Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermogravimetric Analysis (TGA)
2.6. Rheological Characterization
2.7. Mechanical Properties
2.8. X-ray Diffraction
2.9. Determination of the Degree of Crosslinking
2.10. In Vitro Cytotoxicity Test
2.11. Enzymatic In Vitro Degradation
2.12. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Curing Behavior
3.2. Crosslinking Density
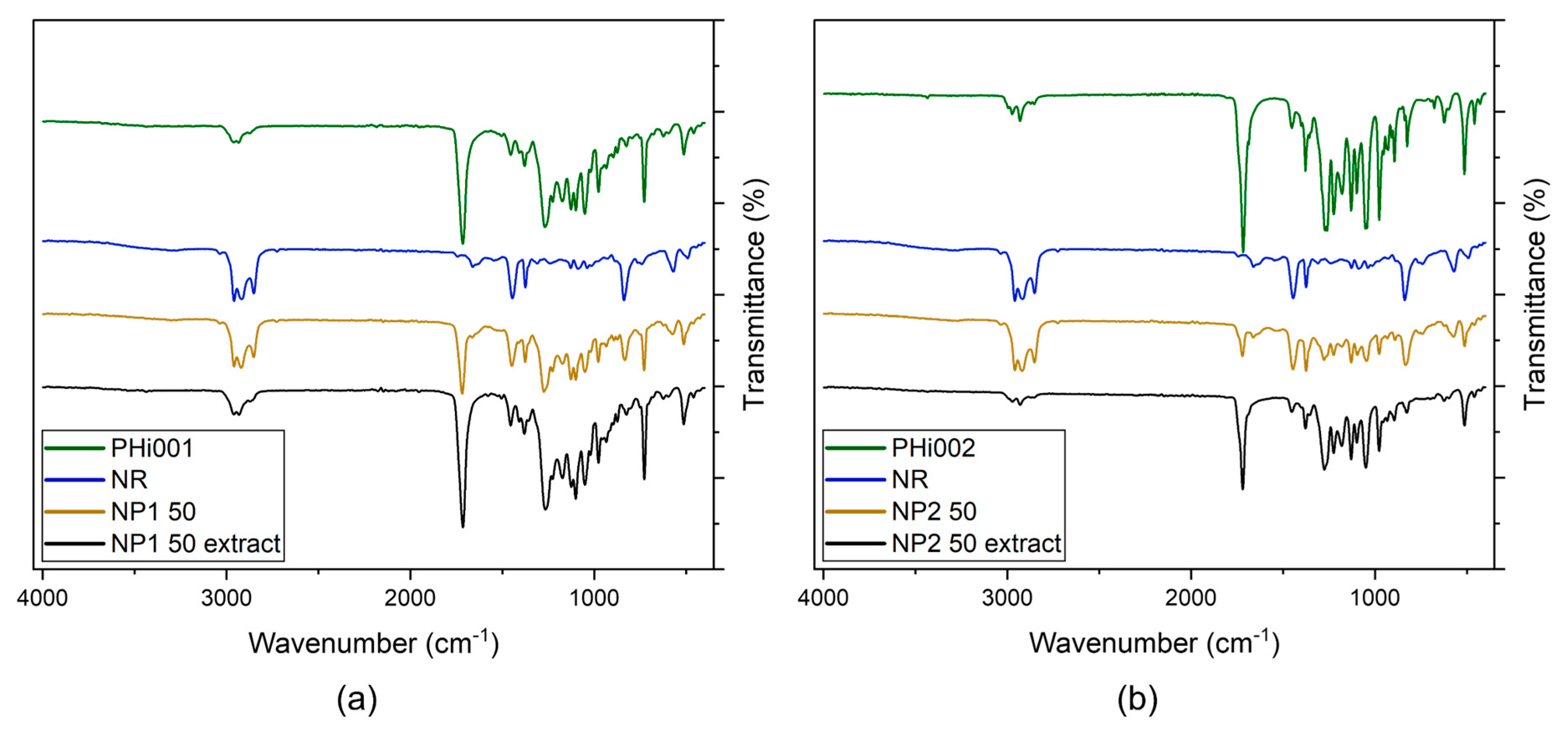
3.3. Fourier-Transformed Infrared (FTIR) Spectroscopy
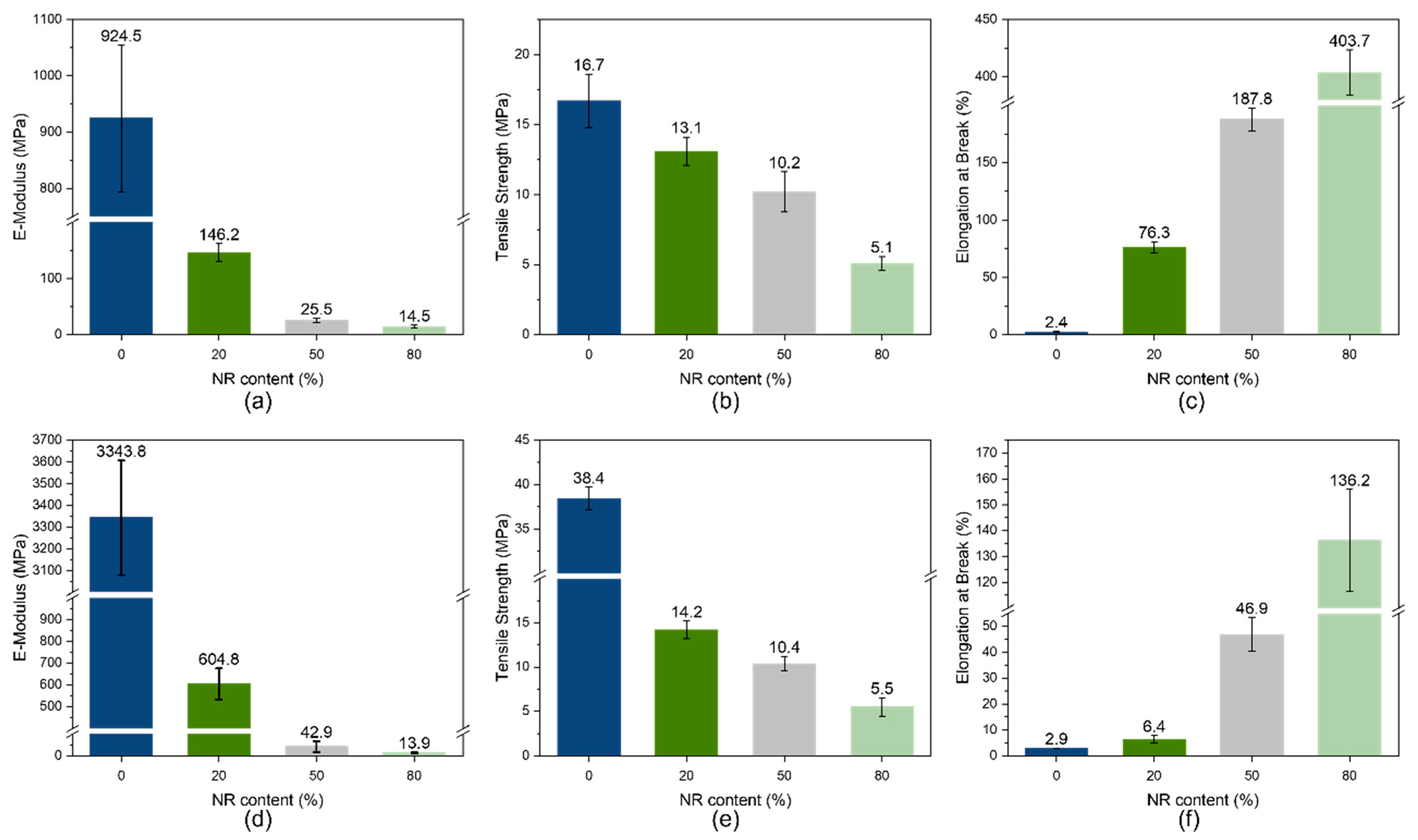
3.4. Mechanical Properties
3.5. Thermal Properties
3.5.1. DSC
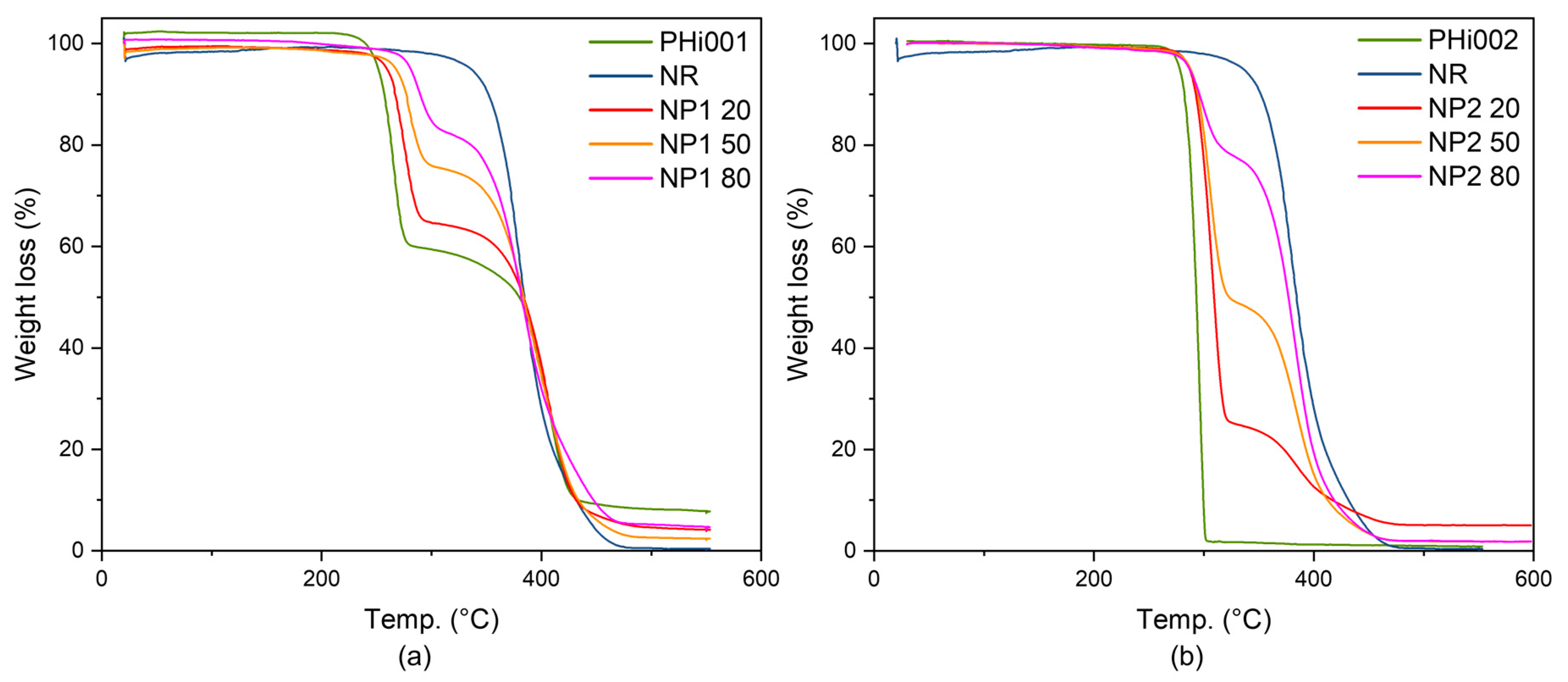
3.5.2. TGA
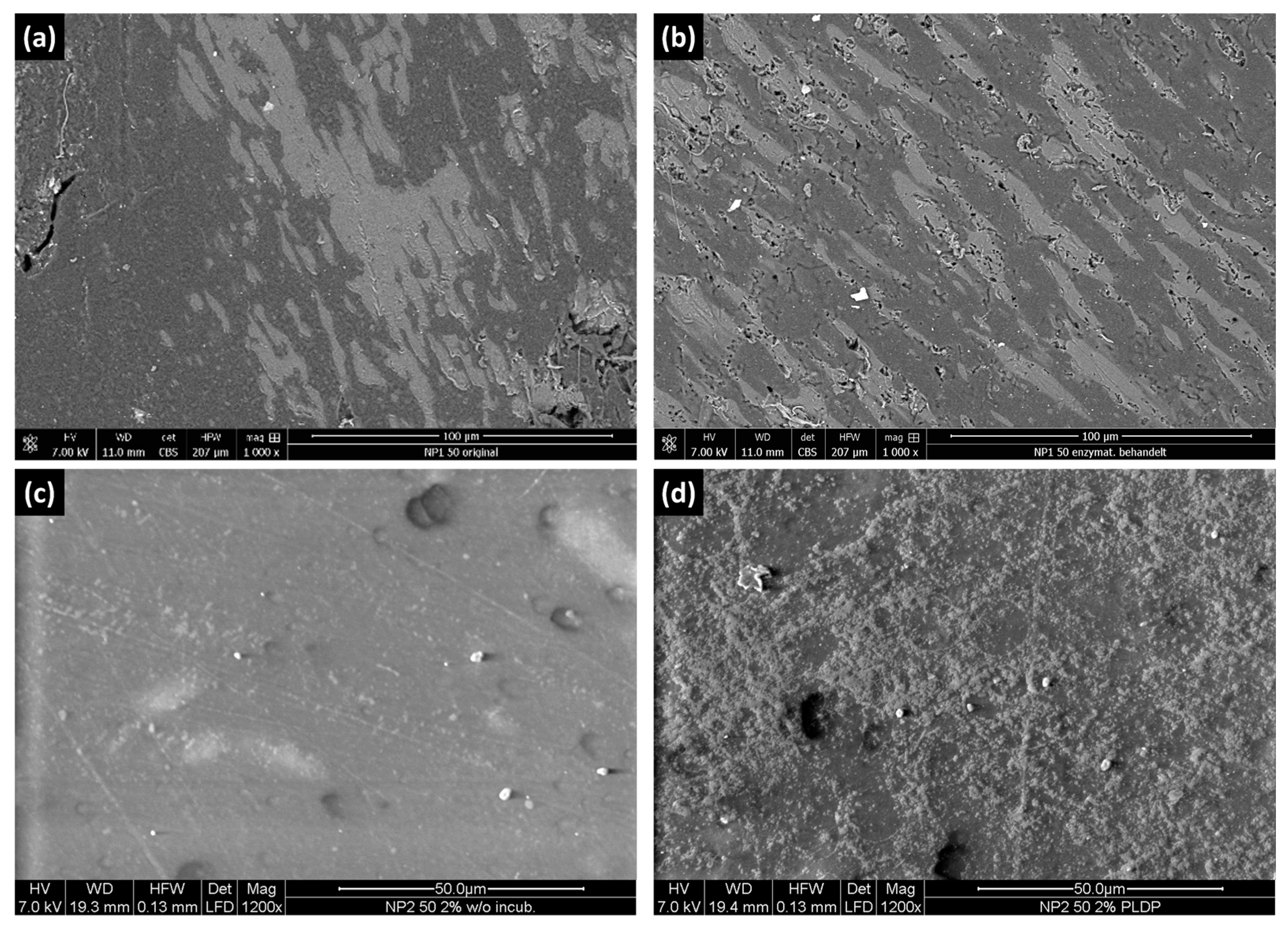
3.6. SEM
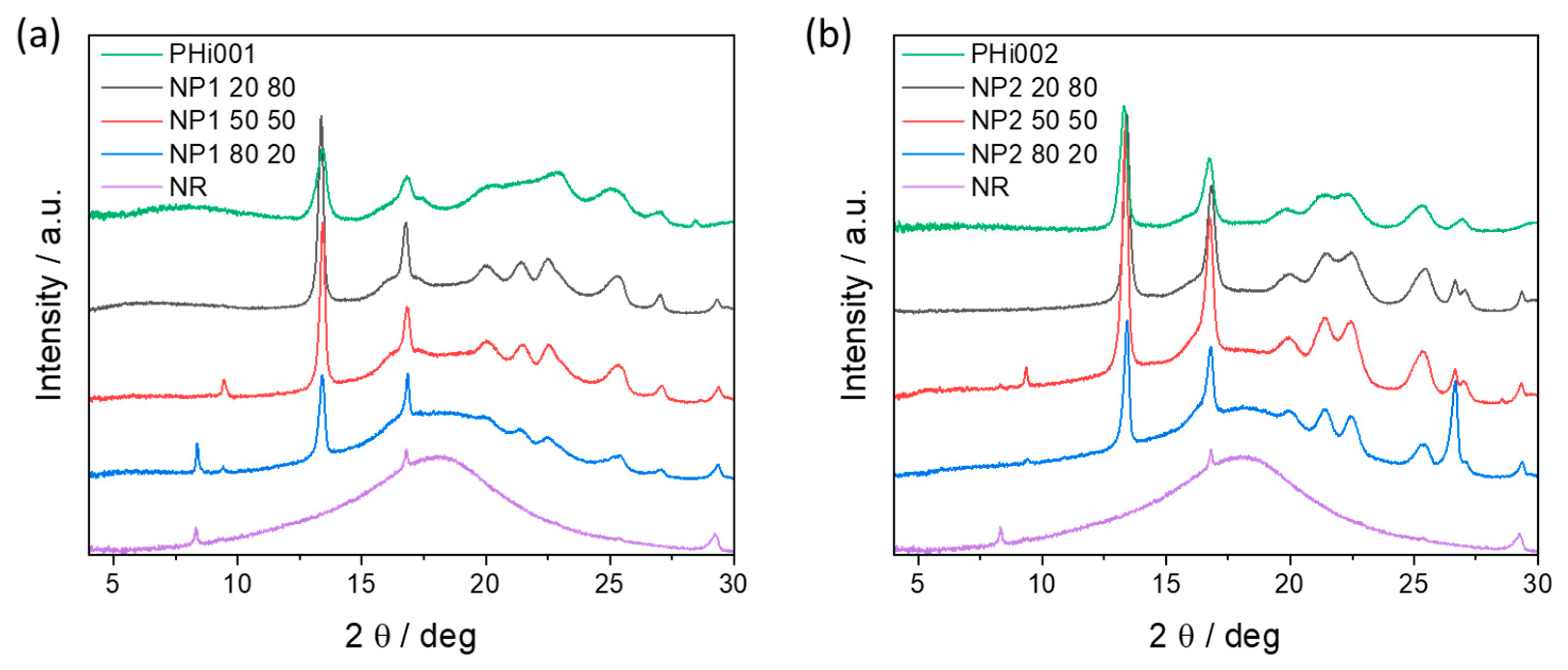
3.7. XRD Characterizations
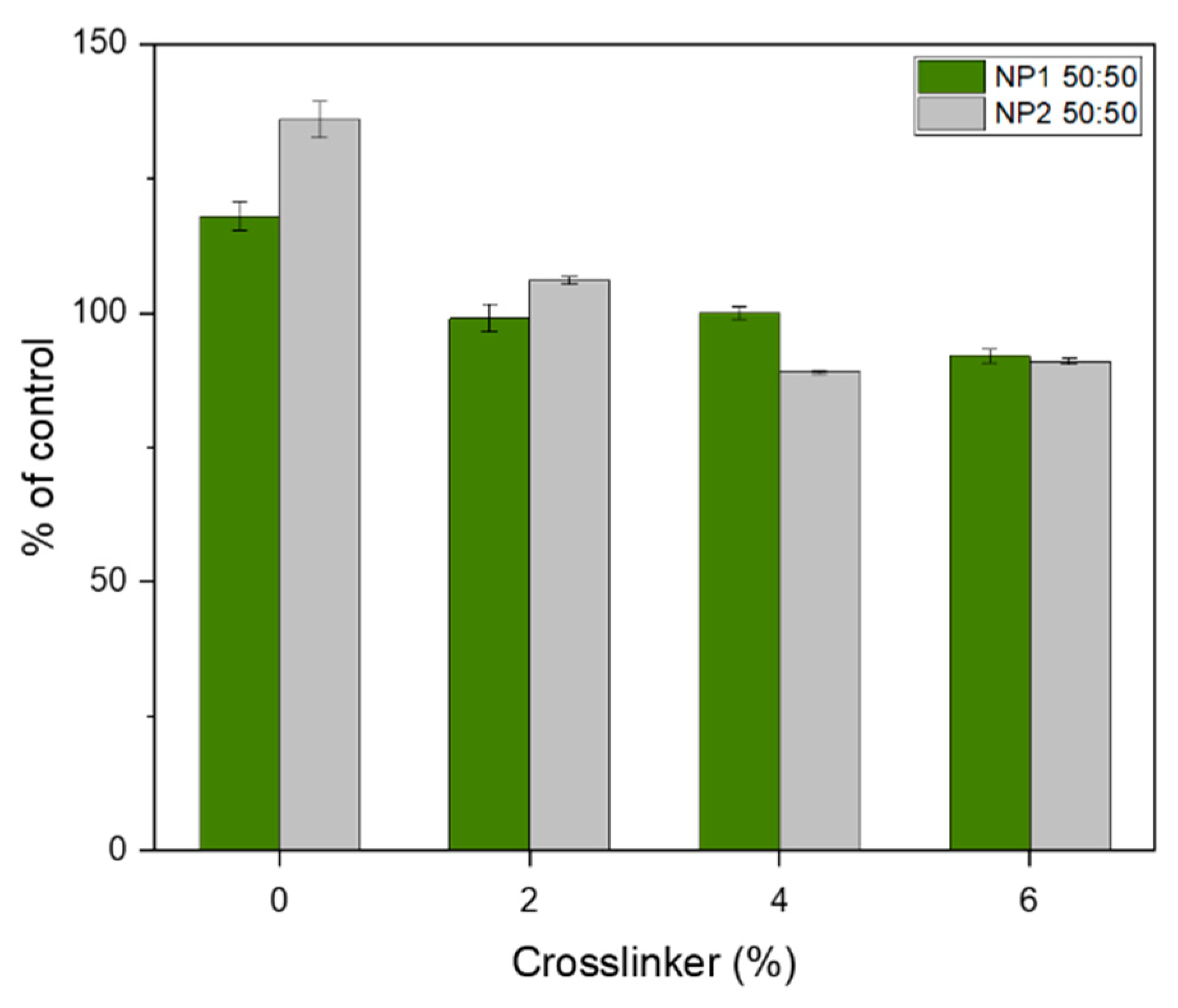
3.8. Toxicity Test
3.9. Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 25 July 2022).
- Plastic Atlas. Available online: https://www.boell.de/en/plasticatlas (accessed on 17 May 2023).
- Williams, A.T.; Rangel-Buitrago, N. The Past, Present, and Future of Plastic Pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Bioplastics—European Bioplastics e.V. Available online: https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf (accessed on 14 January 2023).
- Mukherjee, A.; Koller, M. Polyhydroxyalkanoate (PHA) Biopolyesters—Emerging and Major Products of Industrial Biotechnology. EuroBiotech J. 2022, 6, 49–60. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 2. Bioengineering 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Lambauer, V.; Kratzer, R. Lab-Scale Cultivation of Cupriavidus Necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering 2022, 9, 204. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Maia, A.L.C.; Poletto, F.; de Andrade, F.V.; Soares, D.C.F. Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Current Advances in Synthesis Methodologies, Antitumor Applications and Biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving Biological Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Jost, V.; Kopitzky, R. Blending of Polyhydroxybutyrate-Co-Valerate with Polylactic Acid for Packaging Applications—Reflections on Miscibility and Effects on the Mechanical and Barrier Properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
- Modi, S.J.; Cornish, K.; Koelling, K.; Vodovotz, Y. Fabrication and Improved Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) for Packaging by Addition of High Molecular Weight Natural Rubber. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Mechanical Performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Venoor, V.; Koelling, K.; Cornish, K.; Vodovotz, Y. Bio-Based Blends from Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Natural Rubber for Packaging Applications. J. Appl. Polym. Sci. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (Poly-β-hydroxybutyrate) and Its Enzymatic Degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet Review on the Current Status of Polymer Degradation: A Microbial Approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(Lactic Acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Chengalroyen, M.D.; Dabbs, E.R. Characterization of Rubber Degrading Isolates. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 872–885. [Google Scholar]
- Andler, R. Bacterial and Enzymatic Degradation of Poly(cis-1,4-isoprene) Rubber: Novel Biotechnological Applications. Biotechnol. Adv. 2020, 44, 107606. [Google Scholar] [CrossRef]
- Bode, H.B.; Kerkhoff, K.; Jendrossek, D. Bacterial Degradation of Natural and Synthetic Rubber. Biomacromolecules 2001, 2, 295–303. [Google Scholar] [CrossRef]
- Pant, P.; Harrison, R.M. Estimation of the Contribution of Road Traffic Emissions to Particulate Matter Concentrations from Field Measurements: A Review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Filippi, S.; Cinelli, P.; Mezzetta, A.; Carlozzi, P.; Seggiani, M. Extraction of Polyhydroxyalkanoates from Purple Non-Sulfur Bacteria by Non-Chlorinated Solvents. Polymers 2021, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and Morphology of a Bacterial Thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Rubin Pedrazzo, A.; Anceschi, A.; Appleton, S.L.; Khazaei Monfared, Y.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Nachtnebel, M.; Rastel, M.; Mayrhofer, C.; Zankel, A.; Gahleitner, M.; Poelt, P. The Fracture Behavior of Particle Modified Polypropylene—3D Reconstructions and Interparticle Distances. Polymer 2017, 126, 65–73. [Google Scholar] [CrossRef]
- Zankel, A.; Chernev, B.; Brandl, C.; Poelt, P.; Wilhelm, P.; Nase, M.; Langer, B.; Grellmann, W.; Baumann, H.J. Assessment of Beam Damage in Polymers Caused by in Situ ESEM Analysis Using IR Spectroscopy. Macromol. Symp. 2008, 265, 156–165. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Chen, X.; Pan, J.; Xu, K. Miscibility, Crystallization Kinetics, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV)/Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)(P3/4HB) Blends. J. Appl. Polym. Sci. 2010, 117, 838–848. [Google Scholar] [CrossRef]
- Kennouche, S.; Le Moigne, N.; Kaci, M.; Quantin, J.C.; Caro-Bretelle, A.S.; Delaite, C.; Lopez-Cuesta, J.M. Morphological Characterization and Thermal Properties of Compatibilized Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/Poly(Butylene Succinate) (PBS)/Halloysite Ternary Nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Khan, I.; Poh, B.T. Natural Rubber-Based Pressure-Sensitive Adhesives: A Review. J. Polym. Environ. 2011, 19, 793–811. [Google Scholar] [CrossRef]
- Abidin, Z.; Hanif, H.; Bakar, M.A. Studies on the Properties of Natural Rubber/Poly-3-hydroxybutyrate Blends Prepared by Solvent Casting. Malays. J. Anal. Sci. 2021, 25, 40–52. [Google Scholar]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA-PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Kuciel, S.; Mazur, K.; Jakubowska, P. Novel Biorenewable Composites Based on Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with Natural Fillers. J. Polym. Environ. 2019, 27, 803–815. [Google Scholar] [CrossRef]
- Tao, J.; Song, C.; Cao, M.; Hu, D.; Liu, L.; Liu, N.; Wang, S. Thermal Properties and Degradability of Poly(propylene carbonate)/Poly(β-Hydroxybutyrate-Co-β-Hydroxyvalerate) (PPC/PHBV) Blends. Polym. Degrad. Stab. 2009, 94, 575–583. [Google Scholar] [CrossRef]
- Abraham, E.; Elbi, P.A.; Deepa, B.; Jyotishkumar, P.; Pothen, L.A.; Narine, S.S.; Thomas, S. X-Ray Diffraction and Biodegradation Analysis of Green Composites of Natural Rubber/Nanocellulose. Polym. Degrad. Stab. 2012, 97, 2378–2387. [Google Scholar] [CrossRef]
- Pulungan, A.N.; Wirjosentono, B.; Eddiyanto; Hendrana, S. X-Ray Diffraction and Morphology Studies of Sulfonated Polystyrene and Maleated Natural Rubber Blend with PE-g-MA as Compatibilished. Rasayan J. Chem. 2020, 13, 1112–1123. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Kanwal, A.; Zhang, M.; Sharaf, F.; Li, C. Enzymatic Degradation of Poly (Butylene Adipate Co-Terephthalate) (PBAT) Copolymer Using Lipase B from Candida Antarctica (CALB) and Effect of PBAT on Plant Growth. Polym. Bull. 2022, 79, 9059–9073. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, M.; Weng, Y.; Zhao, Y.; Li, C.; Kanwal, A. Degradation of Poly(butylene adipate-co-terephthalate) by Stenotrophomonas Sp. YCJ1 Isolated from Farmland Soil. J. Environ. Sci. 2021, 103, 50–58. [Google Scholar] [CrossRef]
- Jendrossek, D.; Tomasi, G.; Kroppenstedt, R.M. Bacterial Degradation of Natural Rubber: A Privilege of Actinomycetes? FEMS Microbiol. Lett. 1997, 150, 179–188. [Google Scholar] [CrossRef]
- Yikmis, M.; Steinbüchel, A. Historical and Recent Achievements in the Field of Microbial Degradation of Natural and Synthetic Rubber. Appl. Environ. Microbiol. 2012, 78, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Linh, D.V.; Huong, N.L.; Tabata, M.; Imai, S.; Iijima, S.; Kasai, D.; Anh, T.K.; Fukuda, M. Characterization and Functional Expression of a Rubber Degradation Gene of a Nocardia Degrader from a Rubber-Processing Factory. J. Biosci. Bioeng. 2017, 123, 412–418. [Google Scholar] [CrossRef] [PubMed]



| Blend | Natural Rubber [%] | PHi001 [%] | PHi002 [%] |
|---|---|---|---|
| NP1 20 | 20 | 80 | - |
| NP1 50 | 50 | 50 | - |
| NP1 80 | 80 | 20 | - |
| NP2 20 | 20 | - | 80 |
| NP2 50 | 50 | - | 50 |
| NP2 80 | 80 | - | 20 |
| Material | ΔHm [J/g] | Tg [°C] | Tm [°C] | Tc [°C] | X [%] |
|---|---|---|---|---|---|
| PHi001 | 21.2 | −3.3 | 160 | 50.8 | 15 |
| PHi002 | 92.8 | 5.2 | 168 | 106.2 | 64 |
| NR | −46.6 | ||||
| NP1 20 | 10.6 | −2.2 | 156.1 | 59.5 | 7 |
| NP1 50 | 5.7 | −3.6 | 155.1 | 57.7 | 4 |
| NP1 80 | 0.8 | −3.7 | 158.2 | 54.5 | 0.5 |
| NP2 20 | 41.5 | 2.5 | 160.5 | 98.8 | 28 |
| NP2 50 | 27.4 | −1.9 | 161.4 | 94.3 | 19 |
| NP2 80 | 2.7 | −2.5 | 162 | - | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, C.; Emmerstorfer-Augustin, A.; Rath, T.; Trimmel, G.; Nachtnebel, M.; Stelzer, F. Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation. Polymers 2023, 15, 2593. https://doi.org/10.3390/polym15122593
Frank C, Emmerstorfer-Augustin A, Rath T, Trimmel G, Nachtnebel M, Stelzer F. Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation. Polymers. 2023; 15(12):2593. https://doi.org/10.3390/polym15122593
Chicago/Turabian StyleFrank, Carina, Anita Emmerstorfer-Augustin, Thomas Rath, Gregor Trimmel, Manfred Nachtnebel, and Franz Stelzer. 2023. "Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation" Polymers 15, no. 12: 2593. https://doi.org/10.3390/polym15122593
APA StyleFrank, C., Emmerstorfer-Augustin, A., Rath, T., Trimmel, G., Nachtnebel, M., & Stelzer, F. (2023). Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation. Polymers, 15(12), 2593. https://doi.org/10.3390/polym15122593






