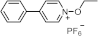
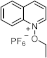
Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems
Abstract
:1. Introduction
2. Single-Component Cationic Photoinitiators
2.1. Ionic Photoinitiators
2.1.1. Aryl Diazonium Salts
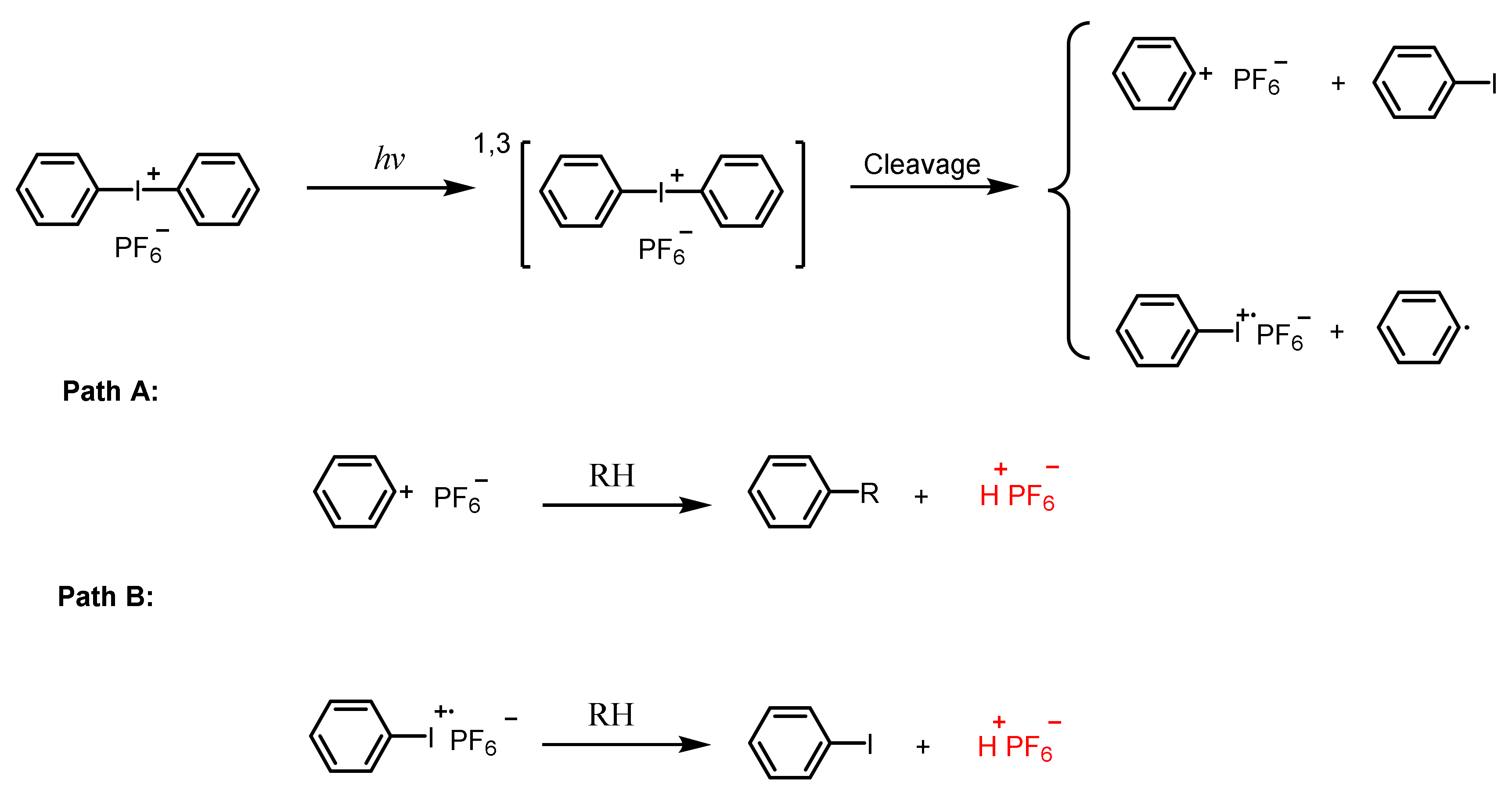
2.1.2. Iodonium Salts
2.1.3. Sulfonium Salts
Triarylsulfonium Salts
Aryl-Alkylsulfonium Salts
Phenacylsulfonium Salts
2.1.4. Phosphonium Salts
2.1.5. Ammonium Salts
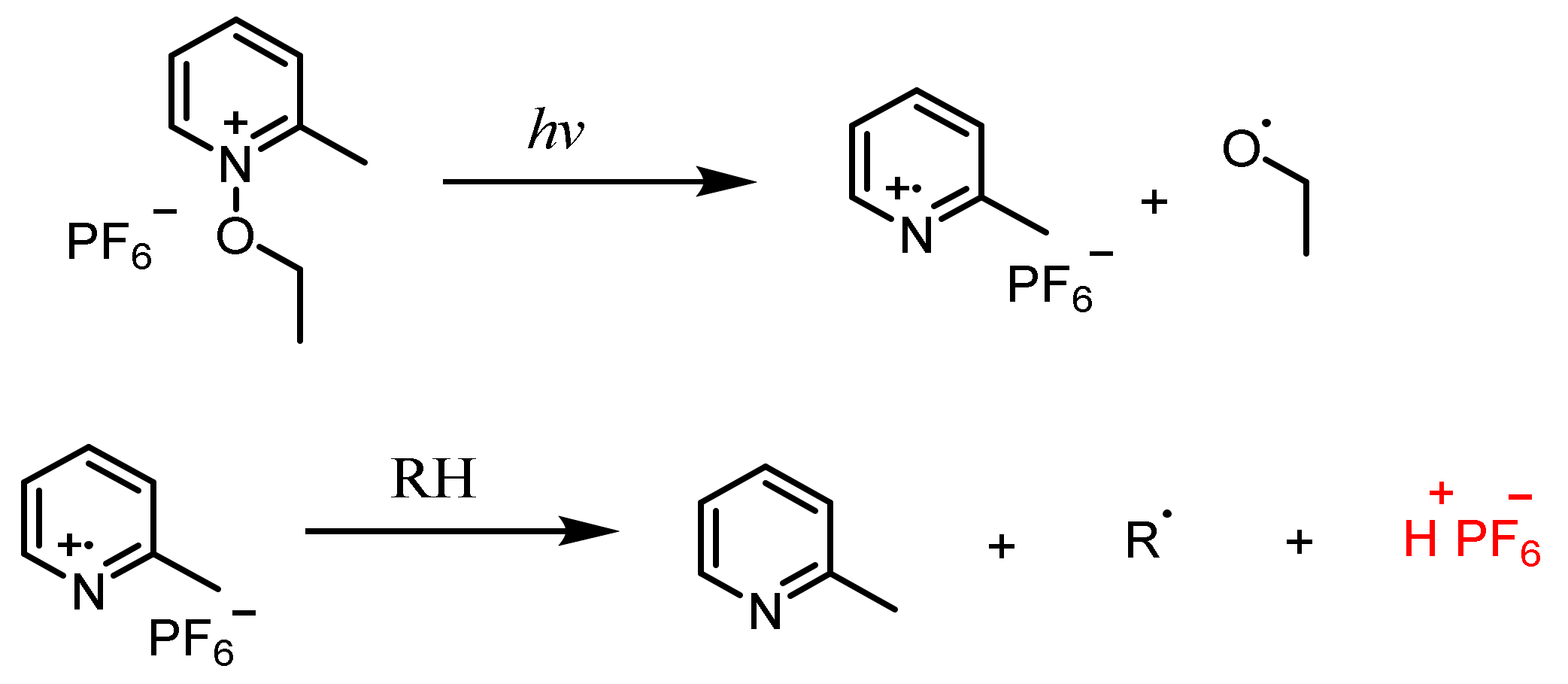
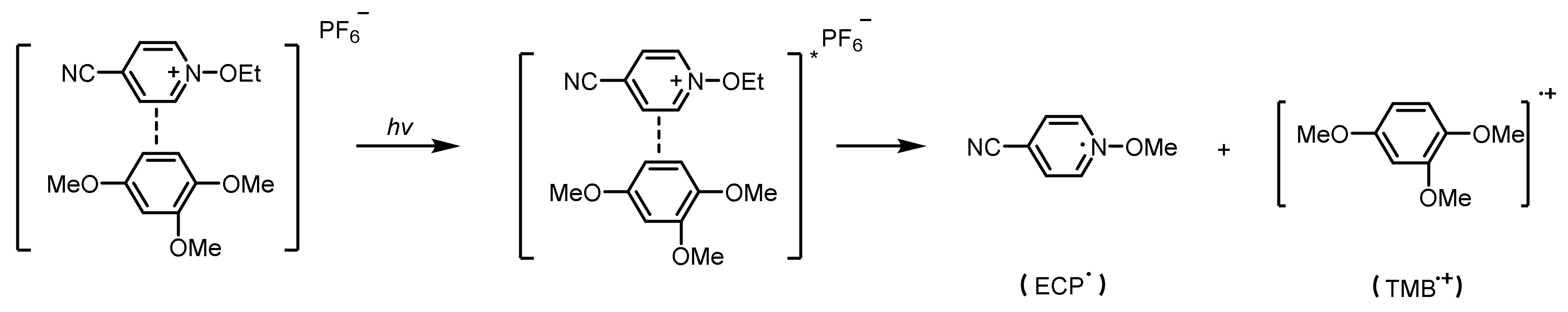
N-Alkoxypyridinium Salts
Phenacyl Ammonium Salts
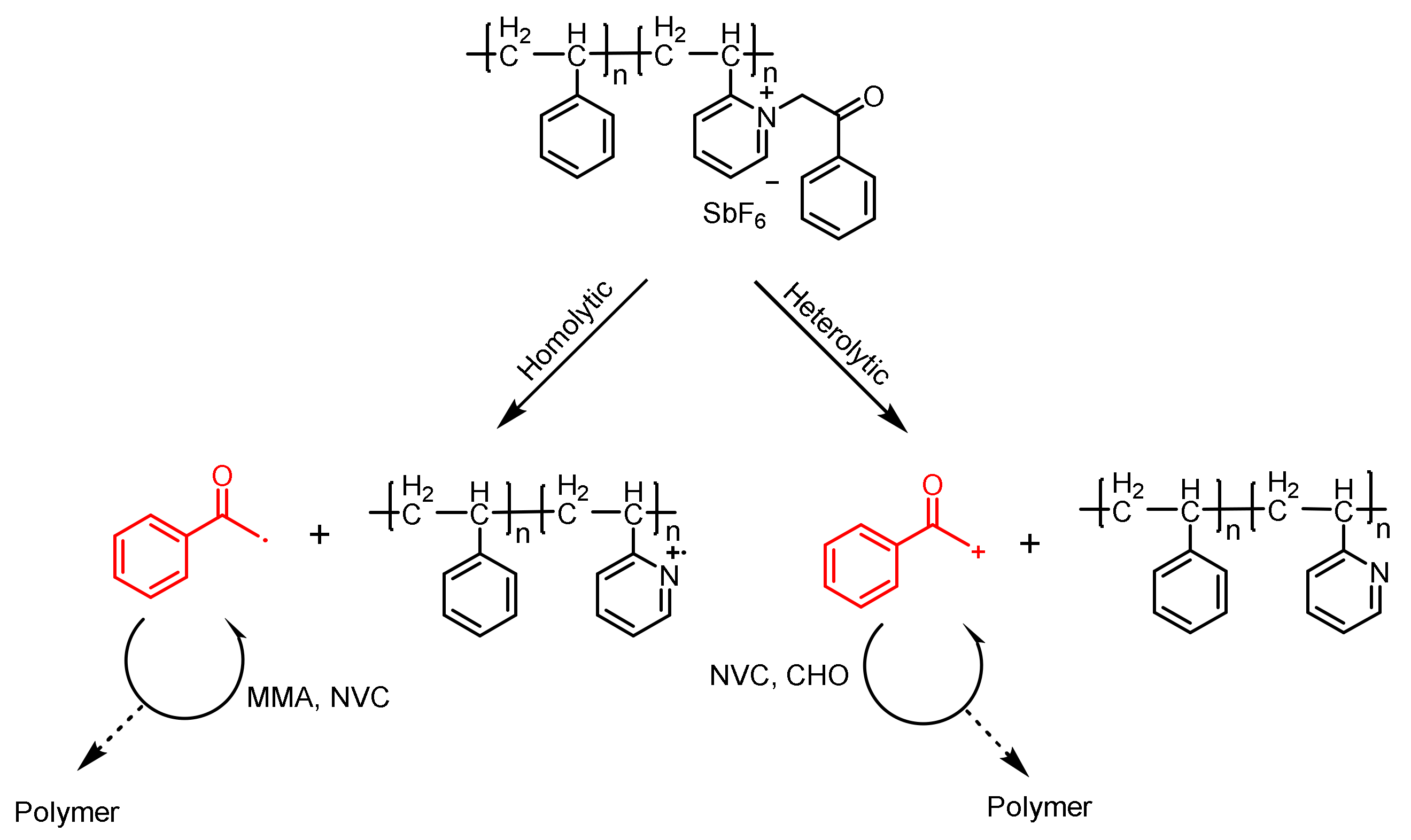
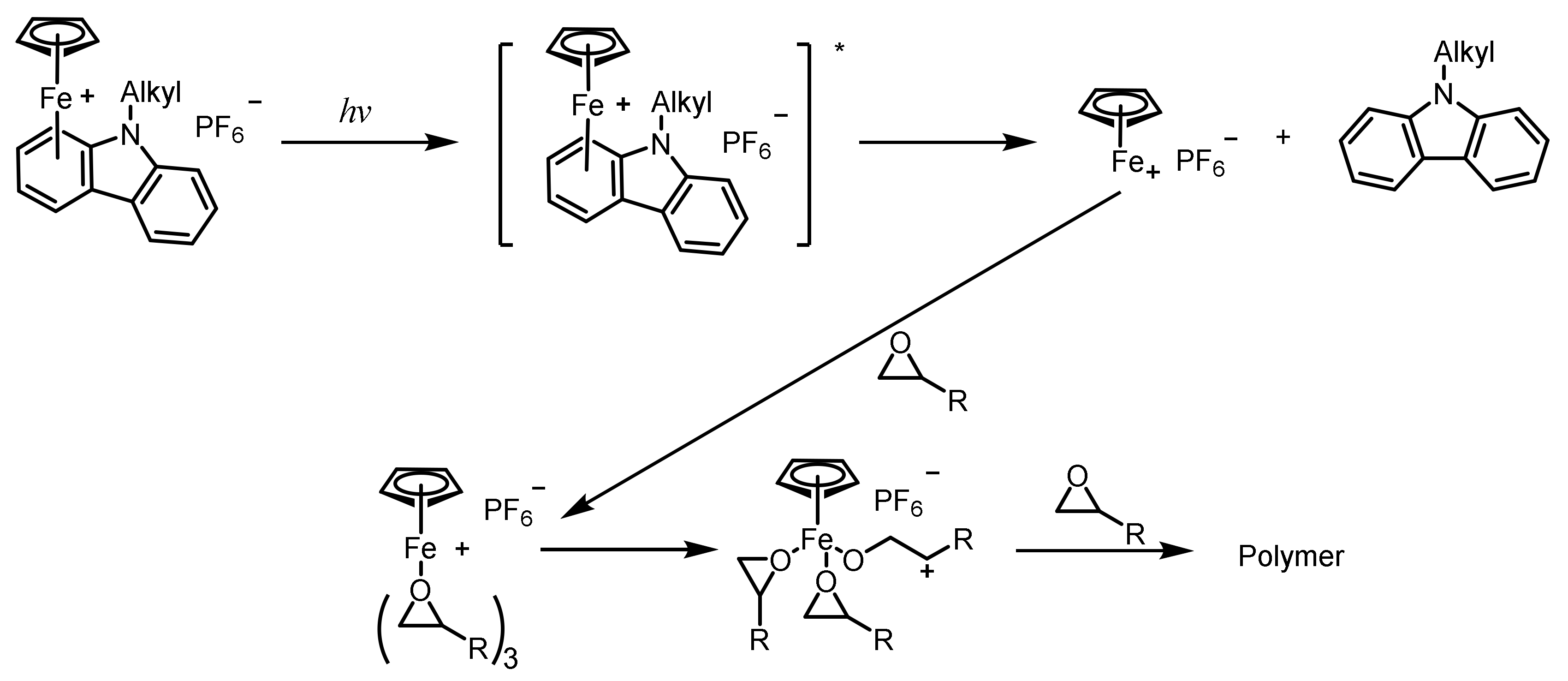
2.1.6. Ferrocenium Salts
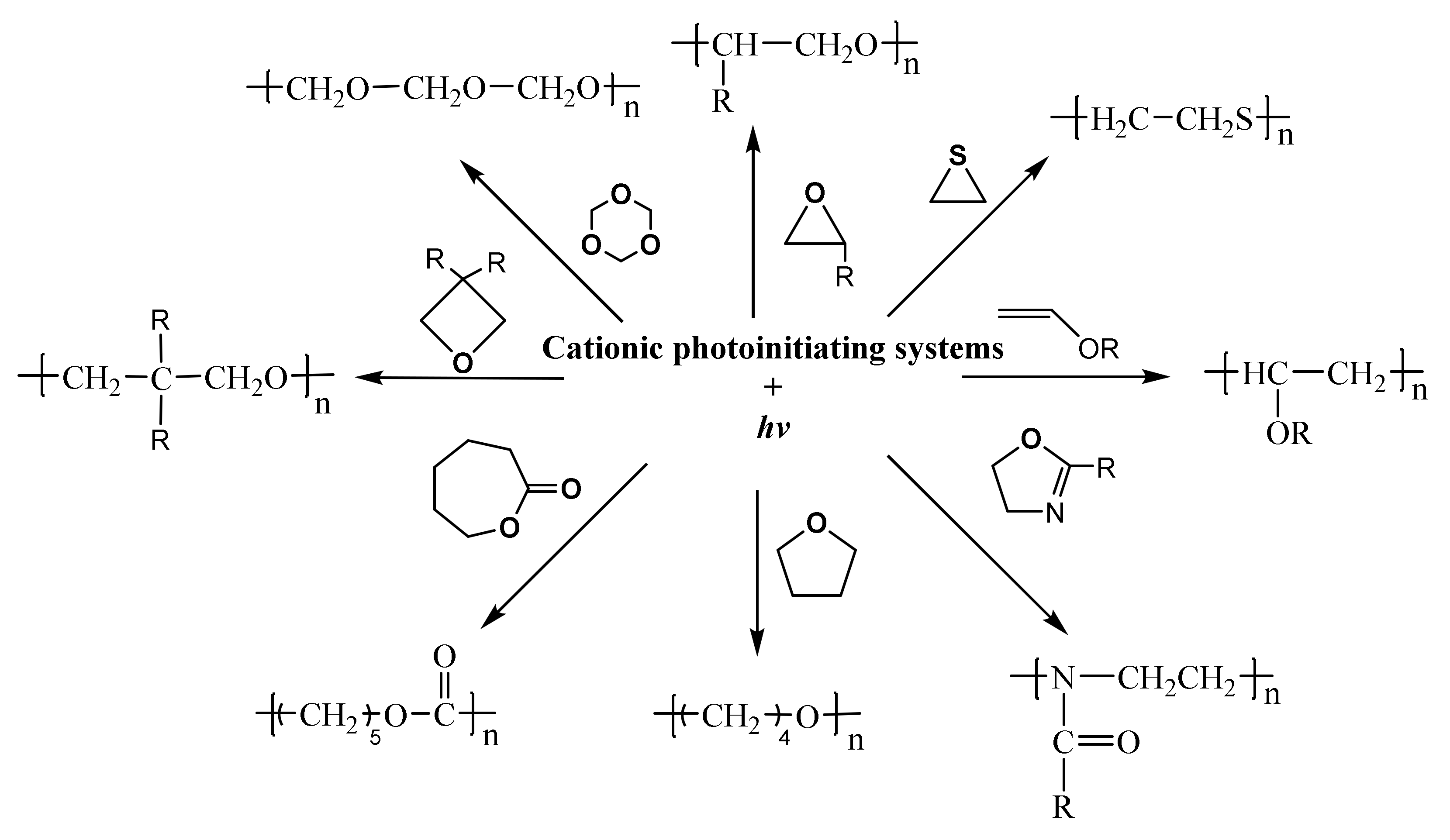
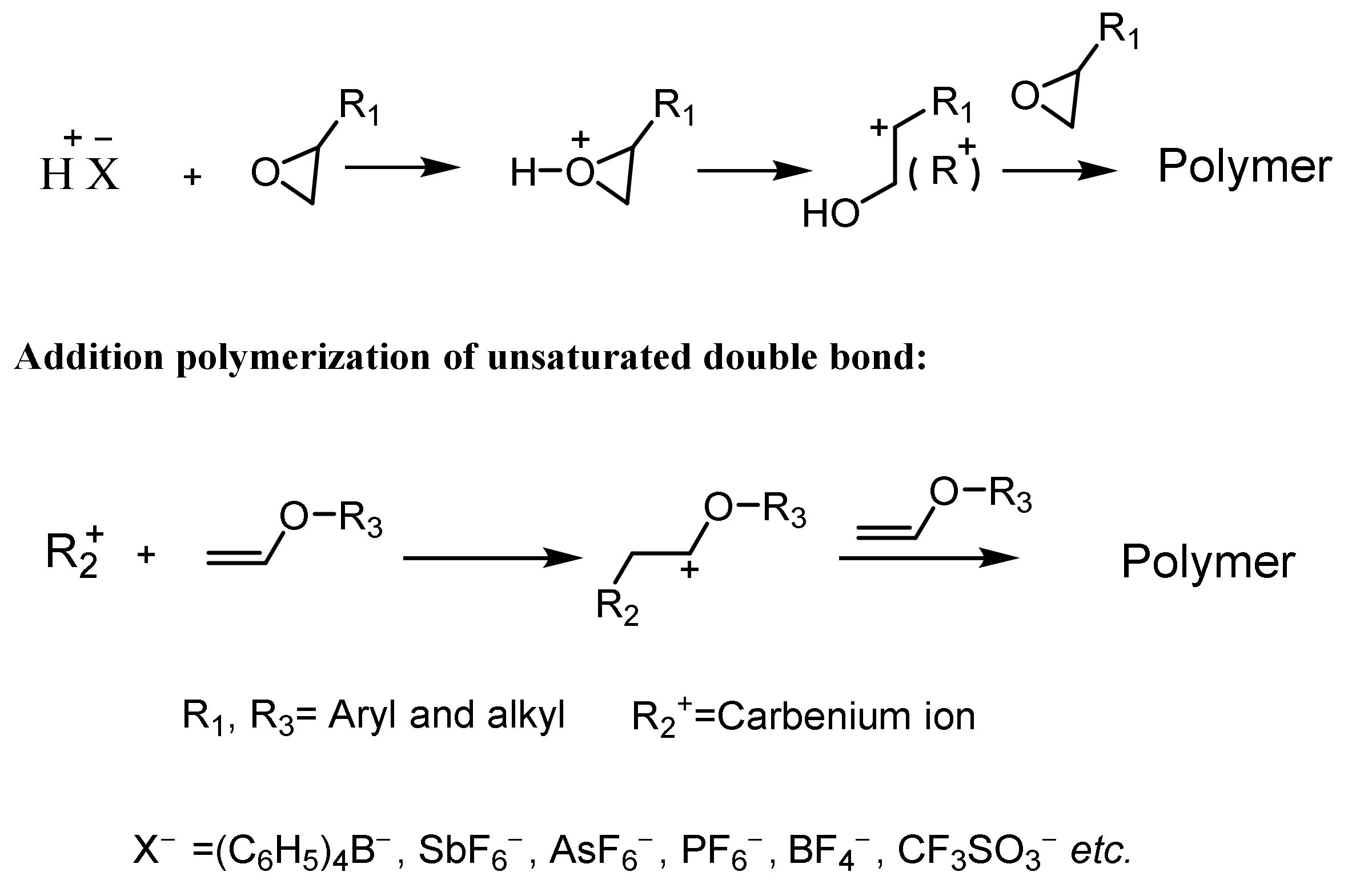
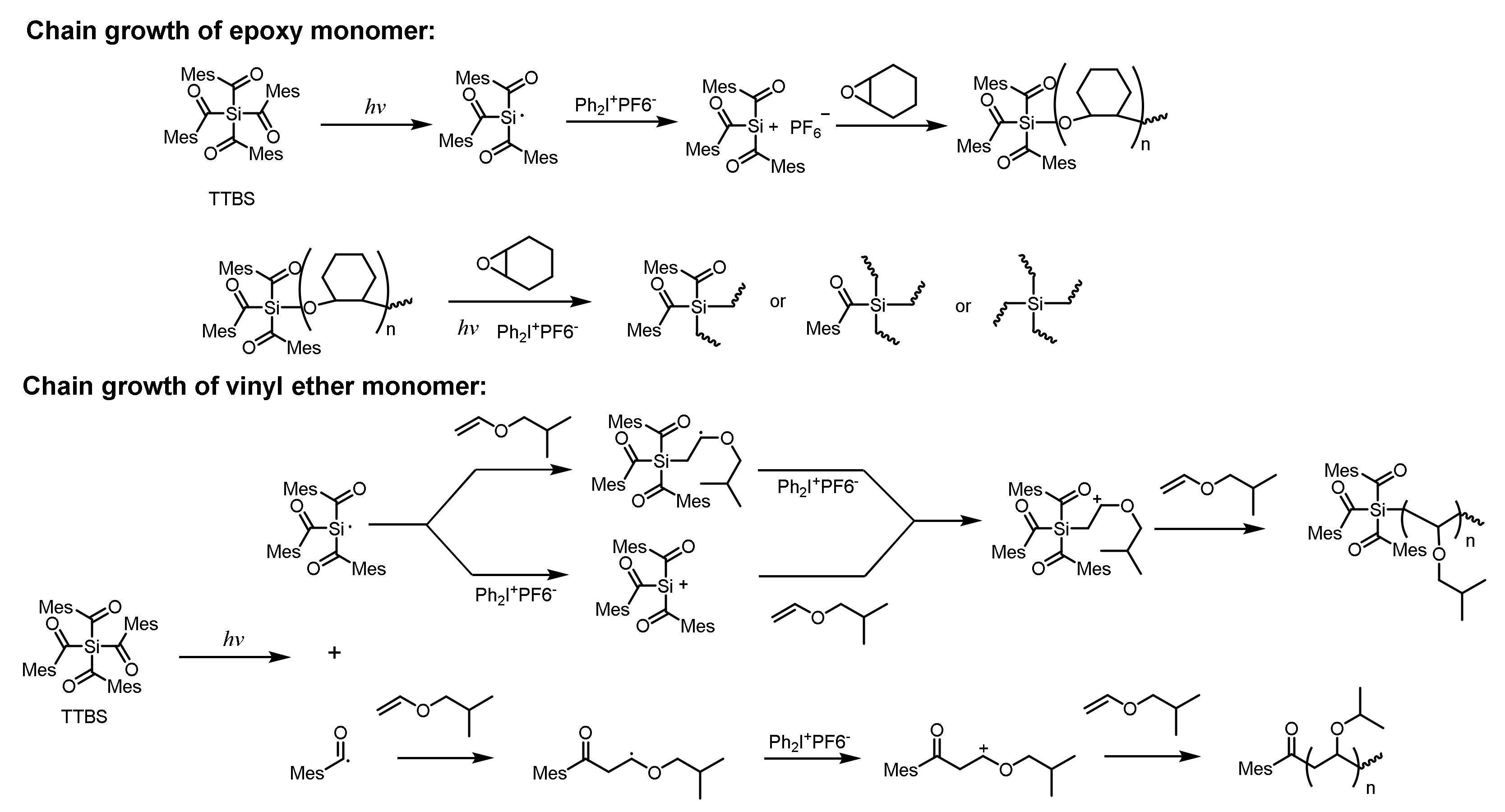
2.2. Nonionic Photoinitiators for Cationic Polymerization
3. Multi-Component Cationic Photoinitiating Systems
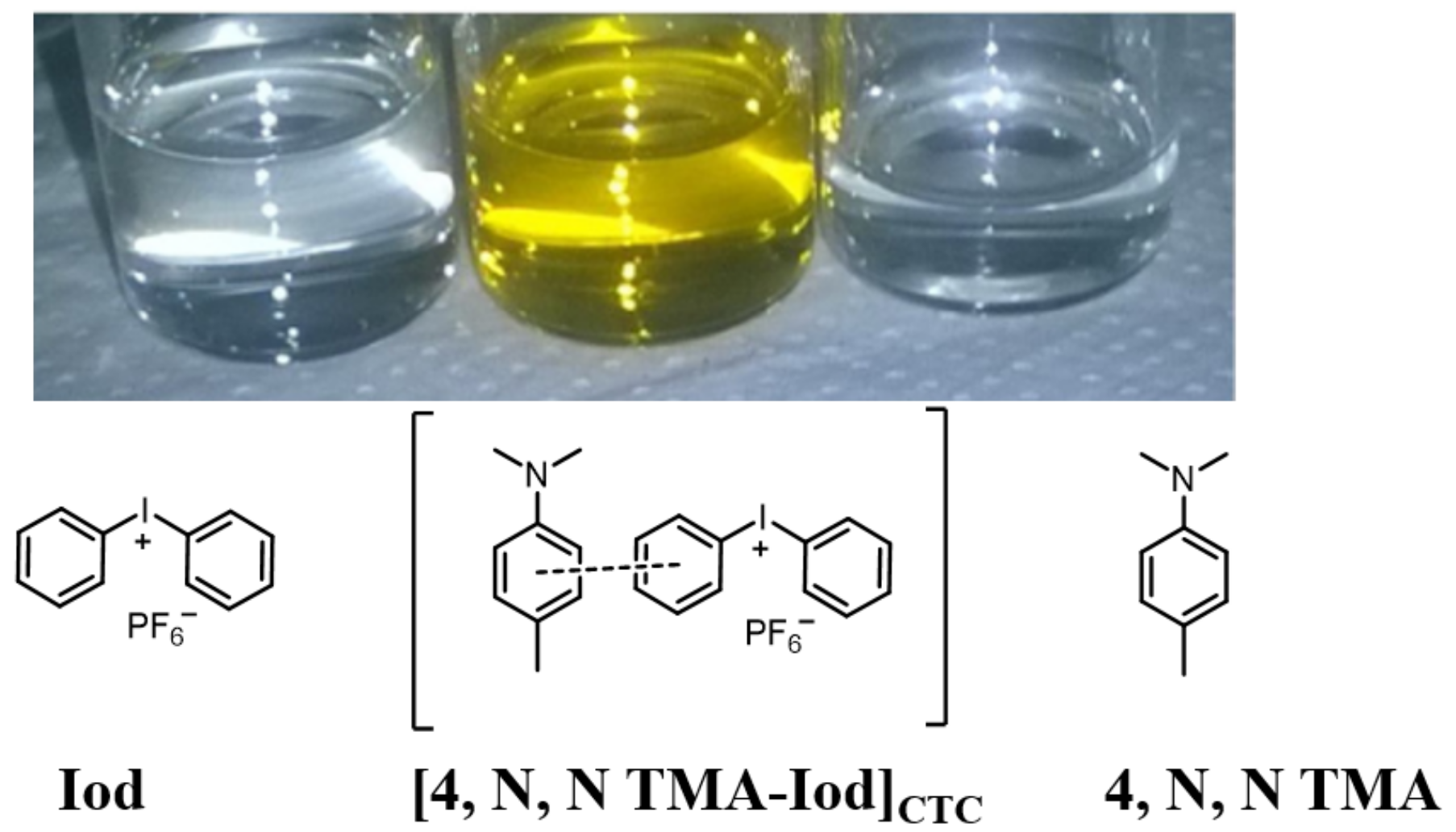
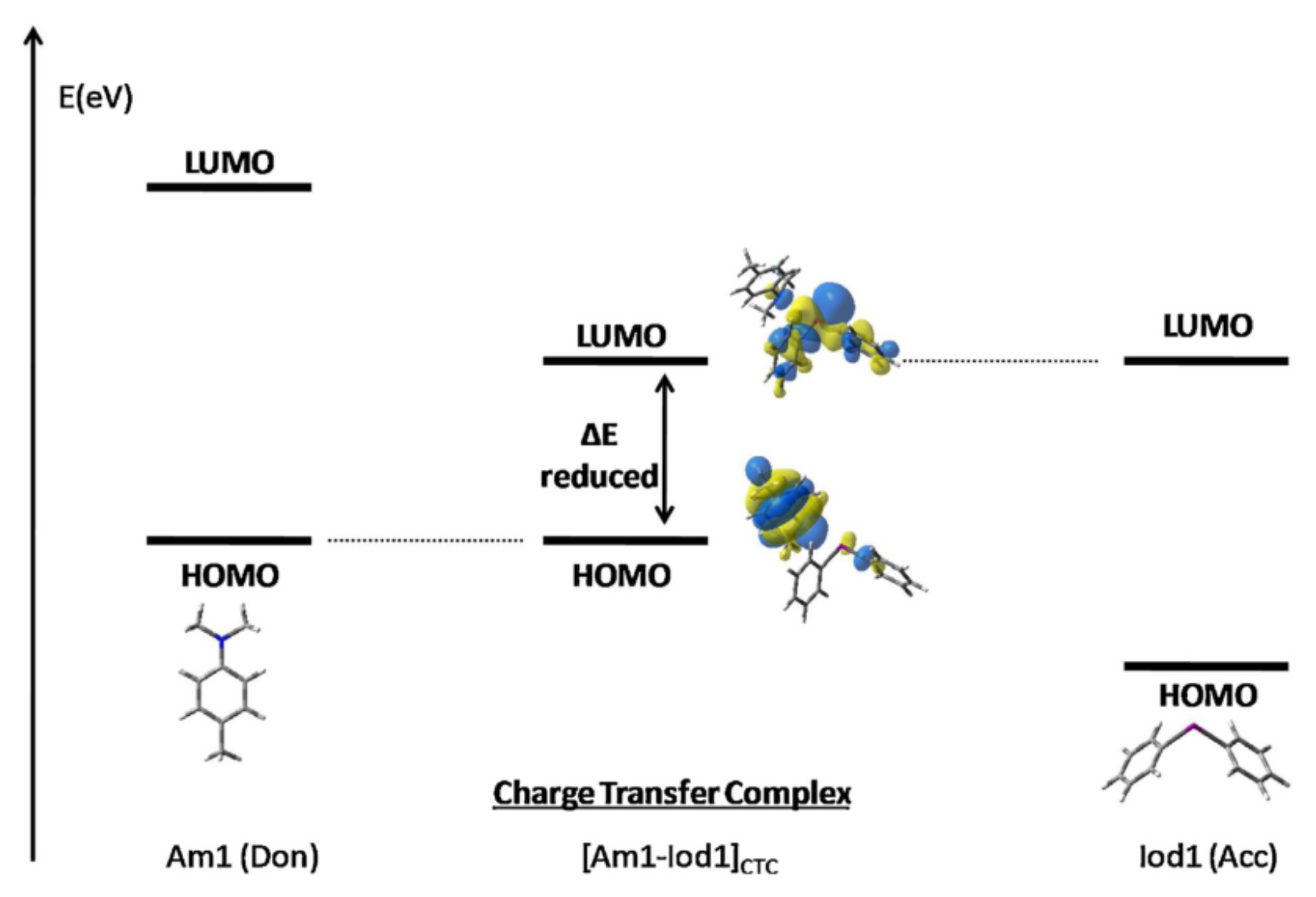
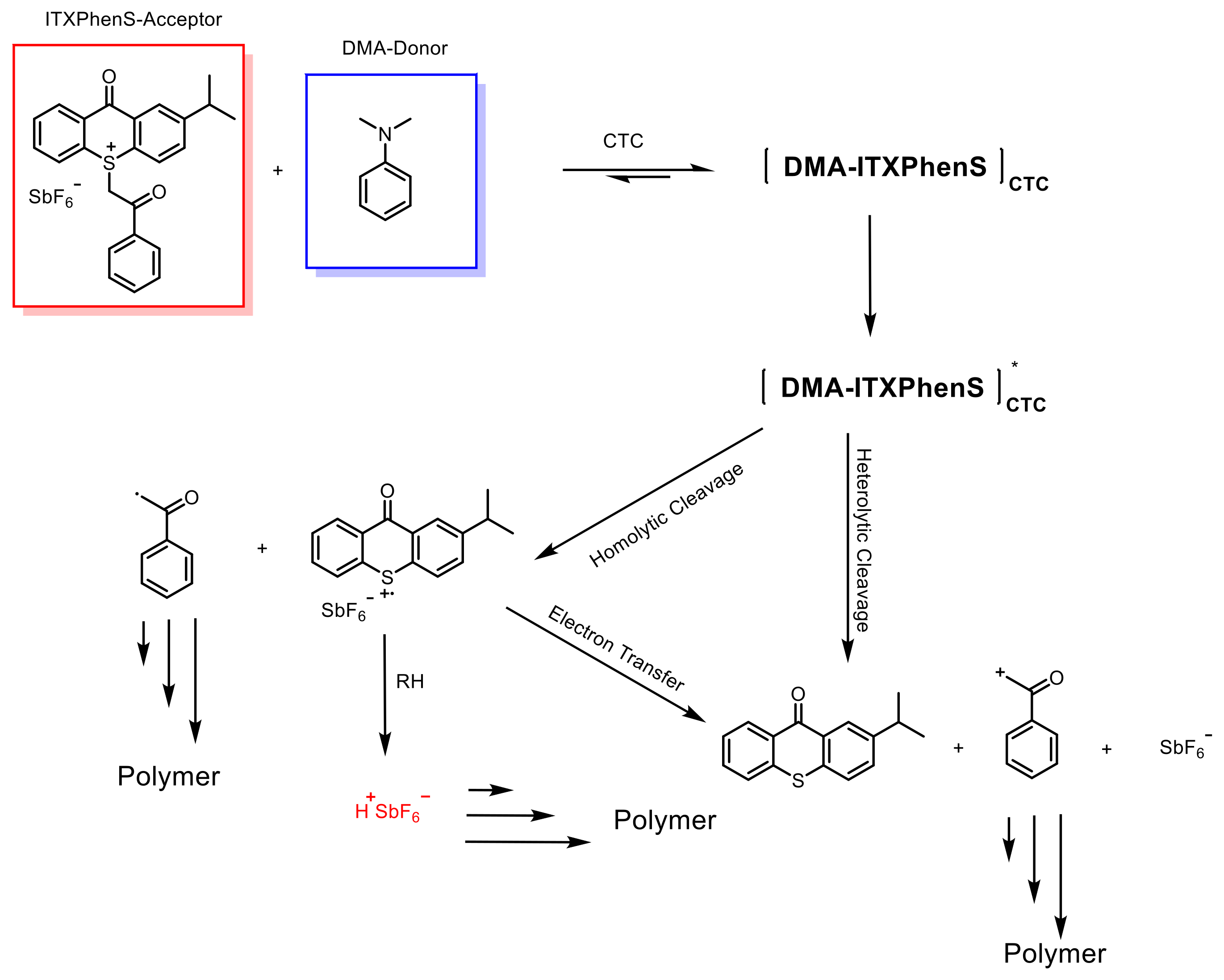
3.1. Electron Transfer within Charge Transfer Complexes (CTC)
3.2. Photosensitization by Electron Transfer in Exciplex
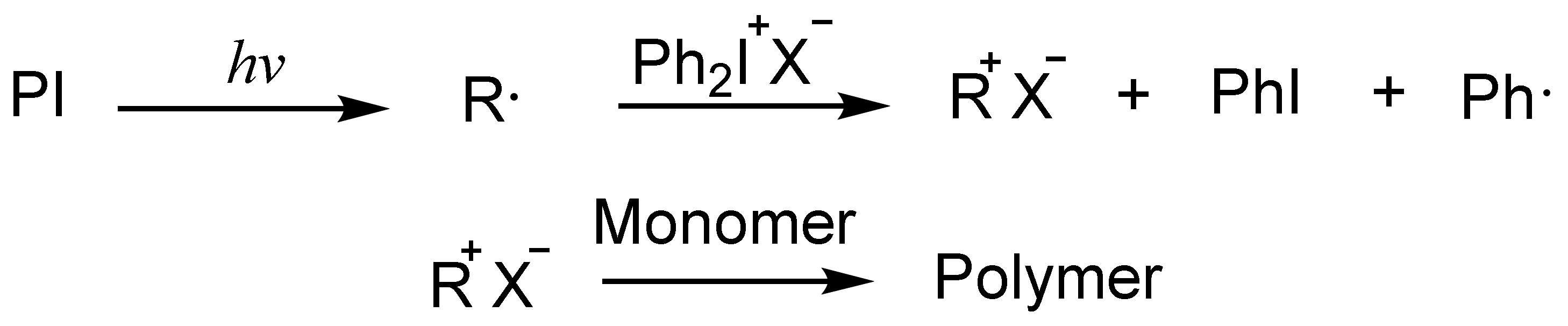
3.3. Free Radical Promotion
3.3.1. Addition-Fragmentation Mechanism
3.3.2. Oxidation of Free Radicals
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Pi, J.; Zhang, Y.; Xu, D.; Yagci, Y.; Liu, R. A new anthraquinone derivative as a near UV and visible light photoinitiator for free-radical, thiol–ene and cationic polymerizations. Polym. Chem. 2021, 12, 3299–3306. [Google Scholar] [CrossRef]
- Cakmakci, E.; Sen, F.; Kahraman, M.V. Isosorbide diallyl based antibacterial thiol–ene photocured coatings containing polymerizable fluorous quaternary phosphonium salt. ACS Sustain. Chem. Eng. 2019, 7, 10605–10615. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Fang, Y.; Meng, Q. A photocuring PUA material with adjustable flexibility used in the fast photochromic coating on ophthalmic lenses. Prog. Org. Coat. 2019, 137, 105330. [Google Scholar] [CrossRef]
- Pan, C.; Song, Y.; Liu, P. Transparent and Flexible Amphiphobic Coatings with Excellent Fold Resistance via Solvent-Free Coating and Photocuring of Fluorinated Liquid Nitrile–Butadiene Rubber. ACS Appl. Mater. Interfaces 2021, 13, 26498–26504. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Corrigan, N.; Boyer, C. Rapid High-Resolution 3D Printing and Surface Functionalization via Type I Photoinitiated RAFT Polymerization. Angew. Chem. Int. Ed. 2021, 60, 8839–8850. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Hoy, Z.X.; Show, P.L.; Huang, N.M.; Lim, H.N.; Foo, C.Y. Single droplet 3D printing of electrically conductive resin using high aspect ratio silver nanowires. Addit. Manuf. 2021, 48, 102473. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhao, Y.; Duan, W.; Liu, B.; Wang, T.; Wang, G. Digital light processing 3D printing of AlSi10Mg powder modified by surface coating. Addit. Manuf. 2021, 39, 101897. [Google Scholar] [CrossRef]
- Chen, M.L.; Chen, C.H.; Huang, Y.F.; Chen, H.C.; Chang, J.W. Cumulative dietary risk assessment of benzophenone-type photoinitiators from packaged foodstuffs. Foods 2022, 11, 152. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Nowakowski, P.; Typek, R.; Dybowski, M.P. Effect of food packaging material on some physicochemical properties of polyacrylate varnish layers. Food Packag. Shelf Life 2019, 21, 100370. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Identification of photoinitiators, including novel phosphine oxides, and their transformation products in food packaging materials and indoor dust in Canada. Environ. Sci. Technol. 2019, 53, 4109–4118. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, L.; Zhao, Y.; You, Z.; Guan, Q. 3D printing preview for stereo-lithography based on photopolymerization kinetic models. Bioact. Mater. 2020, 5, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, H.; Bao, C.; Wan, D.; Jin, M. Fused carbazole–coumarin–ketone dyes: High performance and photobleachable photoinitiators in free radical photopolymerization for deep photocuring under visible LED light irradiation. Polym. Chem. 2022, 13, 3367–3376. [Google Scholar] [CrossRef]
- Hahn, V.; Messer, T.; Bojanowski, N.M.; Curticean, E.R.; Wacker, I.; Schroder, R.R.; Blasco, E.; Wegener, M. Two-step absorption instead of two-photon absorption in 3D nanoprinting. Nat. Photonics 2021, 15, 932–938. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Topa, M.; Hola, E.; Galek, M.; Petko, F.; Pilch, M.; Popielarz, R.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Ortyl, J. One-component cationic photoinitiators based on coumarin scaffold iodonium salts as highly sensitive photoacid generators for 3D printing IPN photopolymers under visible LED sources. Polym. Chem. 2020, 11, 5261–5278. [Google Scholar] [CrossRef]
- Michaudel, Q.; Kottisch, V.; Fors, B.P. Cationic Polymerization: From Photoinitiation to Photocontrol. Angew. Chem. Int. Ed. 2017, 56, 9670–9679. [Google Scholar] [CrossRef]
- Crivello, J.; Kong, S.; Jang, M. Cationic polymerization: New developments and applications. Macromol. Symp. 2004, 217, 47–62. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Shi, S.; Croutxe-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Crivello, J.V. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. Pol. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J. Diaryliodonium salts. A new class of photoinitiators for cationic polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. New photoinitiators for cationic polymerization. Polimery 2012, 57, 510–517. [Google Scholar] [CrossRef]
- Park, C.H.; Takahara, S.; Yamaoka, T. The participation of the anion and alkyl substituent of diaryliodonium salts in photo-initiated cationic polymerization reactions. Polym. Adv. Technol. 2006, 17, 156–162. [Google Scholar] [CrossRef]
- Pobiner, H. Spectrophotometric determinations of aryldiazonium salts of complex halides used as photoinitiators in uv-cured epoxide systems. Anal. Chim. Acta. 1978, 96, 153–163. [Google Scholar] [CrossRef]
- Yağci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Hartwig, A.; Harder, A.; Lühring, A.; Schröder, H. (9-Oxo-9H-fluoren-2-yl)-phenyl-iodonium hexafluoroantimonate (V)—A photoinitiator for the cationic polymerisation of epoxides. Eur. Polym. J. 2001, 37, 1449–1455. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Petko, F.; Galek, M.; Hola, E.; Popielarz, R.; Ortyl, J. One-Component Cationic Photoinitiators from Tunable Benzylidene Scaffolds for 3D Printing Applications. Macromolecules 2021, 54, 7070–7087. [Google Scholar] [CrossRef]
- Topa-Skwarczynska, M.; Galek, M.; Jankowska, M.; Morlet-Savary, F.; Graff, B.; Lalevee, J.; Popielarz, R.; Ortyl, J. Development of the first panchromatic BODIPY-based one-component iodonium salts for initiating the photopolymerization processes. Polym. Chem. 2021, 12, 6873–6893. [Google Scholar] [CrossRef]
- Peng, X.; Yao, M.; Xiao, P. Newly Synthesized Chromophore-linked Iodonium Salts as Photoinitiators of Free Radical Photopolymerization. Macromol. Chem. Phys. 2021, 222, 2100035. [Google Scholar] [CrossRef]
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization by diarylchloronium and diarylbromonium salts. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 563–571. [Google Scholar] [CrossRef]
- Crivello, J.; Lam, J. Tethered sulfonium salt photoinitiators for free radical polymerization. Synth. Commun. 1979, 9, 151–156. [Google Scholar] [CrossRef]
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization with triarylsulfonium salts. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3231–3253. [Google Scholar] [CrossRef]
- Crivello, J.V. Benzophenothiazine and benzophenoxazine photosensitizers for triarylsulfonium salt cationic photoinitiators. J. Polym. Sci. Pol. Chem. 2008, 46, 3820–3829. [Google Scholar] [CrossRef]
- Crivello, J.V. Redox initiated cationic polymerization: Reduction of triarylsulfonium salts by silanes. Silicon 2009, 1, 111–124. [Google Scholar] [CrossRef]
- Jin, M.; Wu, X.; Malval, J.P.; Wan, D.; Pu, H. Dual roles for promoting monomers to polymers: A conjugated sulfonium salt photoacid generator as photoinitiator and photosensitizer in cationic photopolymerization. J. Polym. Sci. Polym. Chem. 2016, 54, 2722–2730. [Google Scholar] [CrossRef]
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization by dialkyl-4-hydroxyphenylsulfonium salts. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 1021–1034. [Google Scholar] [CrossRef]
- Crivello, J.; Lam, J. Complex triarylsulfonium salt photoinitiators. I. The identification, characterization, and syntheses of a new class of triarylsulfonium salt photoinitiators. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 2677–2695. [Google Scholar] [CrossRef]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel photoacid generators for cationic photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Zhang, B.; Li, T.; Kang, Y. Synthesis and characterization of triarylsulfonium salts as novel cationic photoinitiators for UV-photopolymerization. Res. Chem. Intermed. 2017, 43, 6617–6625. [Google Scholar] [CrossRef]
- Yu, J.; Pan, H.; Jiang, S.L.; Gao, Y.J.; Sun, F. Synthesis and performances of polysiloxane-modified 5-arylthianthrenium salt cationic photoinitiators with self-floating capability. Eur. Polym. J. 2017, 97, 338–346. [Google Scholar] [CrossRef]
- Chen, S.; Cao, C.; Shen, X.; Qiu, Y.; Kuang, C.; Wan, D.; Jin, M. One/two-photon sensitive sulfonium salt photoinitiators based on 1, 3, 5-triphenyl-2-pyrazoline. Eur. Polym. J. 2021, 153, 110525. [Google Scholar] [CrossRef]
- Wu, X.; Jin, M.; Xie, J.; Malval, J.P.; Wan, D. Molecular Engineering of UV/Vis Light-Emitting Diode (LED)-Sensitive Donor–π–Acceptor-Type Sulfonium Salt Photoacid Generators: Design, Synthesis, and Study of Photochemical and Photophysical Properties. Chem. Eur. J. 2017, 23, 15783–15789. [Google Scholar] [CrossRef]
- Wu, X.; Jin, M.; Xie, J.; Malval, J.P.; Wan, D. One/two-photon cationic polymerization in visible and near infrared ranges using two-branched sulfonium salts as efficient photoacid generators. Dyes Pigments 2016, 133, 363–371. [Google Scholar] [CrossRef]
- Wu, X.; Malval, J.-p.; Wan, D.; Jin, M. D-π-A-type aryl dialkylsulfonium salts as one-component versatile photoinitiators under UV/visible LEDs irradiation. Dyes Pigments 2016, 132, 128–135. [Google Scholar] [CrossRef]
- Jin, M.; Hong, H.; Xie, J.; Malval, J.-P.; Spangenberg, A.; Soppera, O.; Wan, D.; Pu, H.; Versace, D.-L.; Leclerc, T. π-conjugated sulfonium-based photoacid generators: An integrated molecular approach for efficient one and two-photon polymerization. Polym. Chem. 2014, 5, 4747–4755. [Google Scholar] [CrossRef]
- Xia, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Wan, D.; Pu, H.; Vergote, T.; Morlet-Savary, F.; Chaumeil, H.; Baldeck, P. Enhancement of acid photogeneration through a para-to-meta substitution strategy in a sulfonium-based alkoxystilbene designed for two-photon polymerization. Chem. Mater. 2012, 24, 237–244. [Google Scholar] [CrossRef]
- Meng, X.Y.; Li, L.J.; Huang, Y.X.; Deng, X.; Liu, X.X.; Li, Z.Q. Upconversion nanoparticle-assisted cationic and radical/cationic hybrid photopolymerization using sulfonium salts. Polym. Chem. 2021, 12, 7005–7009. [Google Scholar] [CrossRef]
- Crivello, J.V.; Kong, S. Synthesis and characterization of second-generation dialkylphenacylsulfonium salt photoinitiators. Macromolecules 2000, 33, 825–832. [Google Scholar] [CrossRef]
- Kaya, K.; Kreutzer, J.; Yagci, Y. Diphenylphenacyl sulfonium salt as dual photoinitiator for free radical and cationic polymerizations. J. Polym. Sci. Pol. Chem. 2018, 56, 451–457. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl Phenothiazinium Salt as a New Broad-Wavelength-Absorbing Photoinitiator for Cationic and Free Radical Polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, Y.C.; Ou, Y.; Zhang, J.Y.; Yagci, Y.; Liu, R. Broad wavelength sensitive coumarin sulfonium salts as photoinitiators for cationic, free radical and hybrid photopolymerizations. Prog. Org. Coat. 2023, 174, 107272. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. Structural and mechanistic studies on the photolysis of dialkylphenacylsulfonium salt cationic photoinitiators. Macromolecules 1983, 16, 864–870. [Google Scholar] [CrossRef]
- Ikbal, M.; Jana, A.; Singh, N.P.; Banerjee, R.; Dhara, D. Photoacid generators (PAGs) based on N-acyl-N-phenylhydroxylamines for carboxylic and sulfonic acids. Tetrahedron 2011, 67, 3733–3742. [Google Scholar] [CrossRef]
- Kaya, K.; Kreutzer, J.; Yagci, Y. A Charge-Transfer Complex of Thioxanthonephenacyl Sulfonium Salt as a Visible-Light Photoinitiator for Free Radical and Cationic Polymerizations. ChemPhotoChem 2019, 3, 1187–1192. [Google Scholar] [CrossRef]
- Abu-Abdoun, I.I. Cationic photopolymerization of cyclohexene oxide. Eur. Polym. J. 1992, 28, 73–78. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Aydin, M.; Arsu, N.; Yagci, Y. Photoinitiated polymerization of methyl methacrylate by phenacyl type salts. J. Photochem. Photobiol. A 2003, 159, 151–159. [Google Scholar] [CrossRef]
- Takata, T.; Takuma, K.; Endo, T. Photoinitiated cationic polymerization of epoxide with phosphonium salts as novel photolatent initiators. Makromol. Chem. Rapid Commun. 1993, 14, 203–206. [Google Scholar] [CrossRef]
- Tskuma, K.; Takata, T.; Endo, T. Latent cationic initiator: Photoinitiated polymerization of epoxides and vinyl monomers with phosphonium salts. J. Photopolym. Sci. Tecnol. 1993, 6, 67–74. [Google Scholar]
- Yaḡci, Y.; Kornowski, A.; Schnabel, W. N-alkoxy-pyridinium and N-alkoxy-quinolinium salts as initiators for cationic photopolymerizations. J. Polym. Sci. Pol. Chem. 1992, 30, 1987–1991. [Google Scholar]
- Taskin, O.S.; Erel-Goktepe, I.; Khan, M.; Alyaan, A.; Pispas, S.; Yagci, Y. Polystyrene-b-poly(2-vinyl phenacyl pyridinium) salts as photoinitiators for free radical and cationic polymerizations and their photoinduced molecular associations. J. Photochem. Photobiol. A 2014, 285, 30–36. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Zaim, Ö.; Yagci, Y. Diethoxy-azobis (pyridinium) Salt as Photoinitiator for Cationic Polymerization: Towards Wavelength Tunability by Cis–Trans Isomerization. Macromol. Rapid Comm. 2008, 29, 892–896. [Google Scholar] [CrossRef]
- Karal, O.; Önen, A.; Yaǧcı, Y. Polymeric pyridinium salts as photoinitiators for cationic polymerization. Polymer 1994, 35, 4694–4696. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Schnabel, W. Cationic polymerization initiated by onium ions. Eur. Polym. J. 1997, 33, 1325–1331. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Onen, A.; Bicak, N.; Yagci, Y. Photoinitiated cationic polymerization using a novel phenacyl anilinium salt. Polymer 2002, 43, 2575–2579. [Google Scholar] [CrossRef]
- Kreutzer, J.; Demir, K.D.; Yagci, Y. Synthesis and characterization of a double photochromic initiator for cationic polymerization. Eur. Polym. J. 2011, 47, 792–799. [Google Scholar] [CrossRef]
- Yonet, N.; Bicak, N.; Yagci, Y. Photoinitiated cationic polymerization of cyclohexene oxide by using phenacyl benzoylpyridinium salts. Macromolecules 2006, 39, 2736–2738. [Google Scholar] [CrossRef]
- Yonet, N.; Bicak, N.; Yurtsever, M.; Yagci, Y. Spectroscopic and theoretical investigation of capillary-induced keto–enol tautomerism of phenacyl benzoylpyridinium-type photoinitiators. Polym. Int. 2007, 56, 525–531. [Google Scholar] [CrossRef]
- Li, L.J.; Wan, M.D.; Li, Z.Q.; Luo, Y.C.; Wu, S.F.; Liu, X.X.; Yagci, Y. Coumarinacyl Anilinium Salt: A Versatile Visible and NIR Photoinitiator for Cationic and Step-Growth Polymerizations. ACS Macro Lett. 2023, 12, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Lohse, F.; Zweifel, H. Recent advances in cationic photopolymerization of epoxides. Adv. Polym. Sci. 1986, 78, 69–76. [Google Scholar]
- Tao, W.; Yuli, H.; Shujian, S. Studies on the photosensitivity of cationic polymerization photoinitiator [Cp-Fe-arene] BF4. Chem. J. Chin. Univ. 2003, 24, 735–738. [Google Scholar]
- Nesmeyanov, A.; Vol’Kenau, N.; Bolesova, I. The interaction of ferrocene and its derivatives with aromatic compounds. Tetrahedron Lett. 1963, 4, 1725–1729. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, M.; Li, G.; Chen, Y.; Wang, X.; Wang, T. Naphthoxy bounded ferrocenium salts as cationic photoinitiators for epoxy photopolymerization. Int. J. Photoenergy 2009, 2009, 981068. [Google Scholar] [CrossRef]
- Tao, W.; Pingyu, W.; Lijun, M. Synthesis and characterization of alkoxy and phenoxy-substituted ferrocenium salt cationic photoinitiators. Chin. J. Chem. Eng. 2006, 14, 806–809. [Google Scholar]
- Wang, T.; Chen, J.W.; Li, Z.Q.; Wan, P.Y. Several ferrocenium salts as efficient photoinitiators and thermal initiators for cationic epoxy polymerization. J. Photochem. Photobiol. A 2007, 187, 389–394. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The carbazole-bound ferrocenium salt as a specific cationic photoinitiator upon near-UV and visible LEDs (365–405 nm). Polym. Bull. 2016, 73, 493–507. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P.; Dietlin, C.; Campolo, D.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P.; Lalevée, J. Cationic photoinitiators for near UV and visible LEDs: A particular insight into one-component systems. Macromol. Chem. Phys. 2016, 217, 1214–1227. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Burr, D. Triplet state reactivity of α-sulfonyloxy ketones used as polymerization photoinitiators. Macromolecules 1990, 23, 3615–3619. [Google Scholar] [CrossRef]
- Ikbal, M.; Banerjee, R.; Atta, S.; Dhara, D.; Anoop, A.; Singh, N.P. Synthesis, photophysical and photochemical properties of photoacid generators based on N-hydroxyanthracene-1, 9-dicarboxyimide and their application toward modification of silicon surfaces. J. Org. Chem. 2012, 77, 10557–10567. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.; Okamura, H. i-Line sensitive photoacid generators for UV curing. Prog. Org. Coat. 2009, 64, 175–181. [Google Scholar] [CrossRef]
- Martin, C.J.; Rapenne, G.; Nakashima, T.; Kawai, T. Recent progress in development of photoacid generators. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 41–51. [Google Scholar] [CrossRef]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef]
- Sun, X.; Jin, M.; Wu, X.; Pan, H.; Wan, D.; Pu, H. Bis-substituted thiophene-containing oxime sulfonates photoacid generators for cationic polymerization under UV–visible LED irradiation. J. Polym. Sci. Pol. Chem. 2018, 56, 776–782. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Aguirre-Soto, A.; Lim, C.-H.; Hwang, A.T.; Musgrave, C.B.; Stansbury, J.W. Visible-light organic photocatalysis for latent radical-initiated polymerization via 2e–/1H+ transfers: Initiation with parallels to photosynthesis. J. Am. Chem. Soc. 2014, 136, 7418–7427. [Google Scholar] [CrossRef]
- Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevee, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. Blue-to-Red Light Sensitive Push-Pull Structured Photoinitiators: Indanedione Derivatives for Radical and Cationic Photopolymerization Reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.d.r.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Blue Light Sensitive Dyes for Various Photopolymerization Reactions: Naphthalimide and Naphthalic Anhydride Derivatives. Macromolecules 2014, 47, 601–608. [Google Scholar] [CrossRef]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible light photoinitiating systems by charge transfer complexes: Photochemistry without dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Hizal, G.; Emiroglu, S.E.; Yagci, Y. Photoinitiated radical polymerization using charge transfer complex of N-ethoxy-p-cyano-pyridinium salt and 1,2,4-trimethoxybenzene. Polym. Int. 1998, 47, 391–392. [Google Scholar] [CrossRef]
- Hizal, G.; Yagci, Y.; Schnabel, W. Charge-transfer complexes of pyridinium ions and methyl- and methoxy-substituted benzenes as photoinitiators for the cationic polymerization of cyclohexene oxide and related compounds. Polymer 1994, 35, 2428–2431. [Google Scholar] [CrossRef]
- Garra, P.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.; Lalevée, J. On-demand visible light activated amine/benzoyl peroxide redox initiating systems: A unique tool to overcome the shadow areas in photopolymerization processes. Macromolecules 2016, 49, 9371–9381. [Google Scholar] [CrossRef]
- Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Becht, J.-M.; Fouassier, J.-P.; Lalevee, J. Charge transfer complexes as pan-scaled photoinitiating systems: From 50 μm 3D printed polymers at 405 nm to extremely deep photopolymerization (31 cm). Macromolecules 2018, 51, 57–70. [Google Scholar] [CrossRef]
- Song, Q.Y.; Zhao, K.R.; Xue, T.L.; Zhao, S.; Pei, D.; Nie, J.; Chang, Y.C. Nondiffusion-Controlled Photoelectron Transfer Induced by Host-Guest Complexes to Initiate Cationic Photopolymerization. Macromolecules 2021, 54, 8314–8320. [Google Scholar] [CrossRef]
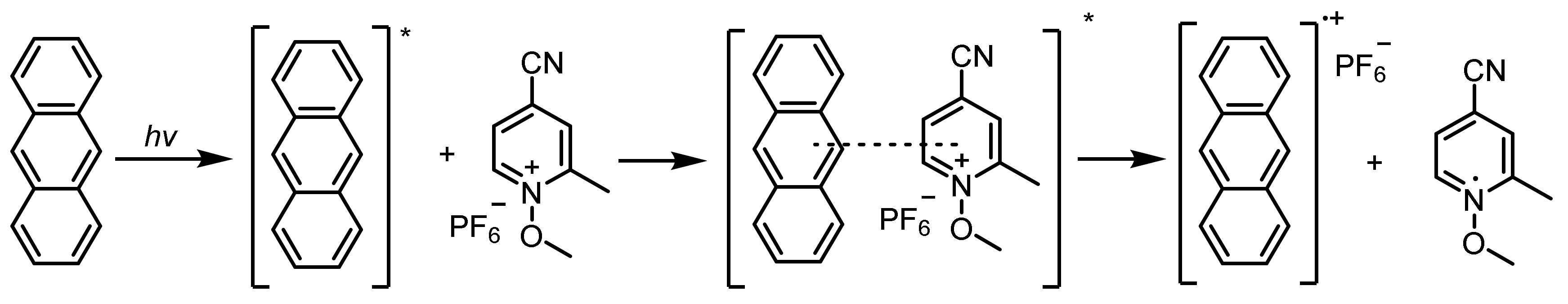
- Crivello, J.V.; Jang, M. Anthracene electron-transfer photosensitizers for onium salt induced cationic photopolymerizations. J. Polym. Sci. Polym. Chem. 2003, 159, 173–188. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on anthracene-based photoinitiators of polymerization. Eur. Polym. J. 2022, 169, 111139. [Google Scholar] [CrossRef]
- Toba, Y. Anthracene-sensitized polymerization of vinyl ethers by onium tetrakis (pentafluorophenyl) borate initiators. J. Polym. Sci. Polym. Chem. 2000, 38, 982–987. [Google Scholar] [CrossRef]
- Dossow, D.; Zhu, Q.Q.; Hizal, G.; Yag, Y.; Schnabel, W. Photosensitized cationic polymerization of cyclohexene oxide: A mechanistic study concerning the use of pyridinium-type salts. Polymer 1996, 37, 2821–2826. [Google Scholar] [CrossRef]
- Hermes, P.; Hermsen, A.; Jäger, M.; Gutmann, J.S.; Strehmel, V.; Strehmel, B. Challenges and limits of upconversion nanoparticles for cationic photopolymerization with UV initiators excited at 980 nm. Polym. Chem. 2022, 13, 4879–4886. [Google Scholar]
- Li, J.; Li, S.; Li, Y.; Li, R.; Nie, J.; Zhu, X. In situ monitoring of photopolymerization by photoinitiator with luminescence characteristics. J. Photochem. Photobiol. A 2020, 389, 112225. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Neumann, M.G. Mechanistic study of tetrahydrofuran polymerization photoinitiated by a sulfonium salt/thioxanthone system. Macromol. Chem. Phys. 2001, 202, 2776–2782. [Google Scholar] [CrossRef]
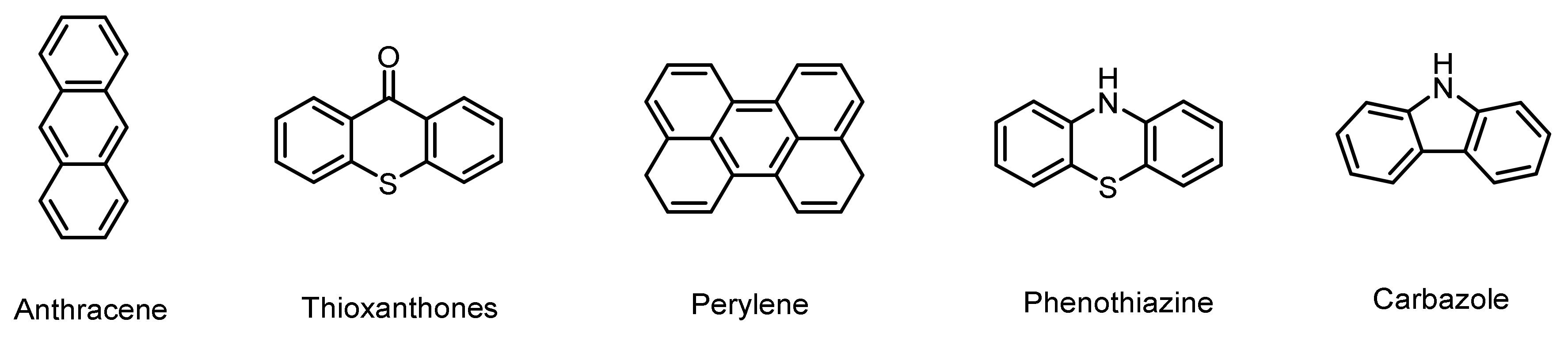
- Dumur, F. Recent advances on perylene-based photoinitiators of polymerization. Eur. Polym. J. 2021, 159, 110734. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. Green-light-induced cationic ring opening polymerization reactions: Perylene-3, 4: 9, 10-bis (dicarboximide) as efficient photosensitizers. Macromol. Chem. Phys. 2013, 214, 1052–1060. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Red-Light-Induced Cationic Photopolymerization: Perylene Derivatives as Efficient Photoinitiators. Macromol. Rapid Comm. 2013, 34, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposite synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Gomurashvili, Z.; Crivello, J.V. Phenothiazine photosensitizers for onium salt photoinitiated cationic polymerization. J. Polym. Sci. Polym. Chem. 2001, 39, 1187–1197. [Google Scholar] [CrossRef]
- Morishima, Y.; Nomura, S.; Kamachi, M. Initiation of cationic polymerization of n-butyl vinyl ether by stable cation radicals of substituted phenothiazines. J. Polym. Sci. Polym. Chem. 1994, 32, 3141–3146. [Google Scholar] [CrossRef]
- Rahal, M.; Abdallah, M.; Bui, T.-T.; Goubard, F.; Graff, B.; Dumur, F.; Toufaily, J.; Hamieh, T.; Lalevée, J. Design of new phenothiazine derivatives as visible light photoinitiators. Polym. Chem. 2020, 11, 3349–3359. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, synthesis and properties of carbazole-indenedione based photobleachable photoinitiators for photopolymerization. J. Photochem. Photobiol. A 2023, 435, 114297. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Arar, A.; Ibrahim-Ouali, M.; Duval, S.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; et al. Carbazole-based compounds as photoinitiators for free radical and cationic polymerization upon near visible light illumination. Photochem. Photobiol. Sci. 2018, 17, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, F.; Hijazi, A.; Duval, S.; Lalevee, J.; Dumur, F. 5,12-Dihydroindolo 3,2-a carbazole: A promising scaffold for the design of visible light photoinitiators of polymerization. Eur. Polym. J. 2022, 162, 110880. [Google Scholar] [CrossRef]
- Li, Y.; Shaukat, U.; Schlogl, S.; Xue, T.L.; Li, J.F.; Nie, J.; Zhu, X.Q. A pyrrole-carbazole photoinitiator for radical and cationic visible light LED photopolymerization. Eur. Polym. J. 2023, 182, 111700. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on carbazole-based photoinitiators of polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Pappas, S.P.; Pappas, B.C.; Gatechair, L.R.; Jilek, J.H.; Schnabel, W. Photoinitiation of cationic polymerization. IV. Direct and sensitized photolysis of aryl iodonium and sulfonium salts. Polym. Photochem. 1984, 5, 1–22. [Google Scholar] [CrossRef]
- Toba, Y.; Saito, M.; Usui, Y. Cationic photopolymerization of epoxides by direct and sensitized photolysis of onium tetrakis (pentafluorophenyl) borate initiators. Macromolecules 1999, 32, 3209–3215. [Google Scholar] [CrossRef]
- Zhao, B.D.; Jia, X.Q.; Liu, J.Q.; Ma, X.Y.; Zhang, H.Q.; Wang, X.N.; Wang, T. Synthesis and Characterization of Novel 1,4-Bis(carbazolyl)benzene Derivatives with Blue-Violet Two-Photon-Excited Fluorescence. Ind. Eng. Chem. Res. 2016, 55, 1801–1807. [Google Scholar] [CrossRef]
- Tang, L.Q.; Nie, J.; Zhu, X.Q. A high performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing. Polym. Chem. 2020, 11, 2855–2863. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, Z.; Zhu, H.; Graff, B.; Knopf, S.; Xiao, P.; Dumur, F.; Lalevée, J. Design of ketone derivatives as highly efficient photoinitiators for free radical and cationic photopolymerizations and application in 3D printing of composites. J. Polym. Sci. 2020, 58, 3432–3445. [Google Scholar] [CrossRef]
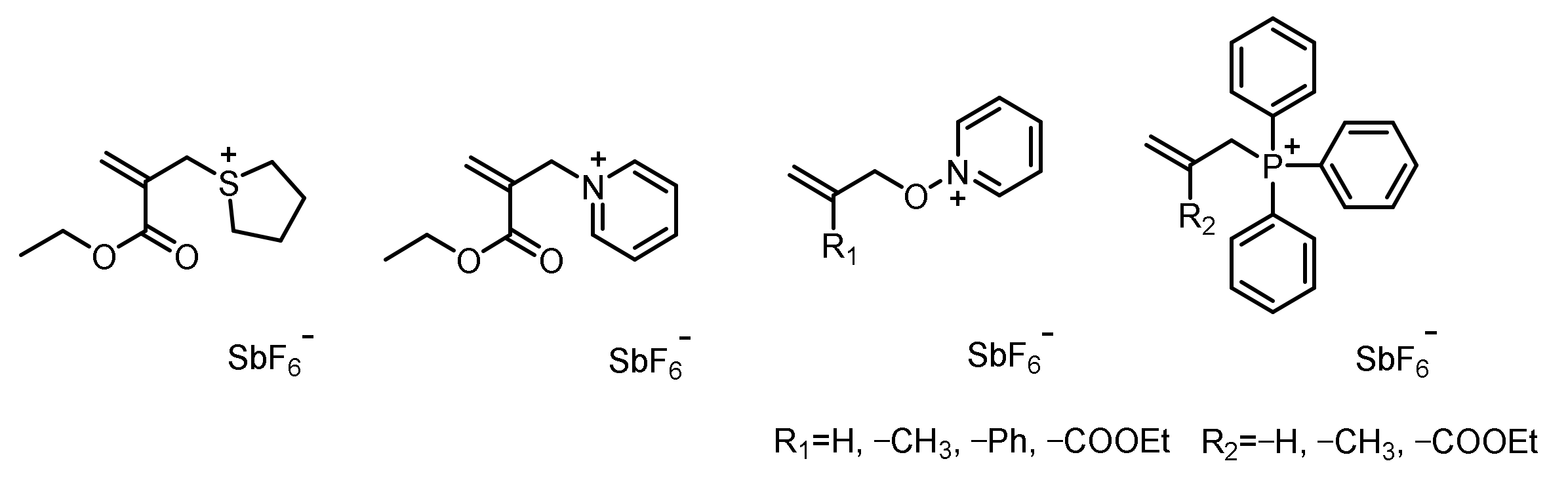
- Atmaca, L.; Onen, A.; Yagci, Y. Allyloxy isoquinolinium salts as initiators for cationic polymerization. Eur. Polym. J. 2001, 37, 677–682. [Google Scholar] [CrossRef]
- Bacak, V.; Reetz, I.; Yagci, Y.; Schnabel, W. Addition–fragmentation type initiation of cationic polymerization using allyloxy-pyridinium salts. Polym. Int. 1998, 47, 345–350. [Google Scholar] [CrossRef]
- Denizligil, S.; Yagci, Y.; McArdle, C. Photochemically and thermally induced radical promoted cationic polymerization using an allylic sulfonium salt. Polymer 1995, 36, 3093–3098. [Google Scholar] [CrossRef]
- Önen, A.; Yaǧcı, Y. Initiation of cationic polymerization by using allyl anilinium salts in the presence of free radical initiators. Macromolecules 2001, 34, 7608–7612. [Google Scholar] [CrossRef]
- Tekin, A.; Yurtsever, M.; Yagci, Y. Structural Effects in the Addition–Fragmentation Reaction of Allylic Onium Salts. Macromol. Theory Simul. 2002, 11, 766–769. [Google Scholar] [CrossRef]
- Yağci, Y.; Önen, A. An allylic pyridinium salt: Radical promoted latent thermal catalyst for cationic polymerization. J. Polym. Sci. Polym. Chem. 1996, 34, 3621–3624. [Google Scholar] [CrossRef]
- Ledwith, A. Possibilities for promoting cationic polymerization by common sources of free radicals. Polymer 1978, 19, 1217–1219. [Google Scholar] [CrossRef]
- Crivello, J.V. Radical-promoted visible light photoinitiated cationic polymerization of epoxides. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 474–483. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Moszner, N.; Yagci, Y. Visible light initiated free radical promoted cationic polymerization using acylgermane based photoinitiator in the presence of onium salts. Macromolecules 2008, 41, 6714–6718. [Google Scholar] [CrossRef]
- Dursun, C.; Degirmenci, M.; Yagci, Y.; Jockusch, S.; Turro, N.J. Free radical promoted cationic polymerization by using bisacylphosphine oxide photoinitiators: Substituent effect on the reactivity of phosphinoyl radicals. Polymer 2003, 44, 7389–7396. [Google Scholar] [CrossRef]
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Polym. Chem. 2019, 57, 1420–1429. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Guan, X.; Liu, R.; Yagci, Y. Near-infrared-induced cationic polymerization initiated by using upconverting nanoparticles and titanocene. Macromol. Rapid Commun. 2019, 40, 1900047. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Blanchard, N.; Fries, C.; Graff, B.; Allonas, X.; Louërat, F.; Fouassier, J.P. Near UV–visible light induced cationic photopolymerization reactions: A three component photoinitiating system based on acridinedione/silane/iodonium salt. Eur. Polym. J. 2010, 46, 2138–2144. [Google Scholar] [CrossRef]
- Telitel, S.; Blanchard, N.; Schweizer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. BODIPY derivatives and boranil as new photoinitiating systems of cationic polymerization exhibiting a tunable absorption in the 400–600 nm spectral range. Polymer 2013, 54, 2071–2076. [Google Scholar] [CrossRef]
- Yaḡci, Y.; Kminek, I.; Schnabel, W. Photochemical cationic polymerization of cyclohexene oxide in solution containing pyridinium salt and polysilane. Eur. Polym. J. 1992, 28, 387–390. [Google Scholar] [CrossRef]
- Schnabel, W. Cationic photopolymerization with the aid of pyridinium-type salts. Macromol. Rapid Comm. 2000, 21, 628–642. [Google Scholar] [CrossRef]
- Sari, E.; Mitterbauer, M.; Liska, R.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using acylsilanes. Prog. Org. Coat. 2019, 132, 139–143. [Google Scholar] [CrossRef]
- Kaya, K.; Seba, M.; Fujita, T.; Yamago, S.; Yagci, Y. Visible light-induced free radical promoted cationic polymerization using organotellurium compounds. Polym. Chem. 2018, 9, 5639–5643. [Google Scholar] [CrossRef]
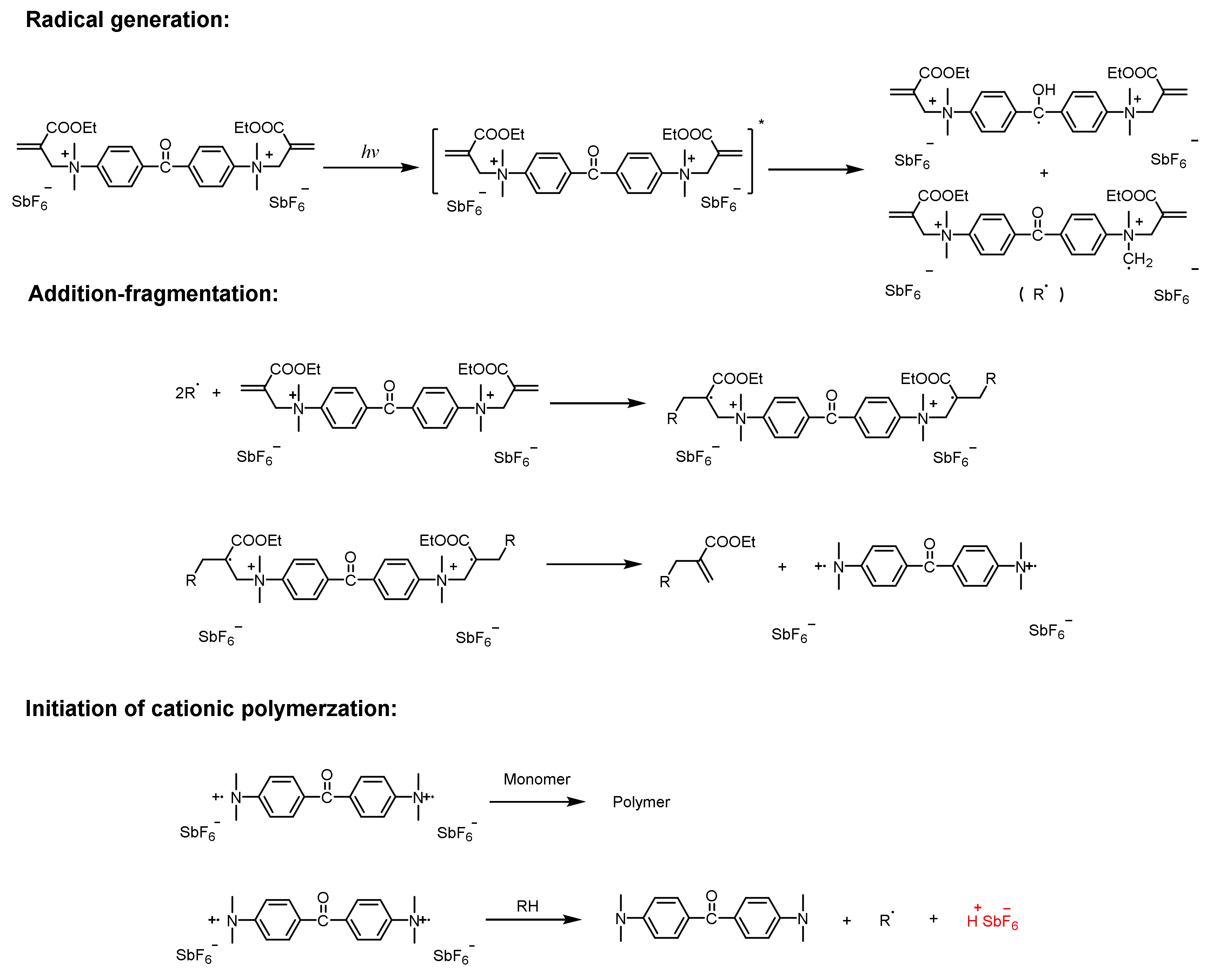
- Zhao, B.; Li, J.; Li, G.; Yang, X.; Lu, S.; Pan, X.; Zhu, J. Fast Living 3D Printing via Free Radical Promoted Cationic RAFT Polymerization. Small 2023, 2207637. [Google Scholar] [CrossRef]
- Zhao, B.W.; Li, J.J.; Pan, X.Q.; Zhang, Z.B.; Jin, G.Q.; Zhu, J. Photoinduced Free Radical Promoted Cationic RAFT Polymerization toward “Living” 3D Printing. ACS Macro Lett. 2021, 10, 1315–1320. [Google Scholar] [CrossRef]








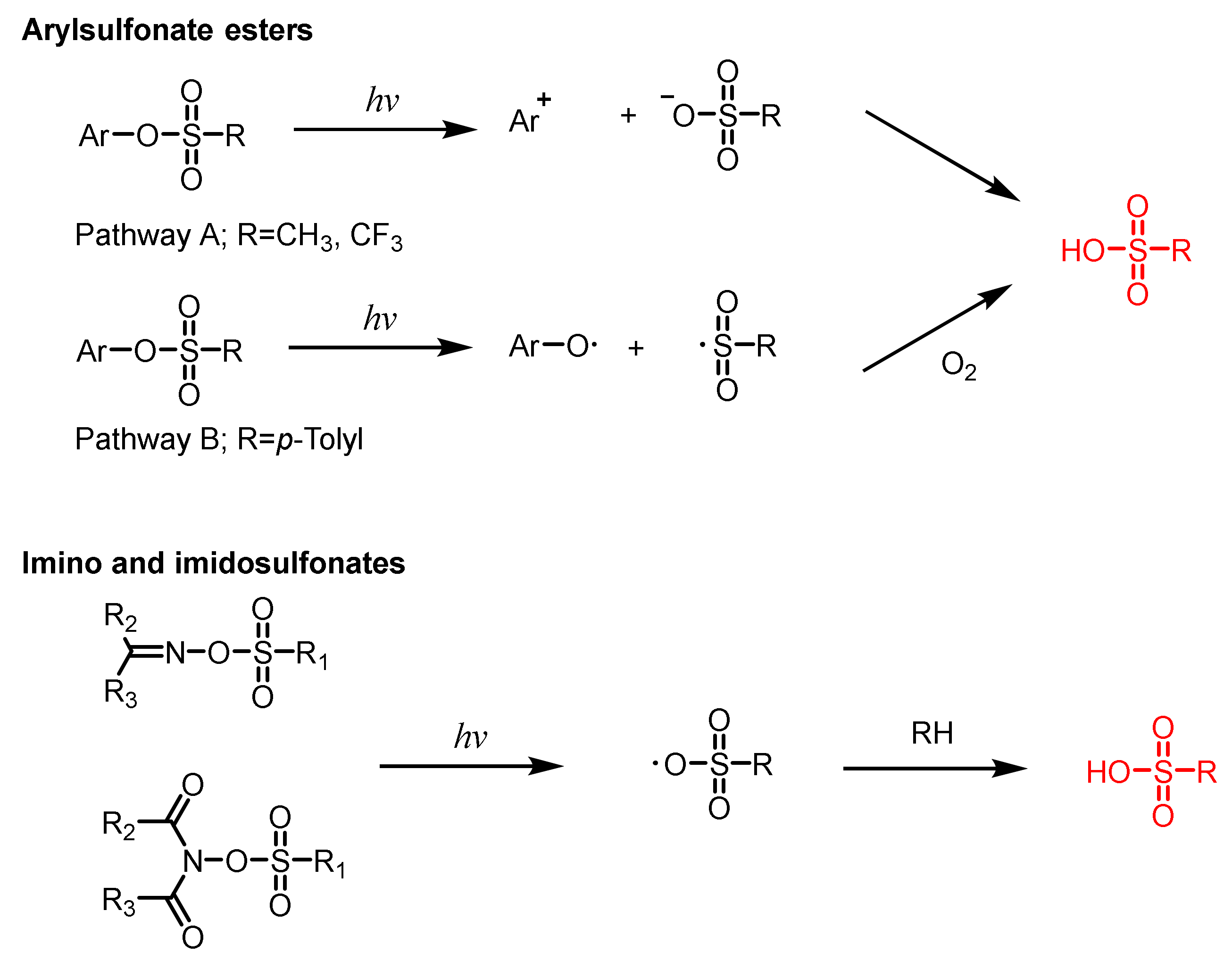
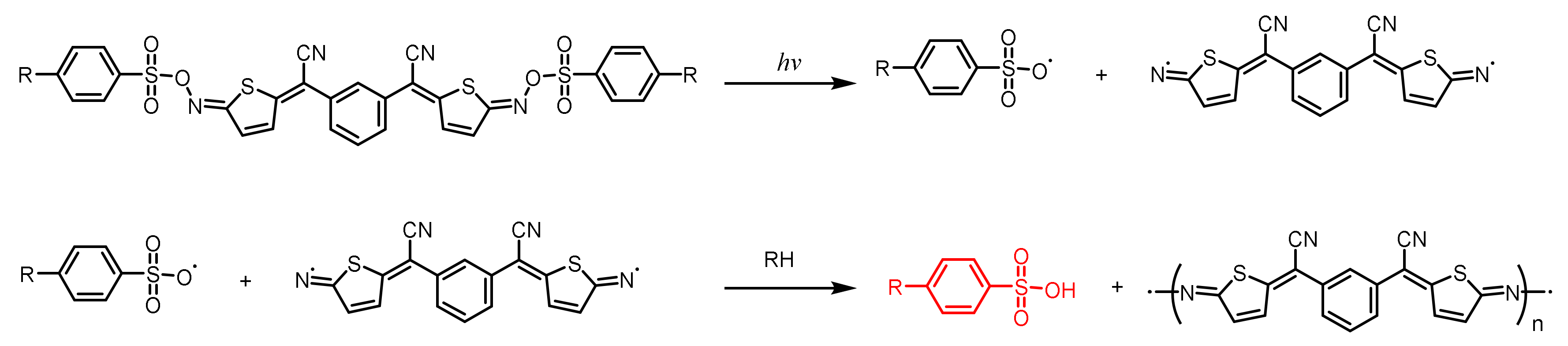
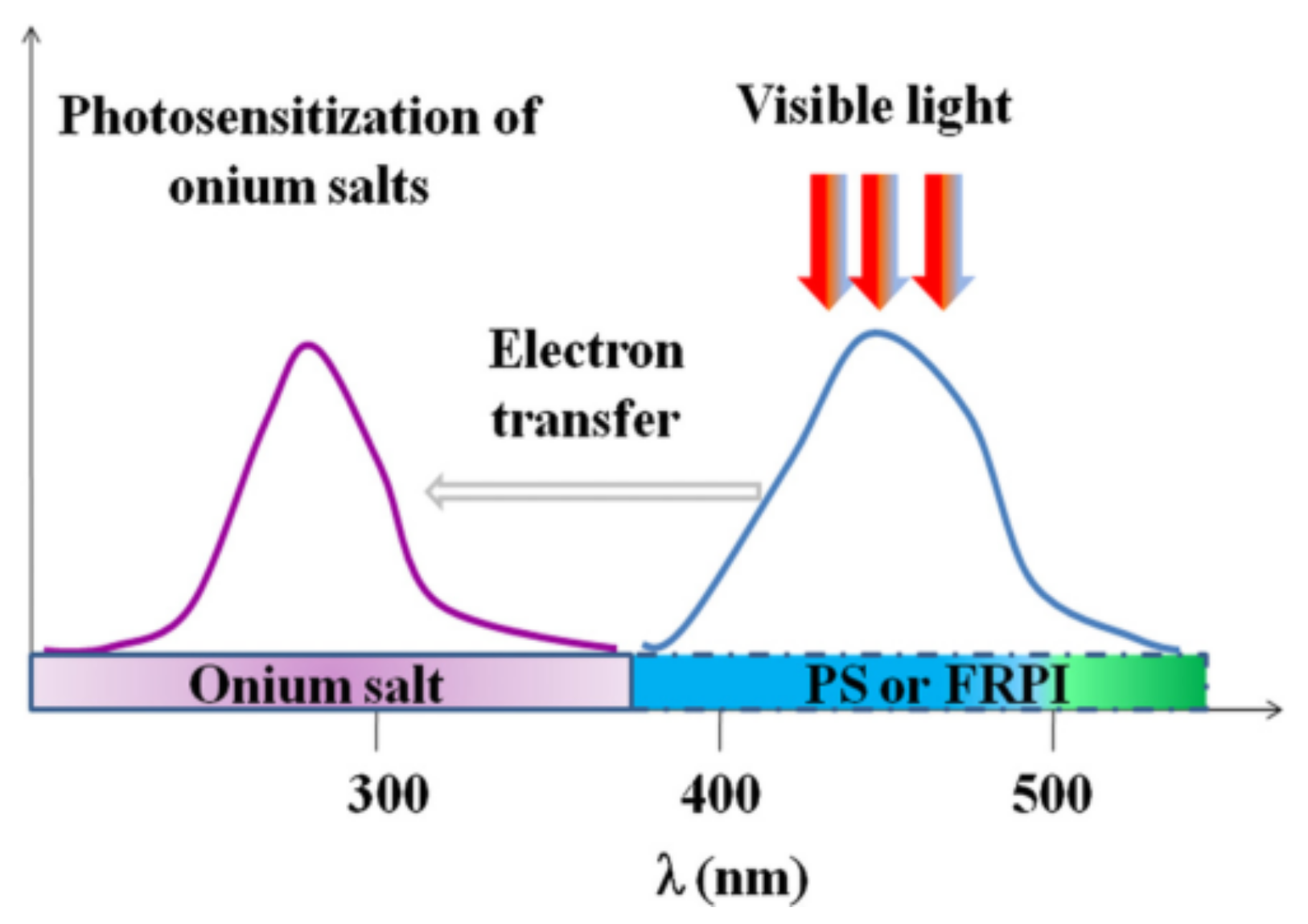
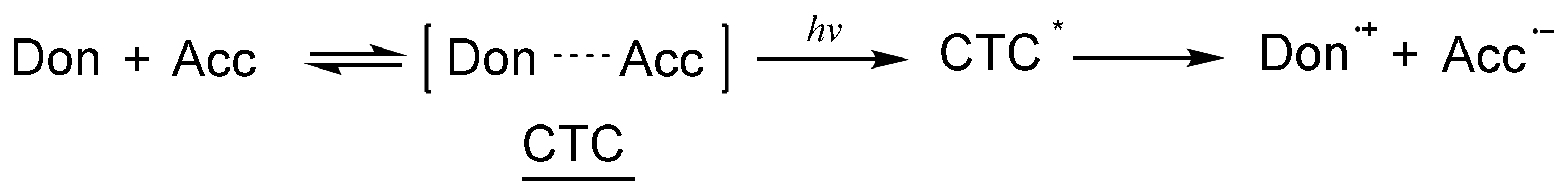



| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
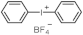 | 227 | 17,800 | [27] |
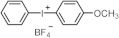 | 246 | 15,400 | [27] |
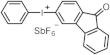 | 267 298 380 394 | 4200 8500 430 400 | [28] |
 | 340 360 | / / | [29] |
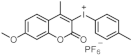 | 349 | 22,704 | [15] |
 | 422 | 8700 | [30] |
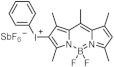 | 475 | 30,869 | [31] |
 | 336 | 5200 | [32] |
 | 344 | 6730 | [32] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
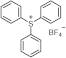 | 227 | 21,000 | [40] |
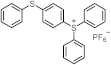 | 230 300 | 24,330 19,500 | [40] |
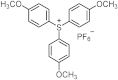 | 255 280 | 21,700 10,100 | [27] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
 | 347 | 36,100 | [49] |
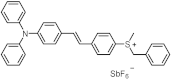 | 397 | 34,400 | [48] |
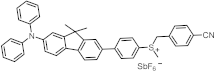 | 378 | 39,400 | [47] |
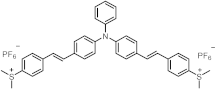 | 413 | 37,000 | [46] |
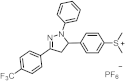 | 372 | 16,700 | [44] |
| Structure | λmax (nm) | ε(L·mol−1·cm−1) | Ref. |
|---|---|---|---|
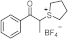 | 255 315 | 11,475 2063 | [55] |
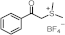 | 250 280 | 10,890 4507 | [51] |
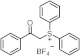 | ~250 | / | [52] |
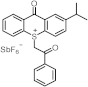 | ~260 ~380 | / / | [57] |
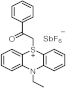 | 514 | 5151 | [53] |
 | 372 | 18,568 | [54] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
 | 280 350 | 38,000 40,000 | [60] |
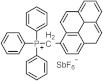 | 280 350 | 30,800 31,200 | [60] |
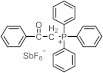 | 257 | 32,000 | [59] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
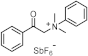 | 249 279 | / | [67] |
 | 249 279 438 | / | [69] |
 | 252 507 | / | [69] |
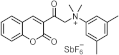 | ~320 ~360 | / | [71] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) | Ref. |
|---|---|---|---|
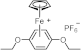 | 243 397 462 | 14,900 136 78 | [76] |
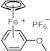 | 242 389 465 | 14,700 136 72 | [76] |
 | 243 397 462 | 19,400 140 75 | [76] |
 | 213 242 376 546 | 21,600 20,100 2380 967 | [77] |
 | 219 259 352 429 | 23,700 28,700 2320 187 | [78] |
| Structure | λmax (nm) | ε (L·mol−1·cm−1) |
|---|---|---|
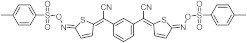 | 425 | 18,900 |
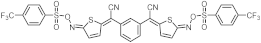 | 426 | 15,900 |
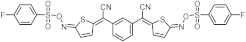 | 423 | 13,400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, L.; Chen, Y.; Pi, J.; Liu, R.; Zhu, Y. Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems. Polymers 2023, 15, 2524. https://doi.org/10.3390/polym15112524
Zhang L, Li L, Chen Y, Pi J, Liu R, Zhu Y. Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems. Polymers. 2023; 15(11):2524. https://doi.org/10.3390/polym15112524
Chicago/Turabian StyleZhang, Liping, Lun Li, Ying Chen, Junyi Pi, Ren Liu, and Yi Zhu. 2023. "Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems" Polymers 15, no. 11: 2524. https://doi.org/10.3390/polym15112524
APA StyleZhang, L., Li, L., Chen, Y., Pi, J., Liu, R., & Zhu, Y. (2023). Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems. Polymers, 15(11), 2524. https://doi.org/10.3390/polym15112524