Facile UV-Induced Surface Covalent Modification to Fabricate Durable Superhydrophobic Fabric for Efficient Oil–Water Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
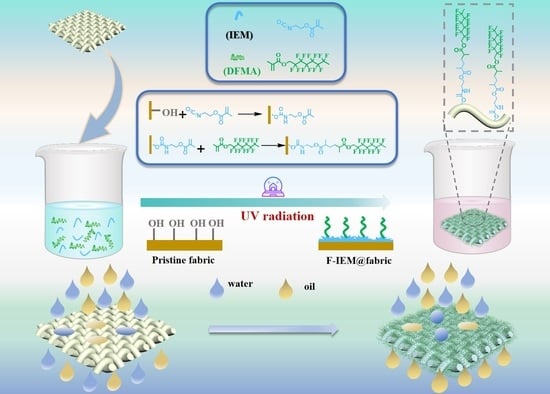
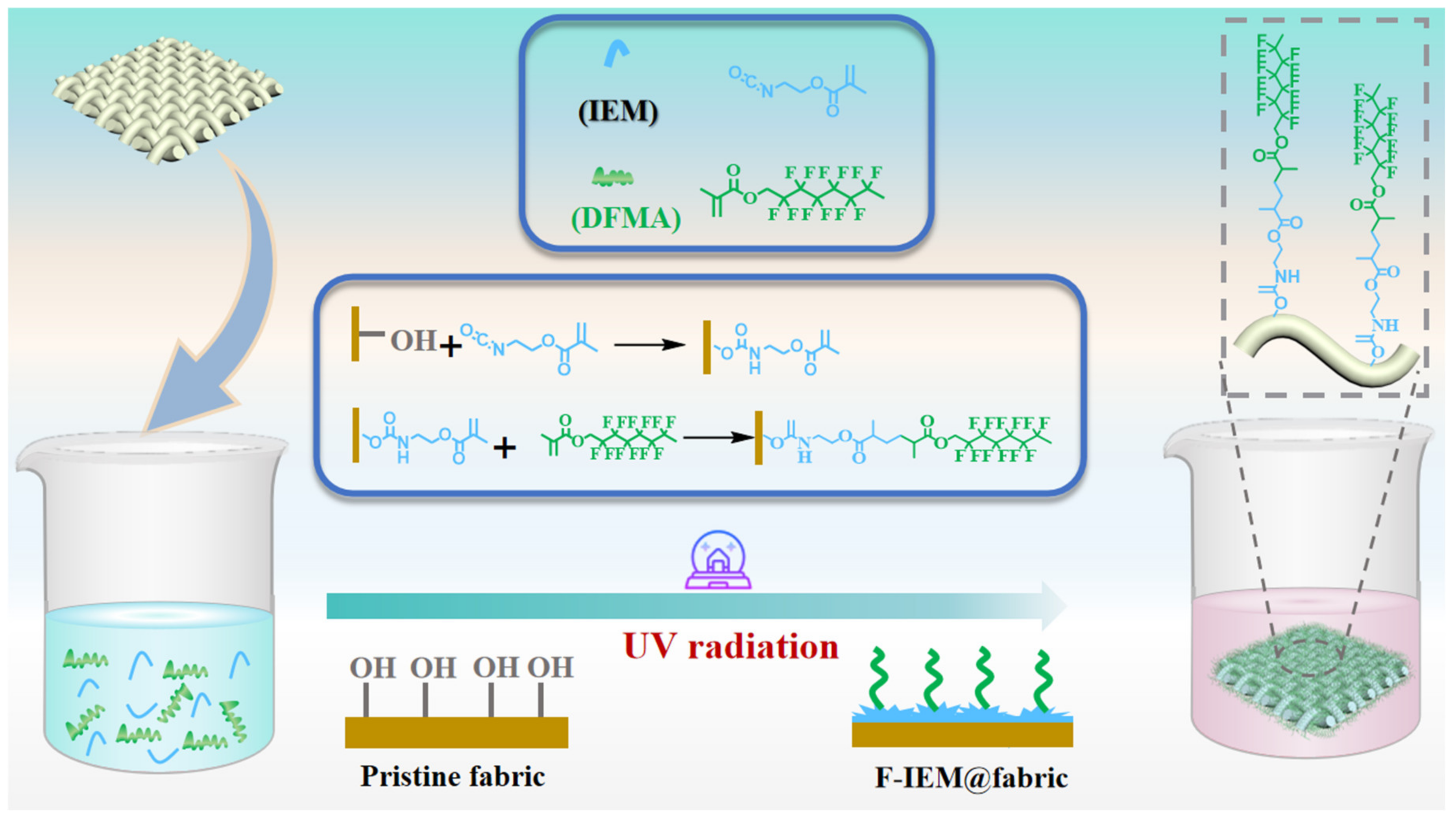
2.2. Fabrication of the Superhydrophobic F-IEM@fabric
2.3. Characterization
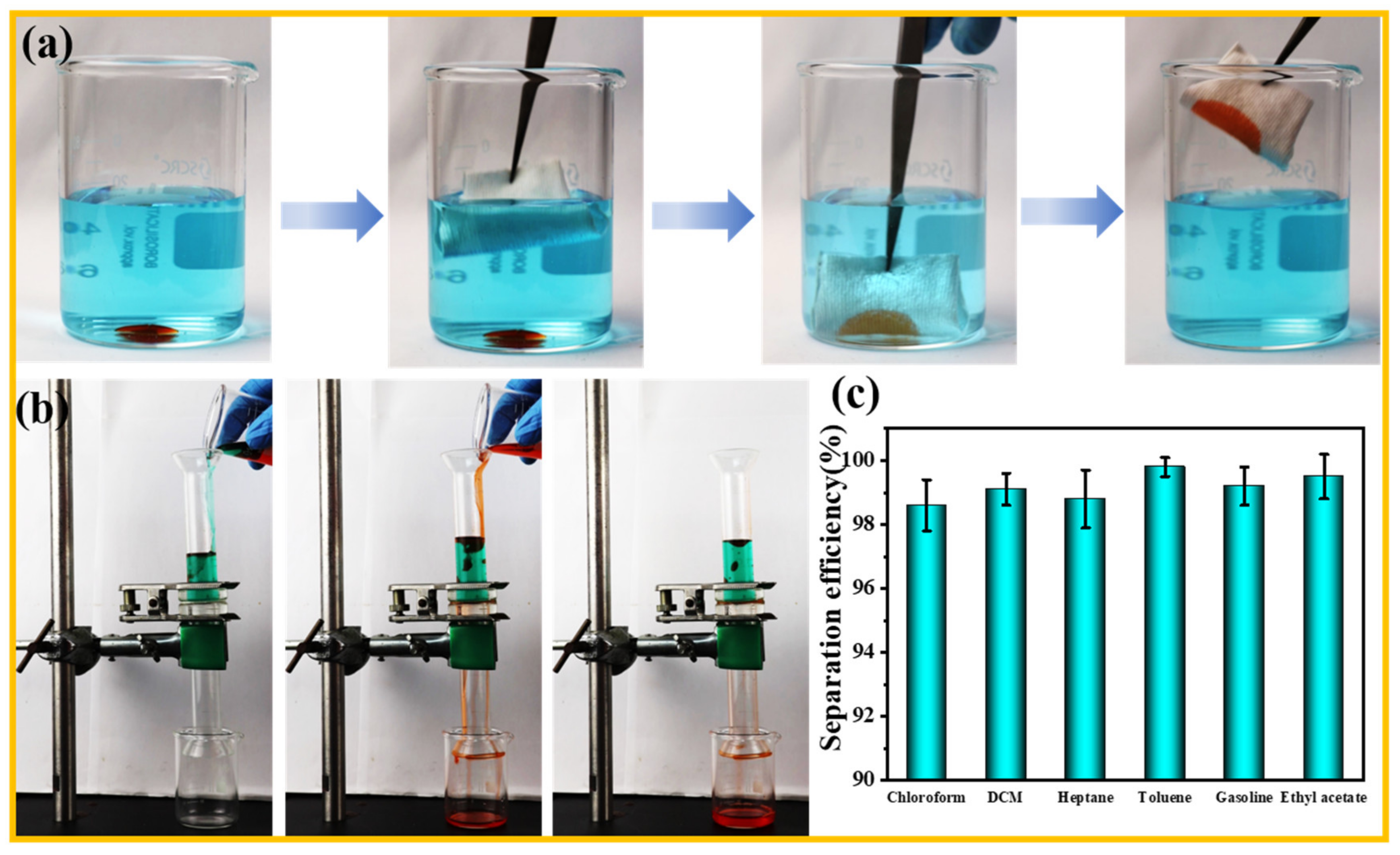
2.4. Oil–Water Separation Efficiency
2.5. Durability of the Superhydrophobic F-IEM@fabric
3. Results and Discussion
3.1. Fabrication of Superhydrophobic F-IEM@fabric
3.2. Surface Morphology of the Superhydrophobic F-IEM@fabric
3.3. Surface Property of the Superhydrophobic F-IEM@fabric
3.4. Breathable Property of Superhydrophobic F-IEM@fabric
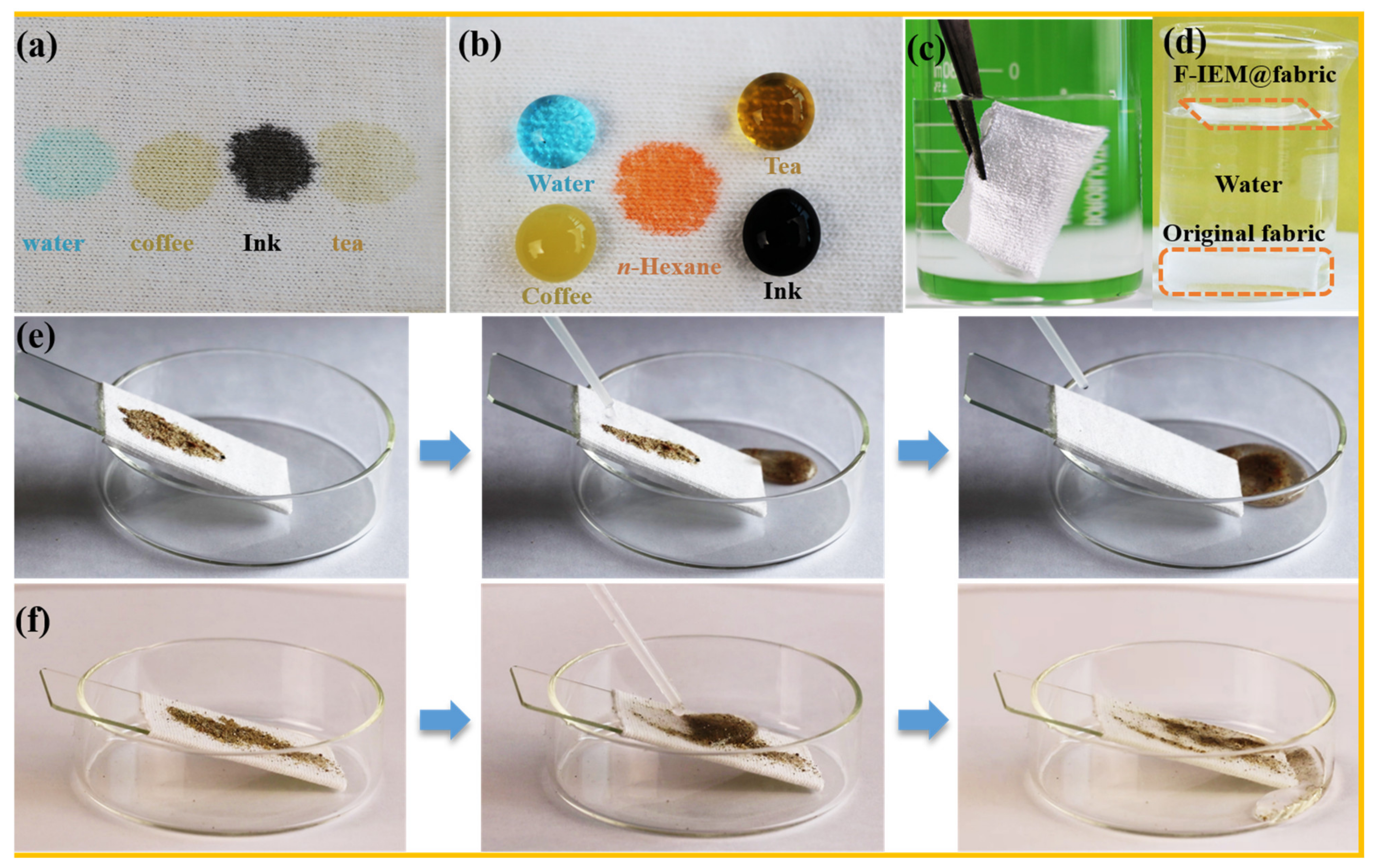
3.5. Oil/Water Separation Performance of F-IEM@fabric
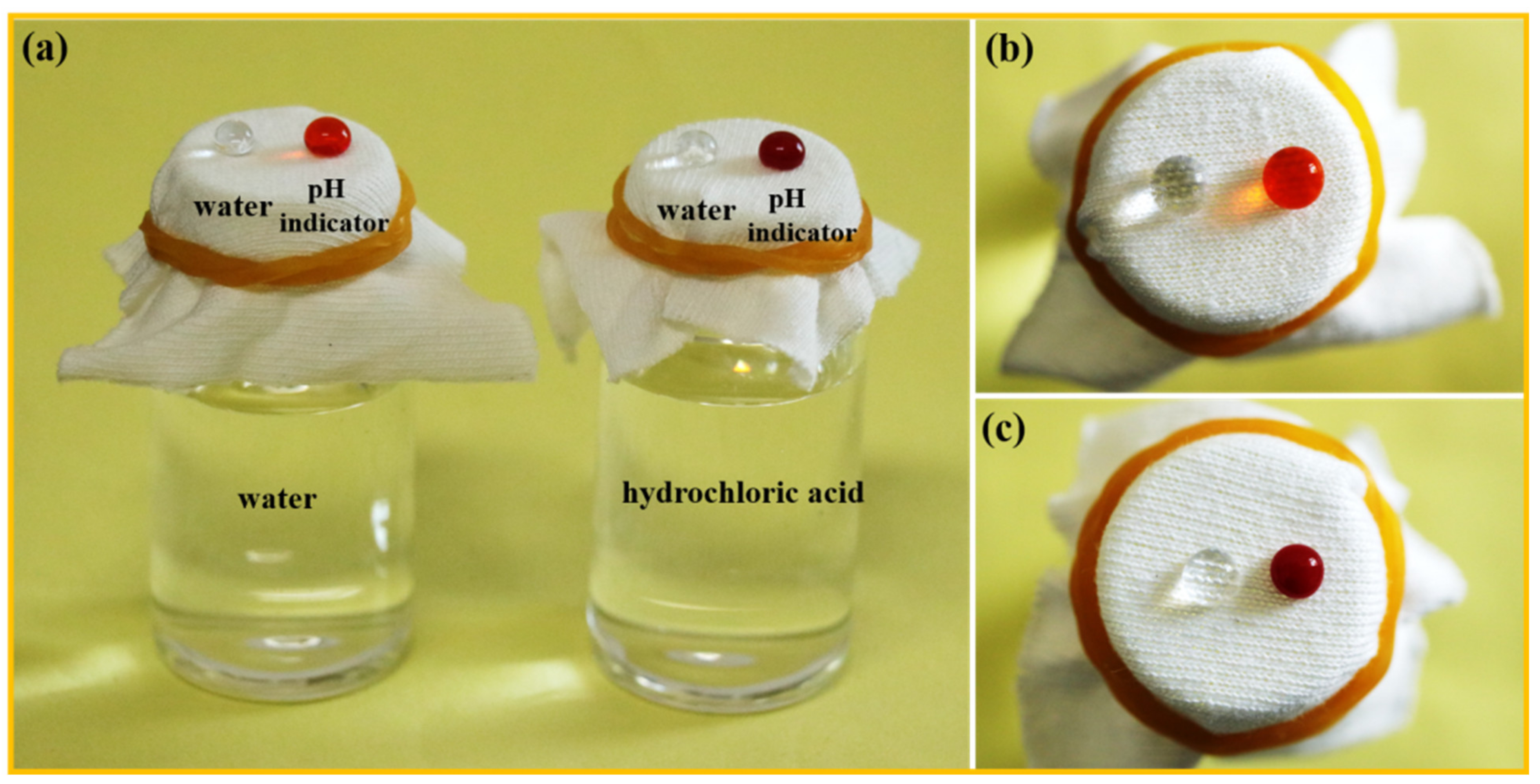
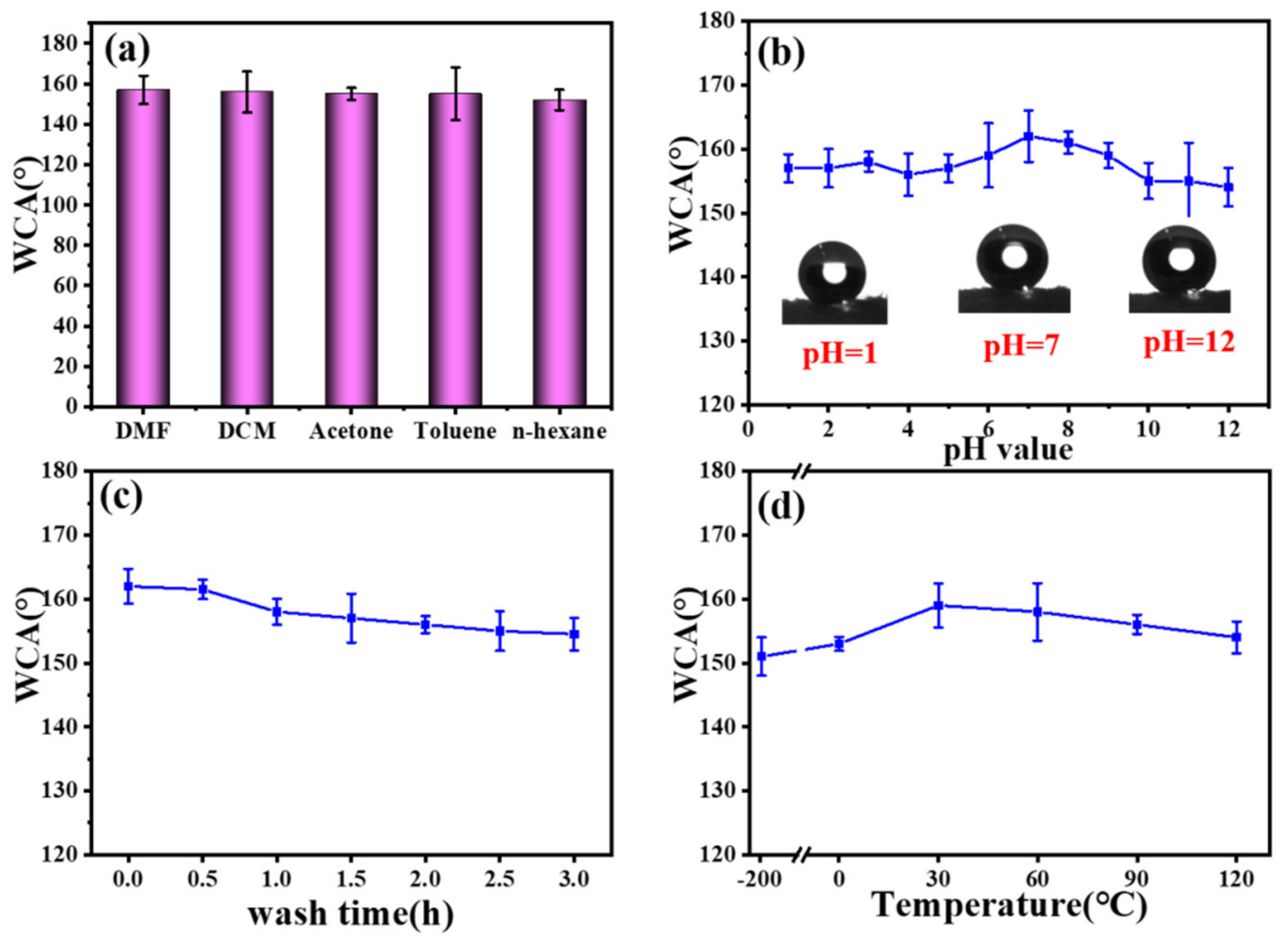
3.6. Tolerance of the F-IEM@fabric in Harsh Environments
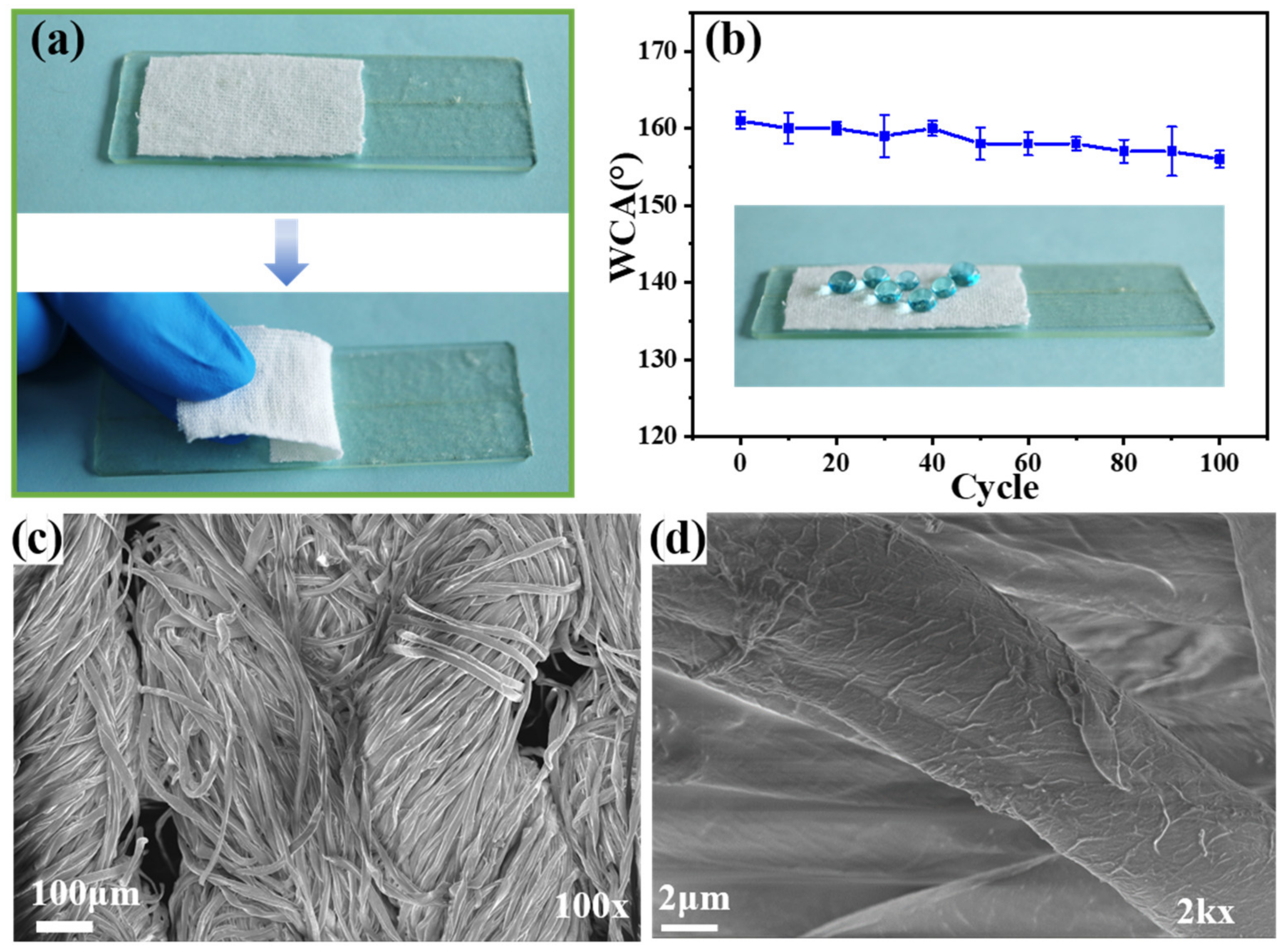
3.7. Mechanical Durability of the F-IEM@fabric
3.8. Method Comparison with Recent Similar Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Daniel, E.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-term ecosystem response to the Exxon Valdez oil spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, P.; Cao, B. Fabrication of Superhydrophobic–Superoleophilic Fabrics by an Etching and Dip-Coating Two-Step Method for Oil–Water Separation. Ind. Eng. Chem. Res. 2016, 55, 5030–5035. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, Z.Z.; Xu, X.H.; Men, X.H.; Zhu, X.T. Facile Fabrication of Superhydrophobic Sponge with Selective Absorption and Collection of Oil from Water. Ind. Eng. Chem. Res. 2013, 52, 9411–9416. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Feng, X.L.; Qu, Y.-X.; Gong, L.-X.; Cao, K.; Zhang, G.-D.; Shi, Y.; Gao, J.-F.; Song, P.; Tang, L.-C. Silane modified MXene/polybenzazole nanocomposite aerogels with exceptional surface hydrophobicity, flame retardance and thermal insulation. Compos. Commun. 2023, 37, 101402. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Zhou, C.; Cheng, J.; Jiang, Z.; Lu, Z.; Miao, J. Underwater superoleophobic mesh based on BiVO4 nanoparticles with sunlight-driven self-cleaning property for oil/water separation. Chem. Eng. J. 2017, 320, 342–351. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. A Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, C.; Cheng, C.; Wang, X. Facile immobilization of Ag nanocluster on nanofibrous membrane for oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 15272–15282. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Van Gelderen, L.; Malmquist, L.M.; Jomaas, G.J.F. Vaporization order and burning efficiency of crude oils during in-situ burning on water. Fuel 2017, 191, 528–537. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Catania, V.; Santisi, S.; Cappello, S.; Botta, L.; Quatrini, P. Polycaprolactone-based scaffold for oil-selective sorption and improvement of bacteria activity for bioremediation of polluted water: Porous PCL system obtained by leaching melt mixed PCL/PEG/NaCl composites: Oil uptake performance and bioremediation efficiency. Eur. Polym. J. 2017, 91, 260–273. [Google Scholar]
- Li, Z.-T.; Lin, B.; Jiang, L.-W.; Lin, E.-C.; Chen, J.; Zhang, S.-J.; Tang, Y.-W.; He, F.-A.; Li, D.-H. Effective preparation of magnetic superhydrophobic Fe3O4/PU sponge for oil-water separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Ge, B.; Zhang, Z.; Zhu, X.; Men, X.; Zhou, X.; Xue, Q. A graphene coated cotton for oil/water separation. Compos. Sci. Technol. 2014, 102, 100–105. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Guan, C.S.; Wang, M.; Li, Y.D.; Zeng, J.B. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef]
- Hu, W.-J.; Xia, Q.-Q.; Pan, H.-T.; Chen, H.-Y.; Qu, Y.-X.; Chen, Z.-Y.; Zhang, G.-D.; Zhao, L.; Gong, L.-X.; Xue, C.-G.; et al. Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil-Water Separation. Polymers 2022, 14, 1628. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Deng, Y.; Wang, C. Thermoresponsive Melamine Sponges with Switchable Wettability by Interface-Initiated Atom Transfer Radical Polymerization for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8967–8974. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y.; Miao, X.; Pei, Y.; Yan, Y.; Xiao, W.; Wu, L. Facile fabrication of superhydrophobic polyurethane sponge towards oil-water separation with exceptional flame-retardant performance. Sep. Purif. Technol. 2019, 229, 115801. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.; Liu, C.; He, S.; Xie, Y.; Wang, Z. Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil-water separation via thiol-ene click chemistry. Appl. Surf. Sci. 2019, 463, 34–44. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, K.; Huang, C.; Yang, K.; Xu, S.; Wang, L.; Pi, P.; Wen, X. Novel pH-Responsive Smart Fabric: From Switchable Wettability to Controllable On-Demand Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 368–376. [Google Scholar] [CrossRef]
- Zhou, M.; Li, M.; Xu, F.; Yang, Y.; Pei, Y.; Yan, Y.; Wu, L. One-Step Covalent Surface Modification to Achieve Oil-Water Separation Performance of a Non-Fluorinated Durable Superhydrophobic Fabric. ACS Omega 2021, 6, 24139–24146. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Rasheed, S.; Ahmed, K.; Musharraf, S.G.; Najam-ul-Haq, M.; Hussain, D. Facile two-step functionalization of multifunctional superhydrophobic cotton fabric for UV-blocking, self cleaning, antibacterial, and oil-water separation. Sep. Purif. Technol. 2023, 306, 122626. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Chen, Z.-Y.; Tang, Y.-H.; Li, Y.-T.; Ma, D.; Zhang, G.-D.; Boukherroub, R.; Cao, C.-F.; Gong, L.-X.; Song, P.; et al. Silicone/graphene oxide co-cross-linked aerogels with wide-temperature mechanical flexibility, super-hydrophobicity and flame resistance for exceptional thermal insulation and oil/water separation. J. Mater. Sci. Technol. 2022, 114, 131–142. [Google Scholar] [CrossRef]
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose nanofiber-poly(ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater. J. Hazard. Mater. 2023, 442, 129955. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef]
- Borah, A.; Gogoi, M.; Goswami, R.; Sarmah, H.; Hazarika, K.K.; Hazarika, S. Thin film nanocomposite membrane incorporated with clay-ionic liquid framework for enhancing rejection of epigallocatechin gallate in aqueous media. J. Environ. Chem. Eng. 2022, 10, 107423. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Bhuyan, C.; Sarmah, H.; Hazarika, S. Functionalized multi-walled carbon nanotube thin-layered hollow fiber membrane for enantioselective permeation of racemic beta-substituted-alpha-amino acids. J. Chem. Sci. 2022, 134, 89. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Akram Raza, M.; Kooij, E.S.; van Silfhout, A.; Poelsema, B. Superhydrophobic Surfaces by Anomalous Fluoroalkylsilane Self-Assembly on Silica Nanosphere Arrays. Langmuir 2010, 26, 12962–12972. [Google Scholar] [CrossRef] [PubMed]
- Berndt, E.; Behnke, S.; Dannehl, A.; Gajda, A.; Wingender, J.; Ulbricht, M. Functional coatings for anti-biofouling applications by surface segregation of block copolymer additives. Polymer 2010, 51, 5910–5920. [Google Scholar] [CrossRef]
- Guo, K.-Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Song, P.; Tang, L.-C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. Part. B 2020, 193, 108017. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Ye, Y. Ultradurable underwater superoleophobic surfaces obtained by vapor-synthesized layered polymer nanocoatings for highly efficient oil–water separation. J. Mater. Chem. A 2017, 5, 14990–14995. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, H.; Wang, H.; Zhao, Y.; Shao, H.; Xu, Z.; Feng, Z.; Liu, D.; Lin, T. Argon Plasma Treatment of Fluorine-Free Silane Coatings: A Facile, Environment-Friendly Method to Prepare Durable, Superhydrophobic Fabrics. Adv. Mater. Interfaces 2017, 4, 1700027. [Google Scholar] [CrossRef]
- Yan, T.; Chen, X.; Zhang, T.; Yu, J.; Jiang, X.; Hu, W.; Jiao, F. A magnetic pH-induced textile fabric with switchable wettability for intelligent oil/water separation. Chem. Eng. J. 2018, 347, 52–63. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Lin, T. Fluoroalkyl Silane Modified Silicone Rubber/Nanoparticle Composite: A Super Durable, Robust Superhydrophobic Fabric Coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Fluorine-Free Waterborne Coating for Environmentally Friendly, Robustly Water-Resistant, and Highly Breathable Fibrous Textiles. ACS Nano 2020, 14, 1045–1054. [Google Scholar] [CrossRef]
- Li, M.; Fang, Y.; Liu, C.; Zhou, M.; Miao, X.; Pei, Y.; Yan, Y.; Xiao, W.; Qiu, H.; Wu, L. Facile generation of highly durable thiol-functionalized polyhedral oligomeric silsesquioxane based superhydrophobic melamine foam. Front. Chem. Sci. Eng. 2022, 16, 1247–1258. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, C.; Li, M.; Miao, X.; Pei, Y.; Yan, Y.; Xiao, W.; Wu, L. Facile Generation of Durable Superhydrophobic Fabrics toward Oil/Water Separation via Thiol-Ene Click Chemistry. Ind. Eng. Chem. Res. 2020, 59, 6130–6140. [Google Scholar] [CrossRef]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem. Eng. J. 2018, 332, 150–159. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, J.; Peng, T.; Jiang, C.; Liu, S.; Li, Y.; Guo, T.; Xie, L.J.A.M.I. Superhydrophobic melamine–formaldehyde sponge functionalized by coupling agent–isocyanate siloxane as efficient absorbents for oil and organic solvents. Adv. Mater. Interfaces 2019, 6, 1900025. [Google Scholar] [CrossRef]
- Xi, G.; Wang, J.; Luo, G.; Zhu, Y.; Fan, W.; Huang, M.; Wang, H.; Liu, X.J.C. Healable superhydrophobicity of novel cotton fabrics modified via one-pot mist copolymerization. Cellulose 2016, 23, 915–927. [Google Scholar] [CrossRef]
- Qiang, S.; Chen, K.; Yin, Y.; Wang, C. Robust UV-cured superhydrophobic cotton fabric surfaces with self-healing ability. Mater. Des. 2017, 116, 395–402. [Google Scholar] [CrossRef]
- Mao, H.; Yang, F.; Wang, C.; Wang, Y.; Yao, D.; Yin, Y. Anthraquinone chromophore covalently bonded blocked waterborne polyurethanes: Synthesis and application. RSC Adv. 2015, 5, 30631–30639. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Chen, S.; Yu, C.; He, Q.S.; Xin, Z. A Robust Polybenzoxazine/SiO2 Fabric with Superhydrophobicity for High-Flux Oil/Water Separation. Ind. Eng. Chem. Res. 2020, 59, 7787–7796. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Wang, Y.; Wu, D.; Liu, Y.; Liu, X. Fabrication of durable hierarchical superhydrophobic fabrics with Sichuan pepper-like structures via graft precipitation polymerization. Appl. Surf. Sci. 2020, 529, 147017. [Google Scholar] [CrossRef]
- Gorzalski, A.S.; Donley, C.; Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 2017, 522, 31–44. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, F.; Weng, D.; Li, X.; Li, Y.; Sun, J. Superhydrophobic Foams with Chemical- and Mechanical-Damage-Healing Abilities Enabled by Self-Healing Polymers. ACS Appl. Mater. Interfaces 2019, 11, 37285–37294. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Y.; Jia, D. Fabrication of a versatile composite material with three-dimensional superhydrophobic for aquatic show. Chem. Eng. J. 2020, 398, 125362. [Google Scholar] [CrossRef]
- Chao, W.; Wang, S.; Li, Y.; Cao, G.; Zhao, Y.; Sun, X.; Wang, C.; Ho, S.-H. Natural sponge-like wood-derived aerogel for solar-assisted adsorption and recovery of high-viscous crude oil. Chem. Eng. J. 2020, 400, 125865. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Wang, Y.; Zhao, X.; Yang, M.; Men, X.; Xue, Q. Colorful superhydrophobic pigments with superior anti-fouling performance and environmental durability. Chem. Eng. J. 2020, 384, 123292. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Ma, L.; Zhang, Y.; Shen, L.; Xiong, S.; Li, K.; Qu, M. Multifunctional conductive cellulose fabric with flexibility, superamphiphobicity and flame-retardancy for all-weather wearable smart electronic textiles and high-temperature warning device. Chem. Eng. J. 2020, 390, 124508. [Google Scholar] [CrossRef]
- Fu, K.; Lu, C.; Liu, Y.; Zhang, H.; Zhang, B.; Zhang, H.; Zhou, F.; Zhang, Q.; Zhu, B. Mechanically robust, self-healing superhydrophobic anti-icing coatings based on a novel fluorinated polyurethane synthesized by a two-step thiol click reaction. Chem. Eng. J. 2021, 404, 127110. [Google Scholar] [CrossRef]
- Deng, Y.; Han, D.; Deng, Y.-Y.; Zhang, Q.; Chen, F.; Fu, Q. Facile one-step preparation of robust hydrophobic cotton fabrics by covalent bonding polyhedral oligomeric silsesquioxane for ultrafast oil/water separation. Chem. Eng. J. 2020, 379, 122391. [Google Scholar] [CrossRef]
- Xue, C.-H.; Guo, X.-J.; Zhang, M.-M.; Ma, J.-Z.; Jia, S.-T. Fabrication of robust superhydrophobic surfaces by modification of chemically roughened fibers via thiol–ene click chemistry. J. Mater. Chem. A 2015, 3, 21797–21804. [Google Scholar] [CrossRef]
- Kleingartner, J.A.; Srinivasan, S.; Truong, Q.T.; Sieber, M.; Cohen, R.E.; McKinley, G.H. Designing Robust Hierarchically Textured Oleophobic Fabrics. Langmuir 2015, 31, 13201–13213. [Google Scholar] [CrossRef]
- Wang, X.; Yu, P.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Superhydrophobic/superoleophilic cotton for efficient oil–water separation based on the combined octadecanoyl chain bonding and polymer grafting via surface-initiated ATRP. ACS Appl. Polym. Mater. 2019, 1, 2875–2882. [Google Scholar] [CrossRef]
- Xi, G.; Fan, W.; Wang, L.; Liu, X.; Endo, T. Fabrication of asymmetrically superhydrophobic cotton fabrics via mist copolymerization of 2, 2, 2-trifluoroethyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1862–1871. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Wang, Y.; Wang, Y.; Ma, P.; Zhang, S.; Chen, M.; Dong, W. Superhydrophobic Composite Cotton Generated from Raspberry-like Nanoparticles and Their Applications in Oil/Water Separation. Ind. Eng. Chem. Res. 2020, 59, 16305–16311. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Si, Y.; Chen, M.; Wu, L. Large-Area Preparation of Robust and Transparent Superomniphobic Polymer Films. Acs Nano 2018, 12, 10338–10346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Duan, J.Z.; Huang, Y.L.; Hou, B.R. Double layered superhydrophobic PDMS-Candle soot coating with durable corrosion resistance and thermal-mechanical robustness. J. Mater. Sci. Technol. 2021, 71, 1–11. [Google Scholar] [CrossRef]
- Zhang, B.B.; Xu, W.C.; Zhu, Q.J.; Hou, B.R. Scalable, fluorine free and hot water repelling superhydrophobic and superoleophobic coating based on functionalized Al2O3 nanoparticles. J. Mater. Sci. Technol. 2021, 66, 74–81. [Google Scholar] [CrossRef]
- Xu, Q.B.; Wang, X.Y.; Zhou, J.; Wang, P.; Zhang, Y.Y.; Chen, Q. Durably Superhydrophobic Cotton Fabric Constructed by Silica Particles and Polydimethylsiloxane via "Mosaic Mode" for Self-Cleaning and Oil-Water Separation. Fibers Polym. 2023, 24, 299–308. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wu, M.; Duan, G.G.; Gong, X. One-step fabrication of eco-friendly superhydrophobic fabrics for high-efficiency oil/water separation and oil spill cleanup. Nanoscale 2022, 14, 1296–1309. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, L.Q.; Li, W.P.; Liu, H.C.; Chen, H.N. Constructing Fluorine-Free and Cost-Effective Superhydrophobic Surface with Normal-Alcohol-Modified Hydrophobic SiO2 Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 858–867. [Google Scholar] [CrossRef]
- Yang, M.P.; Liu, W.Q.; Jiang, C.; He, S.; Xie, Y.K.; Wang, Z.F. Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr. Polym. 2018, 197, 75–82. [Google Scholar] [CrossRef]
- Wang, D.C.; Yang, X.G.; Yu, H.Y.; Gu, J.P.; Qi, D.M.; Yao, J.M.; Ni, Q.Q. Smart nonwoven fabric with reversibly dual-stimuli responsive wettability for intelligent oil-water separation and pollutants removal. J. Hazard. Mater. 2020, 383, 121–123. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, Z.X.; Sun, Y.L.; Yang, H.W.; Zhang, H.J.; Dou, H.Z.; Zhang, L.H. Fabrication of superhydrophobic cotton fabrics using crosslinking polymerization method. Appl. Surf. Sci. 2018, 441, 554–563. [Google Scholar] [CrossRef]
- Lv, L.Z.; Zhao, W.J.; Zhong, X.M.; Fu, H.Q. Fabrication of Magnetically Inorganic/Organic Superhydrophobic Fabrics and Their Applications. ACS Appl. Mater. Interfaces 2020, 12, 45296–45305. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.; Zhang, D.; Zhang, Z.; Fu, F.; Zhang, X.; Yang, Y.; Lin, H.; Chen, Y. Fabrication of robust and self-healing superhydrophobic PET fabrics based on profiled fiber structure. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125686. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Gao, Z.; Qi, X.; He, L.; Huang, W.; Wang, J.; Liu, Z. Photo-responsive Mn-doped TiO2-based superhydrophobic/underwater superoleophobicity membrane for efficient oil-water separation and photothermal decontamination. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131519. [Google Scholar] [CrossRef]
- Li, H.; Miao, S.; Chen, W.; Yang, X.; Li, M.; Xing, T.; Zhao, Y.; Chen, G. Durable superhydrophobic and oleophobic cotton fabric based on the grafting of fluorinated POSS through silane coupling and thiol-ene click reaction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127566. [Google Scholar] [CrossRef]
- Yan, X.J.; Zhu, X.W.; Ruan, Y.T.; Xing, T.L.; Chen, G.Q.; Zhou, C.X. Biomimetic, dopamine-modified superhydrophobic cotton fabric for oil-water separation. Cellulose 2020, 27, 7873–7885. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Zhang, Y.; Wang, P. Preparation of Durable Superhydrophobic Cotton Fabric for Self-cleaning and Oil-water Separation. Fibers Polym. 2022, 23, 1572–1581. [Google Scholar] [CrossRef]
- He, B.B.; Hou, X.; Liu, Y.F.; Hu, J.K.; Song, L.N.; Tong, Z.M.; Zhan, X.L.; Ren, Y.Y.; Liu, Q.; Zhang, Q.H. Design of fluorine-free waterborne fabric coating with robust hydrophobicity, water-resistant and breathability. Sep. Purif. Technol. 2023, 311, 123308. [Google Scholar] [CrossRef]



| IEM Mass Concentration (%) | IEM (mol) | DFMA (mol) | DCM (g) | HMPF (g) |
|---|---|---|---|---|
| 2% | 0.005 | 0.005 | 40 | 0.2 |
| 4% | 0.01 | 0.01 | 40 | 0.2 |
| 6% | 0.015 | 0.015 | 40 | 0.2 |
| 8% | 0.02 | 0.02 | 40 | 0.2 |
| 10% | 0.025 | 0.025 | 40 | 0.2 |
| Samples | Nanoparticles | Fabric Appearance | Operating Steps | Time (h) | Ref. |
|---|---|---|---|---|---|
| P-D-Fabric | SiO2 | milky white | 3 | 9 | [64] |
| PEI/TMSPA/SiO2/DTMS cotton fabric | SiO2 | pure white | 2 | 20 | [65] |
| APTES/IPDI/SiO2 fabric | SiO2 | pure white | 3 | 52 | [66] |
| MCFs | TiO2 | pure white | 4 | 43.5 | [67] |
| DSR-CZPP | ZnO | yellow | 5 | 82 | [68] |
| PA-Ploymer-Al2O3-Fabric | Al2O3 | light yellow | 3 | 30 | [69] |
| Superhydrophobic Fabrics | Co0.8Mg0.2Fe2O4 | brownish black | 3 | 68 | [70] |
| TSP-PET fabric | TiO2(sol) | light yellow | 3 | 30 | [71] |
| Fabric-SMP-S-SiO2@Fe3O4 | MPS-SiO2@Fe3O4 | brown | 2 | 6.5 | [40] |
| Mn@TiO2 membrane | Mn@TiO2 | brown | 3 | 17 | [72] |
| FAS/SH-F-POSS fabric | POSS | pure white | 3 | 11 | [73] |
| Fe/PDA/ODA cotton fabric | PDA | black | 2 | 5.5 | [74] |
| PMT@fabric | ○ | light yellow | 3 | 6 | [75] |
| WSiPU-treated fabrics | ○ | milky white | 2 | 3.5 | [76] |
| F-IEM@fabric | ○ | white | 1 | 1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Liu, X.; Xu, F.; Pei, Y.; Wu, L.; Tang, L.-C. Facile UV-Induced Surface Covalent Modification to Fabricate Durable Superhydrophobic Fabric for Efficient Oil–Water Separation. Polymers 2023, 15, 2505. https://doi.org/10.3390/polym15112505
Zhou M, Liu X, Xu F, Pei Y, Wu L, Tang L-C. Facile UV-Induced Surface Covalent Modification to Fabricate Durable Superhydrophobic Fabric for Efficient Oil–Water Separation. Polymers. 2023; 15(11):2505. https://doi.org/10.3390/polym15112505
Chicago/Turabian StyleZhou, Mengmeng, Xiaohui Liu, Fengjiao Xu, Yongbing Pei, Lianbin Wu, and Long-Cheng Tang. 2023. "Facile UV-Induced Surface Covalent Modification to Fabricate Durable Superhydrophobic Fabric for Efficient Oil–Water Separation" Polymers 15, no. 11: 2505. https://doi.org/10.3390/polym15112505