Synthesis and Properties of the Novel High-Performance Hydroxyl-Terminated Liquid Fluoroelastomer
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Apparatus
2.2.1. Spectral Analyses
2.2.2. Determination of Molar Mass
2.2.3. Thermal Properties
2.2.4. Mechanical Properties
2.2.5. Surface Properties
2.2.6. Oil and Acid Resistances
2.3. Quantitative Analysis via Chemical Titration
2.3.1. Determination of the Carboxyl Content
2.3.2. Determination of the Carboxyl Conversion Rate
2.4. Sample Preparation
2.4.1. Carboxyl-Terminated Liquid Fluoroelastomer
2.4.2. Hydroxyl-Terminated Liquid Fluoroelastomer
2.4.3. Curing
3. Results and Discussion
3.1. Structural Characterisation of the Liquid Fluoroelastomer
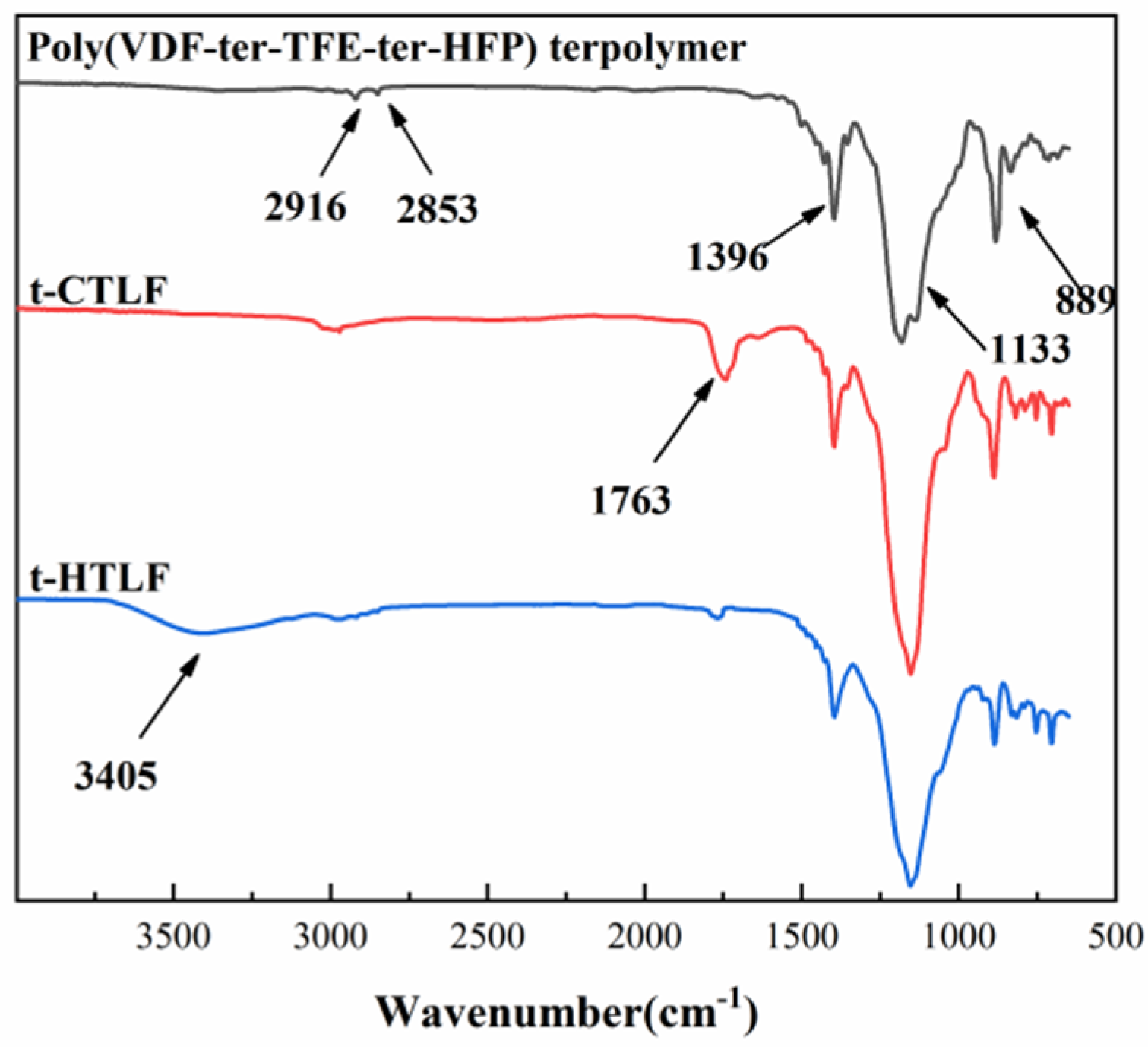
3.1.1. FTIR Spectra of the Liquid Fluoroelastomers
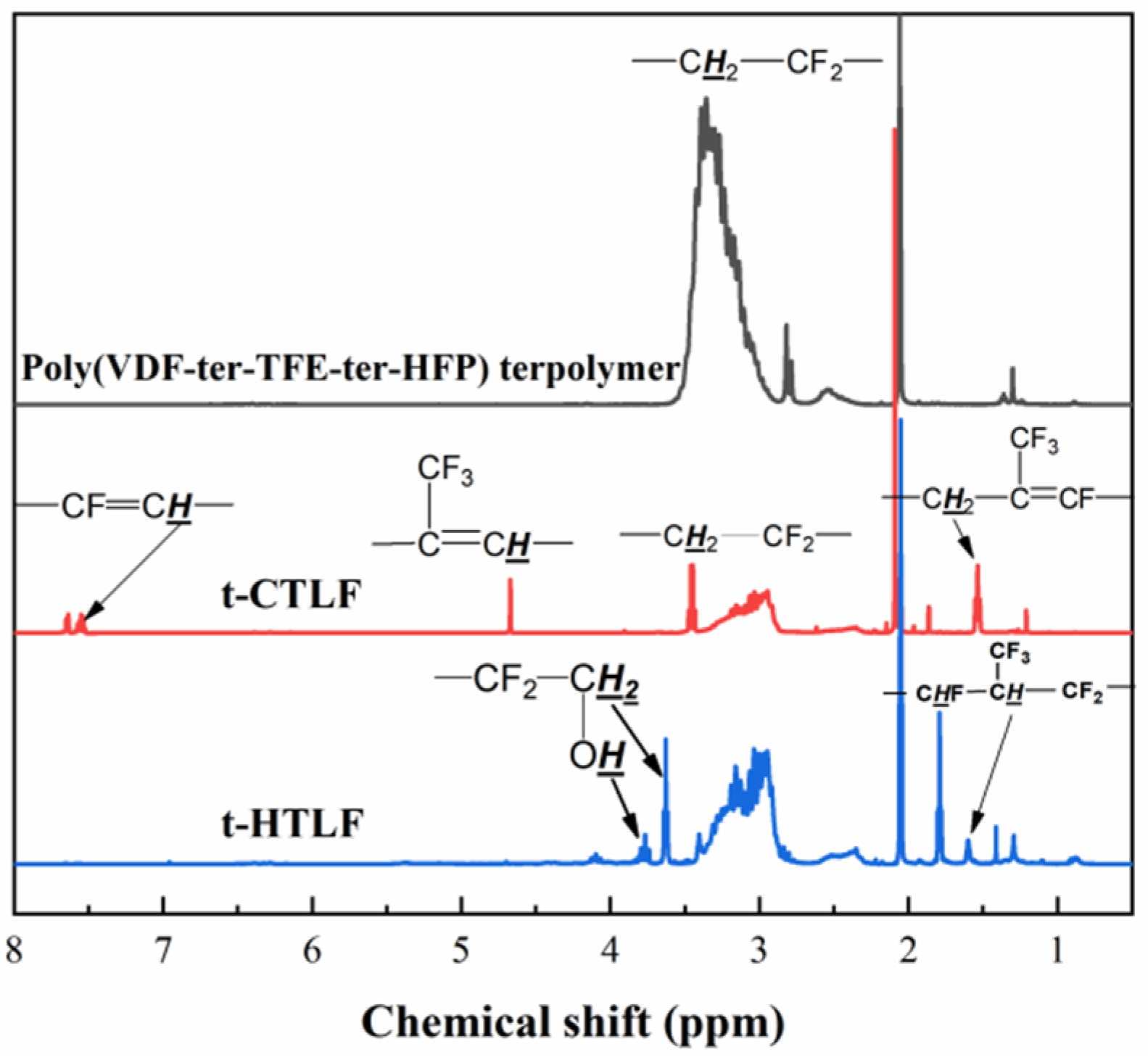
3.1.2. 1H-NMR Spectra of the Liquid Fluoroelastomers
3.1.3. 19F-NMR Spectra of the Liquid Fluoroelastomers
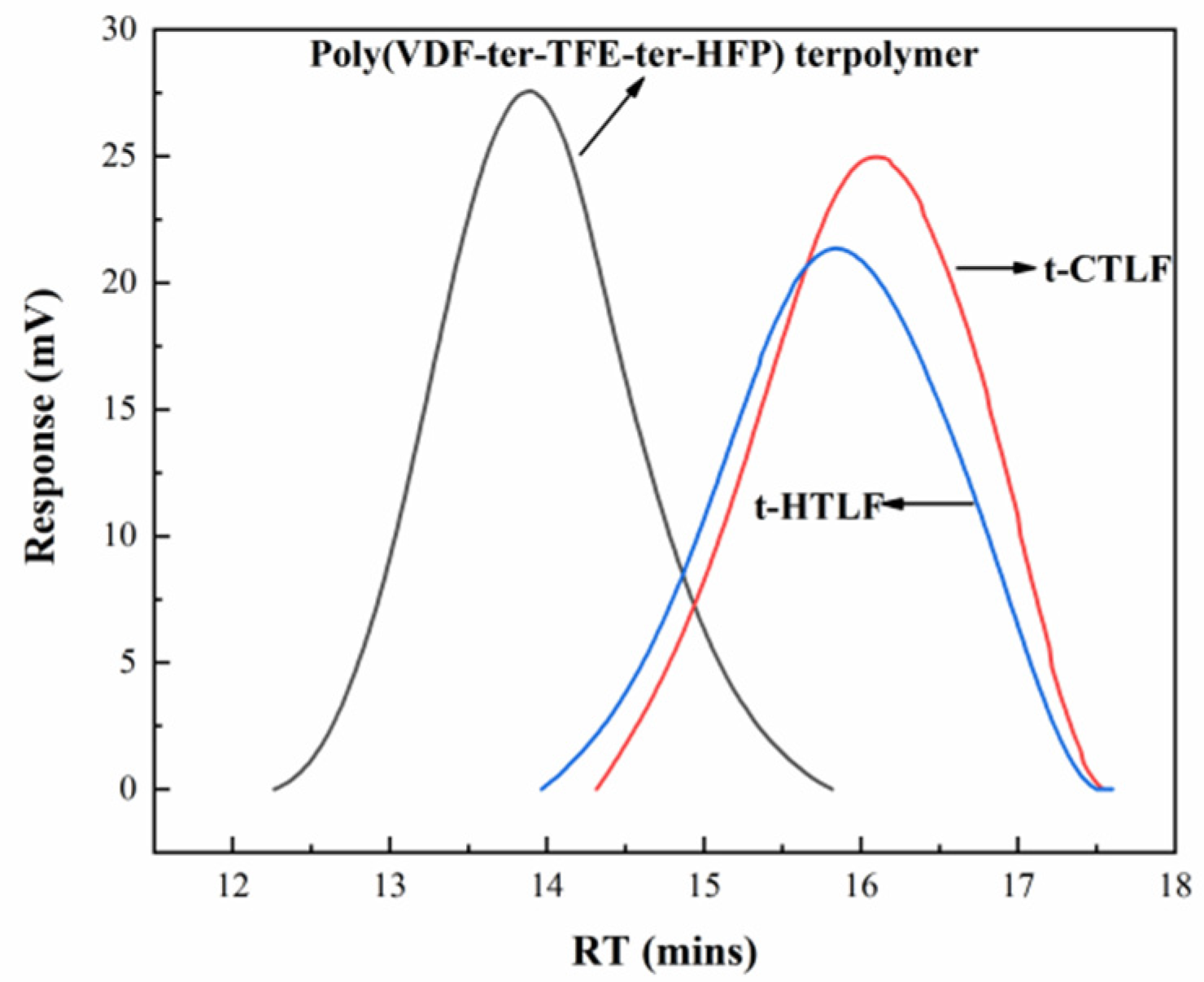
3.1.4. GPC of the Liquid Fluoroelastomers
3.1.5. Reaction Mechanisms
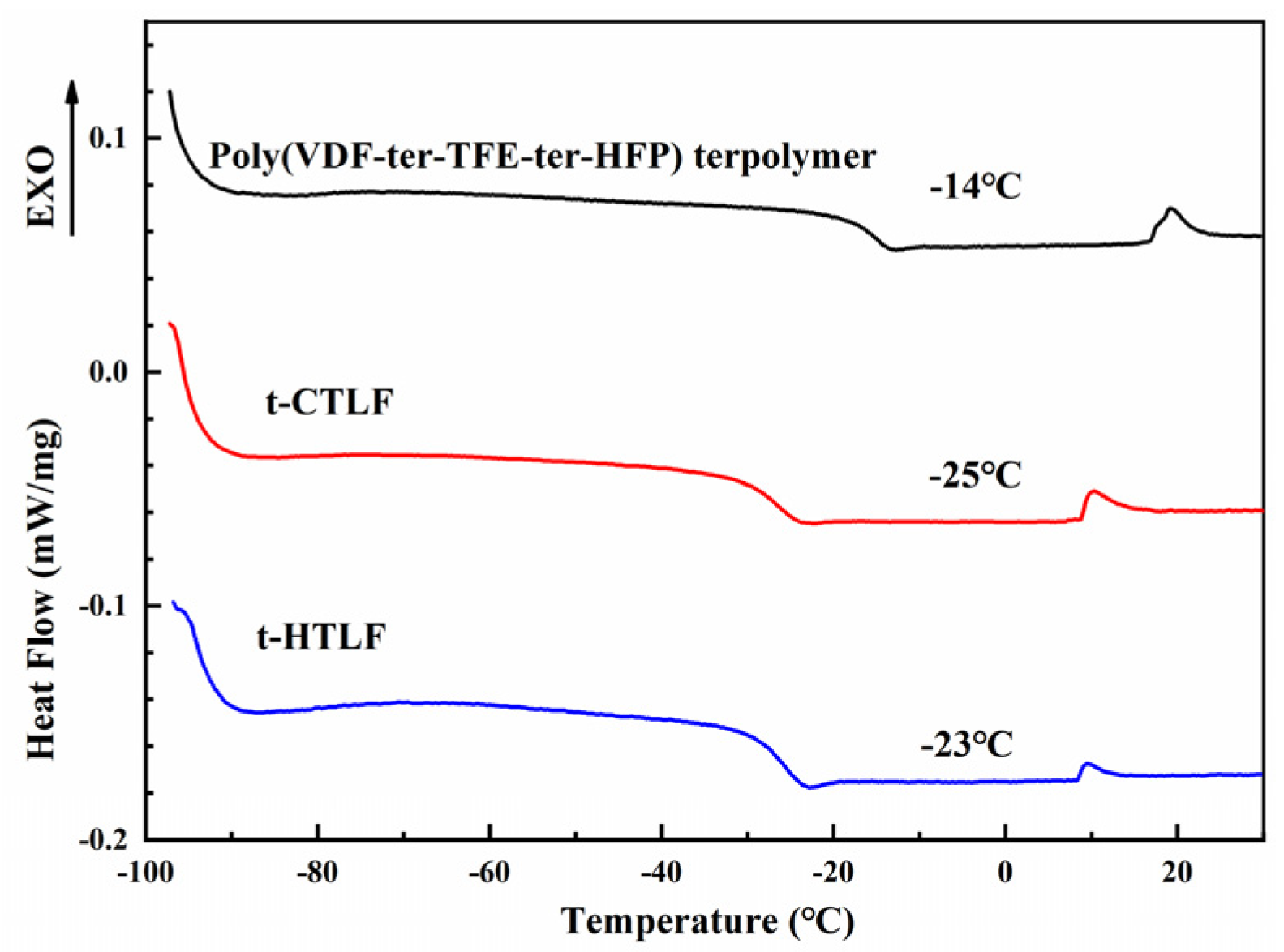
3.2. Thermal Properties of the Liquid Fluoroelastomers
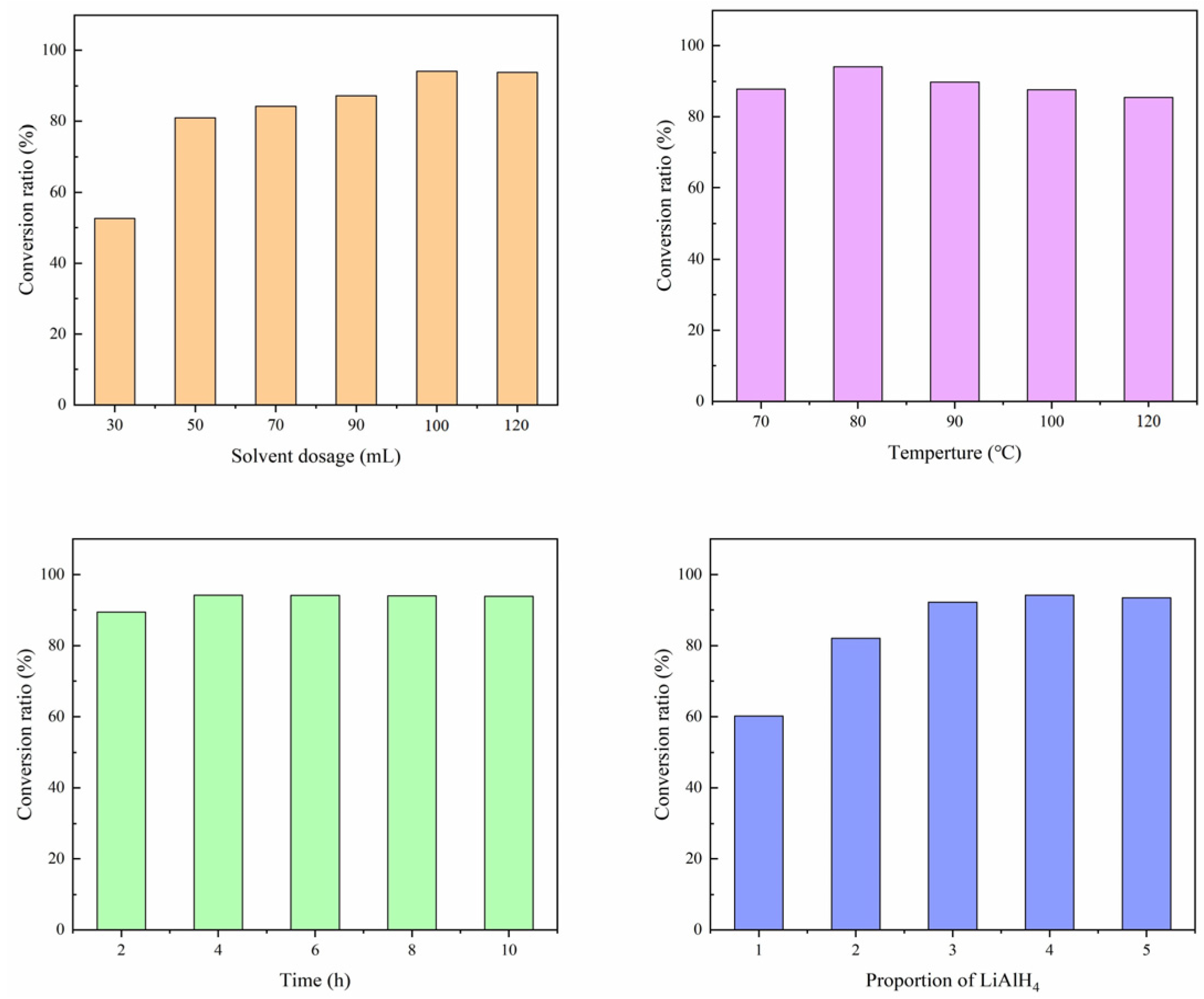
3.3. Factors Influencing the Reaction
3.3.1. Solvent Dosage
3.3.2. Reaction Temperature
3.3.3. Reaction Time
3.3.4. Reductant Ratio
3.4. Curing of the Hydroxyl-Terminated Liquid Fluoroelastomer
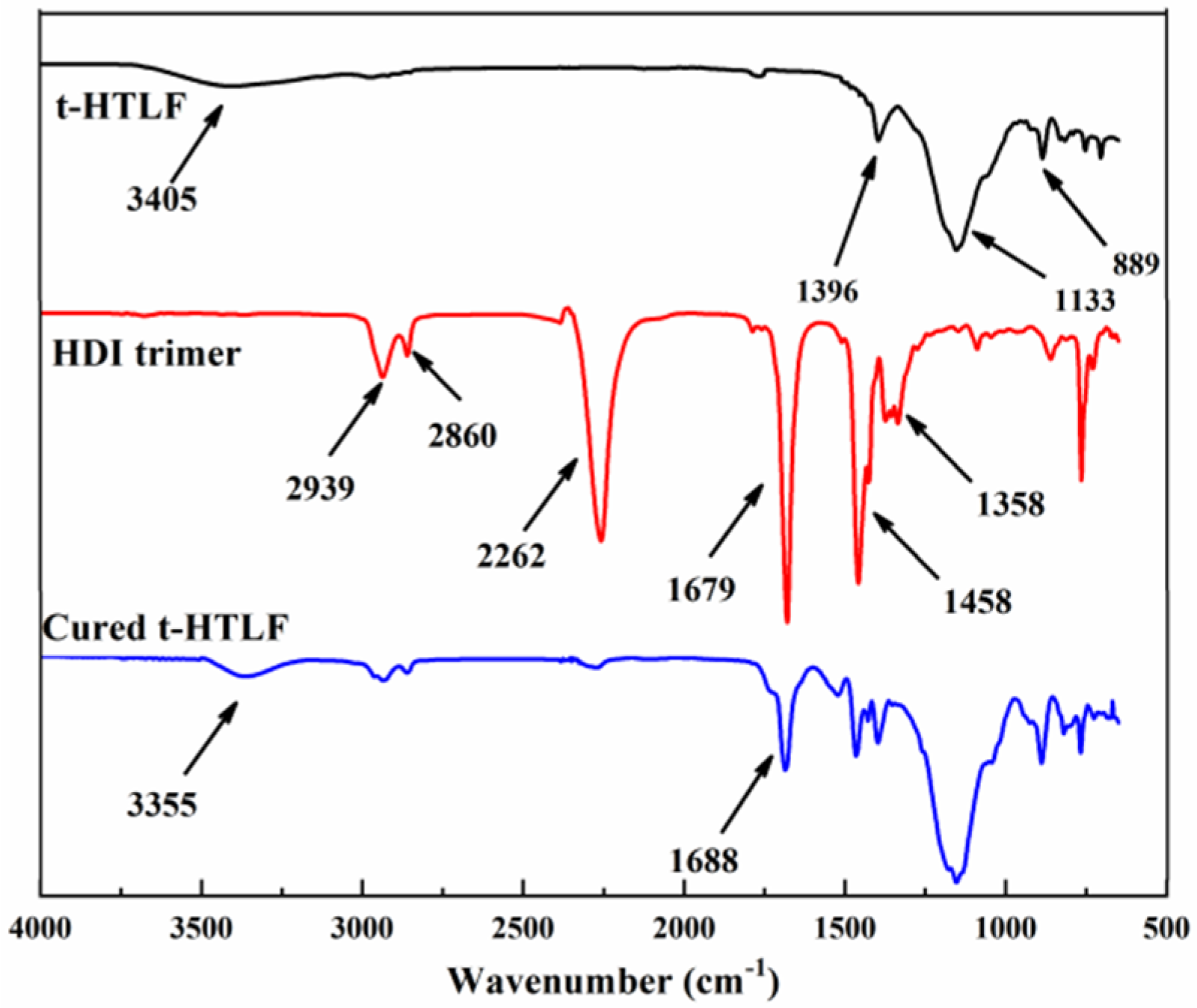
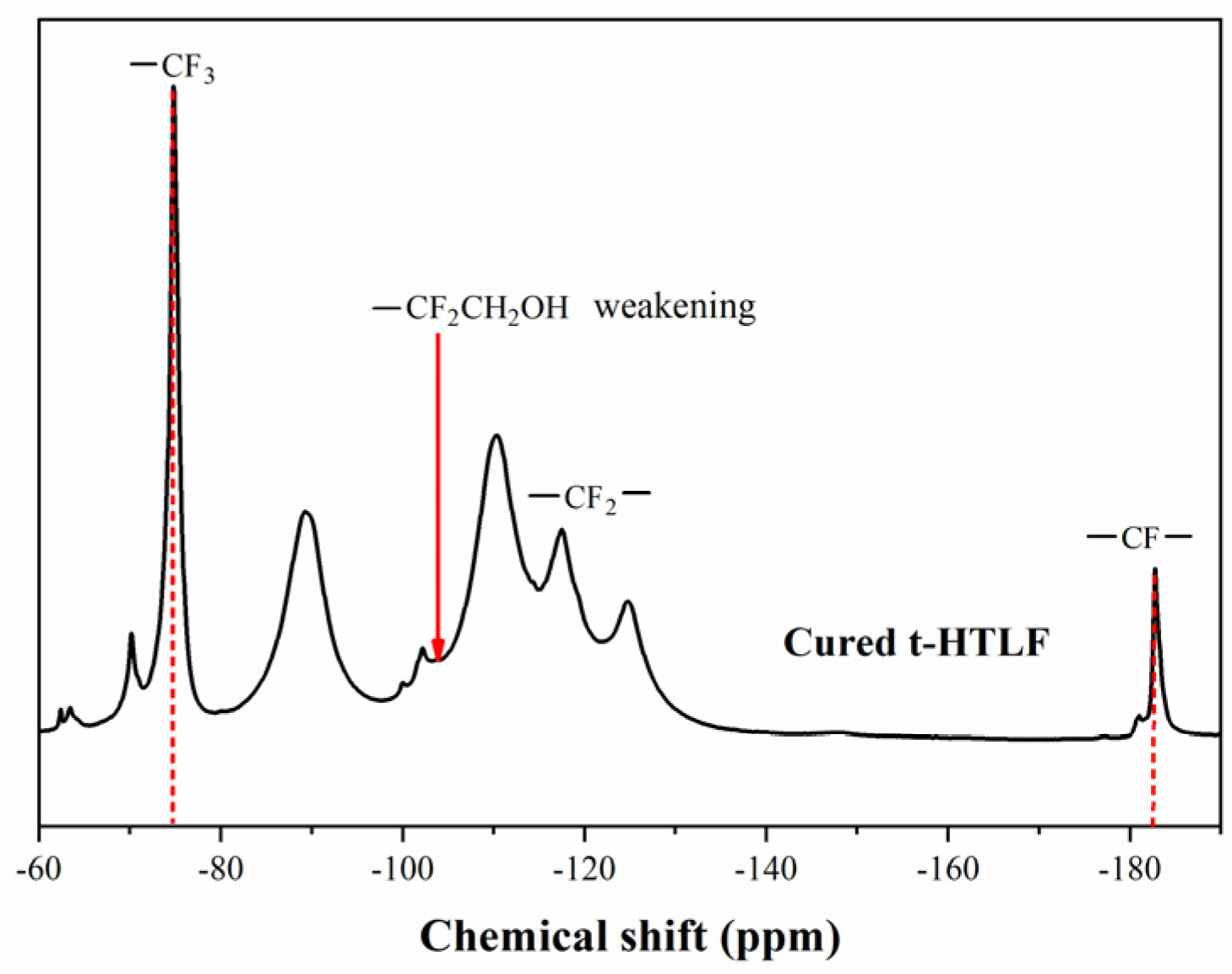
3.4.1. Structural Characterisation
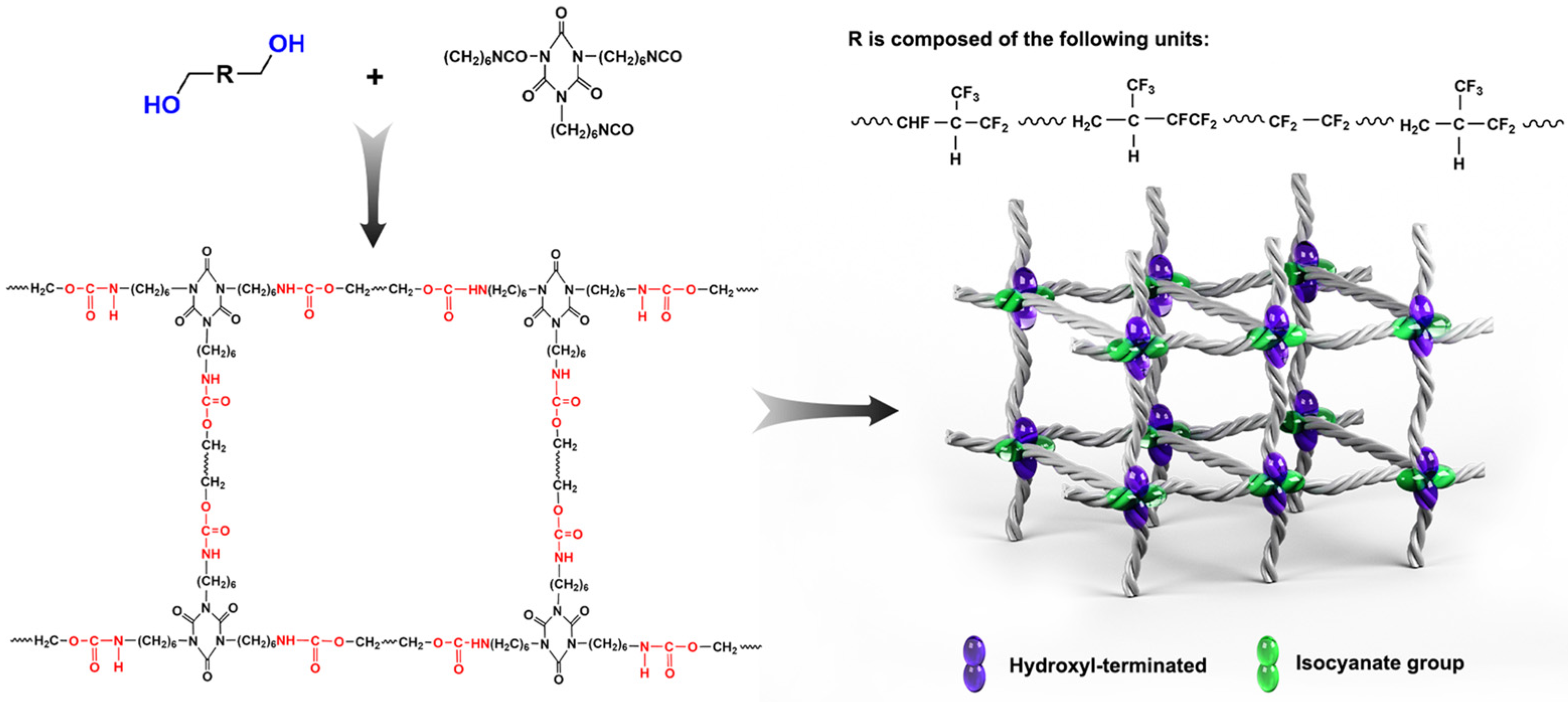
3.4.2. Curing Reaction Mechanism
3.4.3. Mechanical Properties of the Cured Hydroxyl-Terminated Liquid Fluoroelastomer
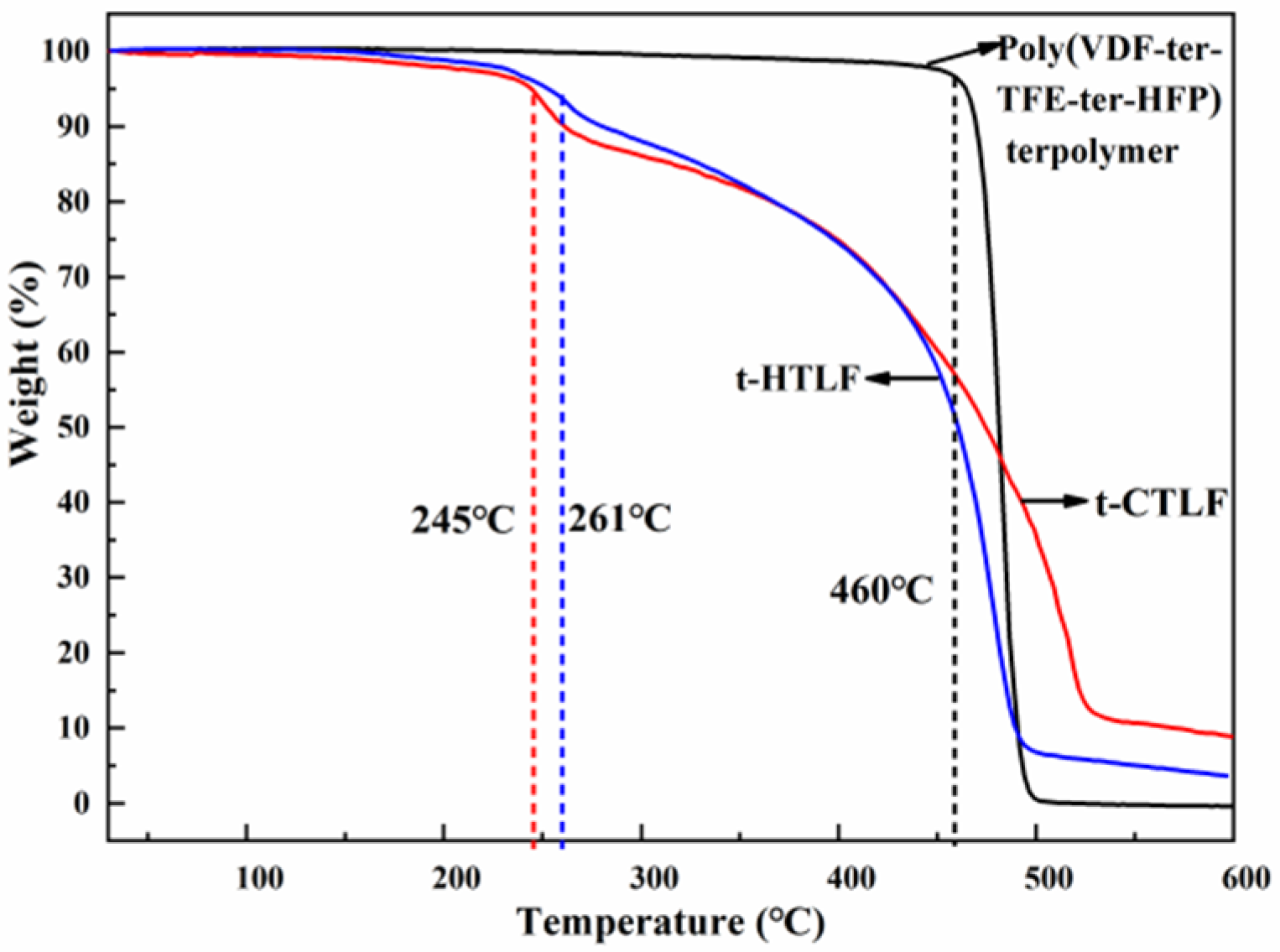
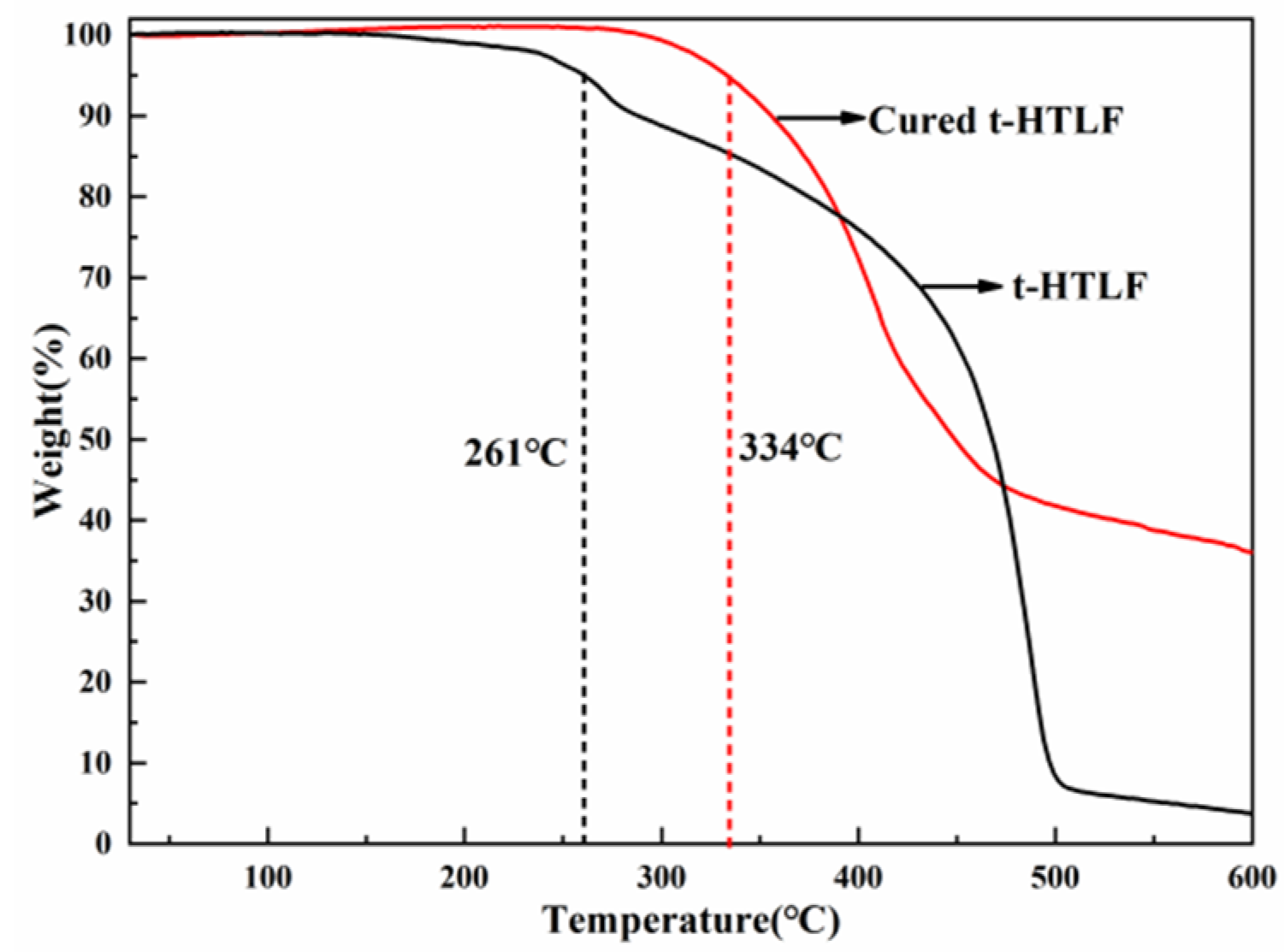
3.4.4. Thermal Stability
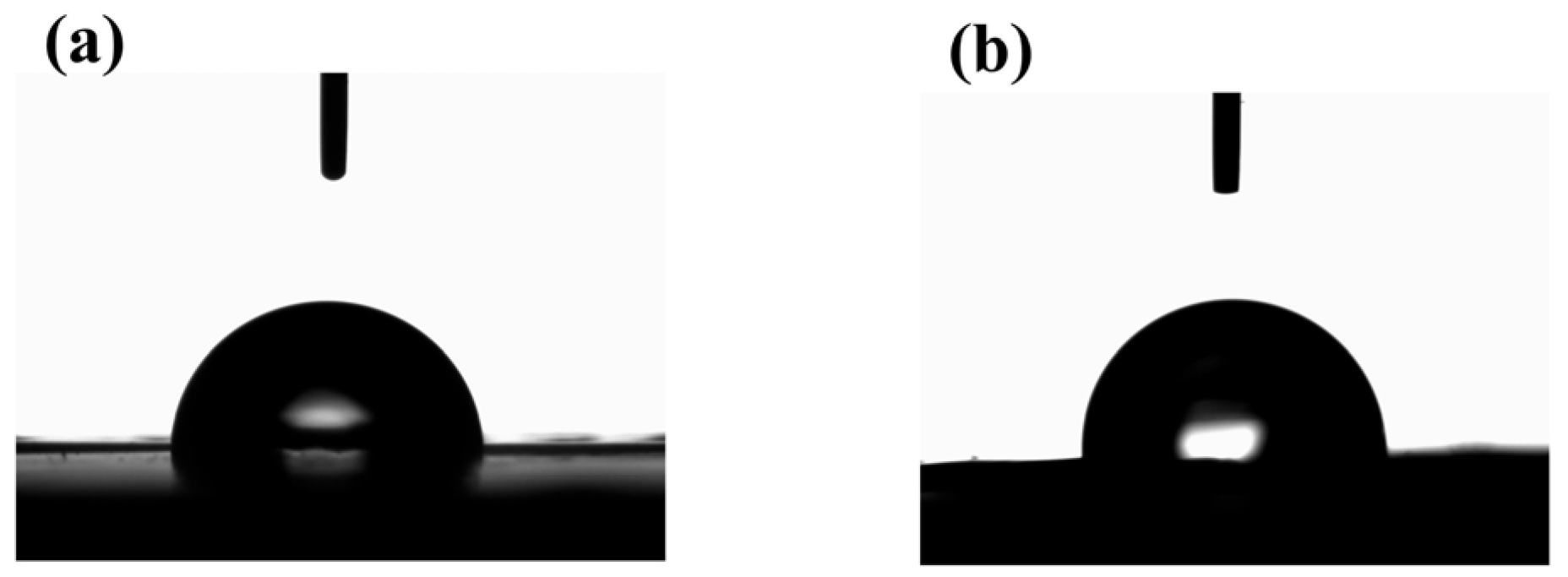
3.4.5. Surface Properties
3.4.6. Oil and Acid Resistances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ameduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Zhao, Y. Progress on research and application of special polymer materials in aerospace industry. Mater. Chin. 2013, 32, 217–228. [Google Scholar]
- Li, D.H.; Liao, M.Y. Dehydrofluorination Mechanisms and Structures of Fluoroelastomersin Alkaline Environments. Mater. Rep. 2018, 32, 1730–1736. [Google Scholar]
- Zhang, Z.Z. Preparation of Fluorinated Polyphosphazene Elastomer, Formulation Optimization and Properties Study. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2015. [Google Scholar]
- Flitney, B. (Ed.) Extending the application of fluorosilicone elastomers. Seal. Technol. 2005, 2005, 6–11. [Google Scholar] [CrossRef]
- Lee, P.C.; Kim, S.Y.; Ko, Y.K.; Ha, J.U.; Jeoung, S.K.; Lee, J.Y.; Kim, M. Durability and Service Life Prediction of Fluorocarbon Elastomer under Thermal Environment. Polymers 2022, 14, 2047. [Google Scholar] [CrossRef]
- Kang, Q.; Li, A.; Zhang, X. Study on mechanical and tribological properties of cerium oxide/fluorine rubber composites. Mater. Today Commun. 2021, 28, 102596. [Google Scholar] [CrossRef]
- Calabrese, L.; Alenza, A. The effect of a liquid rubber modifier on the thermo-kinetic parameters of an epoxy resin during a pultrusion process. Compos. Sci. Technol. 2003, 63, 851–860. [Google Scholar] [CrossRef]
- Maclachlan, J.D. Fluorocarbon elastomers: A technical review. Polym-Plast. Technol. 1978, 11, 41–53. [Google Scholar] [CrossRef]
- Pianca, M.; Bonardelli, P.; Tatò, M.; Cirillo, G.; Moggi, G. Composition and sequence distribution of vinylidene fluoride copolymer and terpolymer fluoroelastomers. Determination by 19F nuclear magnetic resonance spectroscopy and correlation with some properties. Polymer 1987, 28, 224–230. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.F.; You, W.T.; Guo, L.L.; Liu, C.; Zhang, X.A.; Qi, S.C. Synthesis, cure and properties of siloxy-terminater liquid fluoroelastomers. Acta Polym. Sin. 2013, 11, 1430–1437. [Google Scholar]
- Souzy, R.; Boutevin, B.; Ameduri, B. Synthesis and characterizations of novel proton-conducting fluoropolymer electrolyte membranes based on poly (vinylidenefl- uoride-ter-hexafluoropropylene-ter-α -trifluoromethacrylicacid) terpolymers grafted by arylsulfonic acids. Macromolecules 2012, 45, 3145–3160. [Google Scholar] [CrossRef]
- Chalathorn, C.; Kui, X.; He, H.J. Proton-conductive polymer nanocomposite membranes prepared from telechelic fluorinated polymers containing perfluoro- sulfonic acid sidechains. Polym. Sci. Pol. Chem. 2010, 48, 4800–4810. [Google Scholar]
- Li, K.; Liang, S.; Lu, Y. Synthesis of telechelic fluoroelastomers with well-defined functional end groups for cross-linked networks and nanocomposites. Macromolecles 2007, 40, 4121–4123. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.P.; Yue, L.P.; Zheng, X.Q. Synthesis of photo-crosslinked hybrid fluoropolymer and its application as releasing coating for silicone pressure-sensitive adhesives. Appl. Polym. Sci. 2019, 137, 48322–48331. [Google Scholar] [CrossRef]
- Guan, F.X.; Wang, J.; Yang, L.Y. Confinement-induced high-field antiferroelectric-like behavior in a poly(vinylidene fluoride-co-trifluoroethylene-cochlorotrifluoroeth-ylene)-graft-polystyrene graft copolymer. Macromolecules 2011, 44, 2190–2199. [Google Scholar] [CrossRef]
- Li, D.H.; Liao, M.Y. Study on the synthesis of liquid terminal acylfluorine rubber from different acylated reagents. Chin. Rubb/Plast. Technol. Equip. 2017, 43, 11–17. [Google Scholar]
- Li, D.H.; Qi, S.C.; Zhang, X.A.; Liao, M.Y. Preparation, Functionalization and Properties of Low Molecular Fluoropolymers. Prog. Chem. 2016, 28, 13. [Google Scholar]
- Gallagher, G.A. Copolymers of Hexalfuoropropylene Vinylidene Fluoride and Aliphatic, Chain Transfer Agents. US Patent US3069401, 18 December 1962. [Google Scholar]
- Ameduri, B. From vinylidene fluoride (VDF) to the applications of VDF-containing polymers and copolymers: Recent developments and future trends. Chem. Rev. 2001, 109, 6632–6686. [Google Scholar] [CrossRef]
- Tatemoto, M.; Morita, S. Liquid Fluorine-Containing Polymer and its Production with Iodinated Compound. U.S. Patent US4361678, 30 November 1982. [Google Scholar]
- Hung, M.H. Iodine Containing Chain Transfer Agents for Fluoropolymer Polymerizations. U.S. Patent US5231154, 27 July 1993. [Google Scholar]
- Kostov, G.K.; Holan, M.; Ameduri, B.; Hung, M.H. Synthesis and Characterizations of Photo-Cross-Linkable Telechelic Diacrylate Poly(vinylidene fluoride-co-perfluoromethyl vinyl ether) Copolymers. Macromolecules. 2012, 45, 7375–7387. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.F.; Qi, S.C. Synthesis and cure properties of liquid fluoroelastomer with carboxyl end group. Chin. Synth. Rubb. Ind. 2014, 37, 188–193. [Google Scholar]
- Li, D.H.; Liao, M.Y. Study on the dehydrofluorination of vinylidene fluoride (VDF) and hexafluoropropylene (HFP) copolymer. Polym. Degrad. Stabil. 2018, 152, 116–125. [Google Scholar] [CrossRef]
- Li, D.H.; Liao, M.Y. Dehydrofluorination mechanism, structure and thermal stability of pure fluoroelastomer (poly(VDF-ter-HFP-ter-TFE) terpolymer) in alkaline environment. J. Fluor. Chem. 2017, 201, 55–67. [Google Scholar] [CrossRef]
- Mitra, S.; Ghanbari-Siahkali, A.; Kingshott, P.; Almdal, K.; Christensen, A.G. Chemical degradation of fluoroelastomer in an alkaline environment. Polym. Degrad. Stabil. 2003, 83, 195–206. [Google Scholar] [CrossRef]
- Liao, M.Y.; Qi, R.R.; Huo, Y.; Chang, Y.F. Preparation and Characterization of Liquid Carboxyl-Terminated Fluororubberby Microwave Assisted Oxidation Degradation Method. Polym. Mater. Sci. Eng. 2022, 38, 29–36. [Google Scholar]
- Khalilzadeh, M.A.; Hosseini, A.; Tajbakhsh, M.; Mohannazadeh, F. LiAlH4/silica chloride as a new chemoselective system for reduction of carbonyl compounds and phosphine oxides. J. Iran. Chem. Soc. 2008, 5, 699–705. [Google Scholar] [CrossRef]
- Mariappan, P.; Muniappan, T. Methods of enhancement of reactivity and selectivity of sodiumborohydride for applications in organic synthesis. J. Organomet. Chem. 2000, 609, 137–151. [Google Scholar]
- Tale, R.H.; Patil, K.M.; Dapurkar, S.E. An extremely simple, convenient and mild one-pot reduction of carboxylic acids to alcohols using 3,4,5-trifluorophenylboronic acid and sodium borohydride. Tetrahedron. Lett. 2003, 44, 3427–3428. [Google Scholar] [CrossRef]
- Saavedra, J.Z.; Resendez, A.; Rovira, A.; Eagon, S.; Haddenham, D.; Singaram, B. Reaction of InCl3 with various reducing agents: InCl3-NaBH4-mediated reduction of aromatic and aliphatic nitriles to primary amines. J. Org. Chem. 2012, 77, 221–228. [Google Scholar] [CrossRef]
- Aramini, A.; Brinchi, L.; Germani, R.; Savelli, G. Reductions of α,β-Unsaturated Ketones by NaBH4 or NaBH4 + CoCl2: Selectivity Control by Water or by Aqueous Micellar Solutions. Eur. J. Org. Chem. 2000, 2000, 1793–1797. [Google Scholar] [CrossRef]
- Améduri, B.; Balagué, J.; Boutevin, B.; Caporiccio, G.; Gornowicz, G. Synthesis and characterization of fluorinated telomers from vinylidene fluoride, hexafluoropropene and chlorotrifluoroethylene. J. Fluor. Chem. 1992, 58, 168. [Google Scholar] [CrossRef]
- Wu, J.J. Synthesis and Cure of Active-Terminatedliquid Flouroelastomer. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2015. [Google Scholar]
- Li, N.; Wu, J.J.; Wang, Y.Q.; Wu, J.H.; Zhang, J.J.; Huo, J.Z.; Bai, Y.P. Synthesis, curing of hydroxyl-terminated liquid fluoroelastomer: Thermal, chemical resistant and mechanical properties. IOP. Conf. Ser. Mater. Sci. Eng. 2017, 213, 012029. [Google Scholar] [CrossRef]
- Li, D.H. Preparation, Curing, Structures and Properties of End-functionalized Liquid Fluoroelastomers. Ph.D. Thesis, Dalian Maritime University, Dalian, China, 2018. [Google Scholar]
- Chang, Y.F.; Liao, M.Y.; Li, X.Y. Reduction of liquid terminated-carboxyl fluoroelastomers using NaBH4/SmCl3. RSC Adv. 2020, 10, 10932–10938. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Liao, M.Y.; Wen, J.M. Reduction Performance and Mechanism of Liquid Terminated-carboxyl Fluoroelastomers Using NaBH4/MClx Reduction System. Chem. J. Chin. Univ.-Chin. 2022, 43, 318–325. [Google Scholar]
- Song, A.; Zhang, X.; Song, X.; Chen, X.; Yu, C.; He, H.; Li, H.; Wei, W. Construction of Chiral Bridged Tricyclic Benzopyrans: Enantioselective Catalytic Diels-Alder Reaction and a One-Pot Reduction/Acid-Catalyzed Stereoselective Cyclization. Angew. Chem. 2014, 53, 4940–4944. [Google Scholar] [CrossRef]
- Schwager, H.; Wilke, G. Zum Norcaradien-Cycloheptatrien-Strukturproblem. Eur. J. Inorg. Chem. 2010, 120, 79–80. [Google Scholar] [CrossRef]
- Duan, J.Y.; Yang, C.; Kang, H.L.; Li, L.; Yang, F.; Fang, Q.H.; Han, W.C.; Li, D.H. Structure, preparation and properties of liquid fluoroelastomers with different end groups. RSC Adv. 2022, 12, 3108–3118. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.M.; Chang, Y.F.; Liao, M.Y. Reduction of Liquid Carboxyl-terminated Fluorine Rubber by Alkyl Aluminum/Lithium Aluminum Hydride Reduction System. Chin. Rubb. Ind. 2022, 69, 891–898. [Google Scholar]
- Li, A.J.; Feng, B.; Liu, Q.C. Synthesis of 1,3-Aminoalcohols by One-Step Reduction of β-Enaminoketoneswith Lithium Aluminium Hydride. Chin. J. Org. Chem. 2011, 31, 106–109. [Google Scholar]
- Zhai, H. Synthesis of Antibacterials Moxifloxacin. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2007. [Google Scholar]
- Herold, F.; Leubner, O.; Pfeifer, P.; Zakgeym, D.; Drochner, A.; Qi, W.; Etzold, B.J. Synthesis strategies towards amorphous porous carbons with selective oxygen functionalization for the application as reference material. Carbon 2021, 171, 658–670. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zheng, X.; Bai, Y. Towards new environmentally friendly fluoroelastomers: From facile chemical degradation to efficient photo-crosslinkable reaction. Polym. Int. 2019, 12, 68. [Google Scholar] [CrossRef]
- Timperley, C.M.; Arbon, R.E.; Bird, M.; Brewer, S.A.; Willis, C.R. Bis (fluoroalkyl) acrylic and methacrylic phosphate monomers, their polymersand same of their properties. J. Fluor. Chem. 2003, 121, 23–31. [Google Scholar] [CrossRef]
- Saint, L.R.; Manseri, A.; Ameduri, B.; Lebret, B.; Vignane, P. Synthesis and properties of novel fluorotelechelic macrodiols containing vinylidene fluoride, hexafluoropropene and chlorotrifluoroethylene. Macromolecules 2002, 35, 1524–1536. [Google Scholar] [CrossRef]
- Li, D.H.; Liao, M.Y. Preparation of telechelic hydroxyl low molecular weight fluoropolymers. Key Eng. Mater. 2017, 753, 93–98. [Google Scholar] [CrossRef]
- Pianca, M.; Barchiesi, E.; Esposto, G.; Radice, S. End groups in fluoropolymers. J. Fluor. Chem. 1999, 95, 71–84. [Google Scholar] [CrossRef]
- Li, D.H.; Liao, M.Y. Monomers Composition, Molecular Chain Structures and Thermal Propertiesof PLV85540 Fluoroelastomer. Mater. Rep. 2018, 008, 032. [Google Scholar]
- Xing, Q.Y.; Pei, W.W.; Xu, R.Q.; Pei, J. Basic Organic Chemistry, 4th ed.; Peking University Press: Beijing, China, 2017; pp. 537–538. [Google Scholar]
- Lin, S.B.; Li, Z.Y.; Luo, G.; Chen, Y.Y. Methods of reducing carboxylic acid ester to alcohol. Chem. Ind. Eng. Prog. 2014, 33, 1276–1284. [Google Scholar]
- Guo, J.Z.; Huang, P.; Yang, T.; Pei, W. Production and Reaction of Lithium Aluminium Hydride. Chem. Prod. Technol. 2015, 22, 35–37. [Google Scholar]
- Hua, Y.Q.; Jin, R.G. Polymer Physics, 4th ed.; Chemical Industry Press: Beijing, China, 2013; pp. 140–151. [Google Scholar]
- Zeng, N.; Xie, J.J.; Ding, C.; Liu, J.X. Synthesis and properties of blocked isocyanate adducts from methylparaben. Chem. Ind. Eng. Prog. 2014, 33, 470–474. [Google Scholar]
- Savoie, H.R.; Peticolas, W.L. Curing reactions in elastomers. II. Carboxy-terminated polybutadiene. J. Appl. Polym. Sci. 2010, 28, 595–604. [Google Scholar]
- Sun, H.; Ni, W.; Yuan, B.; Wang, T.; Li, P.; Liu, Y.; Wang, L. Synthesis and characterization of emulsion-type curing agent of water-borne epoxy resin. J. Appl. Polym. Sci. 2013, 130, 2652–2659. [Google Scholar] [CrossRef]
- Li, D.H.; Duan, J.Y.; Zhang, C.; Xv, Z.Y.; Fang, Q.H. Multi-stage Curing Mechanisms, Structure and Properties of 26/246Fluoroelastomers. Mater. Rep. 2021, 35, 12202–12208. [Google Scholar]
- Li, X.Y.; Chang, Y.F.; Liao, M.Y. Study on Properties of Carboxyl-terminated Liquid Fluororubber Cured by Aziridine. Mater. Rep. 2020, 34, 20177–20181. [Google Scholar]
- Li, H.; Yao, M.; Lai, J.; Ma, C.P.; Wu, Z.D.; Qiu, Z.M. Synthesis and Application of Hydroxy Terminated Fluorinated Vinyl Polysiloxane. Mater. Rep. 2023, 37, 242–247. [Google Scholar]

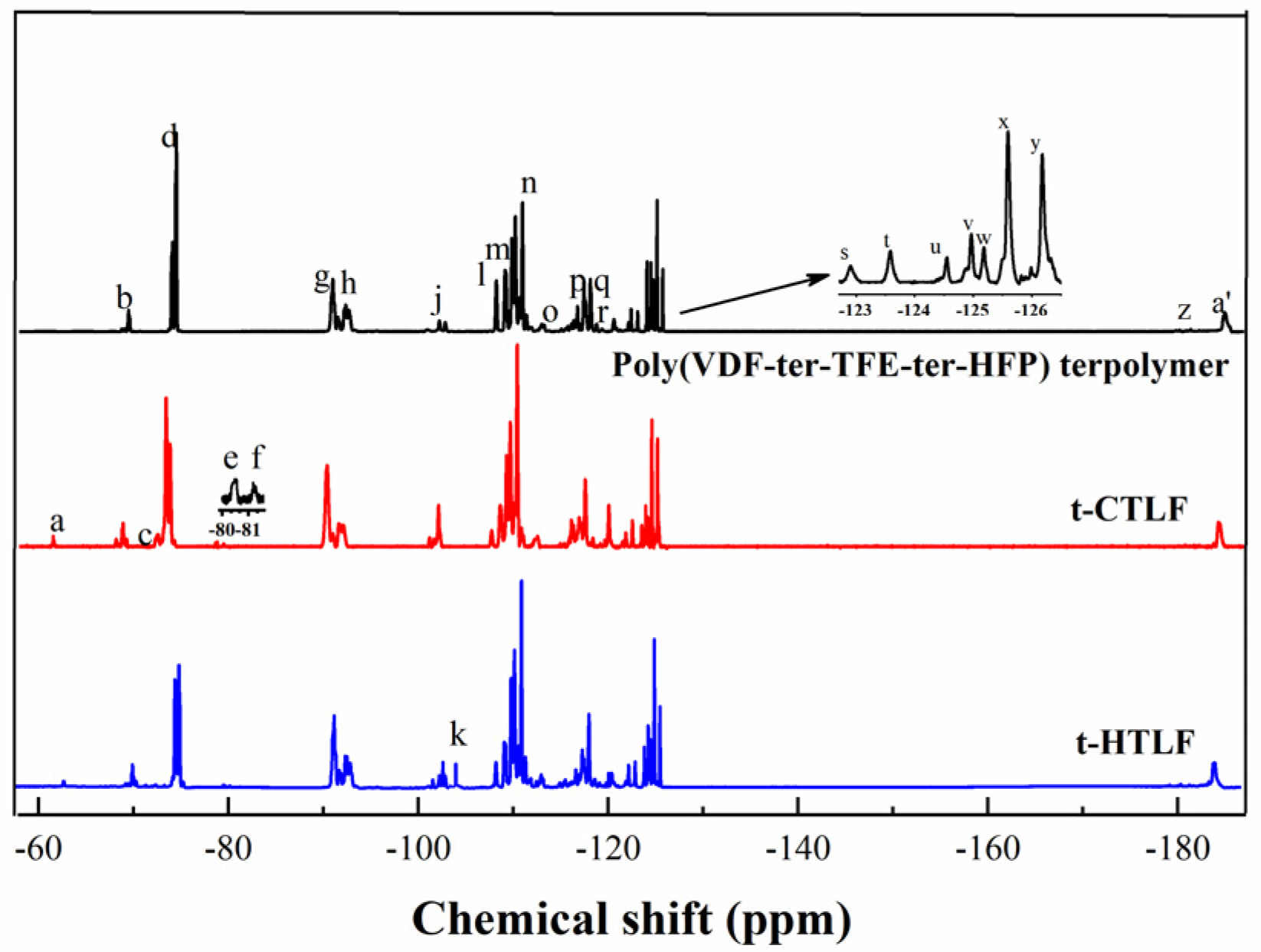


| No. | δ (ppm) | Assignment | No. | δ (ppm) | Assignment |
|---|---|---|---|---|---|
| a | −63.53 | –CF2CF2COOH | o | −113.85 | –CF2CH2CF2CF2CH2– |
| b | −70.76 | –CH2CF2CF(CF3)CF2CH2– | p | −117.26 | –CH2CH2CF2CF2CH2– |
| c | −74.29 | –CF2CH=C(CF3)CF2– | q | −118.68 | –CF2CF2CF(CF3) CH2– |
| d | −75.44 | –CF2CH2CF(CF3)CF2CF2– | r | −121.13 | –CF2CF2CF2CF2CF2– |
| e | −80.53 | –CH=CFCF(CF3)– | s | −122.90 | –CH2CF2CF2CF2CF2– |
| f | −81.19 | –CF=CHCF(CF3)CF2– | t | −123.58 | |
| g | −91.95 | –CF2CH2CF2CH2CF2– | u | −124.54 | –CH2CF2CF2CF2CH2– |
| h | −93.28 | –CF2CH2CF2CH2CF(CF3)– | v | −124.97 | |
| i | −95.64 | –CH2CH2CF2CH2CF2– | w | −125.19 | |
| j | −103.48 | –CF2CH2CF2CF(CF3)CF2– | |||
| k | −104.02 | –CF2CH2OH | x | −125.59 | |
| l | −108.98 | –CF(CF3)CH2CF2CF2CF(CF3)– | |||
| m | −109.85 | –CF2CH2CF2CF2CF(CF3)– | y | −126.18 | |
| n | −111.64 | –CF(CF3)CH2CF2CF2CH2– | z | −182.37 | –CH2CF2CF(CF3)CF2CH2– |
| a′ | −184.32 | –CF2CF2CF(CF3)CH2CF2– |
| Sample | Mn × 103 (g/mol) | PD |
|---|---|---|
| Poly(VDF-ter-TFE-ter-HFP) terpolymer | 93.0 | 1.91 |
| t-CTLF | 3.8 | 1.65 |
| t-HTLF | 4.2 | 1.57 |
| No. | THF (mL) | Mn × 103 (g/mol) | PD | Conversion Ratio (%) |
|---|---|---|---|---|
| 1 | 30 | 4.1 | 1.72 | 52 |
| 2 | 50 | 4.1 | 1.65 | 80 |
| 3 | 70 | 4.1 | 1.66 | 84 |
| 4 | 90 | 4.1 | 1.52 | 87 |
| 5 | 100 | 4.2 | 1.57 | 94 |
| 6 | 120 | 4.2 | 1.55 | 93 |
| No. | Temperture (°C) | Mn × 103 (g/mol) | PD | Conversion Ratio (%) |
|---|---|---|---|---|
| 7 | 70 | 4.2 | 1.52 | 87 |
| 8 | 80 | 4.2 | 1.57 | 94 |
| 9 | 90 | 4.2 | 1.52 | 89 |
| 10 | 100 | 4.2 | 1.60 | 87 |
| 11 | 120 | 4.4 | 1.59 | 85 |
| No. | Time (h) | Mn × 103 (g/mol) | PD | Conversion Ratio (%) |
|---|---|---|---|---|
| 12 | 2 | 3.9 | 1.68 | 89 |
| 13 | 4 | 4.2 | 1.57 | 94 |
| 14 | 6 | 4.3 | 1.61 | 94 |
| 15 | 8 | 4.3 | 1.60 | 94 |
| 16 | 10 | 4.3 | 1.60 | 93 |
| No. | COOH/LiAlH4 | Mn × 103 (g/mol) | PD | Conversion Ratio (%) |
|---|---|---|---|---|
| 17 | 1.00/1.00 | 4.2 | 1.68 | 60 |
| 18 | 1.00/2.00 | 4.1 | 1.98 | 82 |
| 19 | 1.00/3.00 | 4.0 | 1.59 | 92 |
| 20 | 1.00/4.00 | 4.2 | 1.57 | 94 |
| 21 | 1.00/5.00 | 3.9 | 1.61 | 93 |
| Liquid Fluoroelastomer | Mn × 103 (g/mol) | OH (wt%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| Poly(VDF-co-HFP) copolymer | 4.0 | 2.33 | 1.23 | 125 |
| Poly(VDF-ter-TFE-ter-HFP) terpolymer | 4.2 | 3.05 | 2.15 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, C.; Li, P.; Yu, L.; Zhao, S.; Li, L.; Kang, H.; Yang, F.; Fang, Q. Synthesis and Properties of the Novel High-Performance Hydroxyl-Terminated Liquid Fluoroelastomer. Polymers 2023, 15, 2574. https://doi.org/10.3390/polym15112574
Li D, Yang C, Li P, Yu L, Zhao S, Li L, Kang H, Yang F, Fang Q. Synthesis and Properties of the Novel High-Performance Hydroxyl-Terminated Liquid Fluoroelastomer. Polymers. 2023; 15(11):2574. https://doi.org/10.3390/polym15112574
Chicago/Turabian StyleLi, Donghan, Chen Yang, Ping Li, Lu Yu, Shufa Zhao, Long Li, Hailan Kang, Feng Yang, and Qinghong Fang. 2023. "Synthesis and Properties of the Novel High-Performance Hydroxyl-Terminated Liquid Fluoroelastomer" Polymers 15, no. 11: 2574. https://doi.org/10.3390/polym15112574
APA StyleLi, D., Yang, C., Li, P., Yu, L., Zhao, S., Li, L., Kang, H., Yang, F., & Fang, Q. (2023). Synthesis and Properties of the Novel High-Performance Hydroxyl-Terminated Liquid Fluoroelastomer. Polymers, 15(11), 2574. https://doi.org/10.3390/polym15112574






