Biodegradable Cellulose/Polycaprolactone/Keratin/Calcium Carbonate Mulch Films Prepared in Imidazolium-Based Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
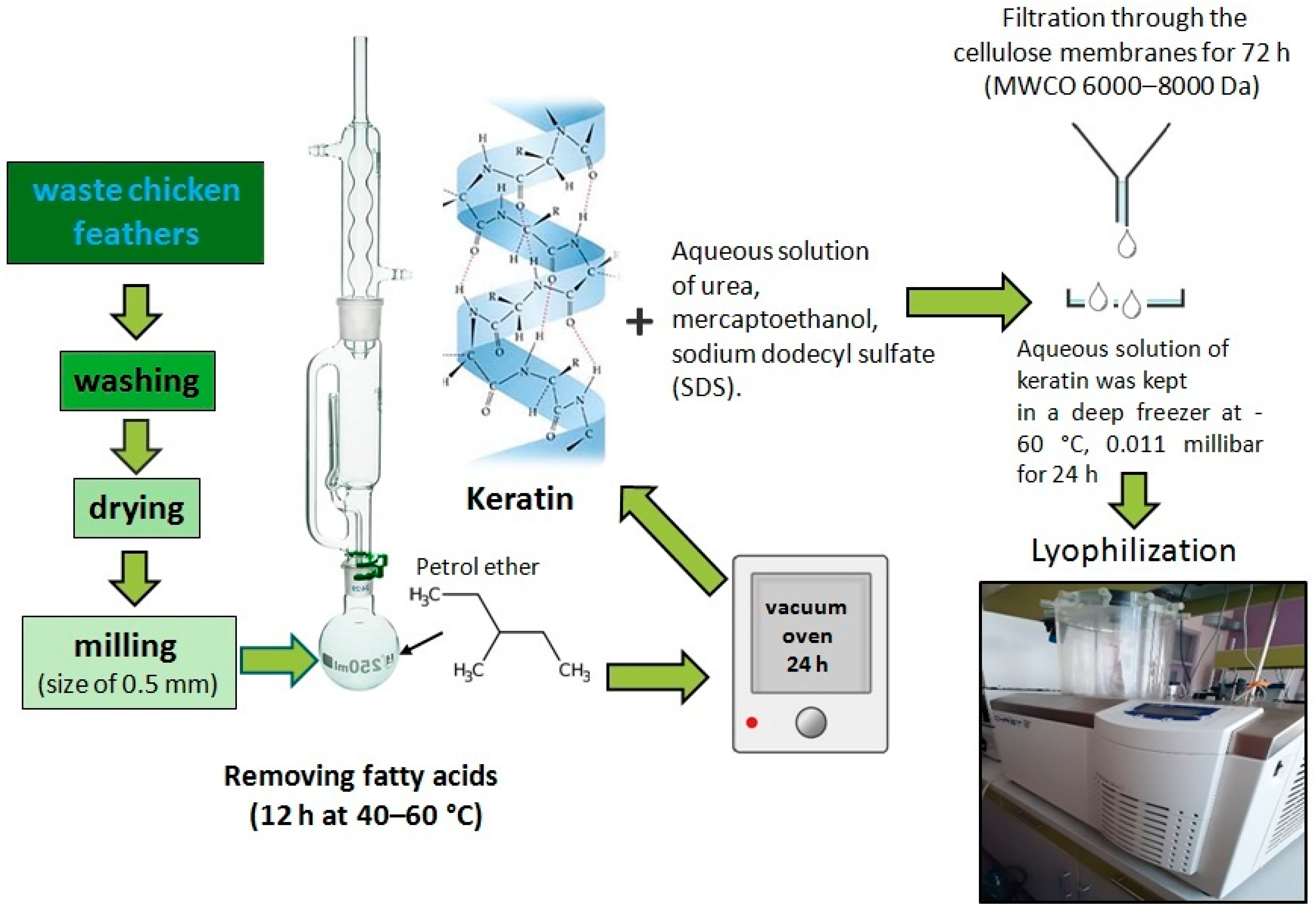
2.2. Extraction of Keratin from Chicken Feather Waste
2.3. Preparation of Regenerated Biocomposite Films
2.4. Methods Used for Characterization
3. Results and Discussion
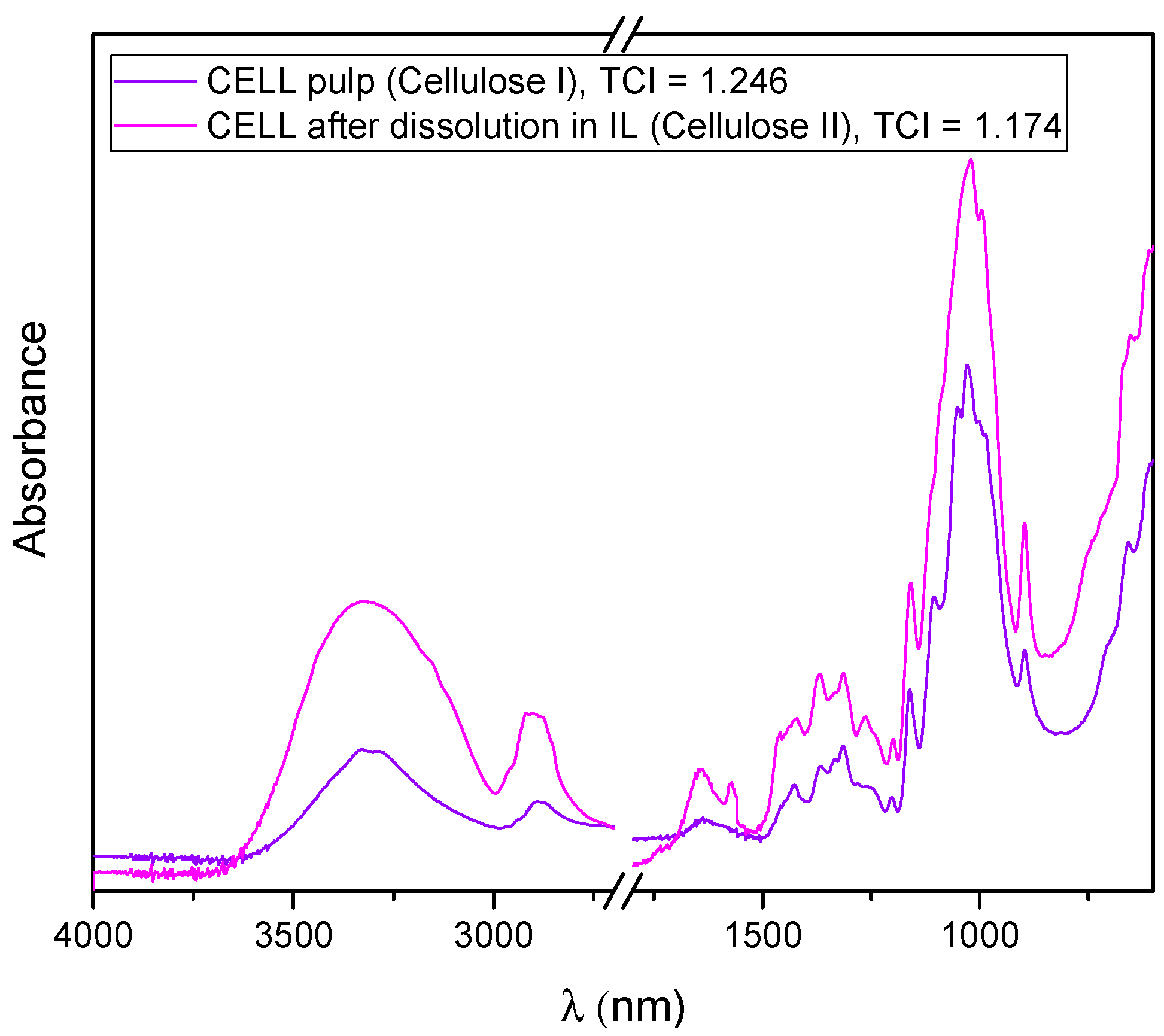
3.1. Cellulose Dissolution in [BMIM][Cl]
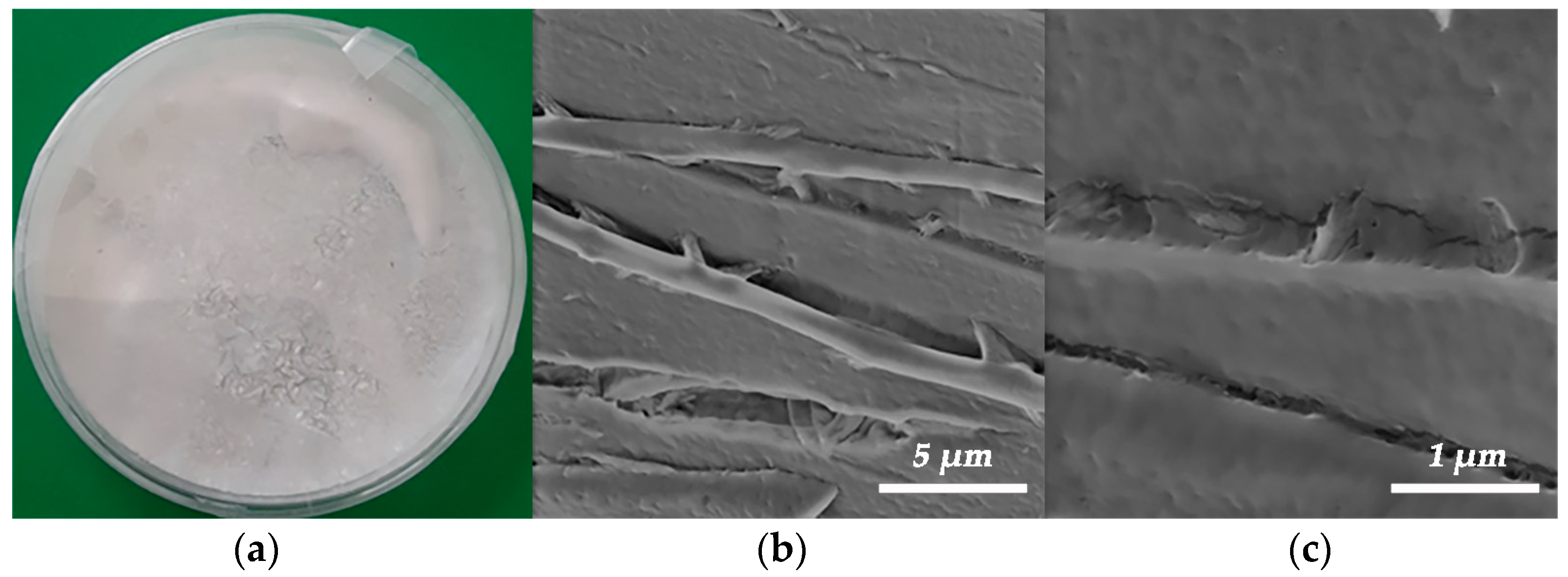
3.2. Keratin Extraction from Chicken Feather Waste
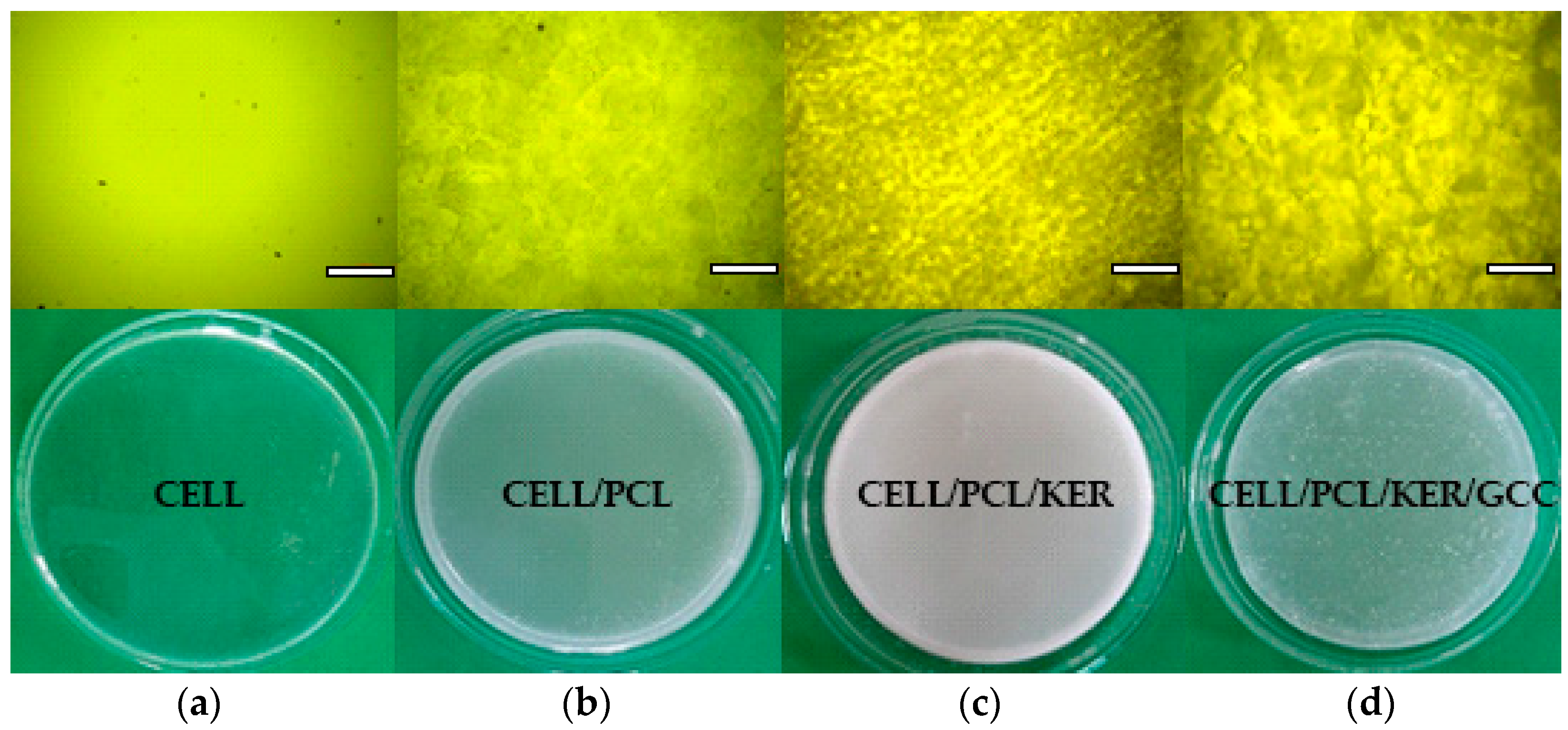
3.3. Appearance of the Prepared Biocomposite Films and Their Optical Properties
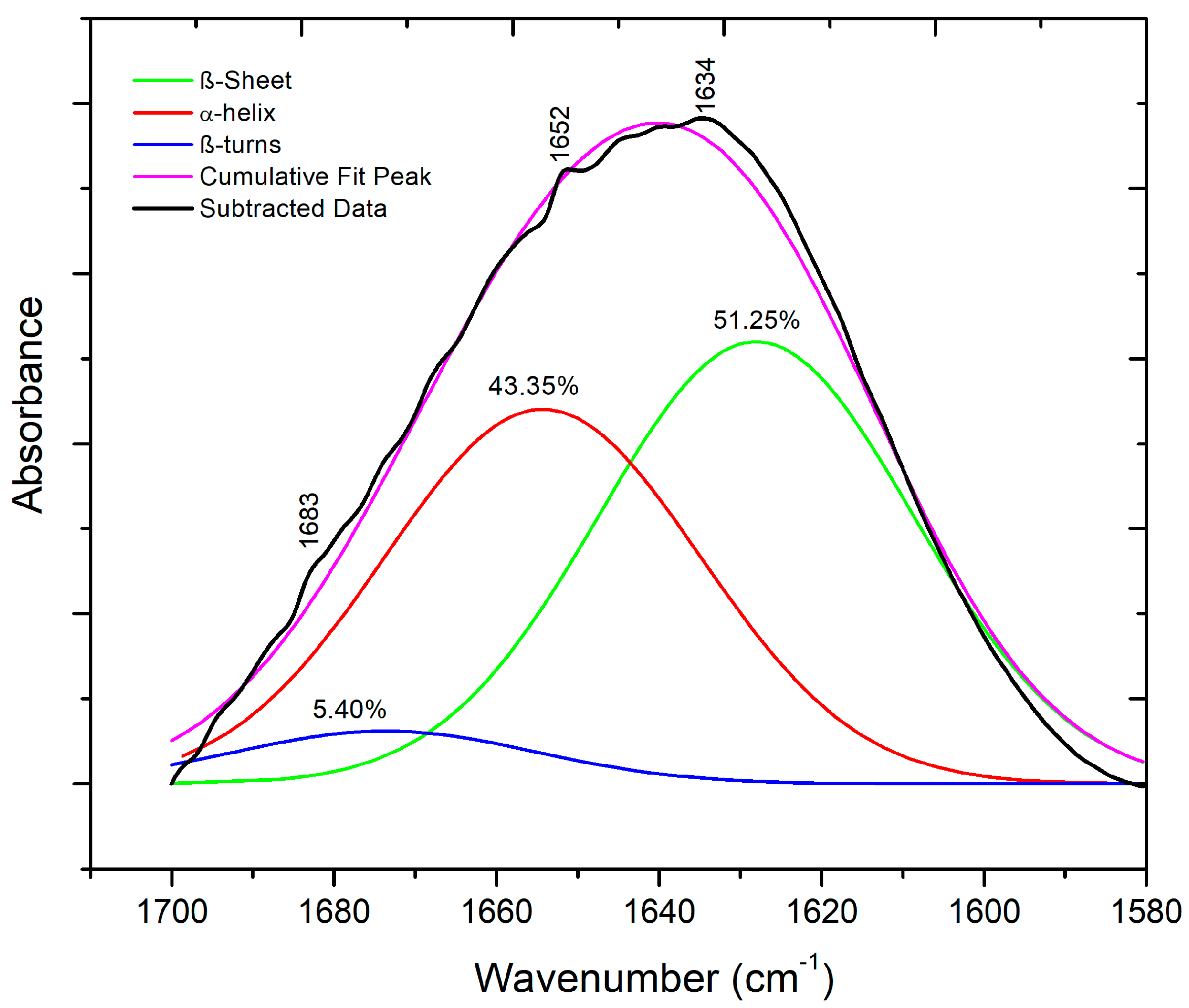
3.4. ATR-FTIR Spectroscopy of Biocomposite Films
3.5. Thermal and Mechanical Properties of the Prepared Biopolymers and Biocomposite Films
3.6. Biodegradability of Developed Cellulose and Biocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adorna, J.A.; Ventura, R.L.G.; Dang, V.D.; Doong, R.-A.; Ventura, J.-R.S. Biodegradable polyhydroxybutyrate/cellulose/calcium carbonate bioplastic composites prepared by heat-assisted solution casting method. J. Appl. Polym. Sci. 2022, 139, 51645. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef] [PubMed]
- Zainul Armir, N.A.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Che Othman, M.H.; Zakaria, S. Regenerated cellulose products for agricultural and their potential: A Review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef]
- Kammiovirta, K.; Jääskeläinen, A.-S.; Kuutti, L.; Holopainen-Mantila, U.; Paananen, A.; Suurnäkki, A.; Orelma, H. Keratin-reinforced cellulose filaments from ionic liquid solutions. RSC Adv. 2016, 6, 88797–88806. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Tominaga, K.; Gotoh, Y. High-strength regenerated cellulose fibers spun from 1-butyl-3-methylimidazolium chloride solutions. J. Appl. Polym. Sci. 2017, 134, 45551. [Google Scholar] [CrossRef]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Fredericks, D.; Patti, A.F.; MacFarlane, D.R. Dissolution of featherkeratin in ionic liquids. Green Chem. 2013, 15, 525–534. [Google Scholar] [CrossRef]
- Keskinen, R.; Suojala-Ahlfors, T.; Sarvi, M.; Hagner, M.; Kaseva, J.; Salo, T.; Uusitalo, R.; Rasa, K. Granulated broiler manure based organic fertilizers as sources of plant available nitrogen. Environ. Technol. Inno. 2020, 18, 100734. [Google Scholar] [CrossRef]
- Li, Q. Perspectives on converting keratin-containing wastes into biofertilizers for sustainable agriculture. Front. Microbiol. 2022, 13, 918262. [Google Scholar] [CrossRef]
- Xiong, R.; Hameed, N.; Guo, Q. Cellulose/polycaprolactone blends regenerated from ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohyd. Polym. 2012, 90, 575–582. [Google Scholar] [CrossRef]
- Al Hosni, A.S.; Pittman, J.K.; Robson, G.D. Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Manag. 2019, 97, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chukwunonso Ossai, I.; Shahul Hamid, F.; Hassan, A. Valorisation of keratinous wastes: A sustainable approach towards a circular economy. Waste Manag. 2022, 151, 81–104. [Google Scholar] [CrossRef]
- Grkovic, M.; Stojanovic, D.B.; Kojovic, A.; Strnad, S.; Kreze, T.; Aleksic, R.; Uskokovic, P.S. Keratin-polyethylene oxide bio-nanocomposites reinforced with ultrasonically functionalized graphene. RSC Adv. 2015, 5, 91280–91287. [Google Scholar] [CrossRef]
- Xu, H.; Pan, W.; Wang, R.; Zhang, D.; Liu, C. Understanding the mechanism of cellulose dissolution in 1-butyl-3-methylimidazolium chloride ionic liquid via quantum chemistry calculations and molecular dynamics simulations. J. Comput. Aided. Mol. Des. 2012, 26, 329–337. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohyd. Polym. 2019, 203, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Isarankura Na Ayutthaya, S.; Tanpichai, S.; Wootthikanokkhan, J. Keratin extracted from chicken feather waste: Extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Srour, B.; Bruechert, S.; Andrade, S.L.A.; Hellwig, P. Secondary Structure Determination by Means of ATR-FTIR Spectroscopy. In Membrane Protein Structure and Function Characterization; Lacapere, J.-J., Ed.; Humana New York: New York, NY, USA, 2018; pp. 195–203. [Google Scholar]
- Sharma, S.; Gupta, A.; Kumar, A.; Kee, C.G.; Kamyab, H.; Saufi, S.M. An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean. Technol. Environ. Policy 2018, 20, 2157–2167. [Google Scholar] [CrossRef]
- Tran, C.D.; Mututuvari, T.M. Cellulose, chitosan and keratin composite materials: Facile and recyclable synthesis, conformation and properties. ACS Sustain. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihajlovic, M.; Rekanovic, E.; Hrustic, J.; Grahovac, M.; Tanovic, B. Methods for management of soil-borne plant pathogens. Pestic. Fitomed. 2017, 32, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Mahadeva, S.K.; Yeol Yang, S.; Kim, J. Effects of solvent systems on its structure, properties and electromechanical behavior of cellulose electro-active paper. Curr. Org. Chem. 2013, 17, 83–88. [Google Scholar] [CrossRef]
- De Silva, R.; Vongsanga, K.; Wang, X.; Byrne, N. Development of a novel regenerated cellulose composite material. Carbohydr. Polym. 2015, 121, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.-A.M.A.; Kamel, S.; El-Sakhawy, M. Thermal behavior and infrared spectroscopy of cellulose carbamates. Polym. Degrad. Stabil. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- Kljun, A.; Benians, T.A.S.; Goubet, F.; Meulewaeter, F.; Knox, K.P.; Blackburn, R.S. Comparative analysis of crystallinity changes in cellulose I polymers using ATR-FTIR, X-ray diffraction, and carbohydrate-binding module probes. Biomacromolecules 2011, 12, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A concise review on the physicochemical properties of biopolymer blends prepared in ionic liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef]
- Rybacki, K.; Love, S.A.; Blessing, B.; Morales, A.; McDermott, E.; Cai, K.; Hu, X.; Salas-de la Cruz, D. Structural and Morphological Properties of Wool Keratin and Cellulose Biocomposites Fabricated Using Ionic Liquids. ACS Mater. Au. 2021, 2, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, A.; Stojanovic, D.B.; Petrovic, M.; Radojević, V.; Uskokovic, P.; Rajilic-Stojanovic, M. Electrospun polycaprolactone nanofibers functionalized with Achillea millefolium extract yield biomaterial with antibacterial, antioxidant and improved mechanical properties. J. Biomed. Mater. Res. Part A 2023, 111, 962–974. [Google Scholar] [CrossRef]
- De Silva, R.; Wang, X.; Byrne, N. Development of a novel cellulose/duck feather composite fibre regenerated in ionic liquid. Carbohydr. Polym. 2016, 153, 115–123. [Google Scholar] [CrossRef]
- Tran, C.D.; Mututuvari, T.M. Cellulose, chitosan, and keratin composite materials. Controlled drug release. Langmuir 2015, 31, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Khaw, Y.Y.; Ching, Y.C.; Gan, S.N.; Ramesh, S.; Ghazali, N.N.N.; Liu, N.S. Poly(lactic acid) composite films reinforced with microcrystalline cellulose and keratin from chicken feather fiber in 1-butyl-3-methylimidazolium chloride. J. Appl. Polym. Sci. 2019, 136, 47642. [Google Scholar] [CrossRef]
- Kostic, M.; Imani, M.; Ivanovska, A.; Radojevic, V.; Dimic-Misic, K.; Barac, N.; Stojanovic, D.; Janackovic, D.; Uskokovic, P.; Barcelo, E.; et al. Extending waste paper, cellulose and filler use beyond recycling by entering the circular economy creating cellulose-CaCO3 composites reconstituted from ionic liquid. Cellulose 2022, 29, 5037–5059. [Google Scholar] [CrossRef]
- Guarás, M.P.; Alvarez, V.A.; Ludueña, L.N. Processing and characterization of thermoplastic starch/polycaprolactone/compatibilizer ternary blends for packaging applications. J. Polym. Res. 2015, 22, 165. [Google Scholar] [CrossRef]
- Filipello-Marchisio, V. Keratinophilic fungi: Their role in nature and degradation of keratinic substrates. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 86–92. [Google Scholar]
- Li, W.; Chen, Y.; Zhang, S. Hydrolysis of abandoned bovine hair by pulping spent liquor and preparation of degradable keratin-based sprayable mulch film. BioResources 2020, 15, 5058–5071. [Google Scholar] [CrossRef]
- Wang, R.; Tong, H. Preparation methods and functional characteristics of regenerated keratin-based biofilms. Polymers 2022, 14, 4723. [Google Scholar] [CrossRef]







| Film Abbreviation | CELL/PCL/KER/GCC Amount (mg) |
|---|---|
| CELL | 1000/0/0/0 |
| PCL | 0/1000/0/0 |
| CELL/PCL | 600/400/0/0 |
| CELL/PCL/KER | 400/400/200/0 |
| CELL/PCL/KER/GCC | 400/400/100/100 |
| Sample | TCI | LOI | HBI |
|---|---|---|---|
| CELL | 1.17 | 0.52 | 1.38 |
| CELL/PCL | 1.92 | 1.07 | 1.83 |
| CELL/PCL/KER | 1.52 | 0.93 | 1.99 |
| CELL/PCL/KER/GCC | 2.13 | 1.43 | 1.70 |
| Sample | Melting Temperature (°C) | Melting Enthalpy (J g−1) | Degree of Crystallinity (%) |
|---|---|---|---|
| PCL | 62.3 | 66.3 | 47.5 |
| CELL/PCL | 61.0 | 9.80 | 7.03 |
| CELL/PCL/KER | 62.6 | 21.9 | 15.7 |
| CELL/PCL/KER/GCC | 68.9 | 30.2 | 21.6 |
| Sample | Tensile Strength (MPa) | Modulus of Elasticity (MPa) | Breaking Strain (%) | Thickness (mm) |
|---|---|---|---|---|
| CELL | 75.3 ± 1.1 | 944.4 ± 2.0 | 6.97 ± 0.82 | 0.19 ± 0.04 |
| CELL/PCL | 2.1 ± 1.6 | 156.0 ± 21.5 | 2.83 ± 1.15 | 0.39 ± 0.03 |
| CELL/PCL/KER | 9.3 ± 0.3 | 599.1 ± 81.9 | 1.52 ± 0.07 | 0.37 ± 0.02 |
| CELL/PCL/KER/GCC | 15.8 ± 0.4 | 687.5 ± 16.6 | 2.03 ± 0.20 | 0.40 ± 0.06 |
| (a) | (b) | (c) | (d) | |
|---|---|---|---|---|
| 0 week |  |  |  |  |
| 1 week |  |  |  |  |
| 2 week |  |  |  |  |
| 3 week |  |  |  |  |
| 4 week |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, D.; Ivanovska, A.; Barać, N.; Dimić-Misić, K.; Kostić, M.; Radojević, V.; Janaćković, D.; Uskoković, P.; Barceló, E.; Gane, P. Biodegradable Cellulose/Polycaprolactone/Keratin/Calcium Carbonate Mulch Films Prepared in Imidazolium-Based Ionic Liquid. Polymers 2023, 15, 2729. https://doi.org/10.3390/polym15122729
Stojanović D, Ivanovska A, Barać N, Dimić-Misić K, Kostić M, Radojević V, Janaćković D, Uskoković P, Barceló E, Gane P. Biodegradable Cellulose/Polycaprolactone/Keratin/Calcium Carbonate Mulch Films Prepared in Imidazolium-Based Ionic Liquid. Polymers. 2023; 15(12):2729. https://doi.org/10.3390/polym15122729
Chicago/Turabian StyleStojanović, Dušica, Aleksandra Ivanovska, Nemanja Barać, Katarina Dimić-Misić, Mirjana Kostić, Vesna Radojević, Djordje Janaćković, Petar Uskoković, Ernest Barceló, and Patrick Gane. 2023. "Biodegradable Cellulose/Polycaprolactone/Keratin/Calcium Carbonate Mulch Films Prepared in Imidazolium-Based Ionic Liquid" Polymers 15, no. 12: 2729. https://doi.org/10.3390/polym15122729
APA StyleStojanović, D., Ivanovska, A., Barać, N., Dimić-Misić, K., Kostić, M., Radojević, V., Janaćković, D., Uskoković, P., Barceló, E., & Gane, P. (2023). Biodegradable Cellulose/Polycaprolactone/Keratin/Calcium Carbonate Mulch Films Prepared in Imidazolium-Based Ionic Liquid. Polymers, 15(12), 2729. https://doi.org/10.3390/polym15122729












