Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration
Abstract
:1. Introduction
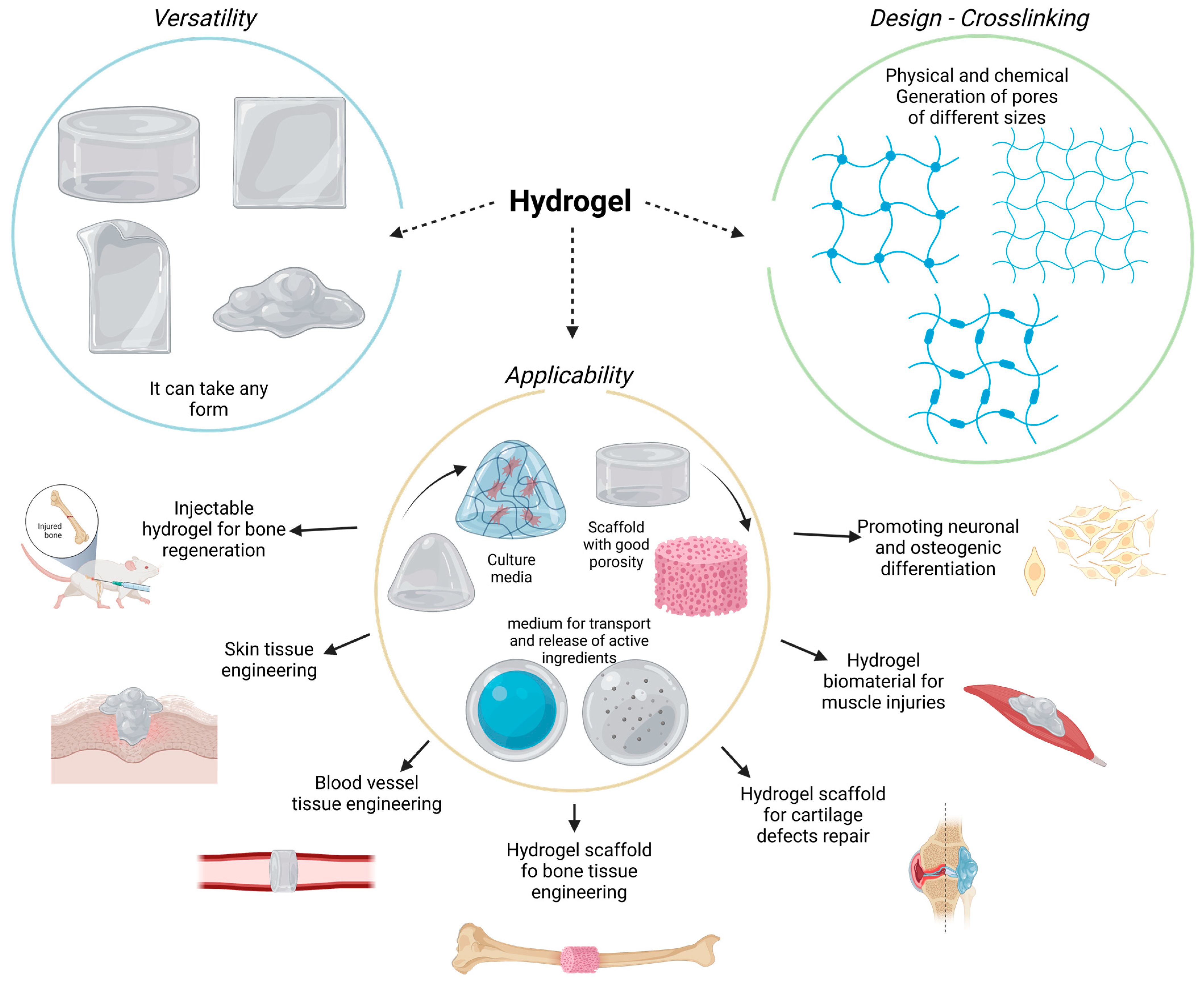
2. Hydrogels
3. Hydrogel Classification
4. Hydrogels for Bone Regeneration
- No cytotoxic and no immunogenic response, in order to avoid a chronic and non-regulable inflammatory reaction;
- Osteoinductive, osteoconductive, osteogenic, and osteocompatible qualities for better bone anchorage and regeneration;
- Mimicking the natural ECM at the implant site;
- Degradable by different enzymes or environmental molecules, leaving sufficient space for new bone formation;
- Resistant and stable during sterilization;
- Controlling the size and interconnection of the pores to optimize the characteristics of drug release, cell growth, oxygen diffusion, and nutrient exchange;
- Patient-friendly injectable form to reduce pain and simplify the administration process.
5. Chitosan as a Carbohydrate Material for Hydrogels
6. Collagen as a Protein Structural Material for Hydrogels
- Fibril-forming;
- Fibril-associated collagens;
- Network-forming collagens;
- Anchoring fibrils;
- Transmembrane collagens;
- Basement membrane collagen;
- Others with unique functions.
7. Gelatin Material for Hydrogels Development
8. Application of Chitosan, Gelatin, and Collagen Hydrogels in BTE
9. Prospects and Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, P.; Yeo, A.; Ramachandran, M. Bone Injury, Healing and Grafting. In Basic Orthopaedic Sciences; CRC Press: Boca Raton, FL, USA, 2017; pp. 205–222. [Google Scholar] [CrossRef]
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355S, S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J.; et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, A.K.; Patel, D.K.; Maiti, P. Nanohybrid Scaffold of Chitosan and Functionalized Graphene Oxide for Controlled Drug Delivery and Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5139–5149. [Google Scholar] [CrossRef]
- Tzagiollari, A.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering 2022, 9, 250. [Google Scholar] [CrossRef]
- Gupta, H.; Zioupos, P. Fracture of bone tissue: The ‘hows’ and the ‘whys’. Med. Eng. Phys. 2008, 30, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef]
- Audigé, L.; Griffin, D.; Bhandari, M.; Kellam, J.; Rüedi, T.P. Path Analysis of Factors for Delayed Healing and Nonunion in 416 Operatively Treated Tibial Shaft Fractures. Clin. Orthop. Relat. Res. 2005, 438, 221–232. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, C.-C.; Wang, C.Y.; Lee, A.K.-X.; Yeh, C.-L.; Lin, C.-P. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-ε-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers 2020, 12, 1455. [Google Scholar] [CrossRef] [PubMed]
- Zyuzkov, G. Targeted Regulation of Intracellular Signal Transduction in Regeneration-Competent Cells: A new Direction for Therapy in Regenerative Medicine. Biointerface Res. Appl. Chem. 2021, 11, 12238–12251. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Fibbe, W.E.; Dazzi, F.; LeBlanc, K. MSCs: Science and trials. Nat. Med. 2013, 19, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Eshghanmalek, M. Biomaterials for repair and regeneration of the cartilage tissue. Bio-Design Manuf. 2019, 2, 41–49. [Google Scholar] [CrossRef]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Shin, M.; Burdick, J.A. Editorial: Special Issue on Advanced Biomedical Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 3993–3996. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef]
- Liao, H.T.; Tsai, M.-J.; Brahmayya, M.; Chen, J.-P. Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. Int. J. Mol. Sci. 2018, 19, 2537. [Google Scholar] [CrossRef] [Green Version]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J. Mater. Chem. B 2015, 3, 7818–7830. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Naseri-Nosar, M.; Ebrahimi-Barough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Wang, L.; Zhang, D.; Zhang, J.; Guan, F.; Yao, M.-H. Dual-enzymatically crosslinked hyaluronic acid hydrogel as a long-time 3D stem cell culture system. Biomed. Mater. 2020, 15, 045013. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.I.; Farrukh, A.; Valbuena-Mendoza, R.; Włodarczyk-Biegun, M.K.; del Campo, A. Thiol-Methylsulfone-Based Hydrogels for 3D Cell Encapsulation. ACS Appl. Mater. Interfaces 2020, 12, 8062–8072. [Google Scholar] [CrossRef]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Gómez-Ríos, D.; Barrera-Zapata, R.; Ríos-Estepa, R. Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus®. Food Bioprod. Process. 2017, 103, 49–57. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.; Jo, D.-H.; Anower, A.K.M.M.; Islam, S.M.S.; Sohn, S. Chitosan as an Immunomodulating Adjuvant on T-Cells and Antigen-Presenting Cells in Herpes Simplex Virus Type 1 Infection. Mediat. Inflamm. 2016, 2016, 4374375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadimitriou, L.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Immunomodulatory Potential of Chitosan-graft-poly(ε-caprolactone) Copolymers toward the Polarization of Bone-Marrow-Derived Macrophages. ACS Biomater. Sci. Eng. 2017, 3, 1341–1349. [Google Scholar] [CrossRef]
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- dos Santos, Z.; Caroni, A.; Pereira, M.; da Silva, D.; Fonseca, J. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Domalik-Pyzik, P.; Chłopek, J.; Pielichowska, K. Chitosan-Based Hydrogels: Preparation, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 1665–1693. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Limón, V.H.V. Caracterización por Espectroscopia Infrarroja con Transformada de Fourier (FT-IR) de Mezclas Acuosas de Ácido Ascórbico y Quitosán Funcionalizadas con Nanoparticulas de Ag, Cu y Au. Ph.D. Thesis, Universidad Autónoma de Baja California, Mexicali, Mexico, 2014. Available online: https://cienciaabierta.uabc.mx/Record/repositorioinstitucional-20.500.12930-1616/Description (accessed on 9 December 2022).
- Velázquez, C.H.H. Desarrollo del Prototipo Funcional de una Membrana de Quitosán con Microcápsulas de Triclosán Para el Tratamiento de la Periodontitis Crónica. Master Thesis, Universidad Autónoma de Baja California, Mexicali, Mexico, 2018. Available online: https://cienciaabierta.uabc.mx/Record/repositorioinstitucional-20.500.12930-1466 (accessed on 4 January 2023).
- Santiago, E.; Pina-Luis, G.; Martinez-Quiroz, M.; Perez-Landeros, O.; Rosas-González, N.; Valdez-Salas, B.; Oropeza-Guzman, M.T. Eco-Friendly Magnetic Nanoscavengers as Emerging Materials for Improving Reclaimed Water Quality. Adv. Sustain. Syst. 2021, 5, 2000236. [Google Scholar] [CrossRef]
- Valderruten, N.; Valverde, J.; Zuluaga, F.; Ruiz-Durántez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Montaño Hurtado, D.H. Desarrollo de Geles Conductoras a Base de Quitosano: Efecto del Agente Entrecruzante. BSc. Thesis, Universidad del Valle, Santiago de Cali, Colombia, 2016. Available online: https://bibliotecadigital.univalle.edu.co/bitstream/handle/10893/14599/CB-0539493.pdf?sequence=1 (accessed on 5 January 2023).
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lapitsky, Y. Salt-Assisted Mechanistic Analysis of Chitosan/Tripolyphosphate Micro- and Nanogel Formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Niyibizi, C.; Eyre, D.R. Structural Characteristics of Cross-Linking Sites in type V Collagen of Bone. Chain Specificities and Heterotypic Links to Type I Collagen. JBIC J. Biol. Inorg. Chem. 1994, 224, 943–950. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, B.; Eikenberry, E.F. Characterization of fibrous forms of collagen. Methods Enzymol. 1982, 82, 127–174. [Google Scholar] [CrossRef]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen as a Biomaterial. Annu. Rev. Biophys. Bioeng. 1974, 3, 231–253. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Cowin, S.C. How Is a Tissue Built?1. J. Biomech. Eng. 2000, 122, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.; Rey, J.; Dobre, O.; González-García, C.; Dalby, M.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Schrieber, R.; Gareis, H. Gelatine Handbook; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Bailey, A.J.; Light, N.D. Connective Tissue in Meat and Meat Products; Elsevier Science Publishers Ltd.: London, UK, 1989. [Google Scholar]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [Green Version]
- Sellers, A.; Reynolds, J.J.; Meikle, M.C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem. J. 1978, 171, 493–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Correction to: Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2018, 29, 39. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Filho, M.D.S.M.S.; Caceres, C.A.; Rosa, M.F.; Morais, J.P.S.; Pinto, A.M.; Azeredo, H.M. Fish gelatin films as affected by cellulose whiskers and sonication. Food Hydrocoll. 2014, 41, 113–118. [Google Scholar] [CrossRef]
- Yang, Z.; Chaieb, S.; Hemar, Y. Gelatin-Based Nanocomposites: A Review. Polym. Rev. 2021, 61, 765–813. [Google Scholar] [CrossRef]
- Yang, Z.; Hemar, Y.; Hilliou, L.; Gilbert, E.P.; McGillivray, D.J.; Williams, M.A.K.; Chaieb, S. Nonlinear Behavior of Gelatin Networks Reveals a Hierarchical Structure. Biomacromolecules 2016, 17, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Tu, Z.-C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.-H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Bode, F.; da Silva, M.A.; Drake, A.F.; Ross-Murphy, S.B.; Dreiss, C.A. Enzymatically Cross-Linked Tilapia Gelatin Hydrogels: Physical, Chemical, and Hybrid Networks. Biomacromolecules 2011, 12, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Anuradha, D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018, 107, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Liu, O.; Liu, Y.; Zhang, Y.-Y.; Li, S.; Feng, X.-B.; Shao, Z.-W.; Yang, C.; Yang, S.-H.; Hong, J.-B. Development of Novel Biocomposite Scaffold of Chitosan-Gelatin/Nanohydroxyapatite for Potential Bone Tissue Engineering Applications. Nanoscale Res. Lett. 2016, 11, 487. [Google Scholar] [CrossRef] [Green Version]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef]
- Nguyen, T.H.M.; Abueva, C.; Van Ho, H.; Lee, S.-Y.; Lee, B.-T. In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel. Carbohydr. Polym. 2018, 180, 246–255. [Google Scholar] [CrossRef]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.-C.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amédée, J.; Montembault, A.; David, L.; et al. Physicochemical modulation of chitosan-based hydrogels induces different biological responses: Interest for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3666–3676. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2022, 110, 266–272. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168. [Google Scholar] [CrossRef]
- Babaei, Z.; Jahanshahi, M.; Rabiee, S.M. The fabrication of nanocomposites via calcium phosphate formation on gelatin–chitosan network and the gelatin influence on the properties of biphasic composites. Mater. Sci. Eng. C 2013, 33, 370–375. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, L.; Wang, J.; Wang, S.; Zou, Y.; Wang, X. Novel fabrication of morphology tailored nanostructures with Gelatin/Chitosan Co-polymeric bio-composited hydrogel system to accelerate bone fracture healing and hard tissue nursing care management. Process. Biochem. 2020, 90, 177–183. [Google Scholar] [CrossRef]
- Peter, M.; Binulal, N.; Nair, S.; Selvamurugan, N.; Tamura, H.; Jayakumar, R. Novel biodegradable chitosan–gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem. Eng. J. 2010, 158, 353–361. [Google Scholar] [CrossRef]
- Sethi, S.; Medha; Kaith, B.S. A review on chitosan-gelatin nanocomposites: Synthesis, characterization and biomedical applications. React. Funct. Polym. 2022, 179, 105362. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Furuya, H.; Kohara, H.; Tabata, Y. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 2011, 32, 2797–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef]
- Kugimiya, F.; Yano, F.; Ohba, S.; Igawa, K.; Nakamura, K.; Kawaguchi, H.; Chung, U.-I. Mechanism of osteogenic induction by FK506 via BMP/Smad pathways. Biochem. Biophys. Res. Commun. 2005, 338, 872–879. [Google Scholar] [CrossRef]
- Han, X.; He, J.; Wang, Z.; Bai, Z.; Qu, P.; Song, Z.; Wang, W. Fabrication of silver nanoparticles/gelatin hydrogel system for bone regeneration and fracture treatment. Drug Deliv. 2021, 28, 319–324. [Google Scholar] [CrossRef]
- Arakawa, C.; Ng, R.; Tan, S.; Kim, S.; Wu, B.; Lee, M. Photopolymerizable chitosan-collagen hydrogels for bone tissue engineering. J. Tissue Eng. Regen. Med. 2014, 11, 164–174. [Google Scholar] [CrossRef]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable chitosan/collagen hydrogels nano-engineered with functionalized single wall carbon nanotubes for minimally invasive applications in bone. Mater. Sci. Eng. C 2021, 128, 112340. [Google Scholar] [CrossRef]
- Gharati, G.; Shirian, S.; Sharifi, S.; Mirzaei, E.; Bakhtirimoghadam, B.; Karimi, I.; Nazari, H. Comparison Capacity of Collagen Hydrogel and Collagen/Strontium Bioglass Nanocomposite Scaffolds with and Without mesenchymal Stem Cells in Regeneration of Critical Sized Bone Defect in a Rabbit Animal Model. Biol. Trace Element Res. 2022, 200, 3176–3186. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Monfared, M.H.; Samani, S.; Farzin, A.; Poursamar, S.A.; Ai, J.; Ebrahimi-Barough, S.; Azami, M. 3D-printed bioactive Chitosan/Alginate/Hardystonite scaffold for bone tissue engineering: Synthesis and characterization. J. Non-Cryst. Solids 2023, 609, 122261. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, K.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, M. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Ma, W.; Chen, H.; Cheng, S.; Wu, C.; Wang, L.; Du, M. Gelatin hydrogel reinforced with mussel-inspired polydopamine-functionalized nanohydroxyapatite for bone regeneration. Int. J. Biol. Macromol. 2023, 240, 124287. [Google Scholar] [CrossRef] [PubMed]
- Lipskas, J.; Deep, K.; Yao, W. Robotic-Assisted 3D Bio-printing for Repairing Bone and Cartilage Defects through a Minimally Invasive Approach. Sci. Rep. 2019, 9, 3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, R.; Wang, X.; Xue, J.; Deng, C.; Feng, C.; Zhuang, H.; Ma, J.; Qin, C.; Wan, L.; et al. 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 2020, 6, eaaz6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Y.; Napiwocki, B.; Mi, H.; Peng, X.; Turng, L.-S. Fabrication of poly(ε-caprolactone) tissue engineering scaffolds with fibrillated and interconnected pores utilizing microcellular injection molding and polymer leaching. RSC Adv. 2017, 7, 43432–43444. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Tamer, T.M.; Omer, A.M.; Baset, W.M.; Abbas, E.; Mohy-Eldin, M.S. Therapeutic potential of two formulated novel chitosan derivatives with prominent antimicrobial activities against virulent microorganisms and safe profiles toward fibroblast cells. Int. J. Pharm. 2023, 634, 122649. [Google Scholar] [CrossRef]
- Tamer, T.M.; Zhou, H.; Hassan, M.A.; Abu-Serie, M.M.; Shityakov, S.; Elbayomi, S.M.; Mohy-Eldin, M.S.; Zhang, Y.; Cheang, T. Synthesis and physicochemical properties of an aromatic chitosan derivative: In vitro antibacterial, antioxidant, and anticancer evaluations, and in silico studies. Int. J. Biol. Macromol. 2023, 240, 124339. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]









| Year | Formulation | Model | Effects | Others | Reference |
|---|---|---|---|---|---|
| ≤2018 | Injectable thermosensitive hydrogel based on CS and glycerophosphate. | In vitro | The pores in the hydrogel permit cell infiltration, new tissue ingrowth, nutrient transport, and active ingredient diffusion, improving scaffold functionality. Biocompatible with MSC. GO improves calcium deposition and osteogenesis. | Injectable thermosensitive property. GO addition improves the physico-chemical properties (protein adsorption, swelling capacity, and control of degradable behavior) | [90] |
| Scaffold of chitosan–gelatin/nanohydroxyapatite (CSG/nHaP) | In vitro | Improves adhesion and growth of MC3T3-E1 cells compared to nHaP alone. The CSG/nHaP scaffolds exhibited significantly higher cell populations compared to the CS scaffolds with the higher porosity of CSG/nHAP. Superior cytocompatibility of the HaP-containing scaffolds. | Less degradability for the added nHAP Increased mineralization in CSG/nHAP | [92] | |
| Glutaraldehyde- or genipin-like cross-likers to CS/gelatin scaffolds. | Mouse model femur implantation | CS/gelation with glutaraldehyde has better properties than that with genipin. The scaffold supports the adhesion, viability, proliferation, and osteogenic differentiation capacity of pre-osteoblasts. The scaffold conducts the formation of the extracellular matrix and the expansion of fibroblasts, which produce collagen. | Minimal inflammatory reaction. A homogeneous cell population of CD90, CD105, and CD73, with negative results for CD45 and CD34. | [93] | |
| Thermosensitive injectable hydrogel of TEMPO-oxidized cellulose nanofibers (TOCNF)/CS | Rat model (Sprague–Dawley male rats) | OCNF enhanced the biocompatibility of hydrogels, both in vitro and in vivo. MC3T3-E1 cells and L929 cells attached to and proliferated on the CS/TOCNF hydrogel better than on the CS hydrogel. | OCNF improved gelation properties. High TOCNF content resulted in faster sol/gel transition, increased porous surface area, and faster degradation. TOCNF hydrogels caused an initial inflammatory response after injection into rats, with the presence of alternatively activated macrophages after 2 weeks. | [94] | |
| CS with high DA grade and low DA grade of | Rat model | Higher DA CS hydrogels were not suitable for the in vitro culture of hMSC or progenitor-derived endothelial cells. Lower-DA CS hydrogel provided better cell adhesion, tissue regeneration, and neovascularization. | Physical hydrogels prepared from highly DA chitosan were softer and degraded quickly in vivo. Lower-DA CS hydrogel provided a more elastic material, induced a shorter inflammatory response than hydrogel with high DA (20%), and was neutralized by ammonia vapors. | [95] | |
| Composite scaffolds of chitosan (CS)–gelatin (CG) with bioactive glass–ceramic nanoparticles (nBGC) | In vitro | A higher number of mineral deposits was present on the nano-composite scaffold, which was increased by elevating the incubation time. The nanocomposite scaffolds provided a healthier environment for cell attachment and spreading. | The degradation and swelling behavior of the nanocomposite scaffolds were decreased, while protein adsorption was increased with the addition of nBGC. | [100] | |
| Gelatin hydrogels incorporating combined stromal cell-derived factor-1 (SDF-1) and bone morphogenetic protein-2 (BMP-2) | Rat model of a critical-sized ulna defect | Enhanced bone regeneration in the presences of SDF-1 and BMP-2 and increased the expression level of chemokine cell-surface receptor-4 (Cxcr4), runt-related factor-2 (Runx2), and osteocalcin genes. Promoted a vascular-like structure. The combined release of SDF-1 and BMP-2 enhanced the recruitment of osteogenic cells and angiogenesis. | - | [103] | |
| Photopolymerizable hidrogel with methacrylated glycol chitosan and semi-interpenetrating collagen with a riboflavin photoinitiator under blue light. | In vitro | Enhanced cellular attachment, spreading, proliferation and osteogenic differentiation of BMSCs seeded on the hydrogels compared to those without Col hydrogels. The mineralization was increased. | Col enhanced the compressive modulus and slowed the degradation rate of the hydrogels. | [107] | |
| CS/HA and CS/alginate hydrogels laden with MC3T3-E1 and processed by 3D bioprinting | Rat model of calvaria bone defects | The hydrogels maintained cell viability and proliferation after printing. The CS/HA hydrogel had peak expression levels for early- and late-stage osteogenic markers. CS and CS/HA hydrogels were mineralized and differentiated after 21 days of culture. | It was shown for the first time that CS and HA can be mixed with cells and printed successfully. The tested groups had viscoelastic properties. CS and CS/HA were stable under physiological stimulation. | [111] | |
| 2019 | CS-sulfonated graphene oxide and CS-graphene oxide scaffold | Mouse model femur implantation | Bone regeneration and increased biocompatibility using graphene oxide. Healthy cellular viability and dense bone morphology. | Well-controlled drug delivery. Antibacterial effect against S. aureus. | [5] |
| Collagen/tacrolimus hydrogel | Rat model of calvaria bone defects | The collagen hydrogel contained 1000 μg of tacrolimus, which was adequate in terms of cell proliferation. In vivo studies provided evidence of the potential of the developed hydrogel for bone healing. | Highly porous structure with interconnected pores Hydrogel showed appropriate swelling, drug release, and blood compatibility behavior. | [34] | |
| Collagen/CS electrospun nanofiber membranes (ECCMs) | Rat model of calvaria bone defects | Better cell proliferation and biocompatibility. Expression of BALP and OC showed that a higher level of osteogenic activity existed in the ECCMs group than other groups at both of the early and late stage. Newly formed bone almost fully filled the cranial defects in the ECCM group after 8 weeks. | Stronger tensile strength was achieved by the ECCMs, in addition to a lower and more stable degradation rate of the membrane. A highly porous and nanofibrous electrospinning structure was present. | [97] | |
| TiO2/gelatin–chitosan hydrogel | In vitro | TiO2-loaded gelatin/chitosan hydrogel showed higher adhesion than gelatin/chitosan expression of osteocalcin and F-actin proteins. Higher mineralization and alkaline phosphatase response. | The addition of TiO2 nanoparticles showed good thermal stability on the hydrogel. | [99] | |
| Composite scaffold of CS/Gel with glutaraldehyde-like cross-linker | Rat model of calvaria bone defects | Supported cell viability and proliferation. Extensive formation of a nHA phase. The concentration of glutaraldehyde significantly affected the expression of specific osteo/odontogenic genes. The CS/Gel scaffold type demonstrated a better biological response. | Increased degradability with 0.1% of glutaraldehyde. | [113] | |
| 2020 | Catecol–chitosan (CA-CS) hydrogels functionalized with zeolitic imidazolate frameworks: 8 nanoparticles (ZIF-8) (CA-CS/Z) | Rat model of calvaria bone defects | Enhanced adhesion and led excellent biocompatibility, osteogenesis, and promotion of bone regeneration. Enhance paracrine of the vascular endothelial growth factor (VEGF). The ZIF-8 NPs released from the hydrogels were also able to up-regulate the production and secretion of ALP, COL 1, and osteocalcin markers, promoting the osteogenic differentiation of rBMSCs. | The bone transplant environment was stabilized. Hydrogels exhibited advanced rheological properties and reliable mechanical strength. Antibacterial activity was present against S. aureus and E. coli. | [102] |
| Thermosensitive hydrogel/nanoparticle system made of CS and glycerol phosphate loaded with vancomycin NPs (VCM) (VCM/Gel) | Rabbit model of chronic osteomyelitis | The VCM-NPs/Gel promoted osteoblast proliferation. The VCM-NPs/Gel showed excellent anti-infection properties and accelerated bone repair under osteomyelitis conditions. | The VCM-NPs had high encapsulation efficiency and drug loading. The VCM-NPs/Gel exhibited sustained release of VCM over 26 days. Antibacterial activity was present against S. aureus. | [114] | |
| 2021 | CS/HA | In vitro | CS/HA scaffolds supported cell proliferation and differentiation. Scaffolds with higher concentrations of HA (60 and 70%) showed an impressive effect on osteogenic differentiation of hMSC towards a mature osteoblast phenotype. | Prevented degradation in an in vitro cell culture model as well as pro-inflammatory events. Showed a good effect on the expression of anti-inflammatory cytokines (IL-10 and IL-4); meanwhile, it was able to decrease pro-inflammatory cytokine (TGF-β) levels. | [96] |
| AgNPs/Gelatin | In vitro | AgNPs/Gel hydrogels are nonhazardous to osteoblasts. Improved survival and spreading of osteoblasts cells on the hydrogel were achieved. | - | [106] | |
| Injectable CS/Col hydrogel using carboxylic acid functionalized single-walled carbon nanotubes (COOH-SWCNTs) as integrators and sodium β-glycerophosphate (β-GP) salt as a cross-linker. | In vitro | The evaluated hydrogels formed a layer of HA on the surface. Hydrogels are non-toxic, increasing cell proliferation and osteogenic differentiation compared to pure CS/Col. | Thermoresponsive with sol–gel transition occurring at physiological temperature. Degradation and swelling properties were found to be composition-dependent. Optimal injectable properties. Enhanced significant mechanical properties. | [108] | |
| 2022 | Collagen hydrogel nanocomposite in combination with 2% strontium (Co/BGSr2%) | Full-thickness bone defect regeneration in the rabbit animal model | The tests (radiographical and histopathological) showed better results, highlighting the Co/BGSr2% + MSCs group. The highest expression level of osteocalcin was detected in Co/BGSr2% + MSCs, especially at the fourth week of post-transplantation. | Large pores. | [109] |
| 2023 | Scaffolds based on Cs/Alg/HD using the direct ink writing 3D printing technique. | In vitro | The addition of HD particles had a positive effect on cell viability and attachment. | Cs/Alg/HD70 demonstrated the highest yield strength (1.38 MPa) and elastic modulus (125.71 MPa), as well as the highest degradation rate and the lowest contact angle. | [112] |
| nHA was incorporated into a dopamine-modified gelatin (Gel-DA) hydrogel system using a polydopamine-like cross-linker | Rat femoral defect model | The hydrogel had excellent cell adhesion and proliferation, leading to the improved biocompatibility. The hydrogel accelerated the bone repair efficiency in in vivo tests. | Adding polydopamine-functionalized nHA increased the compressive strength of the Gel-Da hydrogel. The gelation time of the Gel-DA hydrogels with PHA was controllable. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. https://doi.org/10.3390/polym15132762
Guillén-Carvajal K, Valdez-Salas B, Beltrán-Partida E, Salomón-Carlos J, Cheng N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers. 2023; 15(13):2762. https://doi.org/10.3390/polym15132762
Chicago/Turabian StyleGuillén-Carvajal, Karen, Benjamín Valdez-Salas, Ernesto Beltrán-Partida, Jorge Salomón-Carlos, and Nelson Cheng. 2023. "Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration" Polymers 15, no. 13: 2762. https://doi.org/10.3390/polym15132762





