Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gas, Water Vapor and Liquid Water Permeation System
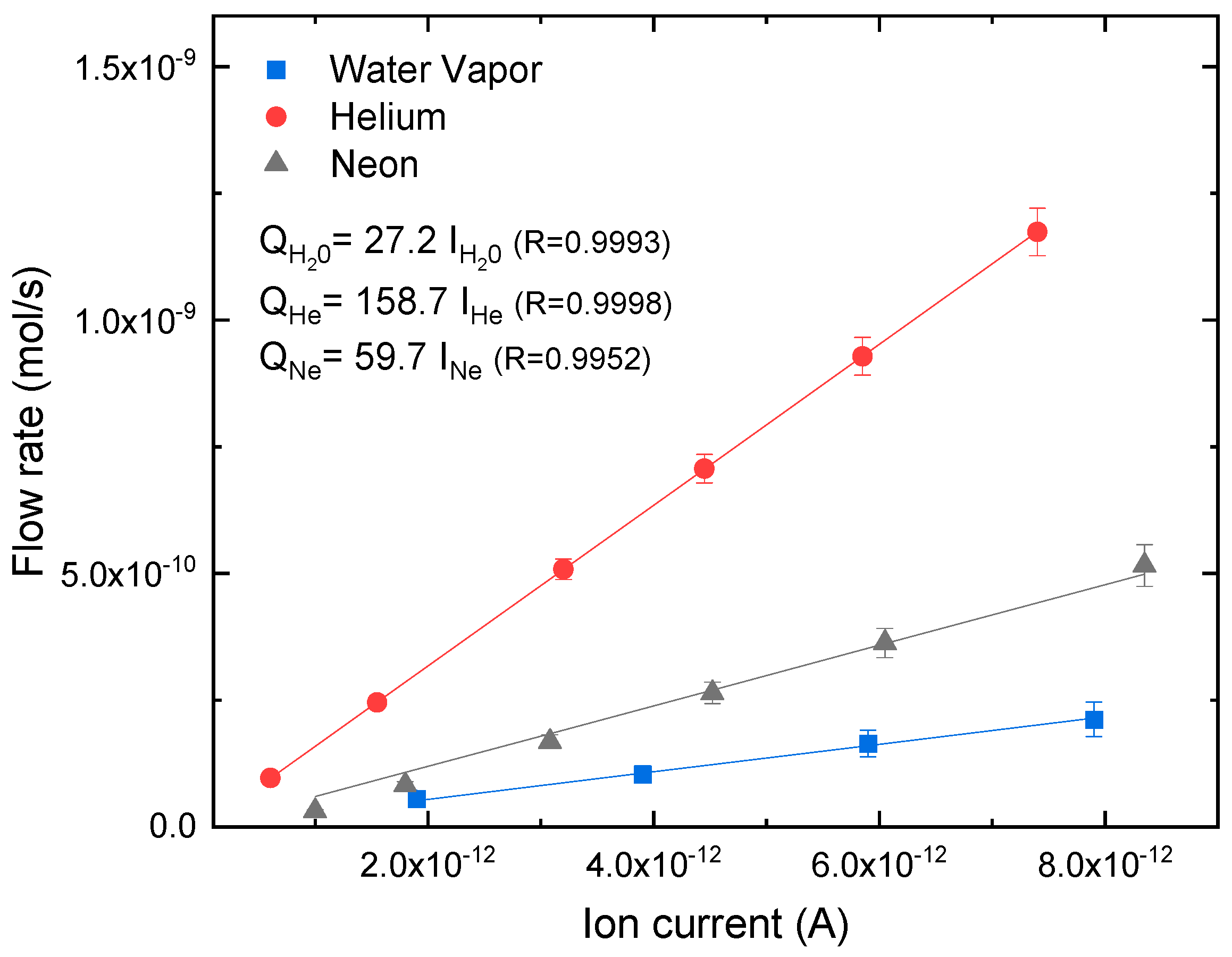
2.2. Calibration of Permeation System
2.3. Water Vapor Permeation ISO 15106-03
2.4. Parylene Film Deposition
2.5. Measurement Procedure
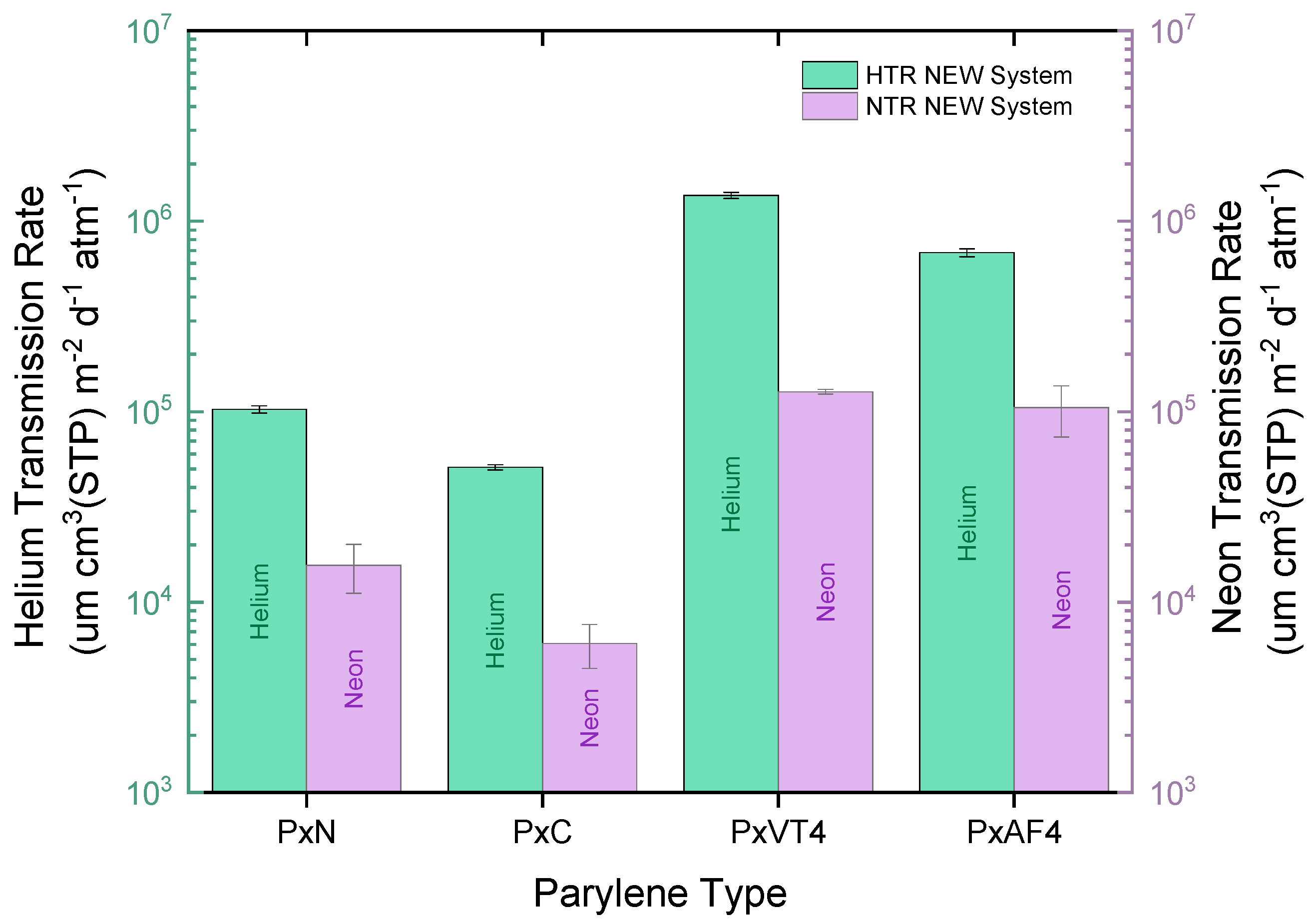
3. Results
3.1. Gas Transmission Rates
3.2. Water Vapor and Water Transmission Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nisato, G.; Bouten, P.C.; Slikkerveer, P.J.; Bennet, W.D.; Graff, G.L.; Rutherford, N.; Wiese, L. Evaluationg High Performance Diffusion Berriers. In 21st Annual Asia Display, 8th International Display Workshop; ITE and SID: Singapore, 2001; p. 1435. [Google Scholar]
- Brewer, P.J.; Goody, B.A.; Kumar, Y.; Milton, M.J.T. Accurate Measurements of Water Vapor Transmission through High-Performance Barrier Layers. Rev. Sci. Instrum. 2012, 83, 075118. [Google Scholar] [CrossRef] [PubMed]
- Beese, H.; Grählert, W.; Kaskel, S.; Grübler, J.; Koch, J.; Pietsch, K. HiBarSens: Measurement of Barrier Properties by Tunable Diode Laser Absorption Spectroscopy (TDLAS). In Proceedings of the 2012 Web Coating & Handling Conference of the Association of Industrial Metallizers, Coaters and Laminators AIMCAL, Myrtle Beach, SC, USA, 21–24 October 2012; p. 848, ISBN 978-1-622-76950-6. [Google Scholar]
- Firon, M.; Cros, S.; Trouslard, P. Method and Device for Measurement of Permeation. U.S. Patent 7,624,621, 1 December 2009. [Google Scholar]
- Morlier, A.; Cros, S.; Garandet, J.P.; Alberola, N. Structural Properties of Ultraviolet Cured Polysilazane Gas Barrier Layers on Polymer Substrates. Thin Solid Film. 2014, 550, 85–89. [Google Scholar] [CrossRef]
- Giacinti Baschetti, M.; Minelli, M. Test Methods for the Characterization of Gas and Vapor Permeability in Polymers for Food Packaging Application: A Review. Polym. Test. 2020, 89, 106606. [Google Scholar] [CrossRef]
- ISO 15106; Plastics—Film and Sheeting—Determination of Water Vapour Transmission Rate. International Organization for Standardization: Geneva, Switzerland, 2003; Voulume 01, p. 13.
- Ranade, A.; D’Souza, N.A.; Wallace, R.M.; Gnade, B.E. High Sensitivity Gas Permeability Measurement System for Thin Plastic Films. Rev. Sci. Instrum. 2005, 76, 013902. [Google Scholar] [CrossRef]
- Yoshida, H.; Ebina, T.; Arai, K.; Kobata, T.; Ishii, R.; Aizawa, T.; Suzuki, A. Development of Water Vapor Transmission Rate Measuring Device Using a Quadrupole Mass Spectrometer and Standard Gas Barrier Films down to the 10−6 g M−2 Day−1 Level. Rev. Sci. Instrum. 2017, 88, 043301. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Yanase, T.; Nagahama, T.; Yoshida, H.; Shimada, T. Accurate and Stable Equal-Pressure Measurements of Water Vapor Transmission Rate Reaching the 10-6 g m-2 Day-1 Range. Sci. Rep. 2016, 6, 35408. [Google Scholar] [CrossRef] [Green Version]
- Gorham, W.F. A New, General Synthetic Method for the Preparation of Linear Poly-p-Xylylenes. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- van den Brand, J.; de Kok, M.; Koetse, M.; Cauwe, M.; Verplancke, R.; Bossuyt, F.; Jablonski, M.; Vanfleteren, J. Flexible and Stretchable Electronics for Wearable Health Devices. Solid. State. Electron. 2015, 113, 116–120. [Google Scholar] [CrossRef]
- Ghosh, A.P.; Gerenser, L.J.; Jarman, C.M.; Fornalik, J.E. Thin-Film Encapsulation of Organic Light-Emitting Devices. Appl. Phys. Lett. 2005, 86, 223503. [Google Scholar] [CrossRef]
- Chen, T.-N.; Wuu, D.-S.; Wu, C.-C.; Chiang, C.-C.; Chen, Y.-P.; Horng, R.-H. Improvements of Permeation Barrier Coatings Using Encapsulated Parylene Interlayers for Flexible Electronic Applications. Plasma Process. Polym. 2007, 4, 180–185. [Google Scholar] [CrossRef]
- Keum, C.; Murawski, C.; Archer, E.; Kwon, S.; Mischok, A.; Gather, M.C. A Substrateless, Flexible, and Water-Resistant Organic Light-Emitting Diode. Nat. Commun. 2020, 11, 6250. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, N.; Kasanuki, H.; Matsuda, N.; Shoda, M.; Ohnishi, S.; Hosoda, S. Contact Sensitivity to Polychloroparaxylene-Coated Cardiac Pacemaker. Pacing Clin. Electrophysiol. 1997, 20, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Bröskamp, S.F.; Franz, G.; Jocham, D. Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene. Coatings 2021, 11, 739. [Google Scholar] [CrossRef]
- Brancato, L.; Decrop, D.; Lammertyn, J.; Puers, R. Surface Nanostructuring of Parylene-C Coatings for Blood Contacting Implants. Materials 2018, 11, 1109. [Google Scholar] [CrossRef] [Green Version]
- Hassler, C.; Von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of Parylene C as an Encapsulation Material for Implanted Neural Prostheses. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef]
- Zeniieh, D.; Ledernez, L.; Urban, G. Parylene-C as High Performance Encapsulation Material for Implantable Sensors. Procedia Eng. 2014, 87, 1398–1401. [Google Scholar] [CrossRef] [Green Version]
- Dolbier, W.R.; Beach, W.F. Parylene-AF4: A Polymer with Exceptional Dielectric and Thermal Properties. J. Fluor. Chem. 2003, 122, 97–104. [Google Scholar] [CrossRef]
- Buchwalder, S.; Borzì, A.; Diaz Leon, J.J.; Bourgeois, F.; Nicolier, C.; Nicolay, S.; Neels, A.; Zywitzki, O.; Hogg, A.; Burger, J. Thermal Analysis of Parylene Thin Films for Barrier Layer Applications. Polymers 2022, 14, 3677. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Caldwell, R.; Diwekar, M.; Tathireddy, P.; Sharma, R.; Solzbacher, F. Long-Term Bilayer Encapsulation Performance of Atomic Layer Deposited Al2O3 and Parylene c for Biomedical Implantable Devices. IEEE Trans. Biomed. Eng. 2013, 60, 2943–2951. [Google Scholar] [CrossRef]
- Hogg, A.; Uhl, S.; Feuvrier, F.; Girardet, Y.; Graf, B.; Aellen, T.; Keppner, H.; Tardy, Y.; Burger, J. Protective Multilayer Packaging for Long-Term Implantable Medical Devices. Surf. Coatings Technol. 2014, 255, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Selbmann, F.; Baum, M.; Wiemer, M.; Gessner, T. Deposition of Parylene C and Characterization of Its Hermeticity for the Encapsulation of MEMS and Medical Devices. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016; pp. 427–432. [Google Scholar] [CrossRef]
- Park, H.; Choi, W.; Oh, S.; Kim, Y.-J.; Seok, S.; Kim, J. A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation. Micromachines 2022, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Arai, K.; Hirata, M.; Akimichi, H. New Leak Element Using Sintered Stainless Steel Filter for In-Situ Calibration of Ionization Gauges and Quadrupole Mass Spectrometers. Vacuum 2012, 86, 838–842. [Google Scholar] [CrossRef]
- Yoshida, H.; Arai, K.; Kobata, T. In-Situ Calibration Method for Ionization Gauges and Quadrupole Mass Spectrometers by Combining the Standard Conductance Element and the Conductance Modulation Method (SCEeCM Method). Vacuum 2014, 101, 433–439. [Google Scholar] [CrossRef]
- Yoshida, H.; Arai, K.; Suzuki, A.; Aizawa, T.; Ishii, R.; Ebina, T. Development of a Gas Permeation Measuring Device and the Evaluation of Gas Barrier Property of Clay-Polymer Nanocomposite Films. Clay Sci. 2018, 22, 95–102. [Google Scholar] [CrossRef]
- Kahouli, A.; Sylvestre, A.; Pairis, S.; Laithier, J.-F. Effect of ClH Aromatic Substitution on Structural and Dielectric Properties of Poly(p-Xylylene). Polymer 2012, 53, 3001–3007. [Google Scholar] [CrossRef]
- Kachroudi, A.; Kahouli, A.; Legrand, J.; Jomni, F. Dielectric and Conduction Mechanisms of Parylene N at High Temperature: Phase-Transition Effect. J. Phys. Chem. A 2015, 119, 6428–6435. [Google Scholar] [CrossRef]
- Menon, P.R.; Li, W.; Tooker, A.; Tai, Y.C. Characterization of Water Vapor Permeation through Thin Film Parylene C. In Proceedings of the TRANSDUCERS 2009–2009 International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 1892–1895. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for BioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Kahouli, A.; Sylvestre, A.; Laithier, J.F.; Lutsen, L.; Pairis, S.; André, E.; Garden, J.L. Structural and Dielectric Properties of Parylene-VT4 Thin Films. Mater. Chem. Phys. 2014, 143, 908–914. [Google Scholar] [CrossRef]
- Yang, N. Parylene AF4 Its Preparations, Characteristics and Applications. Invit. Talk IMID2017 Mater. Transparent Flex. Disp. 2017, 4–5. [Google Scholar] [CrossRef]
- Greenhouse, H.; Lowry, R.K.; Romenesko, B. Hermeticity of Electronic Packages; William Andrew: Norwich, NY, USA, 2011. [Google Scholar]
- Nörenberg, H.; Burlakov, V.M.; Kosmella, H.J.; Smith, G.D.W.; Briggs, G.A.D.; Miyamoto, T.; Tsukahara, Y. Pressure-Dependent Permeation of Noble Gases (He, Ne, Ar, Kr, Xe) through Thin Membranes of Oriented Polypropylene (OPP) Studied by Mass Spectrometry. Polymer 2001, 42, 10021–10026. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Han, B.; Wang, P.; Jin, H.; Hou, Z.; Bai, X. Wettability and Surface Energy of Parylene F Deposited on PDMS. Phys. Lett. A 2020, 384, 126628. [Google Scholar] [CrossRef]
- Wang, Z.; Ou, Y.; Lu, T.-M.; Koratkar, N. Wetting and Electrowetting Properties of Carbon Nanotube Templated Parylene Films. J. Phys. Chem. B 2007, 111, 4296–4299. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchwalder, S.; Nicolier, C.; Hersberger, M.; Bourgeois, F.; Hogg, A.; Burger, J. Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings. Polymers 2023, 15, 2557. https://doi.org/10.3390/polym15112557
Buchwalder S, Nicolier C, Hersberger M, Bourgeois F, Hogg A, Burger J. Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings. Polymers. 2023; 15(11):2557. https://doi.org/10.3390/polym15112557
Chicago/Turabian StyleBuchwalder, Sébastien, Cléo Nicolier, Mario Hersberger, Florian Bourgeois, Andreas Hogg, and Jürgen Burger. 2023. "Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings" Polymers 15, no. 11: 2557. https://doi.org/10.3390/polym15112557
APA StyleBuchwalder, S., Nicolier, C., Hersberger, M., Bourgeois, F., Hogg, A., & Burger, J. (2023). Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings. Polymers, 15(11), 2557. https://doi.org/10.3390/polym15112557









