric acceptor enabling efficient all-polymer solar cells processed under ambient conditions. Adv. Mater. 2016, 28, 8483–8489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, L.; Xu, S.; Liu, Y.; Peng, H.; Zhang, Z.G.; Li, Y.; Zou, Y. A new electron acceptor with meta-alkoxyphenyl side chain for fullerene-free polymer solar cells with 9.3% efficiency. Adv. Sci. 2017, 4, 1700152. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Zhang, H.; Wan, X.; Li, C.; Ke, X.; Feng, H.; Kan, B.; Zhang, H.; Zhang, Q.; Lu, Y.; et al. A new nonfullerene electron acceptor with a ladder type backbone for high-performance organic solar cells. Adv. Mater. 2017, 29, 1604964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 2017, 29, 1700144. [Google Scholar] [CrossRef]
- Bin, H.; Zhang, Z.G.; Gao, L.; Chen, S.; Zhong, L.; Xue, L.; Yang, C.; Li, Y. Non-fullerene polymer solar cells based on alkylthio and fluorine substituted 2D-conjugated polymers reach 9.5% efficiency. J. Am. Chem. Soc. 2016, 138, 4657–4664. [Google Scholar] [CrossRef]
- Guo, B.; Li, W.; Guo, X.; Meng, X.; Ma, W.; Zhang, M.; Li, Y. High efficiency nonfullerene polymer solar cells with thick active layer and large area. Adv. Mater. 2017, 29, 1702291. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhang, L.; Chow, P.C.Y.; Wang, Z.; Zhang, G.; Ma, W.; Yan, H. A wide-bandgap donor polymer for highly efficient non-fullerene organic solar cells with a small voltage loss. J. Am. Chem. Soc. 2017, 139, 6298–6301. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Wang, X.; Wu, Y.; Zhang, Q.; Yan, C.; Ma, W.; You, W.; Zhan, X. Enhancing performance of nonfullerene acceptors via side-chain conjugation strategy. Adv. Mater. 2017, 29, 1702125. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Ma, R.; Liu, T.; Luo, Z.; Guo, Q.; Xiao, Y.; Chen, Y.; Li, X.; Luo, S.; Lu, X.; Zhang, M.; et al. Improving open-circuit voltage by a chlorinated polymer donor endows binary organic solar cells efficiencies over 17%. Sci. China Chem. 2020, 63, 325–330. [Google Scholar] [CrossRef]
- Weng, K.; Ye, L.; Zhu, L.; Xu, J.; Zhou, J.; Feng, X.; Lu, G.; Tan, S.; Liu, F.; Sun, Y. Optimized active layer morphology toward efficient and polymer batch insensitive organic solar cells. Nat. Commun. 2020, 11, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, M.; Zhou, G.; Hao, T.; Xu, J.; Wang, J.; Qiu, C.; Prine, N.; Ali, J.; Feng, W.; et al. Efficient organic solar cell with 16.88% efficiency enabled by refined acceptor crystallization and morphology with improved charge transfer and transport properties. Adv. Energy Mater. 2020, 10, 1904234. [Google Scholar] [CrossRef]
- Chen, S.; Lee, S.M.; Xu, J.; Lee, J.; Lee, K.C.; Hou, T.; Yang, Y.; Jeong, M.; Lee, B.; Cho, Y.; et al. Ultrafast channel II process induced by a 3-D texture with enhanced acceptor order ranges for high-performance non-fullerene polymer solar cells. Energy Environ. Sci. 2018, 11, 2569–2580. [Google Scholar] [CrossRef]
- Bin, H.; Gao, L.; Zhang, Z.G.; Yang, Y.; Zhang, Y.; Zhang, C.; Chen, S.; Xue, L.; Yang, C.; Xiao, M.; et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 2016, 7, 13651. [Google Scholar] [CrossRef]
- Sherborne, G.J.; Gevondian, A.G.; Funes-Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Modular and selective arylation of aryl germanes (C−GeEt3) over C−Bpin, C−SiR3 and halogens enabled by light-activated gold catalysis. Angew. Chem. Int. Ed. 2020, 59, 15543–15548. [Google Scholar] [CrossRef]
- Sharma, S.; Soni, R.; Kurungot, S.; Asha, S.K. Rylene diimide-based alternate and random copolymers for flexible supercapacitor electrode materials with exceptional stability and highpower density. J. Phys. Chem. C 2019, 123, 2084–2093. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J. Design, application, and morphology study of a new photovoltaic polymer with strong aggregation in solution state. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Al-Balushi, R.A.; Rather, J.A.; Munam, A.; Ilmi, R.; Raithby, P.R.; Zhang, Y.; Fu, Y.; Xie, Z.; et al. Synthesis, characterization, and optoelectronic properties of phenothiazine-based organic co-polyynes. New J. Chem. 2021, 45, 15082–15095. [Google Scholar] [CrossRef]
- Jayapal, M.; Haque, A.; Al-Busaidi, I.J.; Al-Rasbi, N.; Al-Suti, M.K.; Khan, M.S.; Al-Balushi, R.; Islam, S.M.; Xin, C.; Wu, W.; et al. Dicopper(I) Complexes Incorporating Acetylide-Functionalized Pyridinyl-Based Ligands: Synthesis, Structural, and Photovoltaic Studies. Inorg. Chem. 2018, 57, 12113–12124. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Islam, J.; Arof, A.K.; Khandaker, M.U.; Zabed, H.M.; Khalil, I.; Rahman, M.R.; Islam, S.M.; Karim, M.R.; Uddin, J. Electrocatalytic and structural properties and computational calculation of PAN-EC-PC-TPAI-I 2 gel polymer electrolytes for dye sensitized solar cell application. RSC Adv. 2021, 11, 22937–22950. [Google Scholar] [CrossRef] [PubMed]






| Materials | λmaxEx (nm) | λonset (nm) | λmaxEm (nm) | ||
|---|---|---|---|---|---|
| Solution | Film | Solution | Film | ||
| PBDB-T-Ge | 477 | 507 | 601 | 666 | 615 |
| Materials | HOMO (eV) | LUMO (eV) | Egopt (eV) a | Egcv (eV) b |
|---|---|---|---|---|
| PBDB-T-C c | −5.23 | −3.18 | - | 2.05 |
| PBDB-T-Si c | −5.45 | −3.58 | 1.81 | - |
| PBDB-T-Ge | −5.45 | −3.64 | 1.86 | 1.81 |
| Y6 | −5.65 | −3.86 | 1.34 | 1.79 |
| Side Groups a | HOMO (eV) | LUMO (eV) | EgDFT (eV) a |
|---|---|---|---|
| Triethyl-C | −5.01 | −2.35 | 2.65 |
| Triethyl-Si | −5.06 | −2.38 | 2.68 |
| Triethyl-Ge | −5.02 | −2.36 | 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abousamra, W.H.; Thomas, D.; Yang, D.; Islam, S.M.; Winstead, C.; Kim, Y.-G. Synthesis and Characterization of the Donor-Acceptor Conjugated Polymer PBDB-T Implementing Group IV Element Germanium. Polymers 2023, 15, 2429. https://doi.org/10.3390/polym15112429
Abousamra WH, Thomas D, Yang D, Islam SM, Winstead C, Kim Y-G. Synthesis and Characterization of the Donor-Acceptor Conjugated Polymer PBDB-T Implementing Group IV Element Germanium. Polymers. 2023; 15(11):2429. https://doi.org/10.3390/polym15112429
Chicago/Turabian StyleAbousamra, Wafaa H., Destinee Thomas, Dan Yang, Shahidul M. Islam, Cherese Winstead, and Young-Gi Kim. 2023. "Synthesis and Characterization of the Donor-Acceptor Conjugated Polymer PBDB-T Implementing Group IV Element Germanium" Polymers 15, no. 11: 2429. https://doi.org/10.3390/polym15112429
APA StyleAbousamra, W. H., Thomas, D., Yang, D., Islam, S. M., Winstead, C., & Kim, Y.-G. (2023). Synthesis and Characterization of the Donor-Acceptor Conjugated Polymer PBDB-T Implementing Group IV Element Germanium. Polymers, 15(11), 2429. https://doi.org/10.3390/polym15112429








