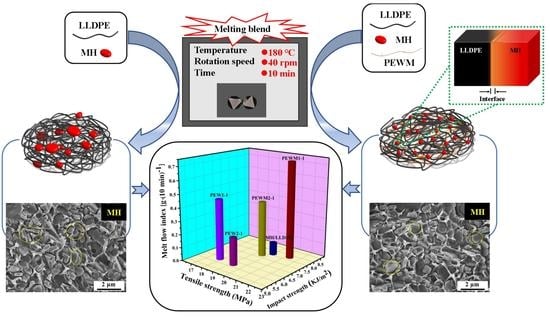
Effect of Functionalized Polyethylene Wax on the Melt Processing and Properties of Highly Filled Magnesium Hydroxide/Linear Low-Density Polyethylene Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
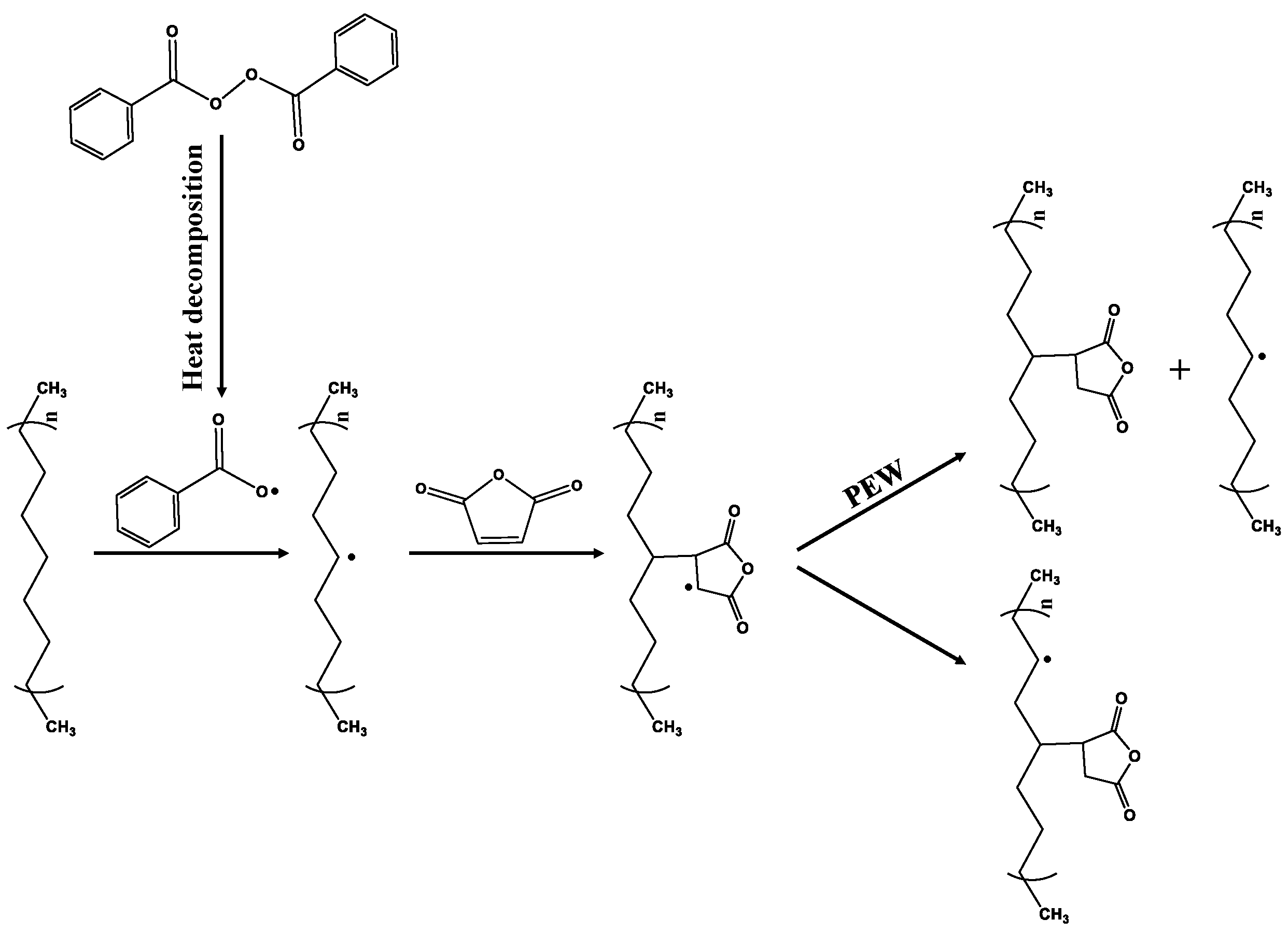
2.2. Preparation and Purification of PEWM
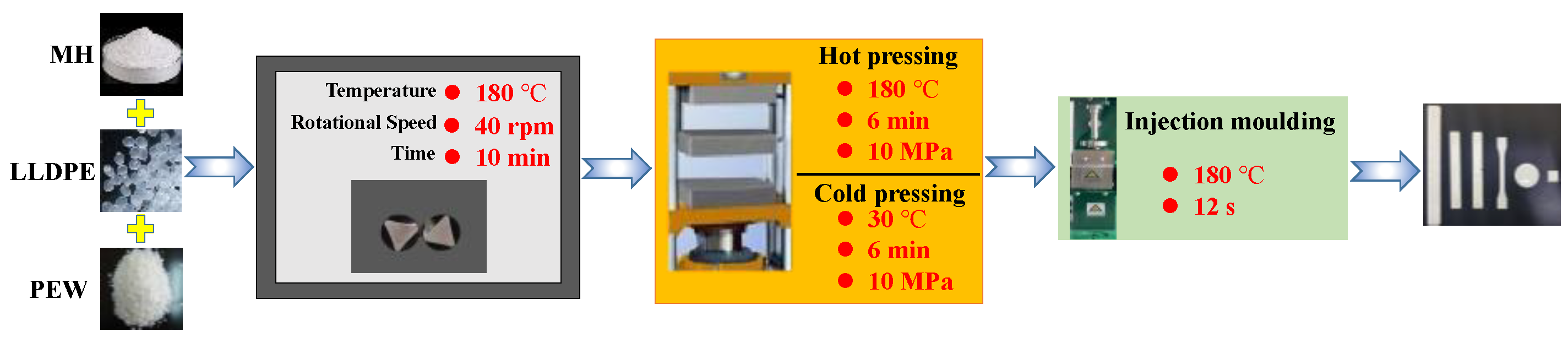
2.3. Preparation of MH/LLDPE Composites
2.4. Characterization
3. Results and Discussion
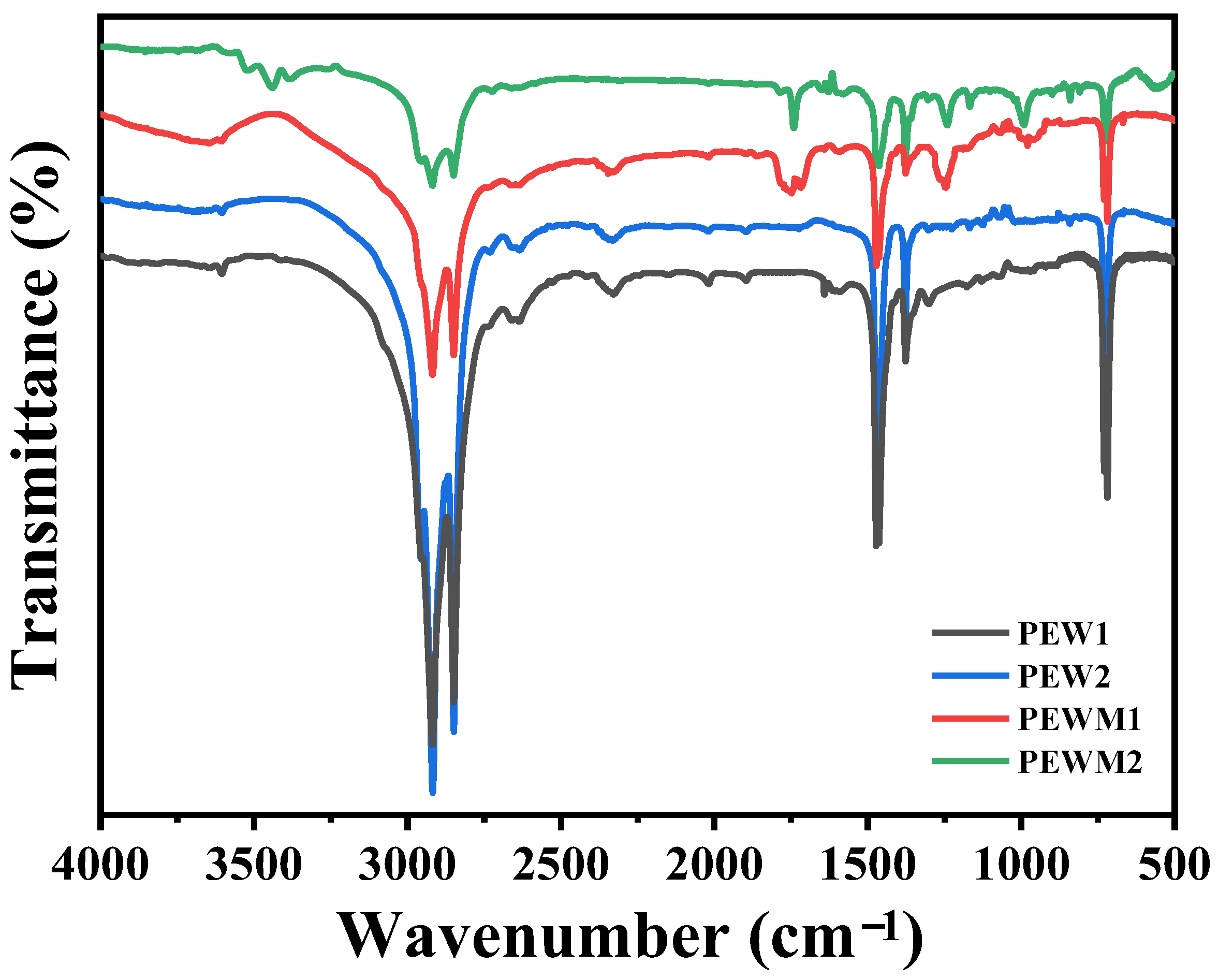
3.1. Characterization of Polyethylene Wax Additives
3.2. Torque Analysis
3.3. Morphology
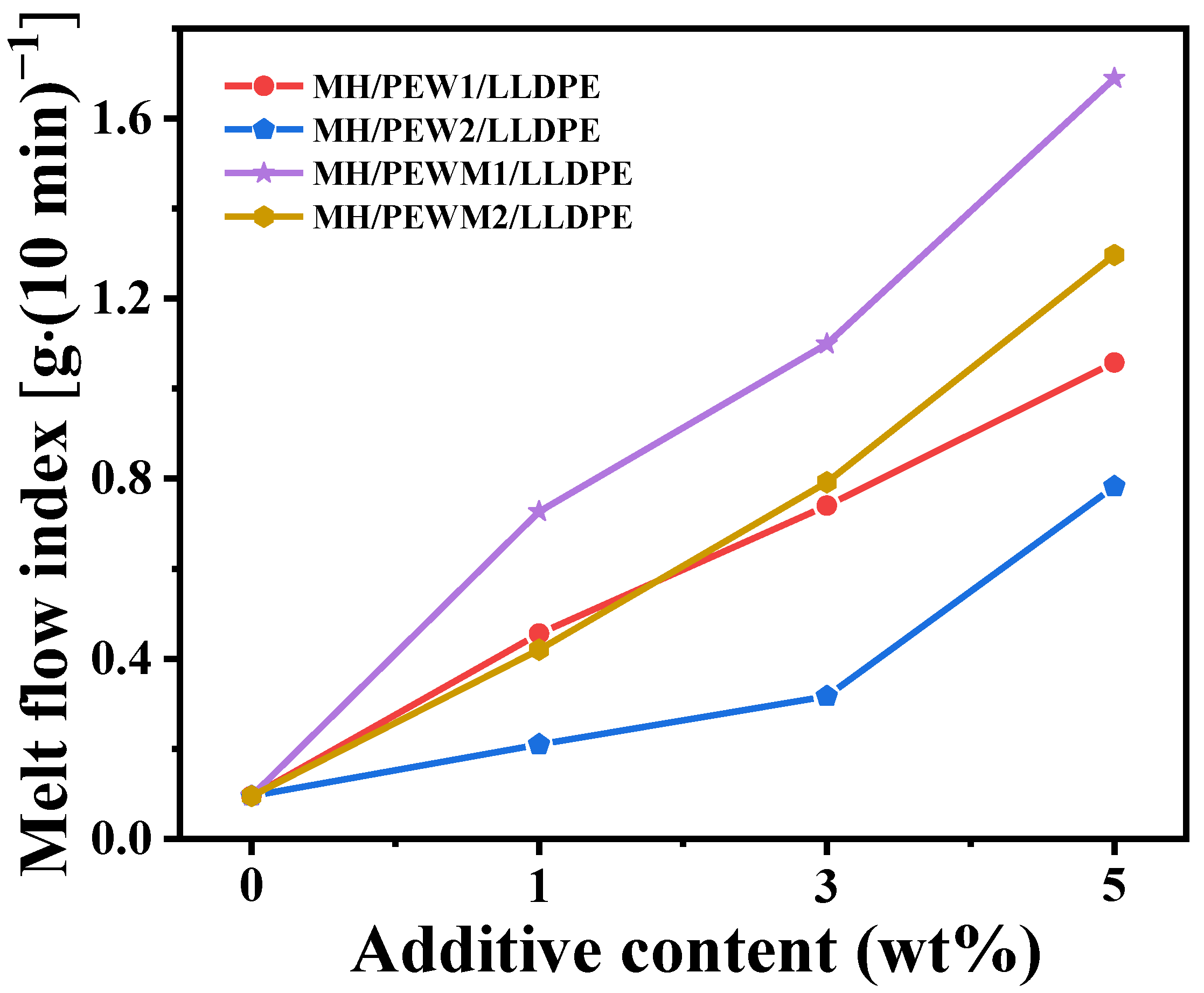
3.4. MFI
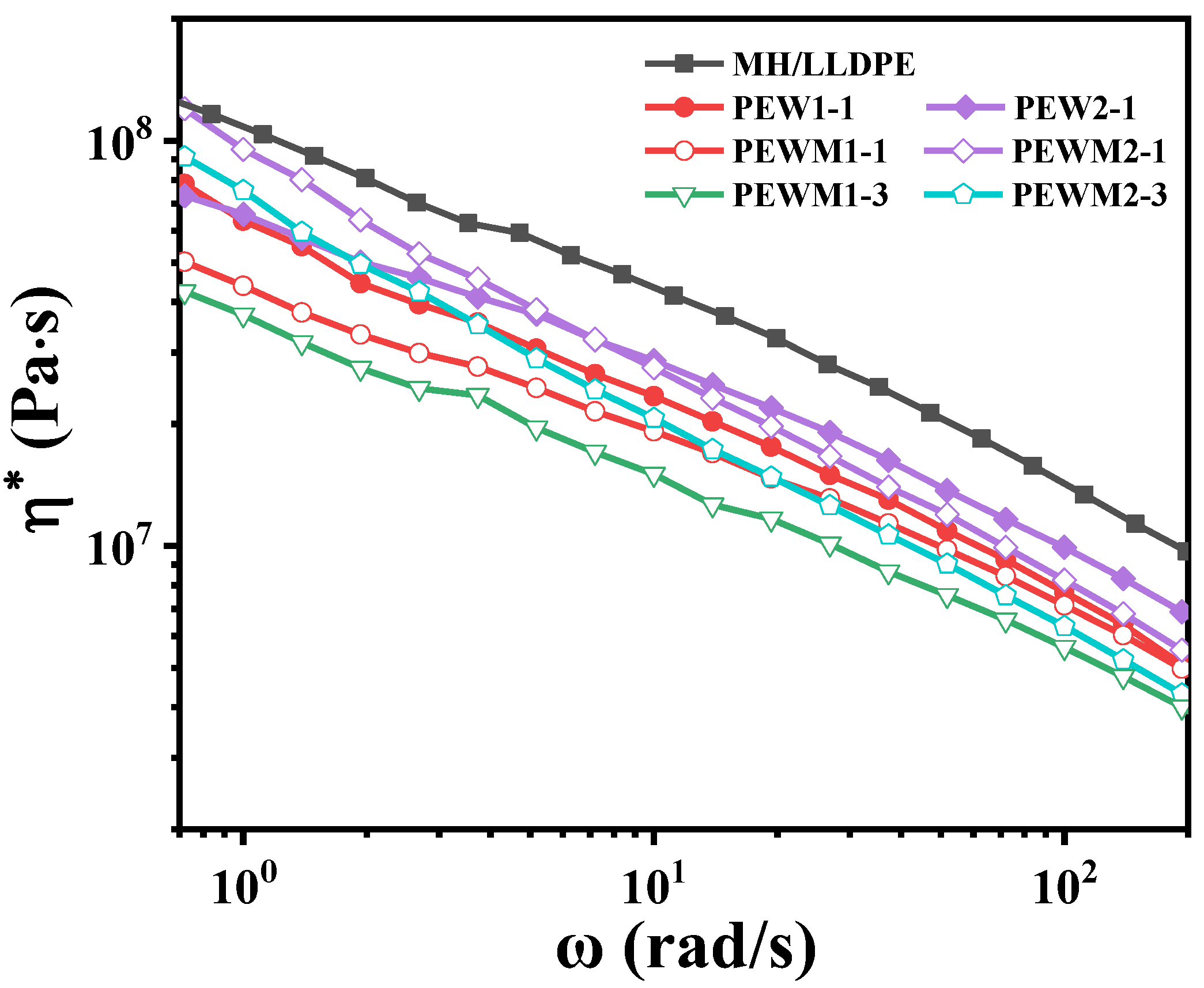
3.5. Dynamic Rheological Properties
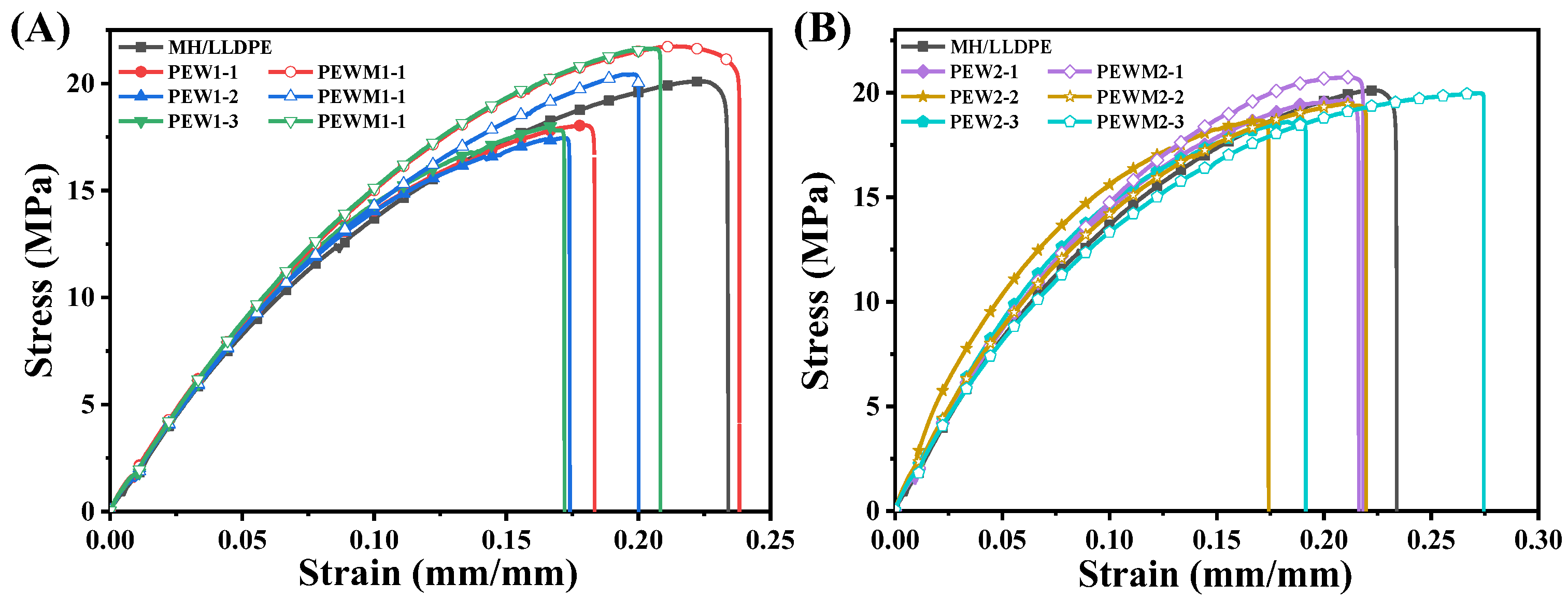
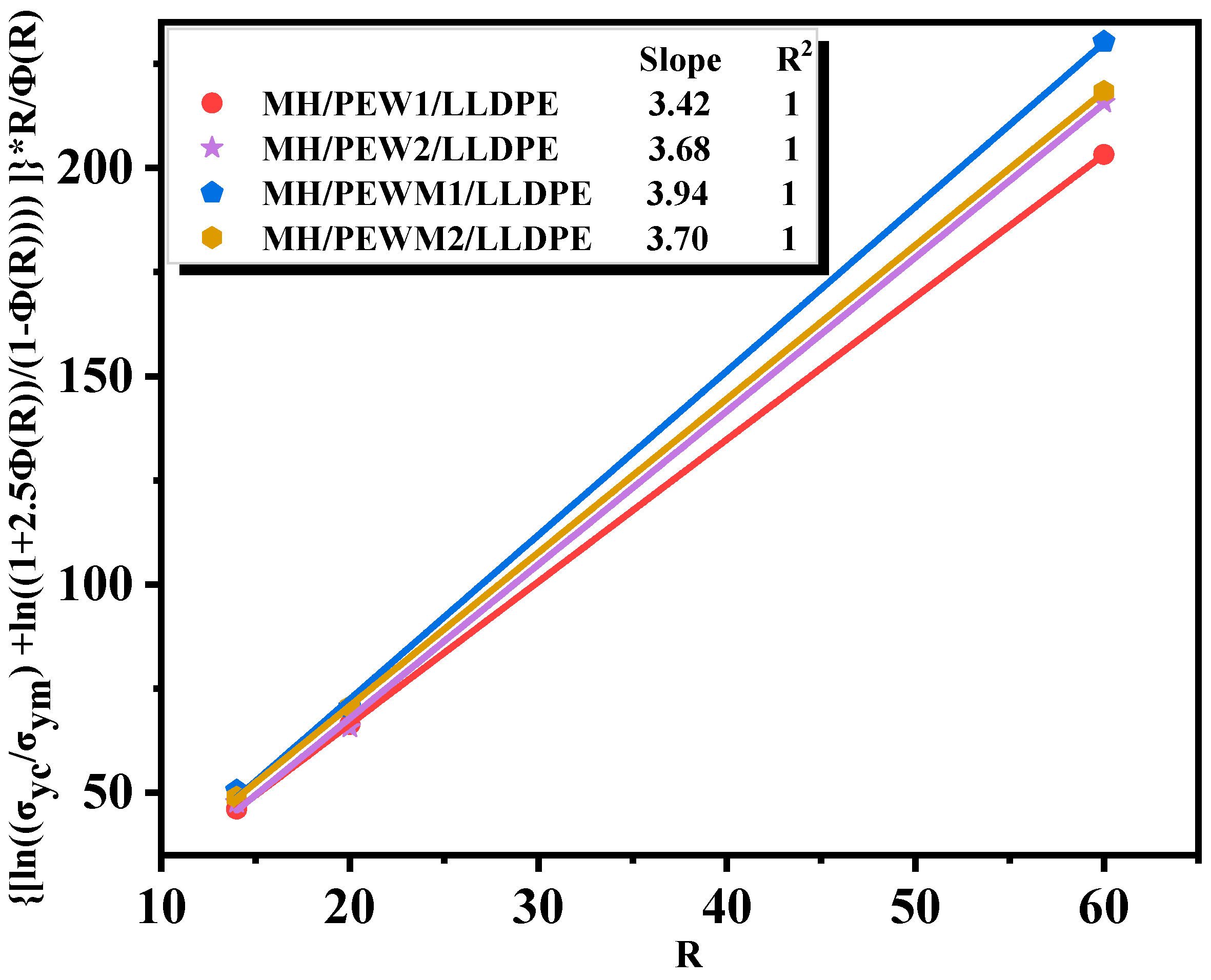
3.6. Mechanical Properties
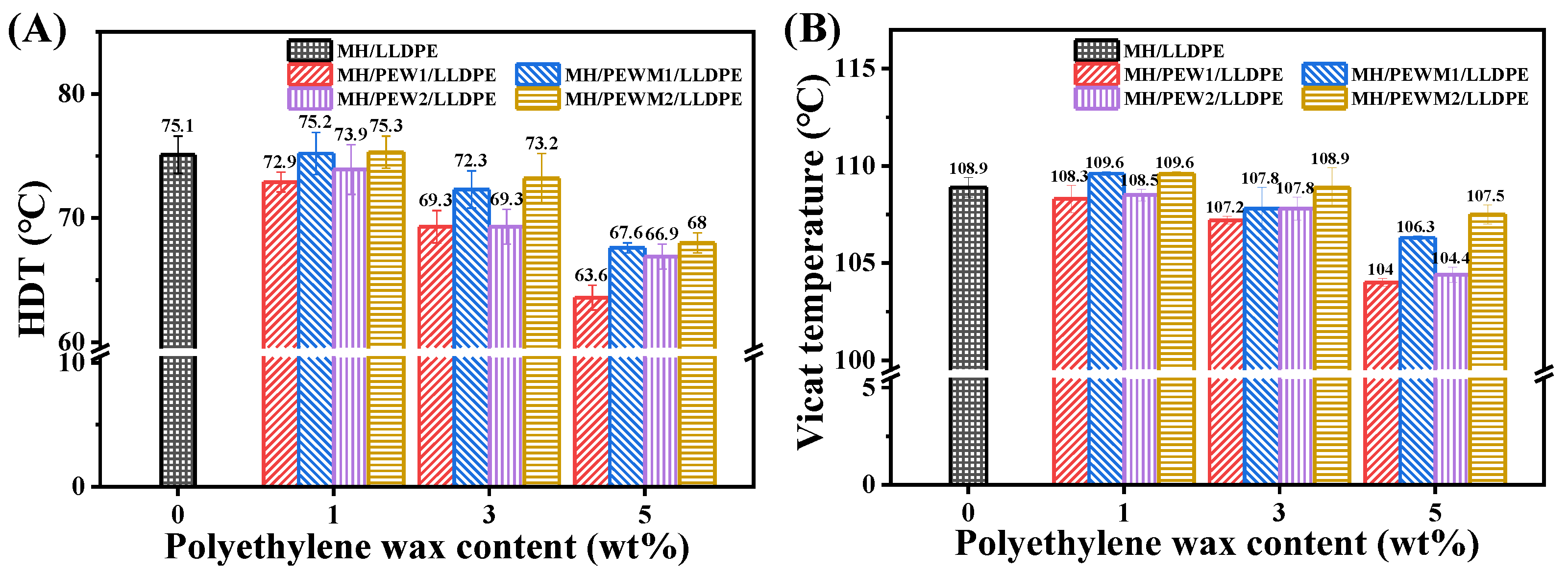
3.7. Thermal Stability
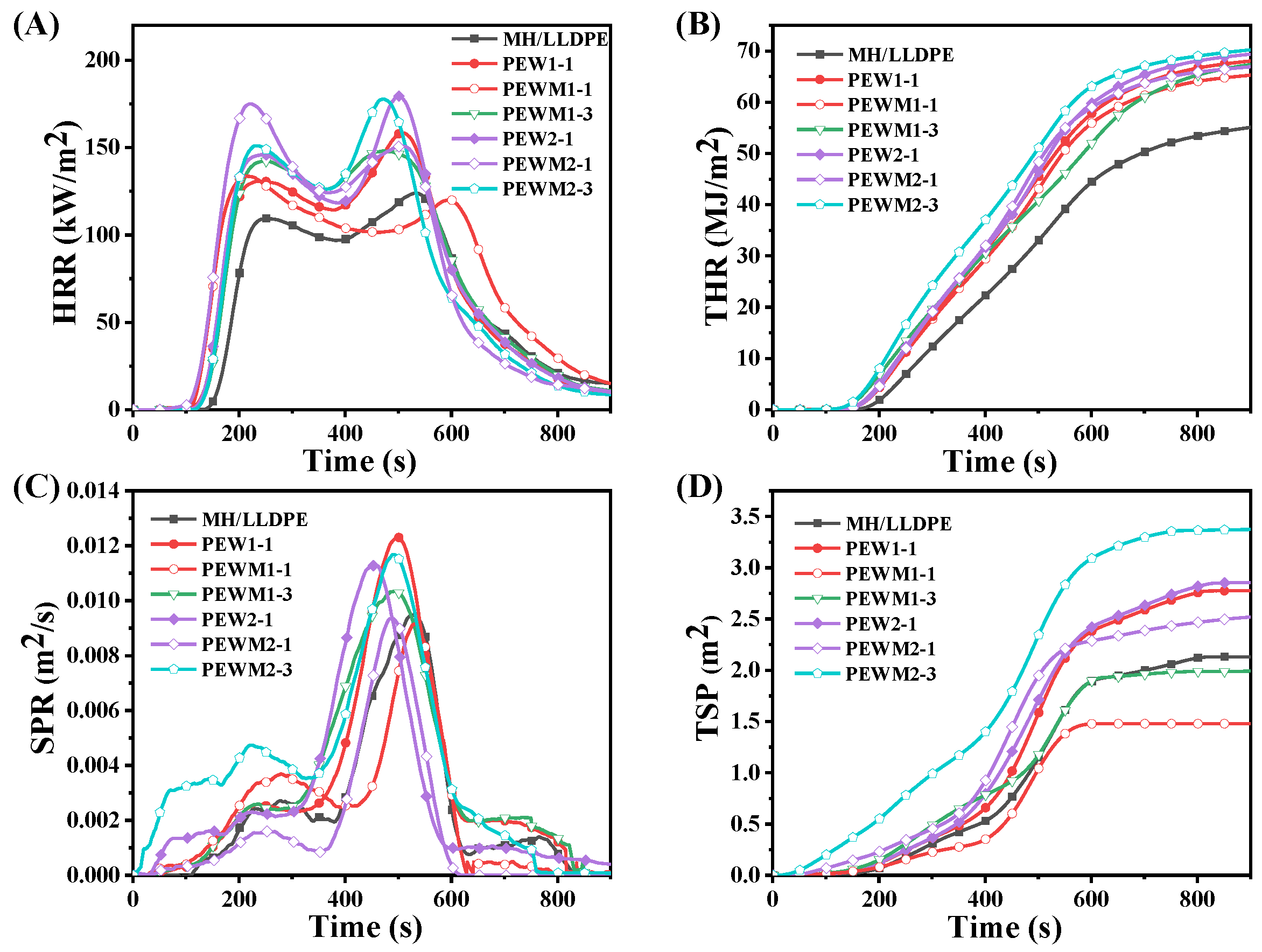
3.8. Flammability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, S.-C.; Liu, H. Melt Rheological Properties of LLDPE/PP Blends Compatibilized by Cross-Linked LLDPE/PP Blends (LLDPE-PP). Polym.-Plast. Technol. Eng. 2013, 52, 841–846. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, R.; Mu, J.; Ding, Y.; Jiang, J. Synergistic flame retardancy of linear low-density polyethylene with surface modified intumescent flame retardant and zinc borate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128400. [Google Scholar] [CrossRef]
- Ou, H.; Xu, J.; Liu, B.; Xue, H.; Weng, Y.; Jiang, J.; Xu, G. Study on synergistic expansion and flame retardancy of modified kaolin to low density polyethylene. Polymer 2021, 221, 123586. [Google Scholar] [CrossRef]
- Seyfi, S.K.; Ali, A.F. Properties of Linear Low Density Polyethylene. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 348–352. [Google Scholar] [CrossRef]
- Adner, D.; Helmy, M.; Otto, T.; Schellenberg, J.; Schadewald, A. A macromolecular halogen-free flame retardant and its effect on the properties of thermoplastic polyesters. Fire Mater. 2018, 43, 169–174. [Google Scholar] [CrossRef]
- Hong, X.D.; Liang, B.; Yang, D.X. Flame Retarded PE with MH/ATH/Microencapsulated Red Phosphorous and its Toughening by Polymeric Compatibilizers. Int. Polym. Process. 2014, 4, 447–453. [Google Scholar] [CrossRef]
- Qin, J.; Liu, N.; Wang, N.; Li, L.; He, W.; Guo, J.; Chen, X.; Zhang, K.; Yu, J. Synergistic Effect of Modified Expanded Graphite and Zinc Borate on the Flammability, Thermal Stability and Crystallization Behavior of LLDPE/EVA Composites with Mg(OH)2/Al(OH)3. Polym. Compos. 2018, 40, E687–E694. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, B.; Fan, W.; Huang, P. Combustion characteristics of halogen-free flame-retarded polyethylene containing magnesium hydroxide and some synergists. J. Appl. Polym. Sci. 2001, 81, 206–214. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef]
- Heinz, M.; Callsen, C.; Stöcklein, W.; Altstädt, V.; Ruckdäschel, H. Halogen-free flame-retardant cable compounds: Influence of magnesium-di-hydroxide filler and coupling agent on EVA/LLDPE blend system morphology. Polym. Eng. Sci. 2021, 62, 461–471. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Kong, X.; Liu, S.; Zhao, J. Flame retardancy effect of surface-modified metal hydroxides on linear low density polyethylene. J. Cent. South Univ. Technol. 2008, 15, 779–785. [Google Scholar] [CrossRef]
- Roza, M.A.; Gennadiy, E.Z. Flammability of polymeric materials. Polymer 2005, 70, 171–229. [Google Scholar]
- Lu, S.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Gul, R.; Islam, A.; Yasin, T.; Mir, S. Flame-retardant synergism of sepiolite and magnesium hydroxide in a linear low-density polyethylene composite. J. Appl. Polym. Sci. 2011, 121, 2772–2777. [Google Scholar] [CrossRef]
- Natrayan, L.; Kaliappan, S.; Baskara Sethupathy, S.; Sekar, S.; Patil, P.; Raja, S.; Velmurugan, G.; Abdeta, D. Investigation on Interlaminar Shear Strength and Moisture Absorption Properties of Soybean Oil Reinforced with Aluminium Trihydrate-Filled Polyester-Based Nanocomposites. J. Nanomater. 2022, 2022, 7588699. [Google Scholar] [CrossRef]
- Aseeva, R.; Zaikov, G. Flammability of polymeric materials. Adv. Polym. Sci. 2005, 70, 171–229. [Google Scholar] [CrossRef]
- Mochane, M.J.; Mokhothu, T.H.; Mokhena, T.C. Synthesis, mechanical, and flammability properties of metal hydroxide reinforced polymer composites: A review. Polym. Eng. Sci. 2021, 62, 44–65. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Xu, S. Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: The effect of filler shape. Compos. Part A Appl. Sci. Manuf. 2018, 113, 1–11. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Sun, J.; Gu, X.; Li, H.; Zhang, S. Improving flame retardant and mechanical properties of ethylene-vinyl acetate by cured compound silicone decorated magnesium hydroxide. J. Mater. Sci. 2022, 57, 2243–2256. [Google Scholar] [CrossRef]
- Harintharavimal, B.; Azman, H.; Nihat, A.I.; Cevdet, K. On the use of magnesium hydroxide towards halogen-free flame-retarded polyamide-6/polypropylene blends. Polym. Degrad. Stab. 2012, 97, 1447–1457. [Google Scholar]
- Albite-Ortega, J.; Sánchez-Valdes, S.; Ramirez-Vargas, E.; Nuñez-Figueredo, Y.; Ramos deValle, L.F.; Martínez-Colunga, J.G.; Graciano-Verdugo, A.Z.; Sanchez-Martínez, Z.V.; Espinoza-Martínez, A.B.; Rodriguez-Gonzalez, J.A.; et al. Influence of keratin and DNA coating on fire retardant magnesium hydroxide dispersion and flammability characteristics of PE/EVA blends. Polym. Degrad. Stab. 2019, 165, 1–11. [Google Scholar] [CrossRef]
- Sangcheol, K. Flame Retardancy and Smoke Suppression of Magnesium Hydroxide Filled Polyethylene. J. Polym. Sci. 2003, 41, 936–944. [Google Scholar]
- Zhao, W.; Kumar Kundu, C.; Li, Z.; Li, X.; Zhang, Z. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Gui, H.; Zhang, X.; Liu, Y.; Dong, W.; Wang, Q.; Gao, J.; Song, Z.; Lai, J.; Qiao, J. Effect of dispersion of nano-magnesium hydroxide on the flammability of flame retardant ternary composites. Compos. Sci. Technol. 2007, 67, 974–980. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Guo, S.; Lu, S.; Luo, Z.; He, M. Surface modification of magnesium hydroxide and its application in flame retardant polypropylene composites. J. Mater. Sci. 2009, 44, 1324–1332. [Google Scholar] [CrossRef]
- Natrayan, L.; Arul Kumar, P.; Baskara Sethupathy, S.; Sekar, S.; Patil, P.; Velmurugan, G.; Thanappan, S. Effect of Nano TiO2 Filler Addition on Mechanical Properties of Bamboo/Polyester Hybrid Composites and Parameters Optimized Using Grey Taguchi Method. Adsorpt. Sci. Technol. 2022, 2022, 6768900. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Noh, J.; Kang, I.; Choi, J.; Fatima, H.; Yoo, P.J.; Oh, K.W.; Park, J. Surface modification of magnesium hydroxide nanoparticles with hexylphosphoric acid to improve thermal stabilities of polyethylene composites. Polym. Bull. 2016, 73, 2855–2866. [Google Scholar] [CrossRef]
- Savas, L.A.; Arslan, C.; Hacioglu, F.; Dogan, M. Effect of reactive and nonreactive surface modifications and compatibilizer use on mechanical and flame-retardant properties of linear low-density polyethylene filled with huntite and hydromagnesite mineral. J. Therm. Anal. Calorim. 2018, 134, 1657–1666. [Google Scholar] [CrossRef]
- Huang, H.-X.; Zhang, J.-J. Effects of filler-filler and polymer-filler interactions on rheological and mechanical properties of HDPE-wood composites. J. Appl. Polym. Sci. 2009, 111, 2806–2812. [Google Scholar] [CrossRef]
- Cao, B.; Zhou, Y.; Wu, Y.; Cai, J.; Guan, X.; Liu, S.; Zhao, J.; Zhang, M. Simultaneous improvement of processability and toughness of highly filled MH/LLDPE composites by using fluorine-containing flow modifiers. Compos. Part A Appl. Sci. Manuf. 2020, 134, 105900. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Pham, T.N.; Gerard, J.F. Synthesis and physical properties of new surfactants based on ionic liquids: Improvement of thermal stability and mechanical behaviour of high density polyethylene nanocomposites. J. Colloid. Interface Sci. 2011, 354, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.L.; Guo, L.M. Surface modification of UHMWPE fibers by means of polyethylene wax grafted maleic anhydride treatment. J. Appl. Polym. Sci. 2018, 135, 46555. [Google Scholar] [CrossRef]
- Bakshi, A.K.; Ghosh, A.K. Processability and physico-mechanical properties of ultrahigh-molecular-weight polyethylene using low-molecular-weight olefin wax. Polym. Eng. Sci. 2022, 62, 2335–2350. [Google Scholar] [CrossRef]
- Mochane, M.J.; Mokhena, T.C.; Motaung, T.E.; Linganiso, L.Z. Shape-stabilized phase change materials of polyolefin/wax blends and their composites. J. Therm. Anal. Calorim. 2019, 139, 2951–2963. [Google Scholar] [CrossRef]
- Shen, C.; Xu, S. Effect of co-monomer on the adhesive performance of PP-g-MAH between cast polypropylene film and aluminum foil. Mater. Res. Express 2020, 7, 015316. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, X.; Liu, X.; Liang, Z.; Ge, Z.; Luo, Y. Preparation and properties of waterborne polyurethane modified by aminoethylaminopropyl polydimethylsiloxane for fluorine-free water repellents. Prog. Org. Coat. 2020, 139, 105407. [Google Scholar] [CrossRef]
- Aroon, V.S. Rheology of Filled Polymer Systems; Kluwer Academic Publishers: Holland, The Netherlands, 1999. [Google Scholar]
- He, S.; He, H.; Li, Y.; Wang, D. Effects of maleic anhydride grafted polyethylene on rheological, thermal, and mechanical properties of ultra high molecular weight polyethylene/poly(ethylene glycol) blends. J. Appl. Polym. Sci. 2015, 132, 42701. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Vassiliou, A.; Pavlidou, E.; Karayannidis, G.P. Compatibilisation effect of PP-g-MA copolymer on iPP/SiO2 nanocomposites prepared by melt mixing. Eur. Polym. J. 2005, 41, 1965–1978. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Liu, X.; Lv, Z.; Li, W. Effect of magnesium oxysulfate (MOS) morphology on the crystallization, mechanical, and rheological properties of polypropylene/MOS composites. J. Thermoplast. Compos. Mater. 2018, 32, 710–726. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.; Liu, X.; Zhu, D.; Dong, Y.; Li, W. Effects of different compatibilizing agents on the interfacial adhesion properties of polypropylene/magnesium oxysulfate whisker composites. Chin. J. Polym. Sci. 2017, 35, 1143–1155. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.; Khanal, S.; Xu, S. Controllable synthesis of hierarchical nanostructured anhydrous MgCO3 and its effect on mechanical and thermal properties of PVC composites. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105926. [Google Scholar] [CrossRef]
- Velmurugan, G.; Natrayan, L. Experimental investigations of moisture diffusion and mechanical properties of interply rearrangement of glass/Kevlar-based hybrid composites under cryogenic environment. J. Mater. Res. Technol. 2023, 23, 4513–4526. [Google Scholar] [CrossRef]
- Ponnusamy, M.; Natrayan, L.; Patil, P.; Velmurugan, G.; Thanappan, S. Multiresponse Optimization of Mechanical Behaviour of Calotropis gigantea/Nano-Silicon-Based Hybrid Nanocomposites under Cryogenic Environment. Adsorpt. Sci. Technol. 2022, 4138179. [Google Scholar] [CrossRef]
- Silva Barbosa Ferreira, E.; Luna, C.B.B.; Araújo, E.M.; Siqueira, D.D.; Wellen, R.M.R. Polypropylene/wood powder/ethylene propylene diene monomer rubber-maleic anhydride composites: Effect of PP melt flow index on the thermal, mechanical, thermomechanical, water absorption, and morphological parameters. Polym. Compos. 2020, 42, 484–497. [Google Scholar] [CrossRef]
- Miguel, A.; Lopez, M.; Jerico, B.; Josem, M.K. Rheological Behavior and Processability of Polypropylene Blends with Rubber Ethylene Propylene Diene Terpolymer. J. Appl. Polym. Sci. 2001, 81, 1–10. [Google Scholar]
- Dai, B.; Wang, Q.; Yan, W.; Li, Z.; Guo, W. Synergistic compatibilization and reinforcement of HDPE/wood flour composites. J. Appl. Polym. Sci. 2016, 133, 42958. [Google Scholar] [CrossRef]
- Saini, D.R.; Shenoy, A.V. Viscoelastic Properties of Highly Loaded Ferrite-Filled Polymeric Systems. Polym. Sci. Eng. Group 1986, 26, 441–445. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Yu, W.; Zhou, C. Dynamic rheological properties of wood polymer composites: From linear to nonlinear behaviors. Polym. Bull. 2010, 66, 683–701. [Google Scholar] [CrossRef]
- Ma, X.; Lu, Y.; Dang, L.; Xu, S. Effects of polyether titanate coupling agent on the flame retardancy and mechanical properties of soft poly(vinyl chloride)/basic magnesium carbonate composites. Polym. Compos. 2020, 41, 3594–3605. [Google Scholar] [CrossRef]
- Zou, S.; Dang, L.; Li, Y.; Lan, S.; Zhu, D.; Li, L. Inorganic-organic dual modification of magnesium borate whisker by magnesium hydrate and dodecyl dihydrogen phosphate and its effect on the fire safety and mechanical properties of epoxy resin. Appl. Surf. Sci. 2022, 589, 153064. [Google Scholar] [CrossRef]




| No. | MH | LLDPE | PEW | PEW-g-MAH |
|---|---|---|---|---|
| MH/LLDPE | 60 | 40 | 0 | 0 |
| PEW1-1 | 60 | 39 | 1 | 0 |
| PEW1-2 | 60 | 37 | 3 | 0 |
| PEW1-3 | 60 | 35 | 5 | 0 |
| PEW2-1 | 60 | 39 | 1 | 0 |
| PEW2-2 | 60 | 37 | 3 | 0 |
| PEW2-3 | 60 | 35 | 5 | 0 |
| PEWM1-1 | 60 | 39 | 0 | 1 |
| PEWM1-2 | 60 | 37 | 0 | 3 |
| PEWM1-3 | 60 | 35 | 0 | 5 |
| PEWM2-1 | 60 | 39 | 0 | 1 |
| PEWM2-2 | 60 | 37 | 0 | 3 |
| PEWM2-3 | 60 | 35 | 0 | 5 |
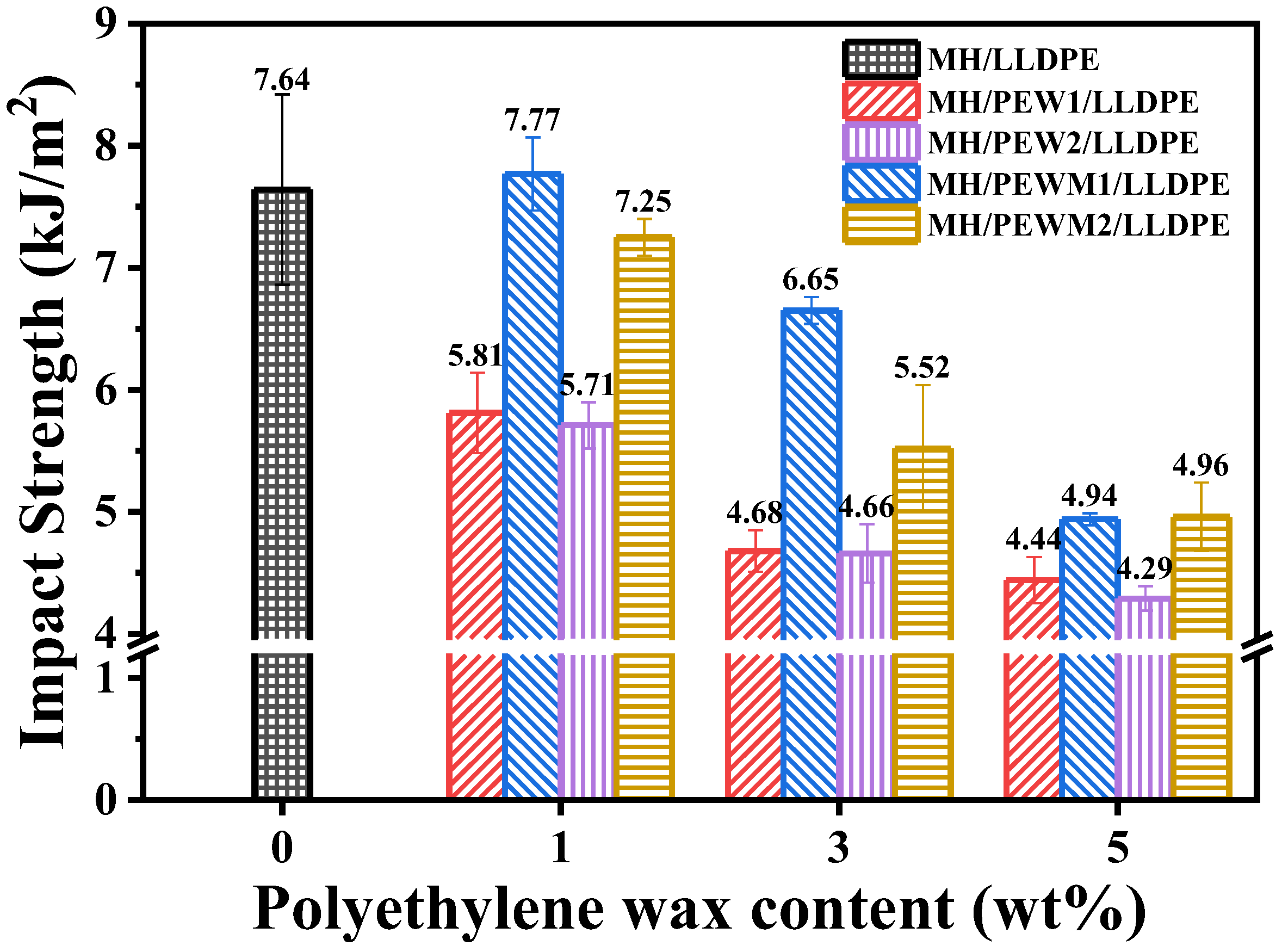
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|
| MH/LLDPE | 20.03 ± 1.10 | 23.42 ± 2.50 | 7.64 ± 0.78 |
| PEW1-1 | 17.85 ± 0.25 | 18.88 ± 0.53 | 5.81 ± 0.33 |
| PEW1-2 | 17.60 ± 0.61 | 16.69 ± 1.17 | 4.68 ± 0.17 |
| PEW1-3 | 17.50 ± 0.72 | 15.68 ± 1.18 | 4.44 ± 0.19 |
| PEW2-1 | 19.23 ± 1.06 | 20.38 ± 1.08 | 5.71 ± 0.19 |
| PEW2-2 | 17.37 ± 0.99 | 16.53 ± 1.76 | 4.66 ± 0.24 |
| PEW2-3 | 18.41 ± 1.02 | 18.76 ± 1.53 | 4.29 ± 0.10 |
| PEWM1-1 | 21.29 ± 0.81 | 24.00 ± 1.54 | 7.77 ± 0.30 |
| PEWM1-2 | 19.21 ± 0.88 | 19.71 ± 1.38 | 6.65 ± 0.11 |
| PEWM1-3 | 20.16 ± 0.88 | 20.08 ± 0.72 | 4.94 ± 0.05 |
| PEWM2-1 | 19.56 ± 0.83 | 21.40 ± 0.68 | 7.25 ± 0.15 |
| PEWM2-2 | 19.25 ± 2.06 | 22.61 ± 1.32 | 5.52 ± 0.52 |
| PEWM2-3 | 19.13 ± 0.80 | 25.80 ± 1.95 | 4.96 ± 0.28 |
| Samples | Temperature (°C) | ||
|---|---|---|---|
| T5% | Tmax | Char Yield (%) | |
| MH/LLDPE | 403.1 | 477.0 | 41.9 |
| PEW1-1 | 396.4 | 475.3 | 41.4 |
| PEW2-1 | 395.3 | 476.1 | 41.2 |
| PEWM1-1 | 403.4 | 477.6 | 42.2 |
| PEWM1-3 | 400.6 | 476.5 | 39.4 |
| PEWM2-1 | 397.7 | 476.8 | 41.3 |
| PEWM2-3 | 362.3 | 476.3 | 41.0 |
| Samples | LOI (%) | Tign (s) | PHRR (kW/m2) | THR (MJ/m2) | PSPR (m2/s) | TSP (m2/kg) | FPI (10−2) | AMLR (g/s) |
|---|---|---|---|---|---|---|---|---|
| MH/LLDPE | 47.0 | 160 | 123.75 | 58.43 | 0.0095 | 2.13 | 129.29 | 1.52 |
| PEW1-1 | 45.0 | 138 | 158.53 | 68.54 | 0.012 | 2.78 | 87.05 | 2.04 |
| PEWM1-1 | 46.0 | 141 | 133.65 | 65.68 | 0.0092 | 1.48 | 105.50 | 1.90 |
| PEWM1-3 | 44.0 | 121 | 148.10 | 68.49 | 0.010 | 2.00 | 81.70 | 1.60 |
| PEW2-1 | 44.7 | 136 | 179.55 | 69.85 | 0.011 | 2.86 | 75.75 | 2.18 |
| PEWM2-1 | 45.8 | 138 | 175.11 | 67.28 | 0.0094 | 2.53 | 78.81 | 2.00 |
| PEWM2-3 | 43.9 | 126 | 177.83 | 70.66 | 0.012 | 3.38 | 70.86 | 2.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Xu, S.; Xu, J.; Pan, T.; Sun, B.; Dang, L. Effect of Functionalized Polyethylene Wax on the Melt Processing and Properties of Highly Filled Magnesium Hydroxide/Linear Low-Density Polyethylene Composites. Polymers 2023, 15, 2575. https://doi.org/10.3390/polym15112575
Li R, Xu S, Xu J, Pan T, Sun B, Dang L. Effect of Functionalized Polyethylene Wax on the Melt Processing and Properties of Highly Filled Magnesium Hydroxide/Linear Low-Density Polyethylene Composites. Polymers. 2023; 15(11):2575. https://doi.org/10.3390/polym15112575
Chicago/Turabian StyleLi, Rujie, Shiai Xu, Jiajun Xu, Tongtong Pan, Beibei Sun, and Li Dang. 2023. "Effect of Functionalized Polyethylene Wax on the Melt Processing and Properties of Highly Filled Magnesium Hydroxide/Linear Low-Density Polyethylene Composites" Polymers 15, no. 11: 2575. https://doi.org/10.3390/polym15112575
APA StyleLi, R., Xu, S., Xu, J., Pan, T., Sun, B., & Dang, L. (2023). Effect of Functionalized Polyethylene Wax on the Melt Processing and Properties of Highly Filled Magnesium Hydroxide/Linear Low-Density Polyethylene Composites. Polymers, 15(11), 2575. https://doi.org/10.3390/polym15112575