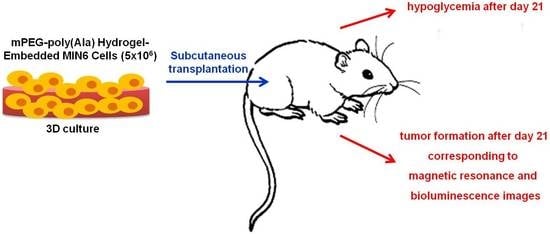
The Image–Histology Correlation of Subcutaneous mPEG-poly(Ala) Hydrogel-Embedded MIN6 Cell Grafts in Nude Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of L-Alanine N-Carboxyanhydride (NCA-Ala) and mPEG-poly(Ala) Diblock Copolymers
2.3. Culture of MIN6 Cells
2.4. SubcutaneousTransplantation of MIN6 Cells
2.5. Histological Analysis of Hydrogel-Embedded MIN6 Cell Grafts
3. Results and Discussion
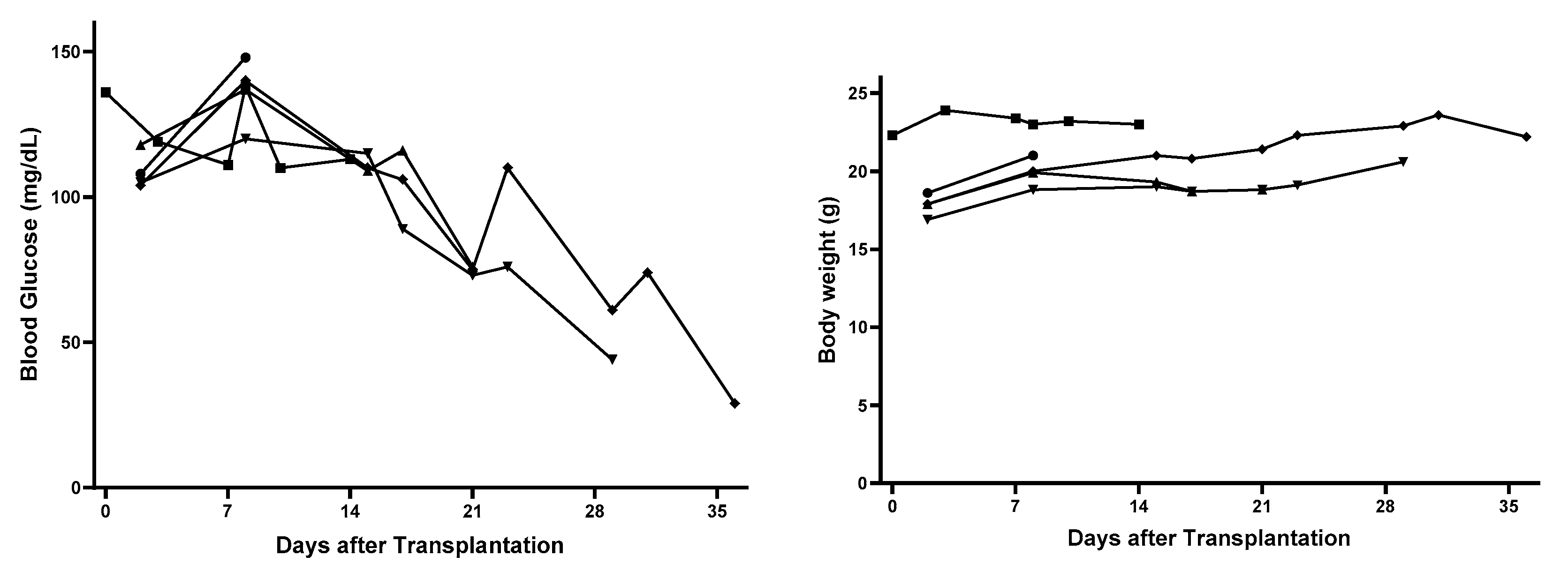
3.1. Changes of Nude Mouse Recipients’ Blood Glucose and Body Weight after Subcutaneous Transplantation of Hydrogel-Embedded MIN6 Cells
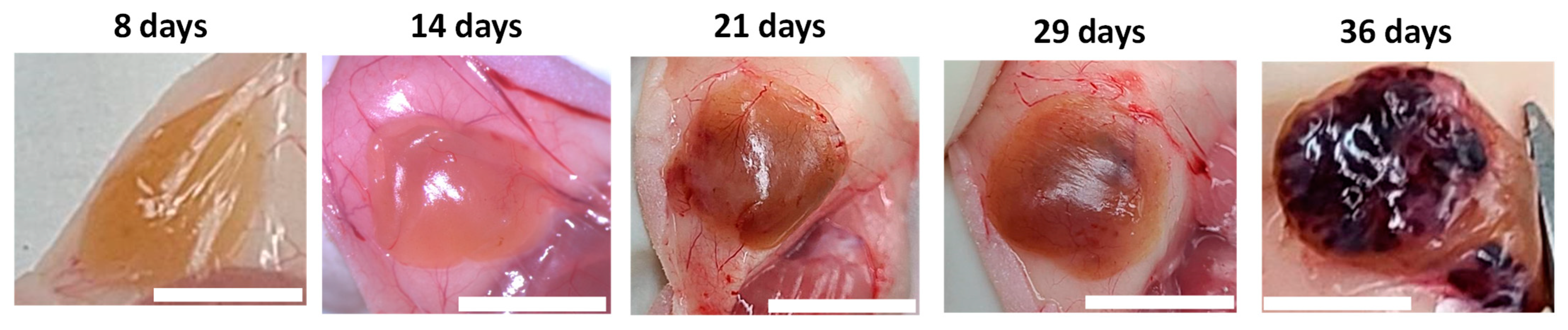
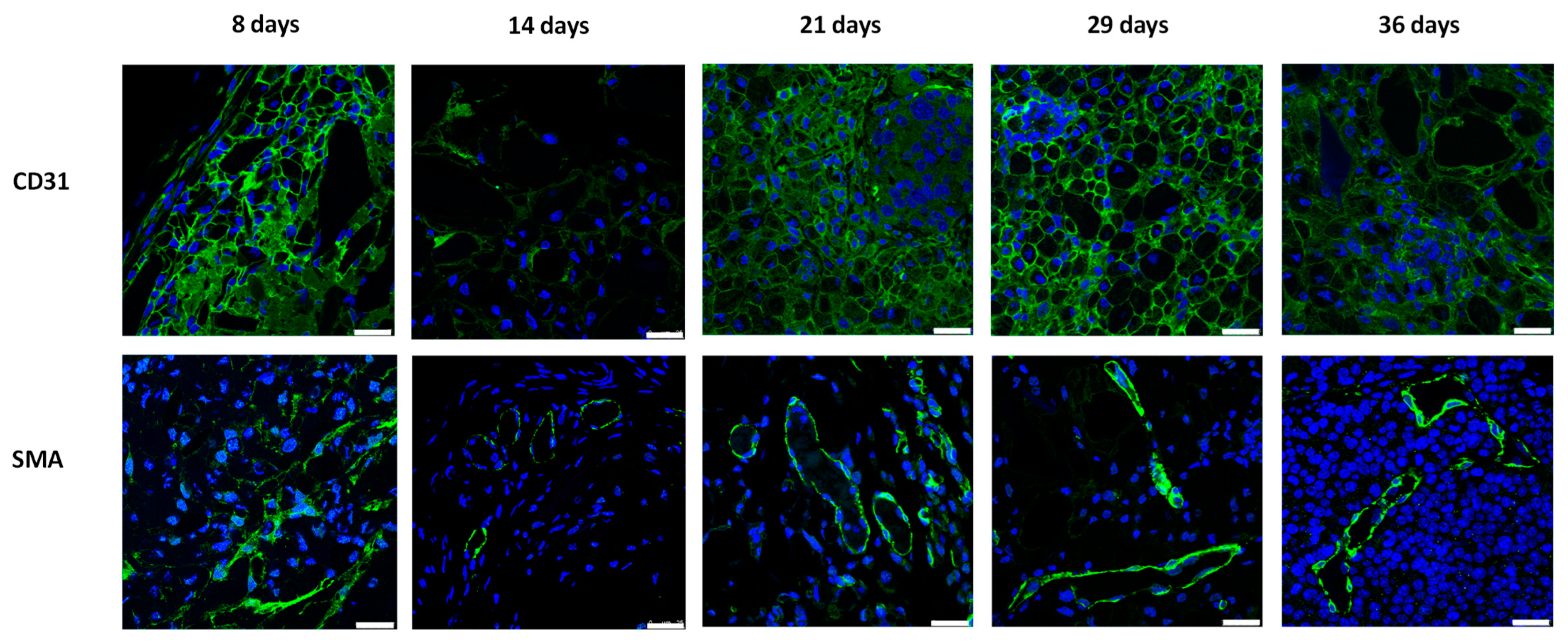
3.2. Vascularization of Subcutaneous Hydrogel-Embedded MIN6 Cell Grafts
3.3. Histological Evolution of Subcutaneous Hydrogel-Embedded MIN6 CELL GRAFTS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfil-Garza, B.A.; Imes, S.; Verhoeff, K.; Hefler, J.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bigam, D.; et al. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 2022, 10, 519–532. [Google Scholar] [CrossRef]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef] [Green Version]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The journey of islet cell transplantation and future development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Rajab, A. Islet Transplantation: Alternative Sites. Curr. Diab. Rep. 2010, 10, 332–337. [Google Scholar] [CrossRef]
- Brusko, T.M.; Russ, H.A.; Stabler, C.L. Strategies for durable β cell replacement in type 1 diabetes. Science 2021, 373, 516–522. [Google Scholar] [CrossRef]
- Kawahara, T.; Kin, T.; Kashkoush, S.; Gala-Lopez, B.; Bigam, D.L.; Kneteman, N.M.; Koh, A.; Senior, P.A.; Shapiro, A.M. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am. J. Transplant. 2011, 11, 2700–2707. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Kin, T.; Shapiro, A.M. A comparison of islet autotransplantation with allotransplantation and factors elevating acute portal pressure in clinical islet transplantation. J. Hepatobiliary Pancreat. Sci. 2012, 19, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Aoki, T.; Yoshimatsu, G.; Tsuchiya, H.; Hata, T.; Katayose, Y.; Egawa, S.; Unno, M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab. Res. Rev. 2014, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Bonner-Weir, S.; Vacanti, J.P.; Ogawa, Y.; Weir, G.C. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 1996, 61, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Hsu, B.R.; Kuo, C.H. Islet transplantation at subcutaneous and intramuscular sites. Transplant. Proc. 2005, 37, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Michael, D. Hunckler and Andrés J. García. Engineered biomaterials for enhanced function of insulin-secreting β-cell organoids. Adv. Funct. Mater. 2020, 30, 2000134. [Google Scholar] [CrossRef]
- Deng, C.; Vulesevic, B.; Ellis, C.; Korbutt, G.S.; Suuronen, E.J. Vascularization of collagen-chitosan scaffolds with circulating progenitor cells as potential site for islet transplantation. J. Control. Release 2011, 152 (Suppl. S1), e196–e198. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive hydrogels and advances in their application in disease therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Alsberg, E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog. Polym. Sci. 2014, 39, 1236–1265. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Anderson, M.A.; Ao, Y.; Khakh, B.S.; Fan, J.; Deming, T.J.; Sofroniew, M.V. Tunable diblock copolypeptide hydrogel depots for local delivery of hydrophobic molecules in healthy and injured central nervous system. Biomaterials 2014, 35, 1989–2000. [Google Scholar] [CrossRef] [Green Version]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering cell-adhesion peptides in tissue engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef]
- Bao, X.; Si, X.; Ding, X.; Duan, L.; Xiao, C. pH-responsive hydrogels based on the self-assembly of short polypeptides for controlled release of peptide and protein drugs. J. Polym. Res. 2019, 26, 278. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lai, P.-L.; Lin, Y.-K.; Peng, S.; Lee, L.-Y.; Chen, C.-N.; Chu, I.-M. A poloxamer-polypeptide thermosensitive hydrogel as a cell scaffold and sustained release depot. Polym. Chem. 2016, 7, 2976–2985. [Google Scholar] [CrossRef]
- Lin, H.-C.; Chen, C.-Y.; Kao, C.-W.; Wu, S.-T.; Chen, C.-L.; Shen, C.-R.; Juang, J.-H.; Chu, I.-M. In situ gelling-polypeptide hydrogel systems for the subcutaneous transplantation of MIN6 cells. J. Polym. Res. 2020, 27, 64. [Google Scholar] [CrossRef]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ohgawara, H.; Miyazaki, J.; Karibe, S.; Tashiro, F.; Akaike, T.; Hashimoto, Y. Embedded-culture of pancreatic beta-cells derived from transgenic mouse insulinoma as a potential source for xenotransplantation using a diffusion chamber. Cell Transplant. 1995, 4, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Sobel, D.O.; Ramasubramanian, B.; Mitnaul, L. Characterization of a mouse model of islet transplantation using MIN-6 cells. Islets 2020, 12, 71–86. [Google Scholar] [CrossRef]
- Yu, C.P.; Juang, J.H.; Lin, Y.J.; Kuo, C.W.; Hsieh, L.H.; Huang, C.C. Enhancement of subcutaneously transplanted β cell survival using 3d stem cell spheroids with proangiogenic and prosurvival potential. Adv. Biosyst. 2020, 4, e1900254. [Google Scholar] [CrossRef]
- Juang, J.-H.; Lin, H.-C.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Wu, S.-T.; Lin, S.-H.; Shen, C.-R.; Wang, J.-J.; Tsai, Z.-T.; et al. Noninvasive tracking of mPEG-poly(Ala) hydrogel-embedded min6 cells after subcutaneous transplantation in mice. Polymers 2021, 13, 885. [Google Scholar] [CrossRef]
- Tsai, Z.-T.; Wang, J.-F.; Kuo, H.-Y.; Shen, C.-R.; Wang, J.-J.; Yen, T.-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: A potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater. 2010, 322, 208–213. [Google Scholar] [CrossRef]
- Cheng, K.; Delghingaro-Augusto, V.; Nolan, C.J.; Turner, N.; Hallahan, N.; Andrikopoulos, S.; Gunton, J.E. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PLoS ONE 2012, 7, e40868. [Google Scholar] [CrossRef] [Green Version]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juang, J.-H.; Wang, J.-J.; Shen, C.-R.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Lin, S.-H.; Wu, S.-T.; Li, W.-C.; Tsai, Z.-T. Magnetic resonance imaging of transplanted porcine neonatal pancreatic cell clusters labeled with chitosan-coated superparamagnetic iron oxide nanoparticles in mice. Polymers 2021, 13, 1238. [Google Scholar] [CrossRef]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant. Proc. 2010, 42, 2104–2108. [Google Scholar] [CrossRef]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Chen, F.R.; Chen, M.H.; Yen, T.C.; Tsai, Z.T. Magnetic resonance imaging of mouse islet grafts labeled with novel chitosan-coated superparamagnetic iron oxide nanoparticles. PLoS ONE 2013, 8, e62626. [Google Scholar] [CrossRef]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Lin, M.Y.; Wu, S.T.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant. Proc. 2010, 42, 4217–4220. [Google Scholar] [CrossRef]
- Tsujimura, M.; Kusamori, K.; Oda, C.; Miyazaki, A.; Katsumi, H.; Sakane, T.; Nishikawa, M.; Yamamoto, A. Regulation of proliferation and functioning of transplanted cells by using herpes simplex virus thymidine kinase gene in mice. J. Control. Release 2018, 275, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Inoue, K.; Hayashi, H.; Wang, W.J.; Setoyama, H.; Gu, Y.J.; Imamura, M.; Iwata, H.; Ikada, Y.; Nozawa, M.; et al. Subcutaneous xenotransplantation of hybrid artificial pancreas encapsulating pancreatic B cell line (MIN6): Functional and histological study. Cell Transplant. 1997, 6, 541–545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, J.-H.; Chen, C.-L.; Kao, C.-W.; Chen, C.-Y.; Shen, C.-R.; Wang, J.-J.; Tsai, Z.-T.; Chu, I.-M. The Image–Histology Correlation of Subcutaneous mPEG-poly(Ala) Hydrogel-Embedded MIN6 Cell Grafts in Nude Mice. Polymers 2023, 15, 2584. https://doi.org/10.3390/polym15122584
Juang J-H, Chen C-L, Kao C-W, Chen C-Y, Shen C-R, Wang J-J, Tsai Z-T, Chu I-M. The Image–Histology Correlation of Subcutaneous mPEG-poly(Ala) Hydrogel-Embedded MIN6 Cell Grafts in Nude Mice. Polymers. 2023; 15(12):2584. https://doi.org/10.3390/polym15122584
Chicago/Turabian StyleJuang, Jyuhn-Huarng, Chen-Ling Chen, Chen-Wei Kao, Chen-Yi Chen, Chia-Rui Shen, Jiun-Jie Wang, Zei-Tsan Tsai, and I-Ming Chu. 2023. "The Image–Histology Correlation of Subcutaneous mPEG-poly(Ala) Hydrogel-Embedded MIN6 Cell Grafts in Nude Mice" Polymers 15, no. 12: 2584. https://doi.org/10.3390/polym15122584