Biobased Castor Oil-Based Polyurethane Foams Grafted with Octadecylsilane-Modified Diatomite for Use as Eco-Friendly and Low-Cost Sorbents for Crude Oil Clean-Up Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of C18-DE Modified Particles
2.3. Synthesis of Polyurethane Foam (PU Foam)
2.4. Synthesis of PU-C18-DE Foam (Modified)
3. Measurements and Characterization:
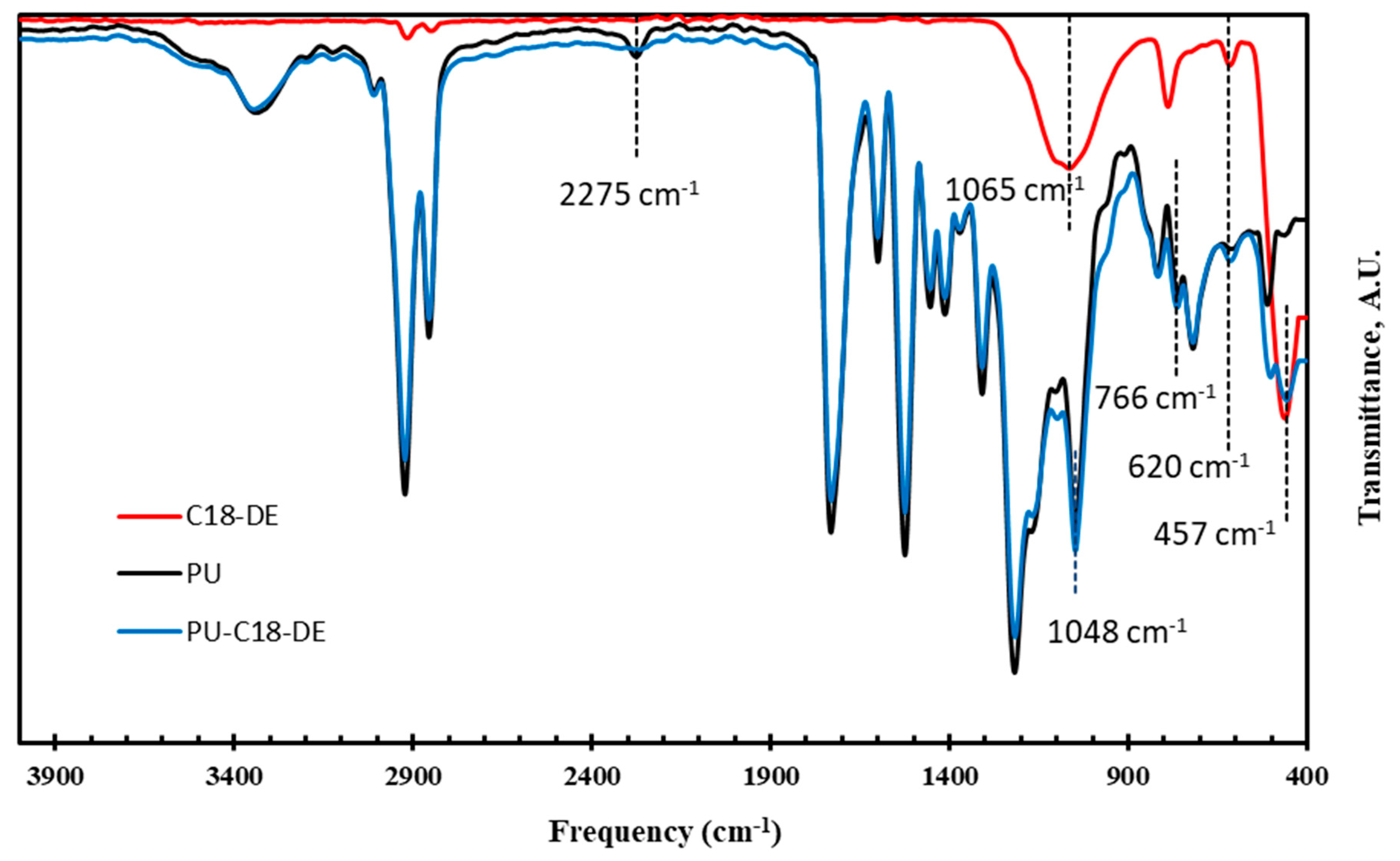
3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.2. Measurement of Thermal Properties
3.3. Morphology and Elemental Composition
3.4. Crystallinity Measurement
3.5. Hydrophobicity
3.6. Determination of Oil Sorption Capacity
4. Results and Discussion
4.1. X-ray Diffraction Analysis of Synthesized Samples
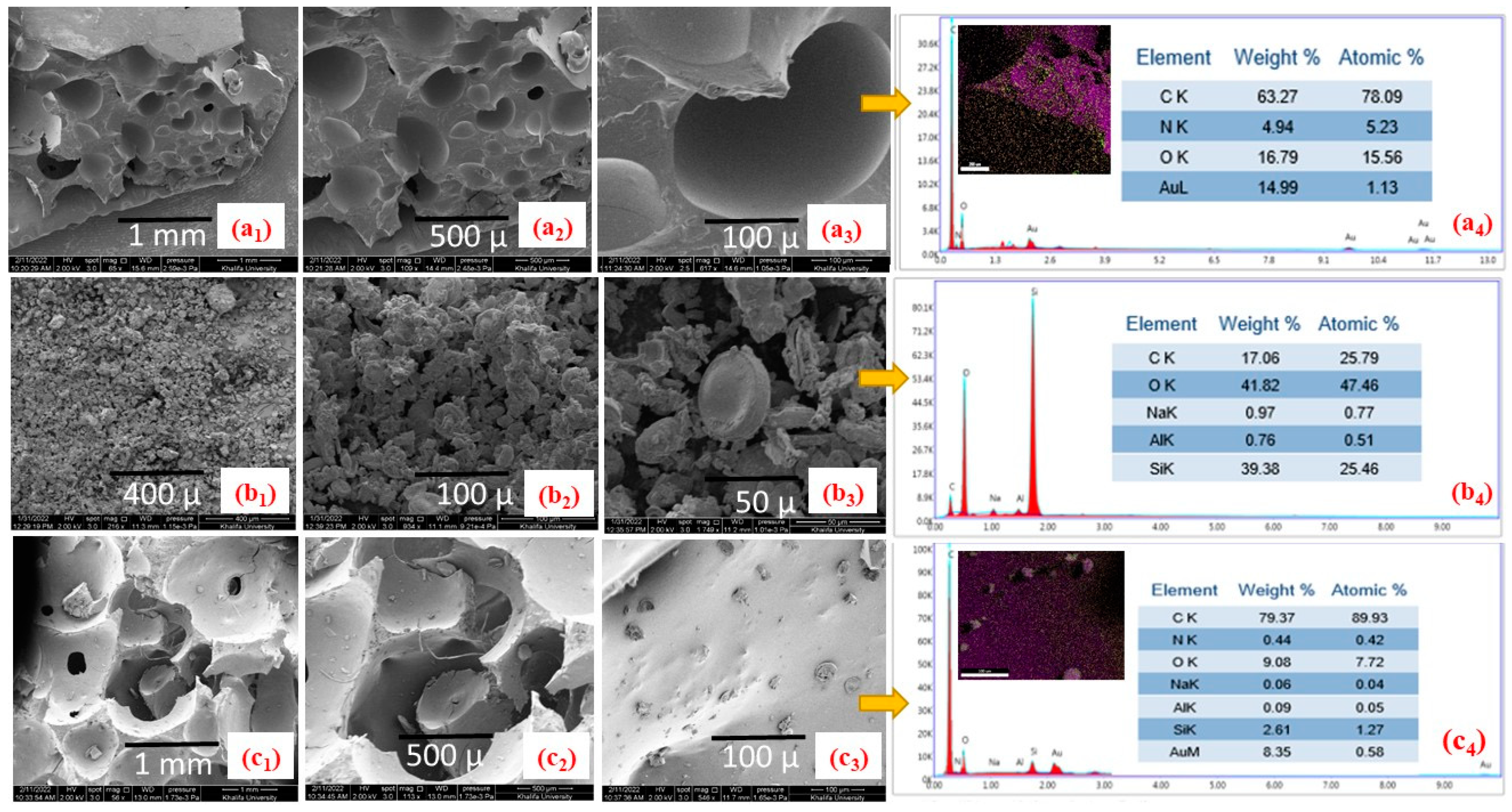
4.2. Scanning Electron Microscopy (SEM)
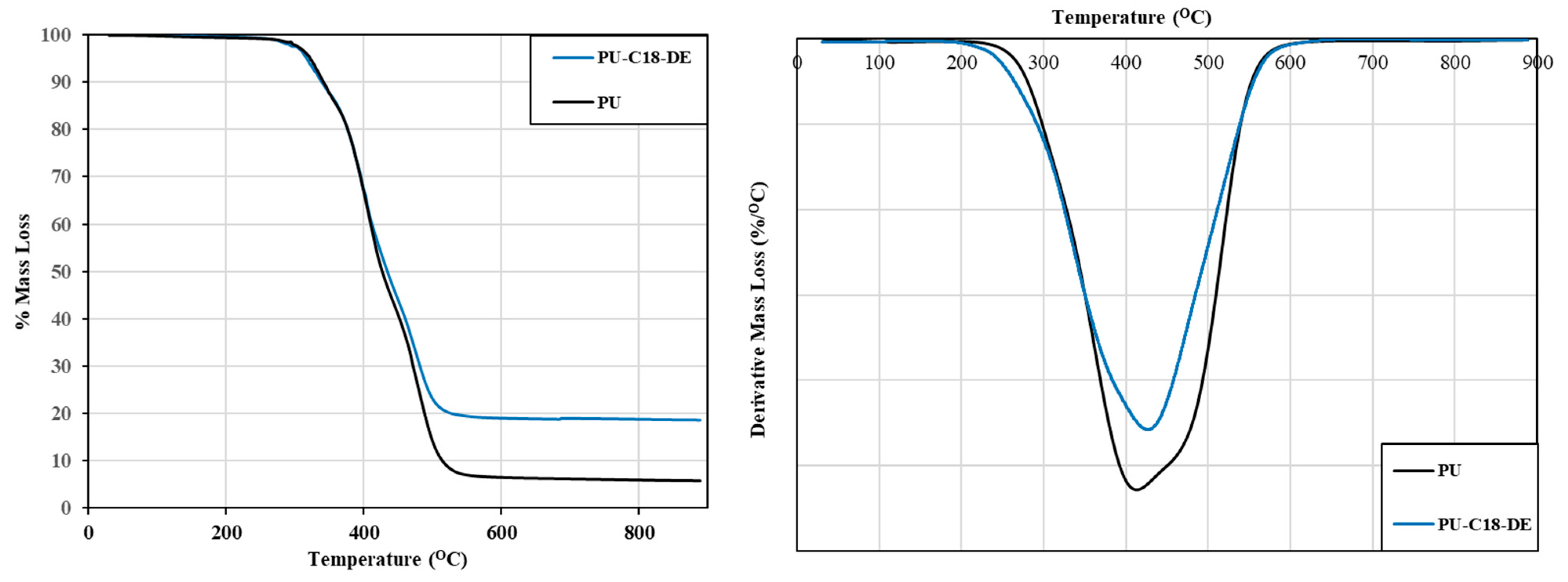
4.3. Thermogravimetric Analysis
4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5. Surface Wettability Measurement
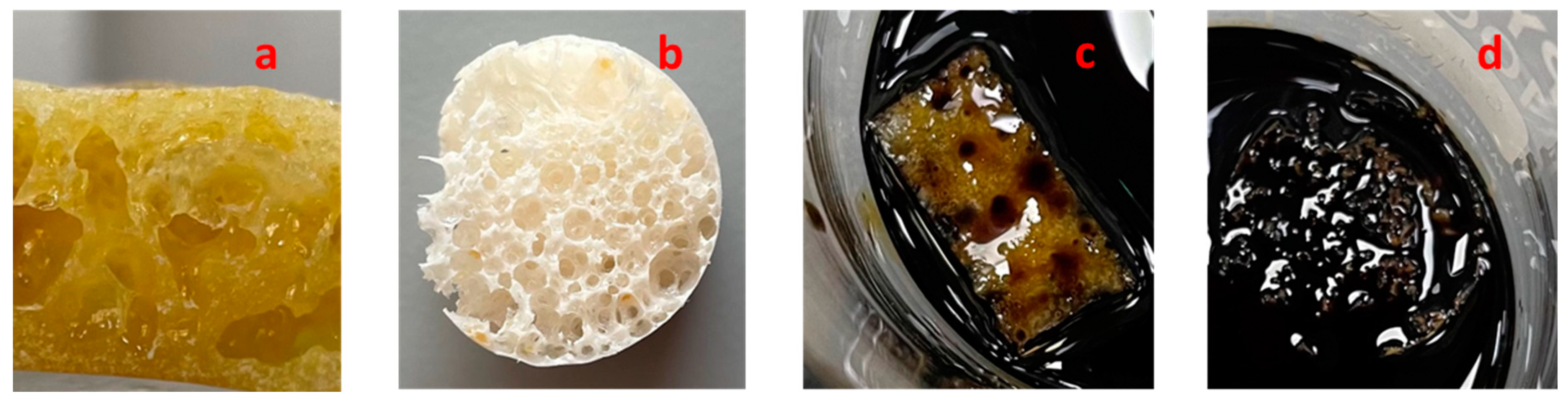
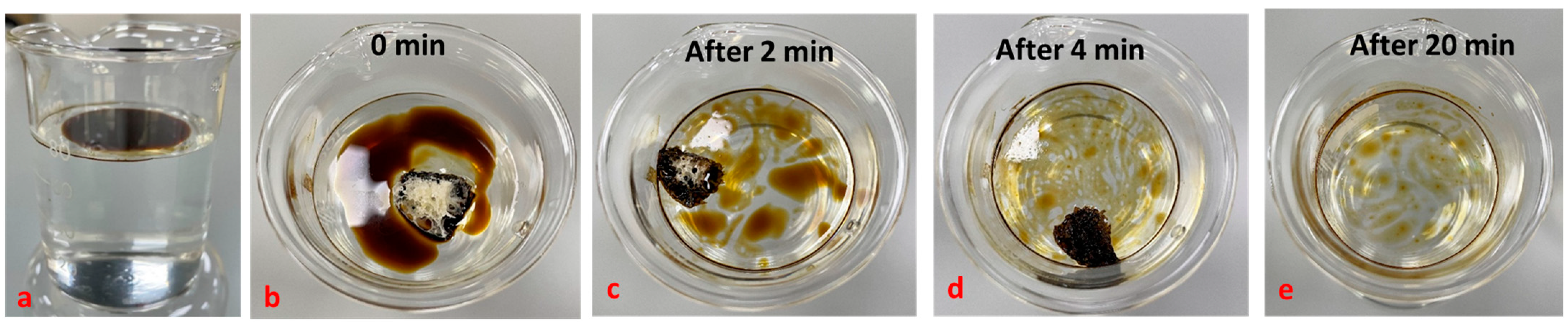
4.6. Determination of Oil Sorption and Water Absorption Capacity of Polymer
4.7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. What Countries Are the Top Producers and Consumers of Oil? Available online: https://www.eia.gov/energyexplained/oil-and-petroleum-products/where-our-oil-comes-from.php (accessed on 27 January 2021).
- The Biggest Oil Producers in the Middle East. Available online: https://www.investopedia.com/articles/investing/101515/biggest-oil-producers-middle-east.asp (accessed on 29 September 2022).
- Action Needed to Prevent Oil Spills off UAE Coast, Says Government Official. Available online: https://www.thenationalnews.com/uae/action-needed-to-prevent-oil-spills-off-uae-coast-says-government-official-1.1047200 (accessed on 12 July 2020).
- Shafir, S.; Van Rijn, J.; Rinkevich, B. Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ. Sci. Technol. 2007, 41, 5571–5574. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.G.; Carls, M.G.; Short, J.W.; Rice, S.D.; Heintz, R.A.; Rau, M.; Di Giulio, R. Assessment of the phototoxicity of weathered Alaska North Slope crude oil to juvenile pink salmon. Chemosphere 2005, 60, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Remediation Technologies for Marine Oil Spills: A Critical Review and Comparative Analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef]
- Teas, C.; Kalligeros, S.; Zanikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G. Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination 2001, 140, 259–264. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Farouq, R.; Mohamed, O.A.; Tony, M.A. Oil spill clean-up using combined sorbents: A comparative investigation and design aspects. Int. J. Environ. Anal. Chem. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Uebe, J.; Ziogas, M. Cellulose aerogel composites as oil sorbents and their regeneration. PeerJ 2021, 9, e11795. [Google Scholar] [CrossRef]
- Tanobe, V.O.A.; Sydenstricker, T.H.D.; Amico, S.C.; Vargas, J.V.C.; Zawadzki, S.F. Evaluation of flexible postconsumed polyurethane foams modified by polystyrene grafting as sorbent material for oil spills. J. Appl. Polym. Sci. 2009, 111, 1842–1849. [Google Scholar] [CrossRef]
- Budlayan, M.L.M.; Patricio, J.N.; Lagare-Oracion, J.P.; Arco, S.D.; Alguno, A.C.; Basilio, A.; Latayada, F.S.; Capangpangan, R.Y. Improvised centrifugal spinning for the production of polystyrene microfibers from waste expanded polystyrene foam and its potential application for oil adsorption. J. Eng. Appl. Sci. 2021, 68, 25. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Schubert, D.W.; Liu, X. Oil–Water Separation Polypropylene Foam with Advanced Solvent-Evaporation Induced Coexistence of Microspheres and Microporous Structure. Macromol. Rapid Commun. 2022, 43, 2200177. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; Finocchi, A.; De Folly D′Auris, A.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A.P. Enhanced Oil Spill Remediation by Adsorption with Interlinked Multilayered Graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, L.; Yang, F. Oleophilic Polyurethane Foams for Oil Spill Cleanup. Proc. Environ. Sci. 2013, 18, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Keshawy, M.; Farag, R.K.; Gaffer, A. Egyptian crude oil sorbent based on coated polyurethane foam waste. Egypt. J. Pet. 2020, 29, 67–73. [Google Scholar] [CrossRef]
- Hwang, U.; Lee, B.; Oh, B.; Shin, H.S.; Lee, S.S.; Kang, S.G.; Kim, D.; Park, J.; Shin, S.; Suhr, J.; et al. Hydrophobic lignin/polyurethane composite foam: An eco-friendly and easily reusable oil sorbent. Eur. Polym. J. 2022, 165, 110971. [Google Scholar] [CrossRef]
- Kong, S.M.; Han, Y.; Won, N.-I.; Na, Y.H. Polyurethane Sponge with a Modified Specific Surface for Repeatable Oil–Water Separation. ACS Omega 2021, 6, 33969–33975. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Yang, F. Hydrophobic modification of polyurethane foam for oil spill clean-up. Mar. Pollut. Bull. 2012, 64, 1648–1653. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Men, X.; Zhu, X. Facile Fabrication of Superhydrophobic Sponge with Selective Absorption and Collection of Oil from Water. Ind. Eng. Chem. Res. 2013, 52, 9411–9416. [Google Scholar] [CrossRef]
- Ng, Z.C.; Roslan, R.A.; Lau, W.J.; Gürsoy, M.; Karaman, M.; Jullok, N.; Ismail, A.F. A Green Approach to Modify Surface Properties of Polyurethane Foam for Enhanced Oil Absorption. Polymers 2020, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiao, C.; Zhao, J.; Chen, K.; Hao, J.; Ji, D. Continuous separation of oil from water surface by a novel tubular unit based on graphene coated polyurethane sponge. Polym. Adv. Technol. 2018, 29, 2317–2326. [Google Scholar] [CrossRef]
- Chen, J.; Yue, X.; Xiao, Z.; Li, H.; Yu, X.; Xiang, J. In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal. Polymers 2020, 12, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harinarayanan, P.; Gregor, F.; Uroš, C. Recent Advances in the Methods for Designing Superhydrophobic Surfaces; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Qiu, S.; Li, Y.; Li, G.; Zhang, Z.; Li, Y.; Wu, T. Robust Superhydrophobic Sepiolite-Coated Polyurethane Sponge for Highly Efficient and Recyclable Oil Absorption. ACS Sustain. Chem. Eng. 2019, 7, 5560–5567. [Google Scholar] [CrossRef]
- Jin, L.; Gao, Y.; Huang, Y.; Ou, M.; Liu, Z.; Zhang, X.; He, C.; Su, B.; Zhao, W.; Zhao, C. Mussel-Inspired and In Situ Polymerization-Modified Commercial Sponge for Efficient Crude Oil and Organic Solvent Adsorption. ACS Appl. Mater. Interfaces 2022, 14, 2663–2673. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-Effective Reduced Graphene Oxide-Coated Polyurethane Sponge as a Highly Efficient and Reusable Oil-Absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef]
- Perera, H.J.; Goyal, A.; Banu, H.; Alhassan, S.M. Low-cost fluorinated diatomaceous earth polyurethane foam for the absorption of oil. MRS Energy Sustain. 2022, 9, 94–104. [Google Scholar] [CrossRef]
- Zimmermann, M.V.G.; Zattera, A.J.; Fenner, B.R.; Santana, R.M.C. Sorbent system based on organosilane-coated polyurethane foam for oil spill clean up. Polym. Bull. 2020, 78, 1423–1440. [Google Scholar] [CrossRef]
- Nikkhah, A.A.; Zilouei, H.; Asadinezhad, A.; Keshavarz, A. Removal of oil from water using polyurethane foam modified with nanoclay. Chem. Eng. J. 2015, 262, 278–285. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.; Zhang, M.; Qiu, F.; Rong, J.; Pan, J. Synthesis and characterization of porous fibers/polyurethane foam composites for selective removal of oils and organic solvents from water. RSC Adv. 2016, 6, 86510–86519. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, Z.; Wang, Z.; Yang, D.; Zhou, D.; Zhang, T. Preparation of biomass carbon/polyurethane foams for selective oil/water absorption. J. Dispers. Sci. Technol. 2020, 41, 1872–1878. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Patro, T.U.; Khakhar, D.V. Polyurethane Foam−Clay Nanocomposites: Nanoclays as Cell Openers. Ind. Eng. Chem. Res. 2006, 45, 7126–7134. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Mir Mohamad Sadeghi, G. Effect of Liquefied Lignin Content on Synthesis of Bio-based Polyurethane Foam for Oil Adsorption Application. J. Polym. Environ. 2020, 28, 892–905. [Google Scholar] [CrossRef]
- Oribayo, O.; Feng, X.; Rempel, G.L.; Pan, Q. Synthesis of lignin-based polyurethane/graphene oxide foam and its application as an absorbent for oil spill clean-ups and recovery. Chem. Eng. J. 2017, 323, 191–202. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Reis, R.L.; Mano, J.F. Superhydrophobic surfaces engineered using diatomaceous earth. ACS Appl. Mater. Interfaces 2013, 5, 4202–4208. [Google Scholar] [CrossRef]
- Sriram, G.; Kigga, M.; Uthappa, U.T.; Rego, R.M.; Thendral, V.; Kumeria, T.; Jung, H.-Y.; Kurkuri, M.D. Naturally available diatomite and their surface modification for the removal of hazardous dye and metal ions: A review. Adv. Colloid Interface Sci. 2020, 282, 102198. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Kamal, S.; Aslam, N. Starch based polyurethanes: A critical review updating recent literature. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The biodegradable cellulose-derived polyol and polyurethane foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Prociak, A.; Malewska, E.; Kurańska, M.; Bąk, S.; Budny, P. Flexible polyurethane foams synthesized with palm oil-based bio-polyols obtained with the use of different oxirane ring opener. Ind. Crops Prod. 2018, 115, 69–77. [Google Scholar] [CrossRef]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Lazdiņa, B.; Misāne, M.; Vilsone, D. Biobased Polyurethanes from Rapeseed Oil Polyols: Structure, Mechanical and Thermal Properties. J. Polym. Environ. 2013, 21, 952–962. [Google Scholar] [CrossRef]
- Macalino, A.D.; Salen, V.A.; Reyes, L.Q. Castor Oil Based Polyurethanes: Synthesis and Characterization. IOP Conf. Ser. Mater. Sci. Eng. 2017, 229, 12016. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tai, W. Castor Oil-Based Polyurethane Resin for Low-Density Composites with Bamboo Charcoal. Polymers 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.C.; dos Santos, V.F.; Monticeli, F.M.; Ornaghi, H.; Barud, H.d.S.; Mulinari, D.R. Efficiency of castor oil–based polyurethane foams for oil sorption S10 and S500: Influence of porous size and statistical analysis. Polym. Polym. Compos. 2021, 29 (Suppl. S9), S1063–S1074. [Google Scholar] [CrossRef]
- Panda, S.S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Preparation, characterization, and properties of castor oil-based flexible polyurethane/Cloisite 30B nanocomposites foam. J. Compos. Mater. 2018, 52, 531–542. [Google Scholar] [CrossRef]
- Martins, L.S.; Silva, N.G.S.; Claro, A.M.; Amaral, N.C.; Barud, H.S.; Mulinari, D.R. Insight on açaí seed biomass economy and waste cooking oil: Eco-sorbent castor oil-based. J. Environ. Manag. 2021, 293, 112803. [Google Scholar] [CrossRef]
- Perera, H.J.; Mortazavian, H.; Blum, F.D. Surface Properties of Silane-Treated Diatomaceous Earth Coatings: Effect of Alkyl Chain Length. Langmuir 2017, 33, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Guangyuan, Y.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S.; Komarneni, S. Mechanism of zeolite X crystallization from diatomite. Mater. Res. Bull. 2018, 107, 132–138. [Google Scholar]
- Parikh, A.N.; Schivley, M.A.; Koo, E.; Seshadri, K.; Aurentz, D.; Mueller, K.; Allara, D.L. n-Alkylsiloxanes: From Single Monolayers to Layered Crystals. The Formation of Crystalline Polymers from the Hydrolysis of n-Octadecyltrichlorosilane. J. Am. Chem. Soc. 1997, 119, 3135–3143. [Google Scholar] [CrossRef]
- Mustafov, S.D.; Sen, F.; Seydibeyoglu, M.O. Preparation and characterization of diatomite and hydroxyapatite reinforced porous polyurethane foam biocomposites. Sci. Rep. 2020, 10, 13308. [Google Scholar] [CrossRef] [PubMed]
- Perera, H.; Goyal, A.; Banu, H.; Alhassan, S. Enhanced oil-spill removal and recovery from water bodies using diatomaceous earth and C18-silane grafted polyurethane. Emerg. Mater. Res. 2022. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Synthesis and characterization of castor oil-based polyurethane for potential application as host in polymer electrolytes. Bull. Mater. Sci. 2015, 38, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Su’Ait, M.S.; Ahmad, K.H.A.; Badri, N.S.; Mohamed, M.Y.A.; Rahman, C.L.; Azanza, R.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrogen Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Jabbary Farrokhi, S.; Pakzad, H.; Fakhri, M.; Moosavi, A. Superhydrophobic home-made polyurethane sponges for versatile and cost-effective oil and water separation. Sep. Purif. Technol. 2021, 276, 119240. [Google Scholar] [CrossRef]
- Wu, F.; Pickett, K.; Panchal, A.; Liu, M.; Lvov, Y. Superhydrophobic Polyurethane Foam Coated with Polysiloxane-Modified Clay Nanotubes for Efficient and Recyclable Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 25445–25456. [Google Scholar] [CrossRef]
- Tavares, L.; Boas, C.; Schleder, G.; Nacas, A.; Rosa, D.; Santos, D. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927. [Google Scholar] [CrossRef]
- Li, Z.-f.; Wang, S.-j.; Li, J.-y. Synthesis and characterization of waterborne polyurethane/organic clay nanocomposites. Front. Mater. Sci. China 2008, 2, 271–275. [Google Scholar] [CrossRef]
- Kucuk, F.; Sismanoglu, S.; Kanbur, Y.; Tayfun, U. Effect of silane-modification of diatomite on its composites with thermoplastic polyurethane. Mater. Chem. Phys. 2020, 256, 123683. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Tan, D.-Y.; Liu, K.-K.; Yu, H.-G.; Zhong, Y.-H.; Yuan, A.-H.; Yu, W.-B.; He, H.-P. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater. 2013, 170, 9–19. [Google Scholar] [CrossRef]
- Dai, M.; Song, P.; Zhang, Y. Preparation and characterization of modified castor oil via photo-click chemistry for UV-curable waterborne polyurethane with enhanced water resistance and low conductive percolation threshold. J. Appl. Polym. Sci. 2021, 138, 49913. [Google Scholar] [CrossRef]
- Visco, A.; Quattrocchi, A.; Nocita, D.; Montanini, R.; Pistone, A. Polyurethane foams loaded with carbon nanofibers for oil spill recovery: Mechanical properties under fatigue conditions and selective absorption in oil/water mixtures. Nanomaterials 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kong, L.Y.; Dai, Y.T.; Yue, X.J.; Rong, J.; Qiu, F.X.; Pan, J.M. Enhanced oils and organic solvents absorption by polyurethane foams composites modified with MnO2 nanowires. Chem. Eng. J. 2017, 309, 7–14. [Google Scholar] [CrossRef]
- Anju, M.; Renuka, N.K. Magnetically actuated graphene coated polyurethane foam as potential sorbent for oils and organics. Arab. J. Chem. 2020, 13, 1752–1762. [Google Scholar] [CrossRef]
- Keshavarz, A.; Zilouei, H.; Abdolmaleki, A.; Asadinezhad, A. Enhancing oil removal from water by immobilizing multi-wall carbon nanotubes on the surface of polyurethane foam. J. Environ. Manag. 2015, 157, 279–286. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, B.; Qiu, F.; Peng, X.; Yue, X.; Yang, D. Preparation of carbon nanotubes/polyurethane hybrids as a synergistic absorbent for efficient oil/water separation. Fib. Polym. 2018, 19, 2195–2202. [Google Scholar] [CrossRef]
- Thomas, S.; Datta, J.; Haponiuk, J.T.; Regunadhan, A. Polyurethane Polymers: Composites and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; 634p. [Google Scholar]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Sobolčiak, P.; Al-Ghouti, M.; Krupa, I. Copolyamide-Clay Nanotube Polymer Composite Nanofiber Membranes: Preparation, Characterization and Its Asymmetric Wettability Driven Oil/Water Emulsion Separation towards Sewage Remediation. Polymers 2021, 13, 3710. [Google Scholar] [CrossRef]
- Jackson, J.; Moallemi, A.; Chiao, M.; Plackett, D. The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds. Polymers 2021, 13, 3899. [Google Scholar] [CrossRef]
- Mardiyati, Y.; Fauza, A.; Rachman, O.; Steven, S.; Santosa, S. A Silica–Lignin Hybrid Filler in a Natural Rubber Foam Composite as a Green Oil Spill Absorbent. Polymers 2022, 14, 2930. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.; Zainal Abidin, Z.; Majid, U.; Abdul Hamid, M.; Abdullah, A.; Othaman, R.; Harun, M. Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation. Polymers 2022, 14, 3325. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shi, D.; Yang, C.; Wu, G. Preparation of polymer-based foam for efficient oil-water separation based on surface engineering. Soft Matter 2022, 18, 3041–3051. [Google Scholar] [CrossRef] [PubMed]


| Chemicals Used | % |
|---|---|
| Polyol | 63.7 |
| Blowing agent: DI water, Pentane | 0.6, 6.4 |
| MDI | 29.3 |
| C18-DE(% by mass of all reactants) | 10 |
| Sample Code | Frequency (cm−1) | |||
|---|---|---|---|---|
| Vibration Bands | Expected Peaks | Observed Peaks | ||
| Pristine PU foam | N-H stretching | 3500–3200 | 3340 | |
| −CH stretching (Asy., Sy.) of the alkyl chain | 2935–2847 | 2924, 2858 | ||
| N=C=O stretching | 2275–2240 | 2275 | ||
| −(C=O) stretching of urethane and ester group | 1745 | 1733 | ||
| amide II: δN–H + νC–N + νC–C; sensitive to chain formation and intermolecular hydrogen bonding | 1600–1500 | 1610 | ||
| NH deformation (stretching vibrations) | 1530–1500 | 1522 | ||
| Isocyanurates | 1420–1410 | 1417 | ||
| C−O−C group | 1250–1210 | 1217 | ||
| C−O stretching | 1070–1030 | 1050 | ||
| CH2 rocking | 730–710 | 717 | ||
| C18-DE | −CH stretching (Asy, Sy) of the alkyl chain | 2935–2847 | 2911, 2847 | |
| Si−O, stretching | 1120–1050 | 1065 | ||
| Si−O−Si Sy. stretching | 795–750 | 791 | ||
| Si−O vib. of polymorphic silica | 640–620 | 628 | ||
| Siloxane bonds, Assy. stretching | 480–450 | 465 | ||
| PU-C18-DE foam | N−H stretching | 3500–3200 | 3352 | |
| −CH stretching (Asy., Sy.) of the alkyl chain | 2935–2847 | 2924, 2856 | ||
| N=C=O stretching | 2275–2240 | 2275 | Disappeared | |
| −(C=O) stretching of urethane and ester group | 1745 | 1733 | ||
| amide II: δN–H + νC–N+ νC–C; sensitive to chain formation and intermolecular hydrogen bonding | 1600–1500 | 1610 | ||
| NH deformation (stretching vibrations) | 1530–1500 | 1522 | ||
| Isocyanates | 1420–1410 | 1417 | ||
| C−O−C group | 1250–1210 | 1217 | ||
| C–O & Si−O−Si stretching | 1070–1030 | 1048 | ||
| Si−OH | 766 | Appeared | ||
| CH2 rocking | 730–710 | 721 | ||
| Si−O−Si vib. of polymorphic silica | 620 | Appeared | ||
| Siloxane bonds, Assy. stretching | 480–450 | 457 | Appeared | |
| Polymer Material | Average Oil Adsorption % | |||
|---|---|---|---|---|
| PU | 1.02 | 1.49 | 0.452 | 45.2% |
| PU-C18-DE | 1.13 | 2.86 | 1.73 | 131.6% |
| Polymer Material | Average Water Absorption % | |||
|---|---|---|---|---|
| PU | 1.2 | 1.89 | 0.575 | 57.5% |
| PU-C18-DE | 1.2 | 1.4 | 0.167 | 16.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, H.J.; Goyal, A.; Alhassan, S.M.; Banu, H. Biobased Castor Oil-Based Polyurethane Foams Grafted with Octadecylsilane-Modified Diatomite for Use as Eco-Friendly and Low-Cost Sorbents for Crude Oil Clean-Up Applications. Polymers 2022, 14, 5310. https://doi.org/10.3390/polym14235310
Perera HJ, Goyal A, Alhassan SM, Banu H. Biobased Castor Oil-Based Polyurethane Foams Grafted with Octadecylsilane-Modified Diatomite for Use as Eco-Friendly and Low-Cost Sorbents for Crude Oil Clean-Up Applications. Polymers. 2022; 14(23):5310. https://doi.org/10.3390/polym14235310
Chicago/Turabian StylePerera, Helanka J., Anjali Goyal, Saeed M. Alhassan, and Hussain Banu. 2022. "Biobased Castor Oil-Based Polyurethane Foams Grafted with Octadecylsilane-Modified Diatomite for Use as Eco-Friendly and Low-Cost Sorbents for Crude Oil Clean-Up Applications" Polymers 14, no. 23: 5310. https://doi.org/10.3390/polym14235310
APA StylePerera, H. J., Goyal, A., Alhassan, S. M., & Banu, H. (2022). Biobased Castor Oil-Based Polyurethane Foams Grafted with Octadecylsilane-Modified Diatomite for Use as Eco-Friendly and Low-Cost Sorbents for Crude Oil Clean-Up Applications. Polymers, 14(23), 5310. https://doi.org/10.3390/polym14235310






