Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis
2.3. Cement
2.4. Experimental Methods
2.4.1. Gel Permeation Chromatography (GPC)
2.4.2. Infrared Spectroscopy
2.4.3. Charge Density
2.4.4. Fluidity
2.4.5. Rheology
2.4.6. Total Organic Carbon (TOC)
2.4.7. Heat of Hydration
2.4.8. SEM
3. Results and Discussion
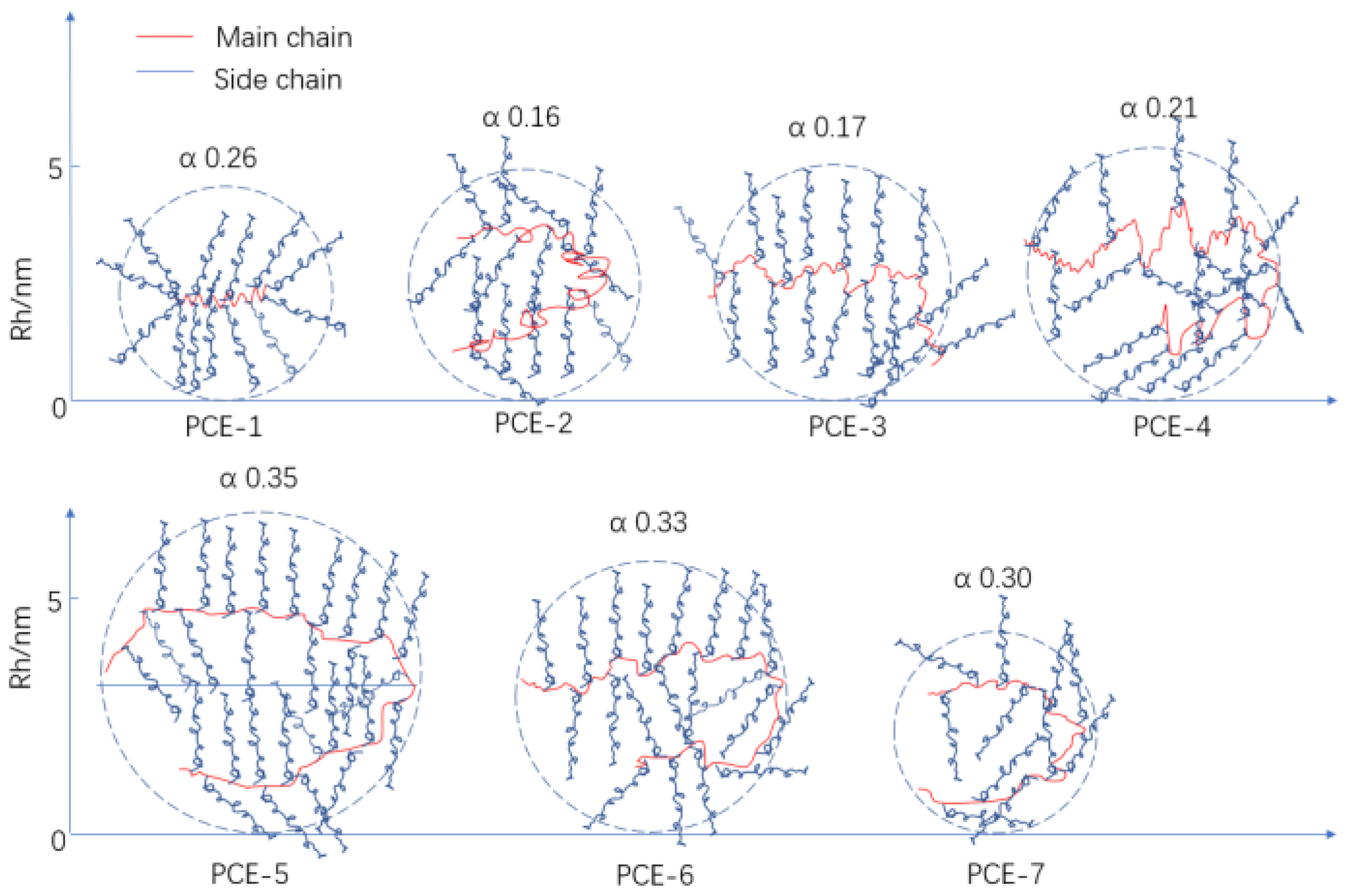
3.1. Gel Permeation Chromatography
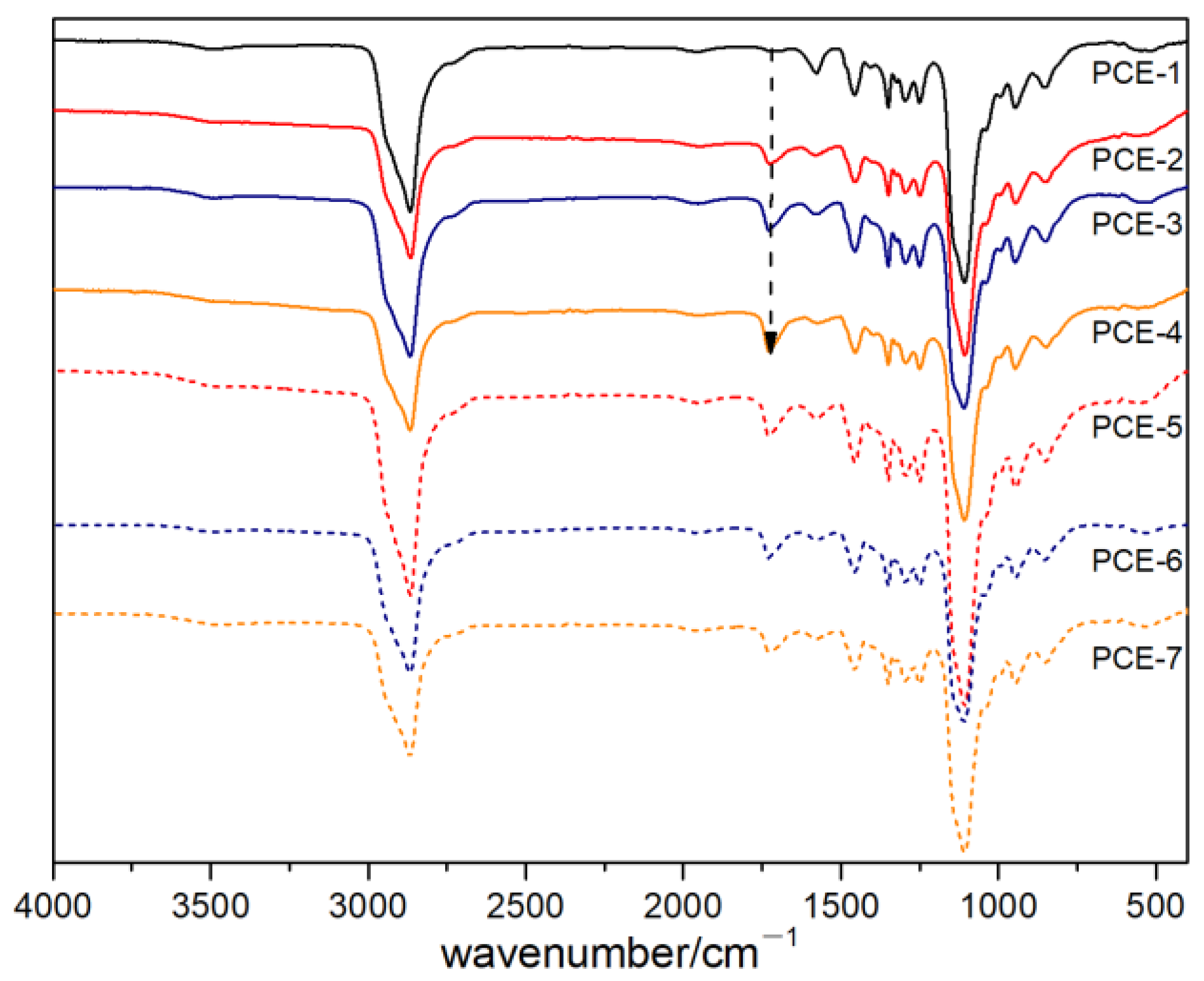
3.2. Infrared Spectral Analysis
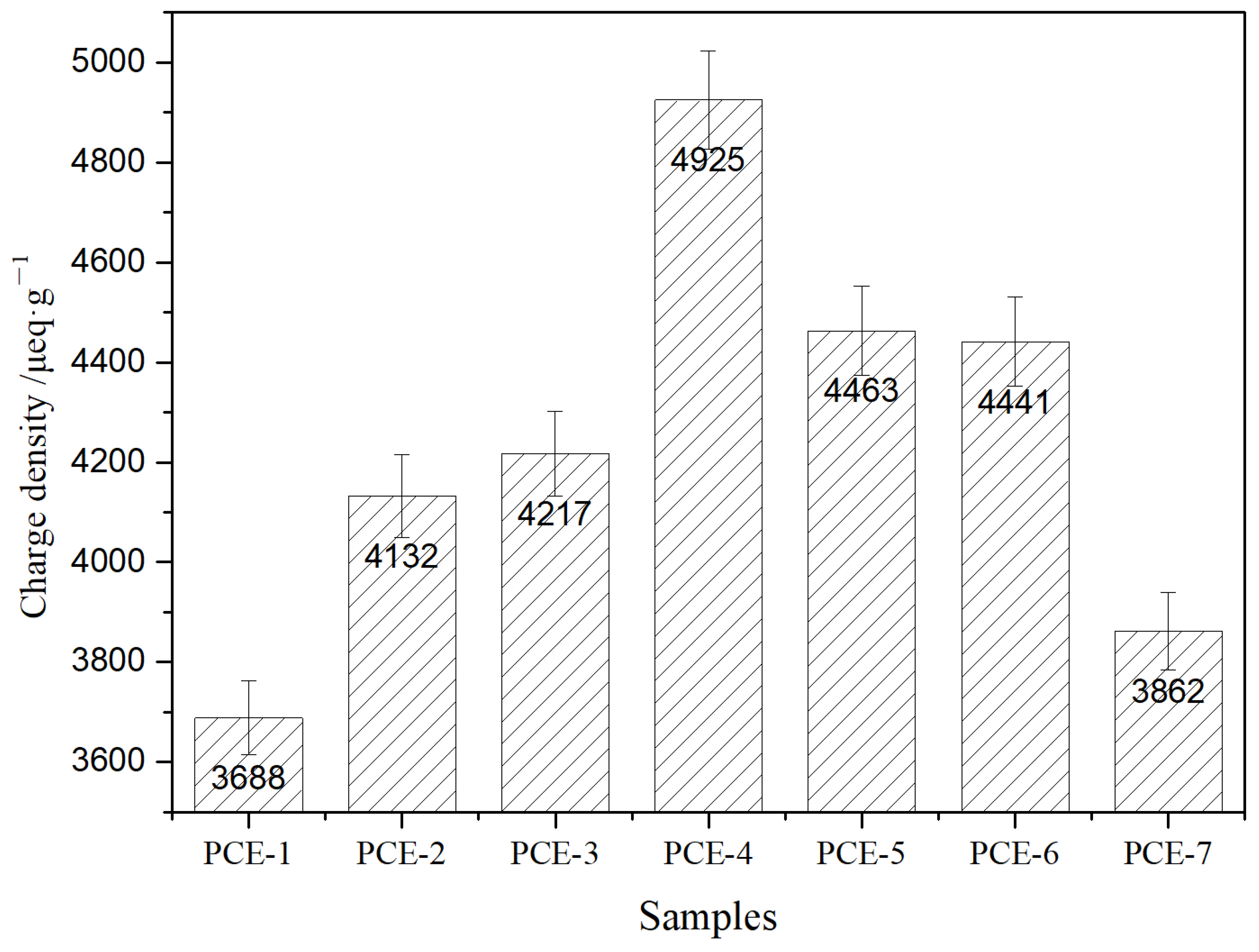
3.3. Charge Density
3.4. Dispersion Performance
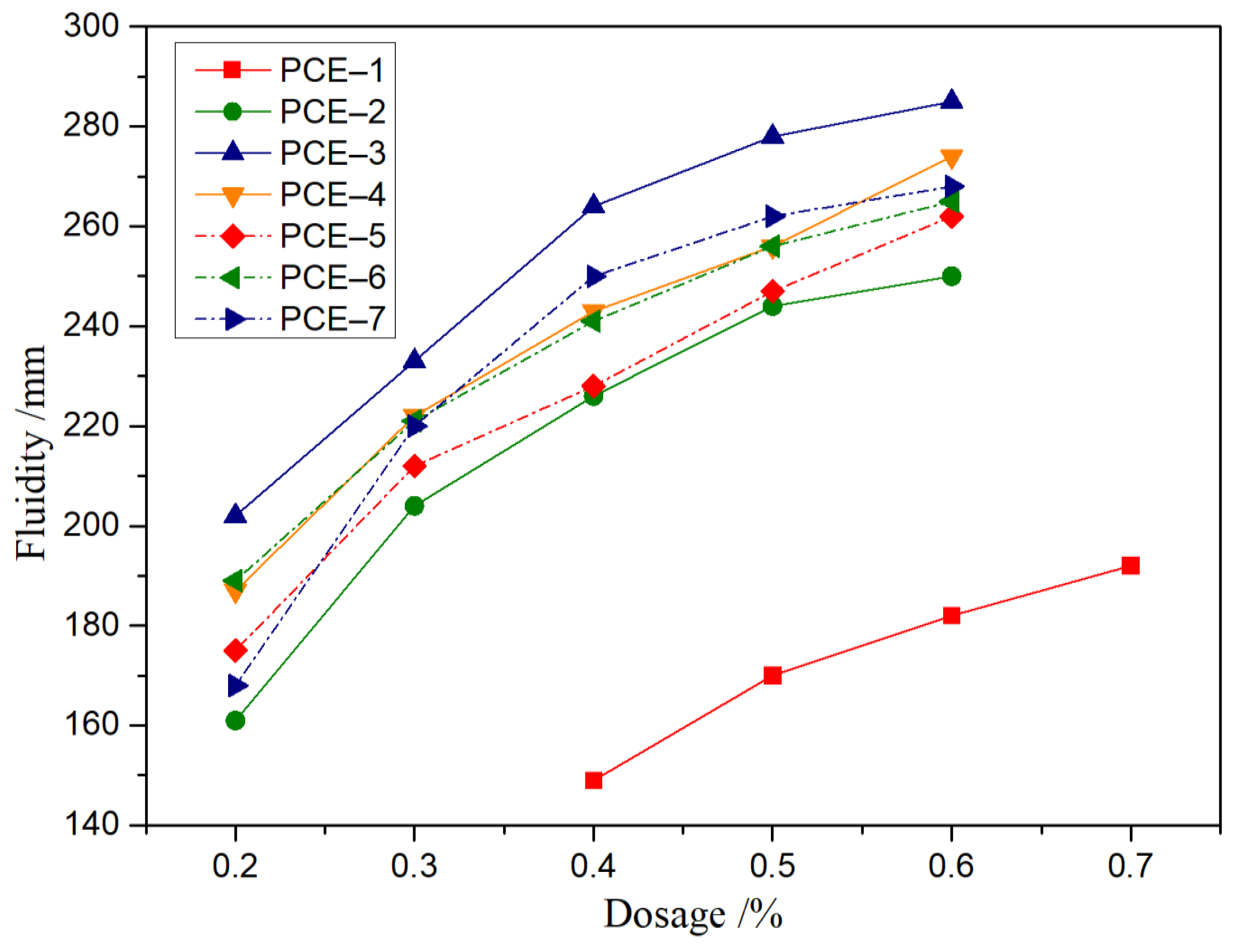
3.4.1. Fluidity
3.4.2. Rheology
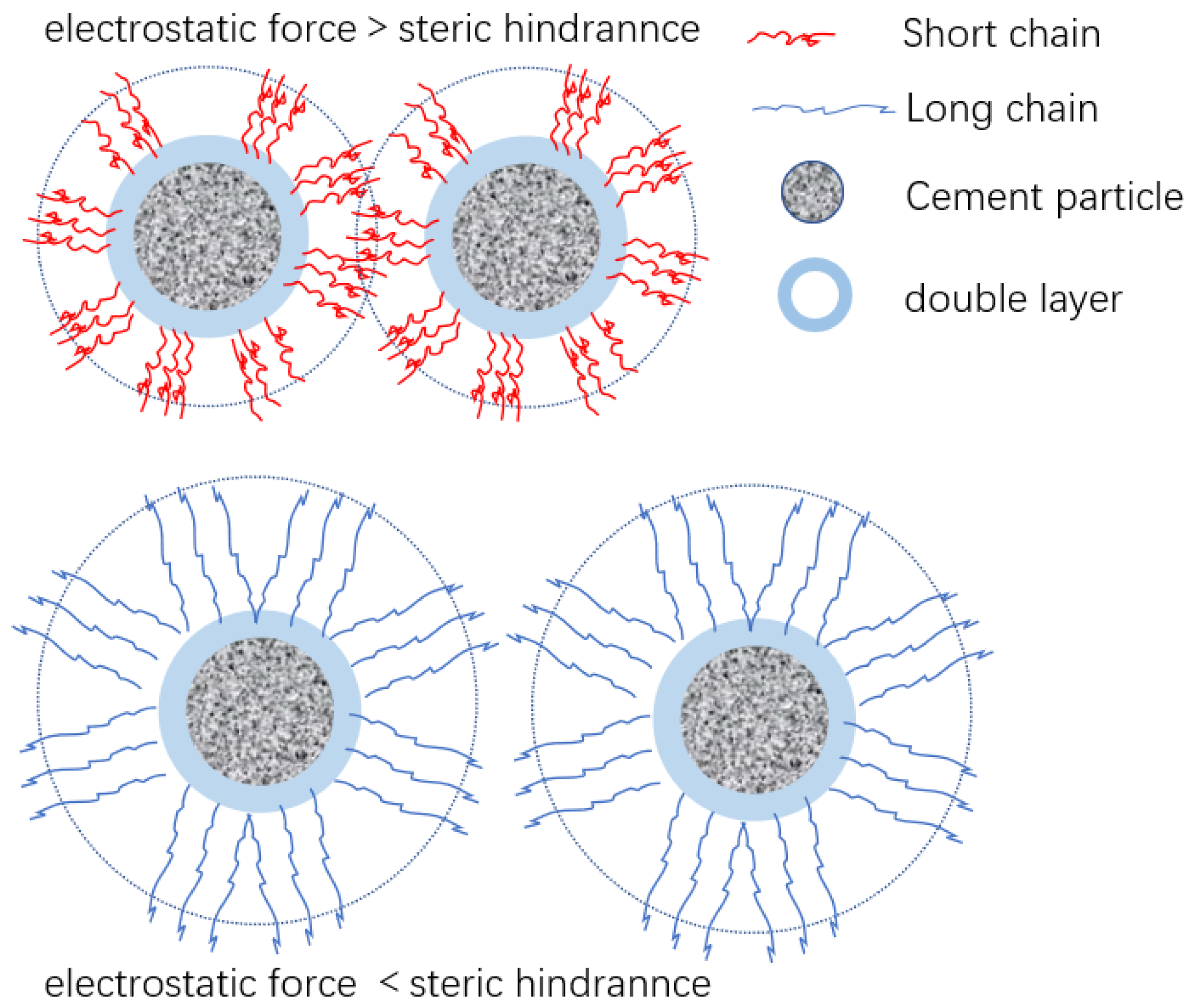
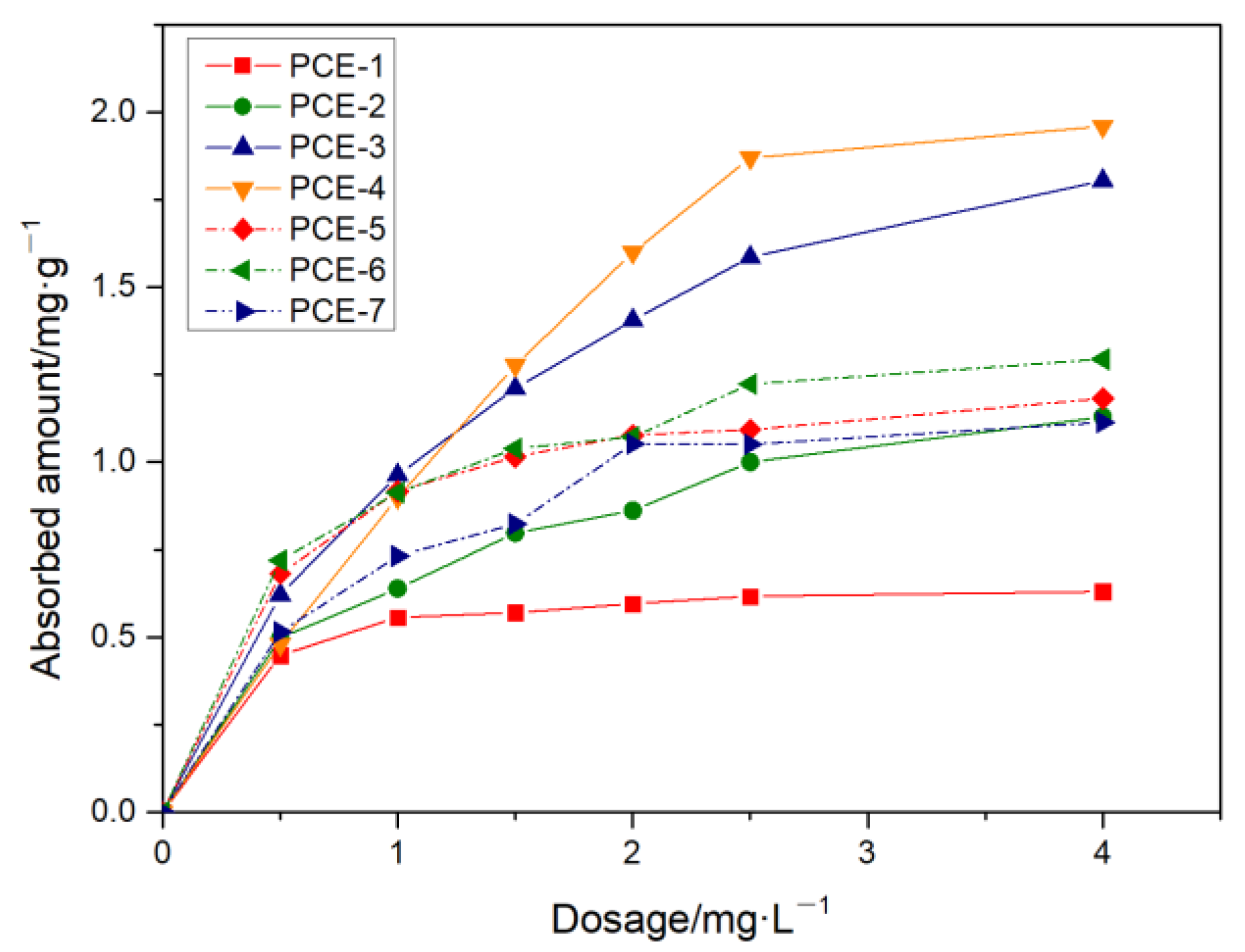
3.5. Adsorption Performance
3.5.1. Adsorption Isotherm Equation
3.5.2. Adsorption Thermodynamics
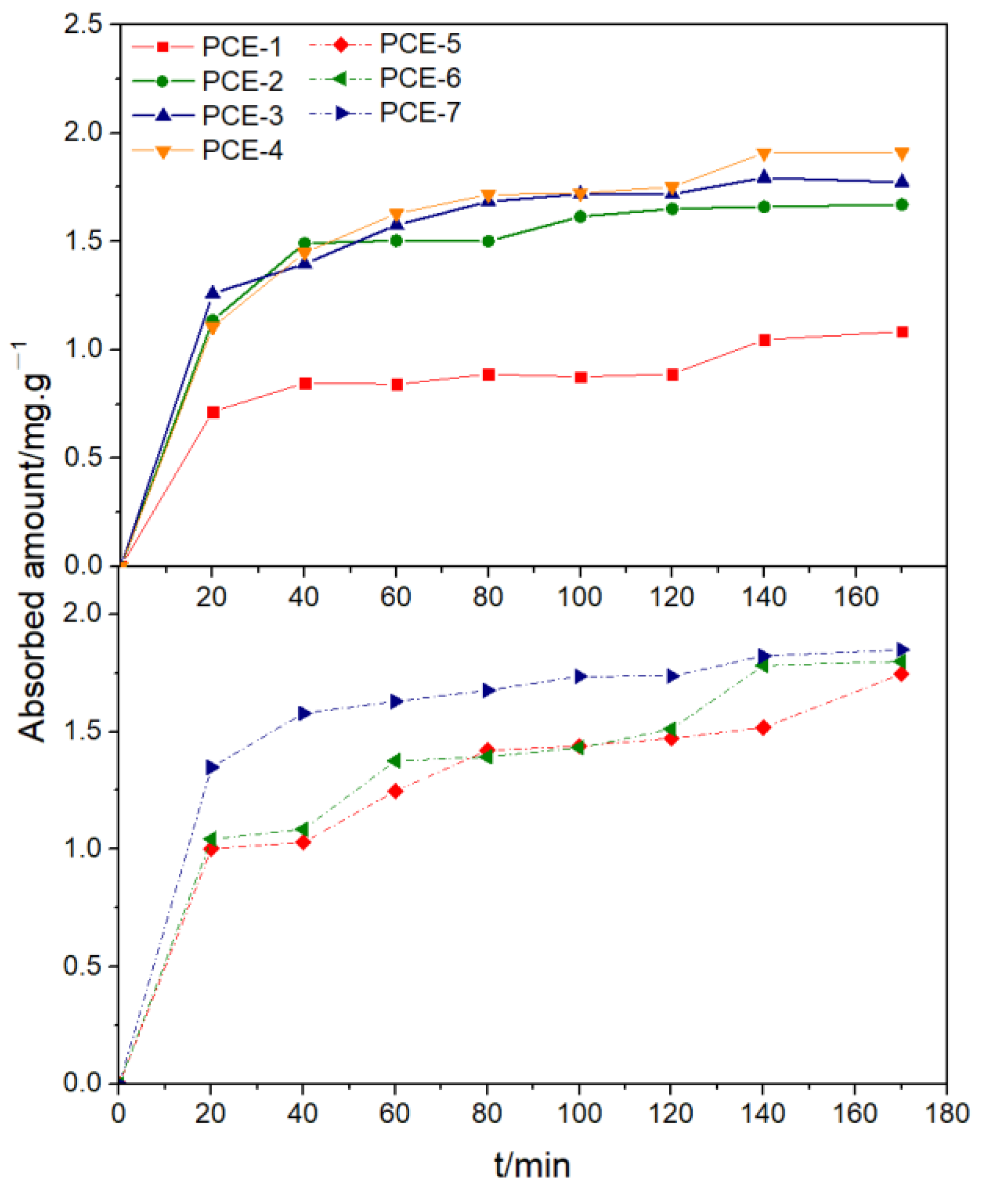
3.5.3. Adsorption Kinetics
3.6. Cement hydration
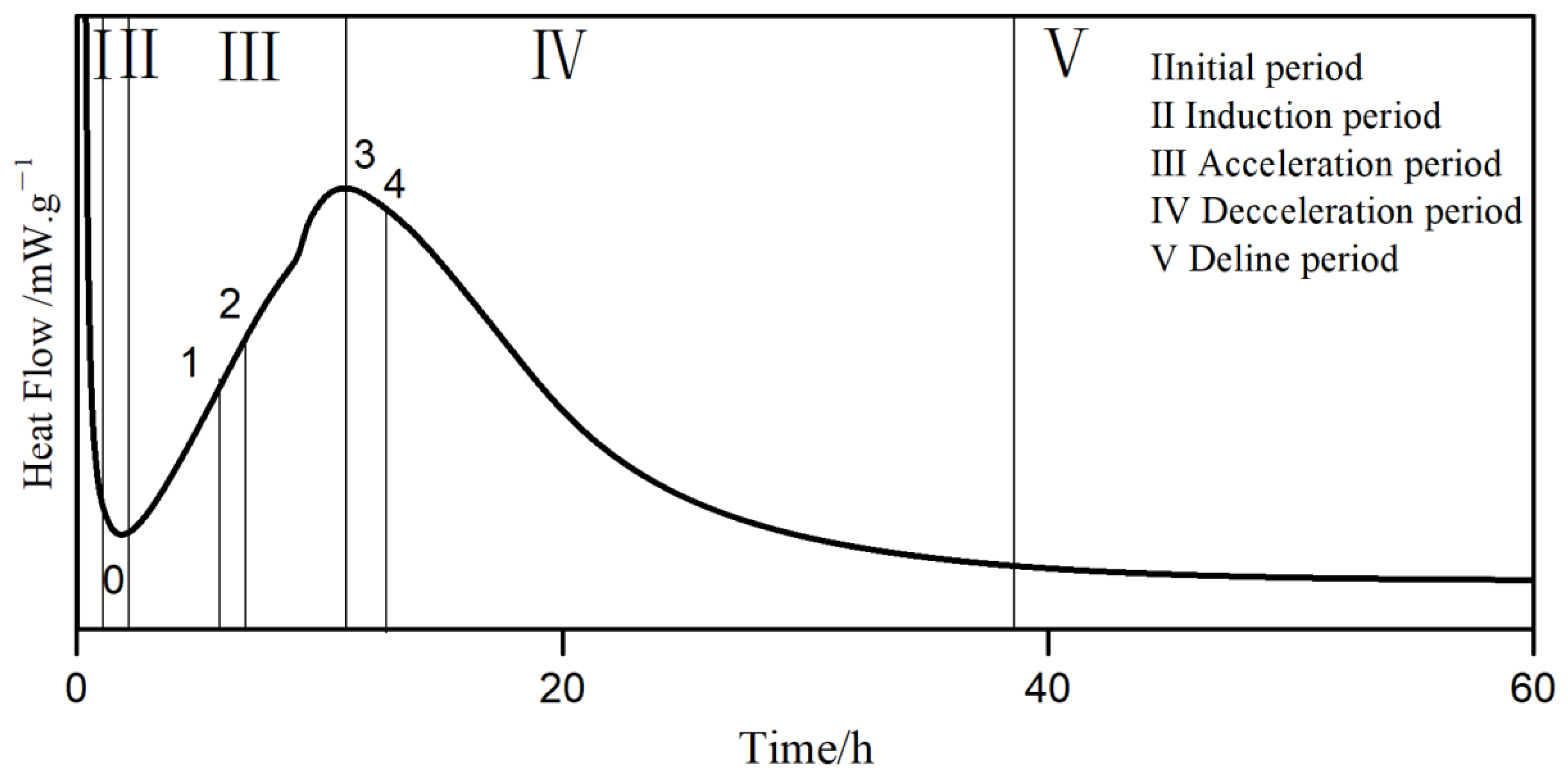
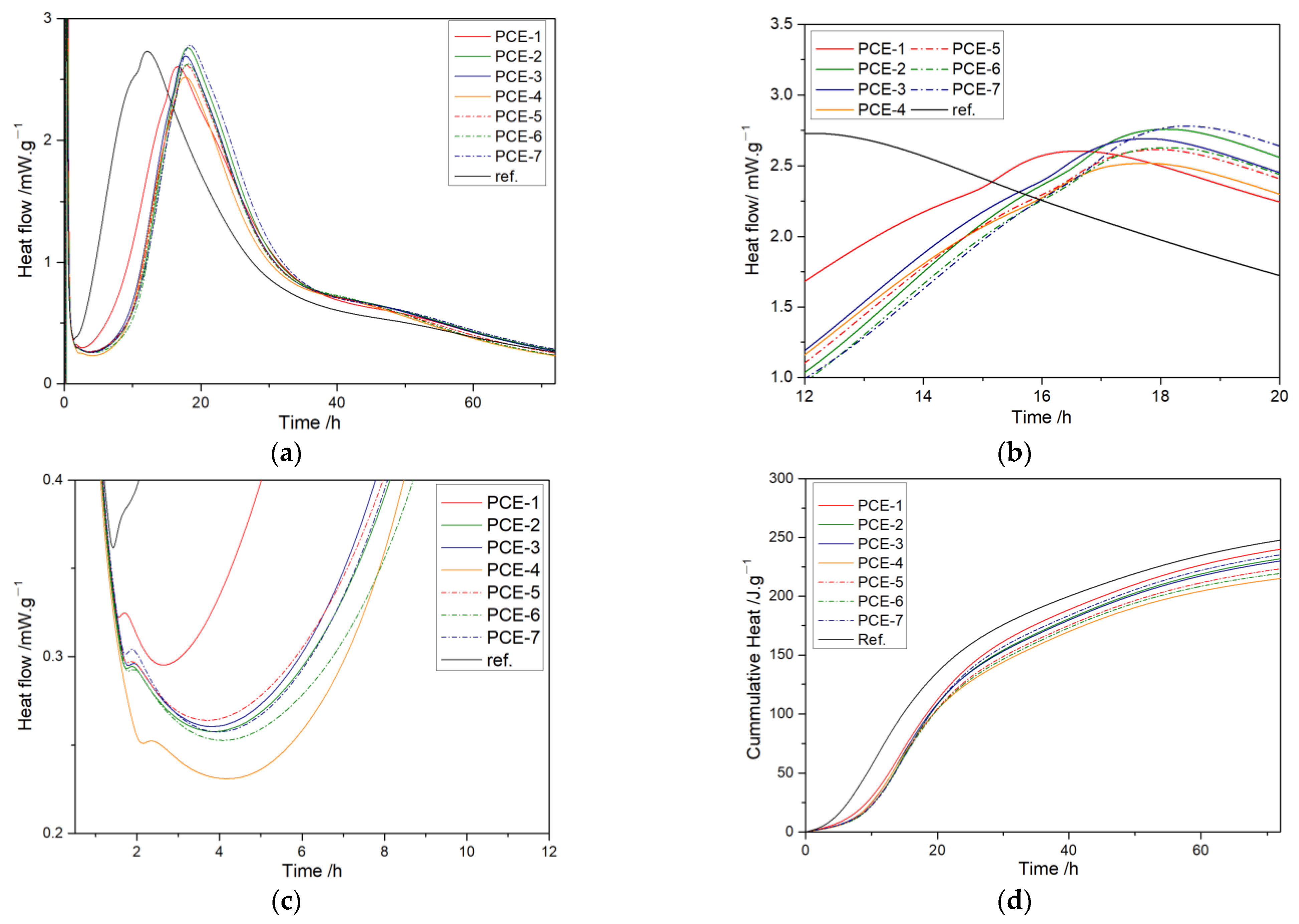
3.6.1. Heat of Hydration
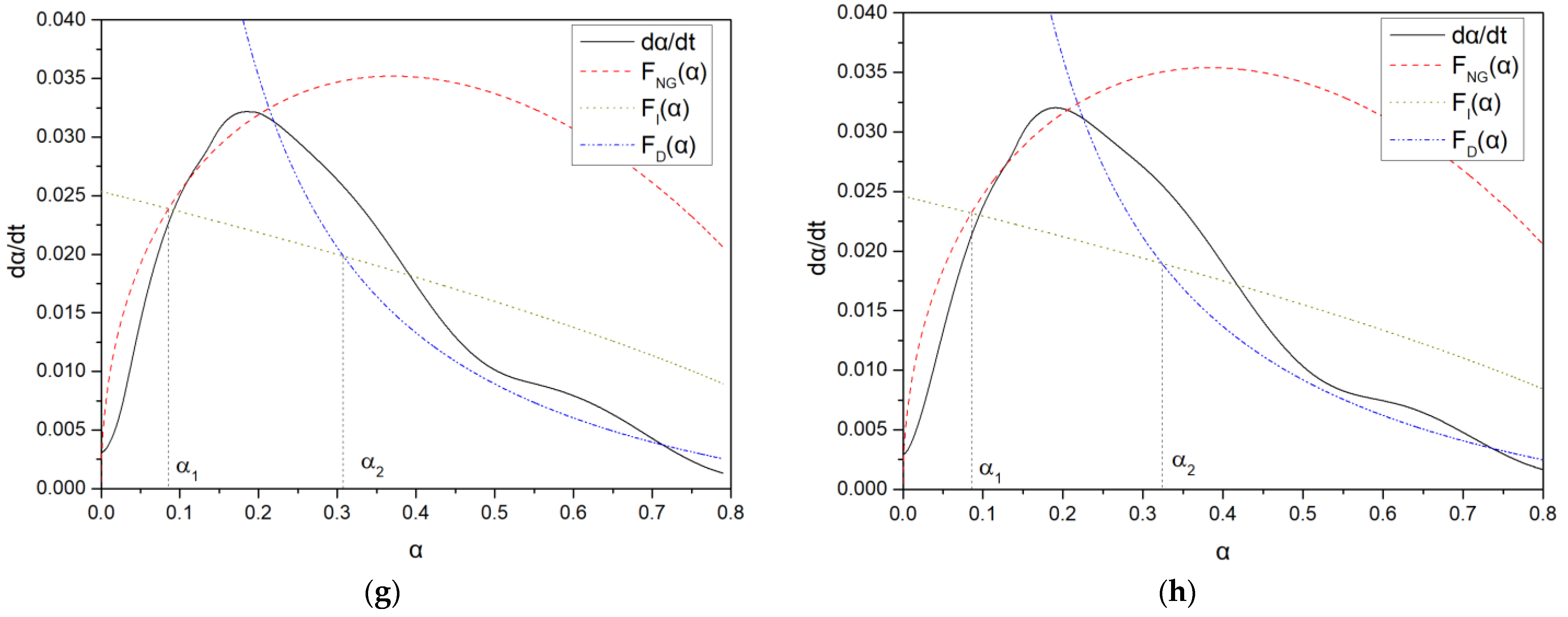
3.6.2. Hydration Kinetics
3.6.3. Hydration Products
3.6.4. Schematic Diagram of Hydration
4. Conclusions
- With the increase of carboxyl group density, the molecular weight, conversion rate, and hydrodynamic radius increased, while the α value first decreased and then increased. With the increase of the main chain polymerization degree, the molecular weight increased rapidly, and the conversion rate remained at around 90%. This indicates that carboxyl group density has a greater impact on the conversion rate during synthesis.
- With the increase of carboxyl group density and main chain polymerization degree, the fluidity of the paste showed a trend of first increasing and then decreasing, consistent with the rheological parameters. PCE-3 had optimal dispersion and rheological properties.
- The adsorption amount increased with the increase in dosage until it reached a steady state. When the carboxyl group density was small or the main chain polymerization degree was large, it showed single-molecule layer adsorption, while the adsorption effect weakened when the carboxyl group density was maximum. The main chain polymerization degree was the determining factor for the adsorption rate on cement, and the charge density was the determining factor for the equilibrium adsorption amount. The smaller the main chain polymerization degree, the faster the adsorption rate, and the smaller the charge density, the higher the equilibrium adsorption amount.
- With the increase of carboxyl group density, the induction period time gradually increased, and the increase slowed down. PCE-4 had the shortest acceleration period. With the increase of the main chain polymerization degree, the induction period time first increased and then decreased, opposite to the acceleration period.
- Cement hydration kinetics: during the NG stage, the blank sample had the smallest n value and more needle-shaped products were formed, while the addition of PCE samples resulted in a larger n value and domination of C-S-H products, indicating a delay in the early stage of acceleration period. With the increase of carboxyl group density, PCE-4 had the smallest n value, and the nucleation amount was the smallest in the I process. With the increase of the main chain polymerization degree, PCE-7 had the largest n value, indicating that ion concentration had the greatest influence on its nucleation and growth agent.
- PCE-4 had fewer clustered C-S-H, needle-shaped, and blocky AFt, but more obvious needle-shaped products, with fewer quantities and more inclined toward needle-shaped, which is consistent with the hydration kinetics analysis. In PCE with different main chain polymerization degrees, there were fewer needle-shaped products, and the products tended toward clustered and blocky AFt, consistent with the analysis results of the n value. The addition of PCE had a significant impact on the hydration induction period and acceleration period, improving the hydration degree of 3d hydration samples and benefitting the development of later strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandran, V.S. Concrete Admixtures Handbook: Properties, Science, and Technology; William Andrew Publishing/Noyes: New York, NY, USA, 1995. [Google Scholar]
- Sha, S.N.; Wang, M.; Shi, C.J.; Xiao, Y.C. Influence of the structures of polycarboxylate superplasticizer on its performance in cement-based materials-A review. Constr. Build. Mater. 2020, 233, 117257. [Google Scholar] [CrossRef]
- Flatt, R.J.; Schober, I.; Raphael, E.; Plassard, C.; Lesniewska, E. Conformation of Adsorbed Comb Copolymer Dispersants. Langmuir 2009, 23, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Pourchet, S.; Liautaud, S.; Rinaldi, D.; Pochard, I. Effect of the repartition of the PEG side chains on the adsorption and dispersion behaviors of PCP in presence of sulfate. Cem. Concr. Res. 2012, 42, 431–439. [Google Scholar] [CrossRef]
- Plank, J.; Schroefl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. J. Adv. Concr. Technol. 2009, 7, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.M.; Zhang, Y.R.; Hou, S.S. Study on the rheological properties of Portland cement pastes with polycarboxylate superplasticizer. Rheol. Acta 2013, 52, 707–718. [Google Scholar] [CrossRef]
- Oproiu, C.; Voivu, G.; Nicoara, A.I.; Badanoiu, A.I. The Influence of Partial Substitution of Raw Materials with Heavy Ash on the Main Properties of Portland Cements. Rev. Chim. 2018, 69, 860–863. [Google Scholar] [CrossRef]
- Li, F.L.; Sun, Q.Y.; Zhu, Q. Experimental Study on Limit of Stone-powder of Gravel-crushed Proto-machine-made Sand for Concrete. J. YR Sci. Res. Inst. 2010, 27, 66–69. [Google Scholar]
- Cui, J.Y.; He, Z.; Zhang, G.Z.; Cai, X.H. Rheological properties of sprayable ultra-high performance concrete with different viscosity-enhancing agents. Constr. Build. Mater. 2022, 321, 126154. [Google Scholar] [CrossRef]
- Liu, G.L. Effect of Silt Content of the Sand on the Performance of Concrete with Polycarboxylic Acid High Efficiency Water Reducing Agent. Constr. Des. Proj. 2018, 10, 24–25. [Google Scholar]
- Zhou, D.L.; Ran, Q.P.; Jiang, J.; Liu, J.P.; Miao, C.W. Influence of Temperature on Properties of Different Ester-Type Polycarboxylate Graft Copolymers. J. Southeast Univ. Nat. Sci. Ed. 2010, 40, 133–137. [Google Scholar]
- Toyoharu, N. Effect of Chemical Structure on Steric Stabilization of Polycarboxylate-based Superplasticizer. J. Adv. Concr. Technol. 2006, 4, 225–232. [Google Scholar]
- Wu, F.L.; Song, J. On Synthesis of Polyester-Type and Polyether-Type Composite Superplasticizer with Clay Tolerance and Action Mechanism of Bentonite. J. Southwest China Norm. Univ. Nat. Sci. Ed. 2019, 44, 70–76. [Google Scholar]
- He, Y.; Zhang, X.; Wang, Y.T.; Kong, Y.N.; Ji, T.; Shui, L.L.; Wang, X.F.; Wang, H.R. Effect of PCEs with Different Functional Groups on the Performance of Cement Paste. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 1163–1169. [Google Scholar] [CrossRef]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Götz, T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effect of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Feng, H.; Feng, Z.J.; Wang, W.S.; Deng, Z.L.; Zheng, B.C. Impact of polycarboxylate superplasticizers (PCEs) with novel molecular structures on fluidity, rheological behavior and adsorption properties of cement mortar. Constr. Build. Mater. 2021, 292, 123285. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, Q.Q.; Liu, J.P. Effects of molecular weight of polycarboxylate superplasticizers on rheological properties of cement pastes. J. Southeast Univ. Nat. Sci. Ed. 2020, 50, 482–487. [Google Scholar]
- Yoshioka, K.; Tazawa, E.; Kawai, K.; Enohata, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Li, Y.W.; Yang, C.L.; Zhang, Y.F.; Zheng, J.; Guo, H.L.; Lu, M.G. Study on dispersion, adsorption and flow retaining behaviors of cement mortars with TPEG-type polyether kind polycarboxylate superplasticizers. Constr. Build. Mater. 2014, 64, 324–332. [Google Scholar] [CrossRef]
- Liu, J.P.; Yu, Y.H.; Ran, Q.P.; Zhou, D.L.; Qiao, M. Adsorption Characteristics of Comb-like polycarboxylate on pure cement component. J. Build. Mater. 2012, 15, 596–600, 611. [Google Scholar]
- Mollah, M.Y.A.; Adams, W.J.; Schennach, R.; Cocke, D.L. A review of cement–superplasticizer interactions and their models. Adv. Cem. Res. 2000, 12, 153–161. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T.L. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Weckwerth, S.A.; Temme, R.L.; Flatt, R.J. Experimental method and thermodynamic model for competitive adsorption between polycarboxylate comb copolymer. Cem. Concr. Res. 2022, 151, 106523. [Google Scholar] [CrossRef]
- Tian, H.W.; Kong, X.M.; Su, T.; Wang, D.M. Comparative study of two PCE Superplasticizers with varied charge density in Portland cement and sulfoaluminate cement systems. Cem. Concr. Res. 2019, 115, 43–58. [Google Scholar] [CrossRef]
- Zhao, X.Z. Principles of Chemical Reaction Kinetics; Higher Education Press: Beijing, China, 1984; pp. 1–2. [Google Scholar]
- Krstulović, R.; Dabić, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Han, J. Study on the Hydration Properties of MultiComponent Composite Cementitious System with Low Water-binder Ratio. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2021. [Google Scholar]
- Han, F.H. Study on the Hydration Characteristics and Kinetics of Composite Binder. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2015. [Google Scholar]
- Li, Z.P. Study on Optimization Design and Hydration Performance of Composite Cementitious Materials. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2021. [Google Scholar]
- Zhang, H.; Yang, Z.H.; Su, Y.F. Hydration kinetics of cement-quicklime system at different temperatures. Thermochim. Acta 2019, 673, 1–11. [Google Scholar] [CrossRef]
- Han, F.H.; Zhang, Z.Q.; Wang, D.M.; Yan, P.Y. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Lei, B.; Wu, L.; Song, G.Q. Cement Hydration Kinetics Research Based on the Multi-Phase Hydration Model, Advanced Materials Research. Adv. Mater. Res. 2011, 168, 26–30. [Google Scholar]
- Cai, B.X. Fundamentals of Physical Chemistry; Science Press: Beijing, China, 2001. [Google Scholar]
- Scrivener, K.L.; Juilland, P.; Monteiro, P. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Han, F.H.; Wang, D.M.; Yan, P.Y. Hydration Kinetics of Composite Binder Containing Different Content of Slag or Fly Ash. J. Chin. Ceram. Soc. 2014, 42, 613–620. [Google Scholar]
- Ohama, Y. Principle of latex modification and some typical properties of latex-modified mortars and concretes adhesion; binders (materials); bond (paste to aggregate); carbonation; chlorides; curing; diffusion. J. Am. Concr. Inst. 1987, 84, 511–518. [Google Scholar]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoefferg, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Shui, L.L.; Wang, Y.T.; Gu, M.D.; Wang, X.F.; Wang, H.R.; Lei, P. Effects of PCEs with various carboxylic densities and functional groups on the fluidity and hydration performances of cement paste. Constr. Build. Mater. 2019, 202, 656–668. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Academic Press Ltd.: London, UK, 1990; p. 775. [Google Scholar]




| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | MnO | TiO2 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| 63.79 | 19.80 | 5.12 | 3.65 | 2.30 | 2.49 | 0.30 | 0.31 | 0.12 | 0.16 | 1.85 |
| C3S | C2S | C3A | C4AF |
|---|---|---|---|
| 58.93 | 16.42 | 7.81 | 10.88 |
| X50 (μm) | <3 μm (%) | 3~32 μm (%) | 32~65 μm (%) | >65 μm (%) | >80 μm (%) |
|---|---|---|---|---|---|
| 14.581 | 18.903 | 67.724 | 13.229 | 0.144 | 0.000 |
| Samples | n(AA): n(TPEG) | SHP/% | Mn/Da | Mw/Da | Conversion/% | PDI | Rh/nm | Mark–Houwink α | ρs | Lm |
|---|---|---|---|---|---|---|---|---|---|---|
| PCE-1 | 1.5 | 1.5 | 22,461 | 36,142 | 68.3 | 1.61 | 4.62 | 0.26 | 0.31 | 14.41 |
| PCE-2 | 3.0 | 1.5 | 24,335 | 42,692 | 87.1 | 1.75 | 5.00 | 0.16 | 0.23 | 16.32 |
| PCE-3 | 4.5 | 1.5 | 25,247 | 44,843 | 89.6 | 1.78 | 5.10 | 0.17 | 0.17 | 16.46 |
| PCE-4 | 6 | 1.5 | 26,263 | 47,852 | 90.3 | 1.82 | 5.46 | 0.21 | 0.13 | 16.90 |
| PCE-5 | 4.5 | 0.5 | 48,328 | 92,690 | 90.0 | 1.92 | 6.94 | 0.35 | 0.16 | 34.03 |
| PCE-6 | 4.5 | 1.0 | 35,489 | 60,722 | 90.9 | 1.71 | 5.85 | 0.33 | 0.17 | 22.29 |
| PCE-7 | 4.5 | 2.0 | 19,123 | 30,452 | 90.0 | 1.59 | 4.37 | 0.30 | 0.19 | 11.18 |
| Sample | Solid Content/% | Flowability/mm | Plastic Viscosity/mPa·s | Yield Stress/MPa | Thixotropy Area/Pa·s·cm³ |
|---|---|---|---|---|---|
| PCE-1 | 0.2 | 149 | 0.9862 | 14.2700 | 312.46 |
| PCE-2 | 0.2 | 189 | 0.8232 | 7.6964 | 358.67 |
| PCE-3 | 0.2 | 216 | 0.7303 | 3.9429 | 268.65 |
| PCE-4 | 0.2 | 197 | 0.7425 | 7.0267 | 286.8 |
| PCE-5 | 0.2 | 177 | 0.7442 | 8.9846 | 304.65 |
| PCE-6 | 0.2 | 209 | 0.7752 | 3.3746 | 278.45 |
| PCE-7 | 0.2 | 195 | 0.7568 | 7.2311 | 315.44 |
| Sample | Langmuir | Freundlich | Temkin | R-P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe/mg·g−1 | KL/L·mg−1 | R2 | KF/(mg·g−1)·(L·mg−1)1/n | nF | R2 | kT/ L·mol−1 | bT/g·mol·mg−1·J−1 | R2 | kRP/ L·g−1 | R2 | |
| PCE-1 | 0.8295 | 1.8620 | 0.9911 | 0.5202 | 3.4413 | 0.9714 | 27.8690 | 15,494.73 | 0.9910 | 3.7907 | 0.9901 |
| PCE-2 | 1.4175 | 0.8915 | 0.9908 | 0.6622 | 2.4951 | 0.9858 | 8.7044 | 7897.65 | 0.9635 | 2.5646 | 0.9903 |
| PCE-3 | 2.5466 | 0.6221 | 0.9790 | 0.9758 | 2.1070 | 0.9702 | 5.5048 | 4225.72 | 0.9707 | 1.5732 | 0.9910 |
| PCE-4 | 3.3376 | 0.4169 | 0.9594 | 0.9823 | 1.7645 | 0.9638 | 3.5942 | 3183.23 | 0.9832 | 1.3805 | 0.9592 |
| PCE-5 | 1.3109 | 2.2301 | 0.9993 | 0.8833 | 4.2279 | 0.9890 | 41.6606 | 10,423.41 | 0.9944 | 2.7751 | 0.9983 |
| PCE-6 | 1.4486 | 1.7891 | 0.9935 | 0.9062 | 3.6167 | 0.9973 | 25.3350 | 8750.36 | 0.9964 | 4.8559 | 0.9964 |
| PCE-7 | 1.3966 | 1.1434 | 0.9878 | 0.7340 | 2.8581 | 0.9728 | 10.8635 | 7980.01 | 0.9838 | 1.3491 | 0.9578 |
| PCE | T/K | KL/L·mg−1 | RL | ∆G/kJ·mol−1 | ∆H/kJ·mol−1 | ∆S/J·mol−1·K−1 |
|---|---|---|---|---|---|---|
| PCE-1 | 298 | 0.7552 | 0.3463 | −24.14 | −18.11 | 20.46 |
| 308 | 0.6536 | 0.3797 | −24.58 | |||
| 318 | 0.6317 | 0.3877 | −25.29 | |||
| 328 | 0.4002 | 0.4999 | −24.84 | |||
| PCE-2 | 298 | 0.1975 | 0.5584 | −21.93 | −14.31 | 17.08 |
| 308 | 0.1682 | 0.6687 | −21.47 | |||
| 318 | 0.1575 | 0.7175 | −21.56 | |||
| 328 | 0.1411 | 0.7392 | −21.93 | |||
| PCE-3 | 298 | 0.4266 | 0.4839 | −23.00 | −19.30 | 12.69 |
| 308 | 0.3577 | 0.5279 | −23.32 | |||
| 318 | 0.3461 | 0.5361 | −23.99 | |||
| 328 | 0.2121 | 0.6535 | −23.41 | |||
| PCE-4 | 298 | 0.8287 | 0.3463 | −24.76 | −24.70 | 0.69 |
| 308 | 0.7061 | 0.3797 | −25.18 | |||
| 318 | 0.4656 | 0.3877 | −24.89 | |||
| 328 | 0.3447 | 0.4999 | −24.86 | |||
| PCE-5 | 298 | 0.9363 | 0.2993 | −26.57 | −11.99 | 48.92 |
| 308 | 2.4663 | 0.1396 | −26.46 | |||
| 318 | 0.6908 | 0.3667 | −27.55 | |||
| 328 | 8.9483 | 0.0428 | −26.78 | |||
| PCE-6 | 298 | 2.9798 | 0.1183 | −28.68 | −15.70 | 43.57 |
| 308 | 2.4454 | 0.1406 | −29.13 | |||
| 318 | 2.0337 | 0.1644 | −29.59 | |||
| 328 | 1.6645 | 0.1938 | −29.97 | |||
| PCE-7 | 298 | 3.7597 | 0.0962 | −29.32 | −37.62 | 25.50 |
| 308 | 1.1893 | 0.2517 | −29.64 | |||
| 318 | 0.5980 | 0.4008 | −29.78 | |||
| 328 | 0.8160 | 0.3290 | −29.11 |
| Sample | PFO | PSO | Elovich | ID | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1/ min−1 | qe1/ mg·g−1 | h0/ mg·(g·min)−1 | R2 | qe2/ mg·g−1 | k2/ min−1 | h0/ mg2·(g2·min)−1 | R2 | a/ mg·g−1·min−1 | b/ g·mg−1 | R2 | kID/ mg·g−1·min−0.5 | CID/ mg·g−1 | R2 | |
| PCE-1 | 0.944 | 0.0629 | 0.0594 | 0.9311 | 1.0462 | 0.0896 | 0.0980 | 0.9568 | 0.8618 | 6.7907 | 0.9708 | 0.0715 | 0.2163 | 0.8090 |
| PCE-2 | 1.617 | 0.0592 | 0.0957 | 0.9892 | 1.7749 | 0.0543 | 0.1709 | 0.9934 | 2.2001 | 4.3047 | 0.9876 | 0.1177 | 0.4001 | 0.7736 |
| PCE-3 | 1.721 | 0.0546 | 0.0940 | 0.9799 | 1.8991 | 0.0460 | 0.1660 | 0.9947 | 1.5468 | 3.7758 | 0.9953 | 0.1268 | 0.4024 | 0.8045 |
| PCE-4 | 1.820 | 0.0418 | 0.0760 | 0.9863 | 2.0849 | 0.0270 | 0.1175 | 0.9958 | 0.4146 | 2.7034 | 0.9925 | 0.1394 | 0.3300 | 0.8712 |
| PCE-5 | 1.545 | 0.0343 | 0.0531 | 0.9381 | 1.7997 | 0.0249 | 0.0806 | 0.9658 | 0.2147 | 2.8412 | 0.9779 | 0.1207 | 0.2256 | 0.9145 |
| PCE-6 | 1.634 | 0.0331 | 0.0541 | 0.9241 | 1.9148 | 0.0221 | 0.0810 | 0.9554 | 0.2020 | 2.6038 | 0.9692 | 0.1283 | 0.2248 | 0.9179 |
| PCE-7 | 1.747 | 0.0682 | 0.1191 | 0.9874 | 1.8936 | 0.0629 | 0.2254 | 0.9973 | 5.7160 | 4.5343 | 0.9980 | 0.1259 | 0.4609 | 0.7607 |
| Sample | t0/h | q0/ mW·g−1 | Q0/J·g−1 | t1/h | t2/h | K2/ mW·g−1·h−1 | q2/ mW·g−1 | t3/h | t4/h | q3/ mW·g−1 | Q3/J·g−1 | Q0–3/J·g−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 1.42 | 0.36 | 2.14 | 6.93 | 6.01 | 0.36 | 1.49 | 12.12 | 14.62 | 2.73 | 76.86 | 74.72 |
| PCE-1 | 2.64 | 0.30 | 3.24 | 10.12 | 11.52 | 0.29 | 1.55 | 16.60 | 19.31 | 2.60 | 85.87 | 82.63 |
| PCE-2 | 3.82 | 0.26 | 4.15 | 10.41 | 12.95 | 0.36 | 1.52 | 17.77 | 18.08 | 2.69 | 92.26 | 88.11 |
| PCE-3 | 4.12 | 0.25 | 4.46 | 10.85 | 13.49 | 0.38 | 1.56 | 18.09 | 18.54 | 2.76 | 93.42 | 88.96 |
| PCE-4 | 4.14 | 0.23 | 4.15 | 10.09 | 12.75 | 0.34 | 1.41 | 17.82 | 18.58 | 2.52 | 89.37 | 85.21 |
| PCE-5 | 3.73 | 0.26 | 4.03 | 10.44 | 13.09 | 0.35 | 1.47 | 17.99 | 18.56 | 2.62 | 89.91 | 85.88 |
| PCE-6 | 4.09 | 0.25 | 4.27 | 10.78 | 4.09 | 0.37 | 1.50 | 18.08 | 18.15 | 2.63 | 90.06 | 85.80 |
| PCE-7 | 3.92 | 0.26 | 4.21 | 10.73 | 14.21 | 0.36 | 1.70 | 18.44 | 19.10 | 2.78 | 95.85 | 91.65 |
| Sample | Qmax/J·g−1 | t50/h | NG | I | D | α1 | α2 | α3 | Δα1 | Δα2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KNG/h−1 | n | KI/μm·h−1 | KD/μm2·h−1 | ||||||||
| Blank | 333.33 | 24.73 | 0.0404 | 1.59 | 0.0085 | 0.0019 | 0.01 | 0.09 | 0.30 | 0.08 | 0.29 |
| PCE-1 | 322.58 | 25.26 | 0.0374 | 1.80 | 0.0077 | 0.0019 | 0.01 | 0.09 | 0.33 | 0.08 | 0.32 |
| PCE-2 | 303.03 | 23.36 | 0.0419 | 1.82 | 0.0085 | 0.0020 | 0.01 | 0.09 | 0.31 | 0.08 | 0.30 |
| PCE-3 | 303.03 | 23.39 | 0.0425 | 1.85 | 0.0086 | 0.0021 | 0.01 | 0.09 | 0.32 | 0.08 | 0.31 |
| PCE-4 | 270.27 | 19.89 | 0.0431 | 1.67 | 0.0092 | 0.0025 | 0.02 | 0.09 | 0.35 | 0.08 | 0.34 |
| PCE-5 | 294.12 | 22.62 | 0.0423 | 1.90 | 0.0084 | 0.0020 | 0.01 | 0.09 | 0.32 | 0.07 | 0.31 |
| PCE-6 | 294.12 | 25.29 | 0.0429 | 1.86 | 0.0085 | 0.0020 | 0.02 | 0.09 | 0.31 | 0.07 | 0.29 |
| PCE-7 | 312.50 | 24.28 | 0.0421 | 1.94 | 0.0082 | 0.0020 | 0.01 | 0.09 | 0.32 | 0.07 | 0.31 |
| Sample | Hydration Time 2 h | Hydration Time 12 h | Hydration Time 1 d | Hydration Time 3 d | ||||
|---|---|---|---|---|---|---|---|---|
| Hydration Degree | Morphology | Hydration Degree | Morphology | Hydration Degree | Morphology | Hydration Degree | Morphology | |
| Blank | 0.0101 | Corrosion pit | 0.2272 | Subtle hydration product, no needle-like | 0.4650 | Needle-like, length 1~2 μm | 0.7433 | Gel substrate |
| PCE-1 | 0.0072 | Corrosion pit | 0.1383 | 0.4230 | Fuzzy, non-needle-like | 0.7440 | substrate, needle-like | |
| PCE-2 | 0.0064 | Corrosion pit | 0.1227 | 0.4336 | Needle-like, length 1 μm | 0.7653 | substrate, needle-like | |
| PCE-3 | 0.0065 | Corrosion pit | 0.1319 | 0.4327 | Needle-like, few, length 1 μm | 0.7591 | substrate, needle-like | |
| PCE-4 | 0.0064 | Corrosion pit | 0.1488 | 0.4571 | Needle-like, few, length 1 μm | 0.7957 | Gel substrate, rod-like, needle-like | |
| PCE-5 | 0.0067 | Corrosion pit | 0.1267 | 0.4306 | Needle-like, few, length 1 μm | 0.7596 | Gel substrate, rod-like, needle-like | |
| PCE-6 | 0.0064 | Corrosion pit | 0.1246 | 0.4256 | Needle-like, length 1 μm | 0.7463 | Gel substrate, rod-like, needle-like | |
| PCE-7 | 0.0062 | Corrosion pit | 0.1159 | 0.4262 | Needle-like, length 2 μm | 0.7532 | Gel substrate, rod-like, needle-like | |
| Sample | Hydration Time 2 h | Hydration Time 12 h |
|---|---|---|
| Blank | 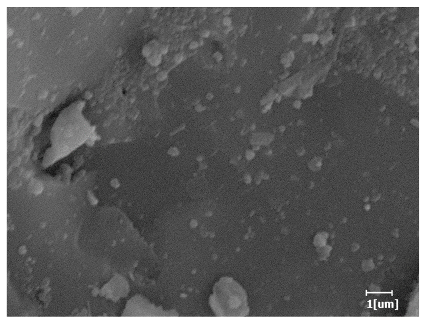 |  |
| PCE-1 |  | 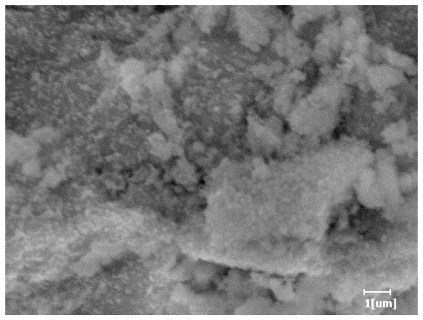 |
| PCE-2 | 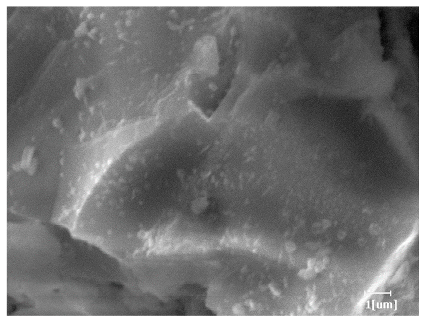 |  |
| PCE-3 |  | 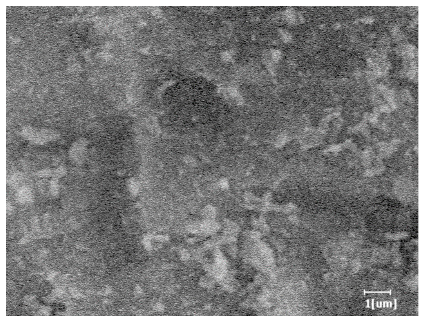 |
| PCE-4 |  | 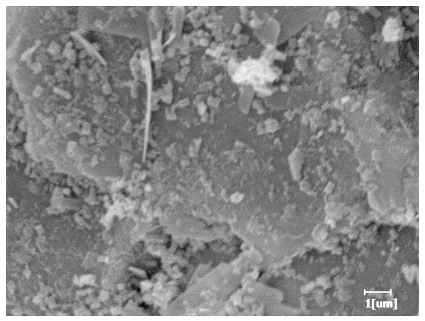 |
| PCE-5 |  | 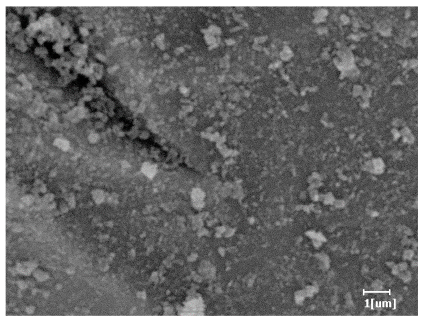 |
| PCE-6 | 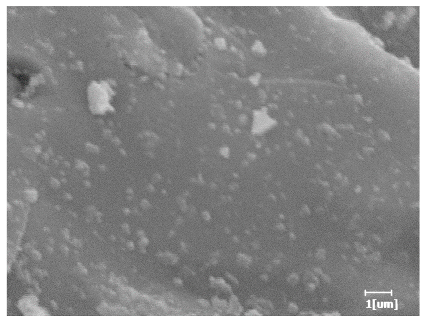 |  |
| PCE-7 | 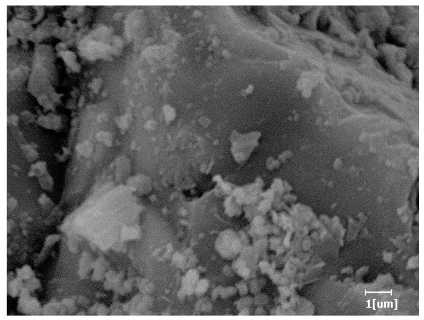 | 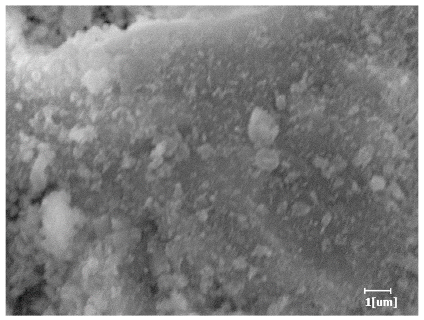 |
| Sample | Hydration Time 1 d | Hydration Time 3 d |
|---|---|---|
| Blank |  | 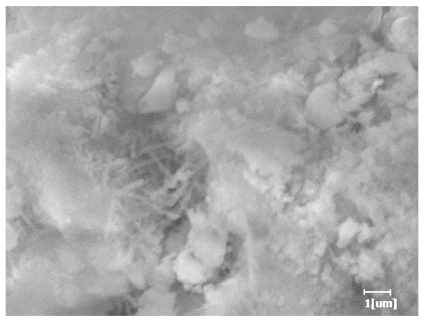 |
| PCE-1 |  |  |
| PCE-2 |  | 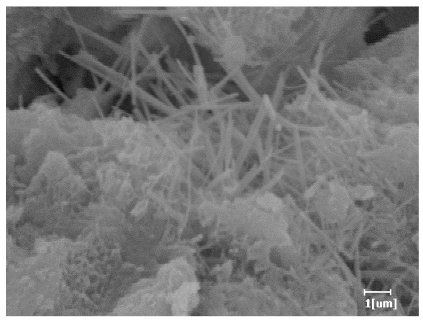 |
| PCE-3 | 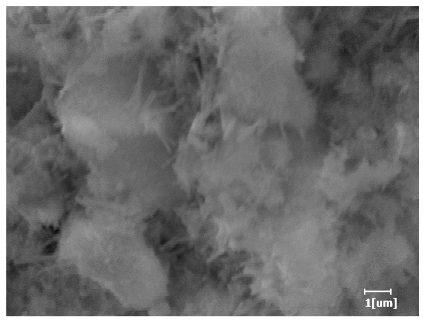 |  |
| PCE-4 | 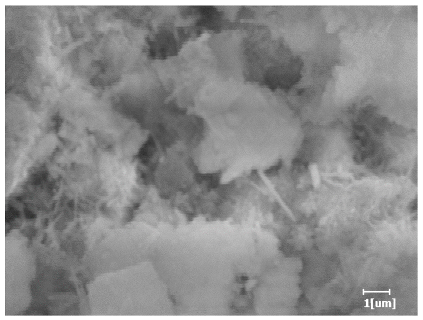 |  |
| PCE-5 | 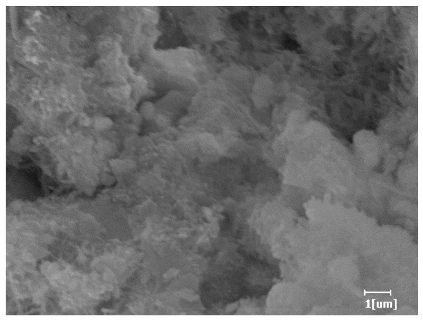 |  |
| PCE-6 |  |  |
| PCE-7 | 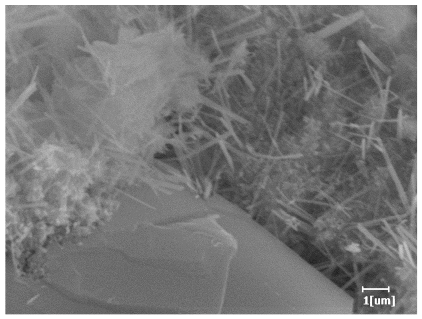 | 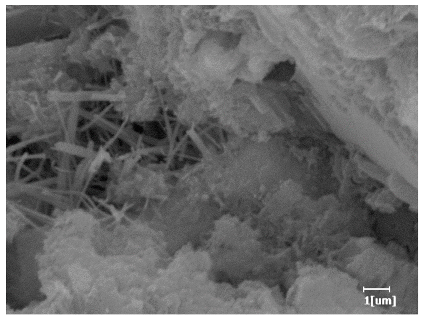 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Lin, Z.; Yan, D.; Zhang, X.; Ma, X.; Lai, J.; Liu, Y.; Chen, Z.; Wang, Z. Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure. Polymers 2023, 15, 2496. https://doi.org/10.3390/polym15112496
Fang Y, Lin Z, Yan D, Zhang X, Ma X, Lai J, Liu Y, Chen Z, Wang Z. Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure. Polymers. 2023; 15(11):2496. https://doi.org/10.3390/polym15112496
Chicago/Turabian StyleFang, Yunhui, Zhijun Lin, Dongming Yan, Xiaofang Zhang, Xiuxing Ma, Junying Lai, Yi Liu, Zhanhua Chen, and Zhaopeng Wang. 2023. "Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure" Polymers 15, no. 11: 2496. https://doi.org/10.3390/polym15112496
APA StyleFang, Y., Lin, Z., Yan, D., Zhang, X., Ma, X., Lai, J., Liu, Y., Chen, Z., & Wang, Z. (2023). Study on the Effect of Polycarboxylate Ether Molecular Structure on Slurry Dispersion, Adsorption, and Microstructure. Polymers, 15(11), 2496. https://doi.org/10.3390/polym15112496







