Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Analysis
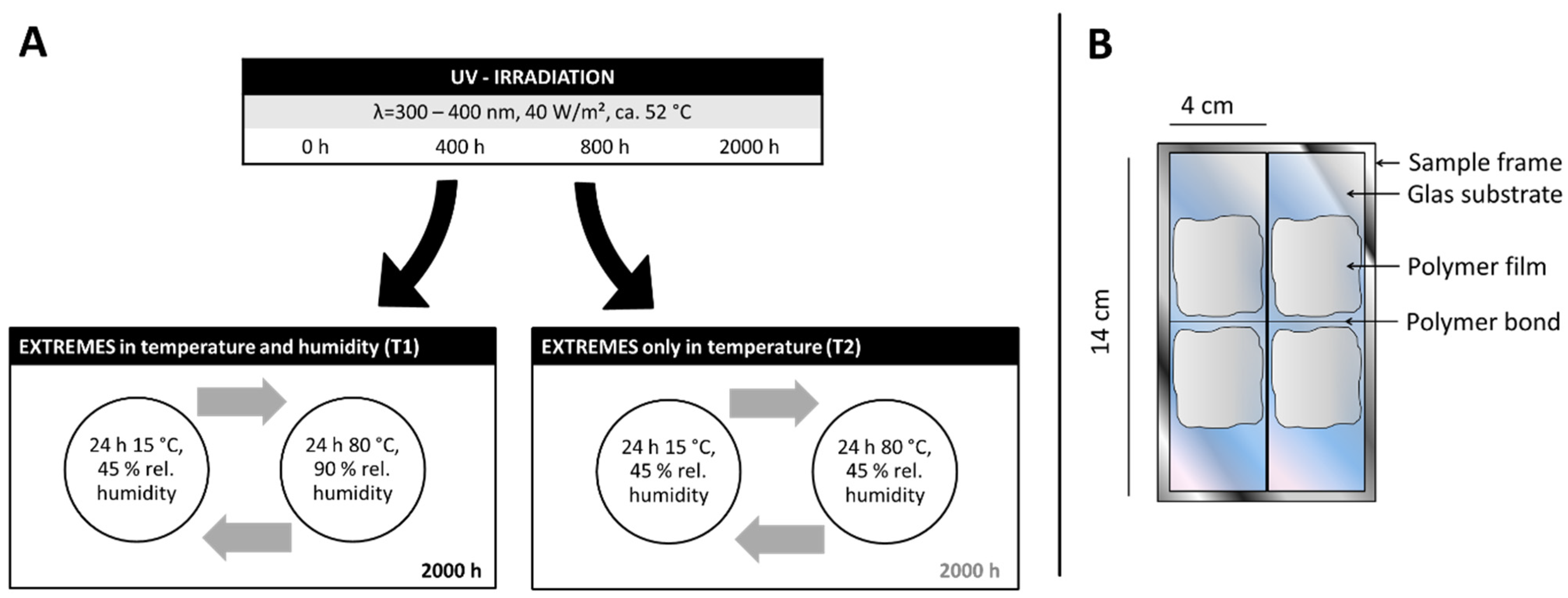
2.2. Artificial Aging
2.2.1. Model Materials
- A deeper understanding of the parameters influencing the aging of the polymer materials used on the Naumburg Cathedral (sunlight, UV content, and changes in temperature and humidity) and their associated changes in properties.
- On the basis of the knowledge gained, the condition of the samples taken in the cathedral is to be assessed in a more profound way because the corresponding measurements cannot be carried out on the original material for the most part. It is particularly important for future restoration work to assess whether a material is already on the verge of failure or is likely to be able to withstand the current conditions for a certain time.
2.2.2. Aging Conditions
3. Results and Discussion
3.1. Naturally Aged Historical Polymers
3.2. Results after Artificial Aging and Discussion
3.2.1. Colorimetry
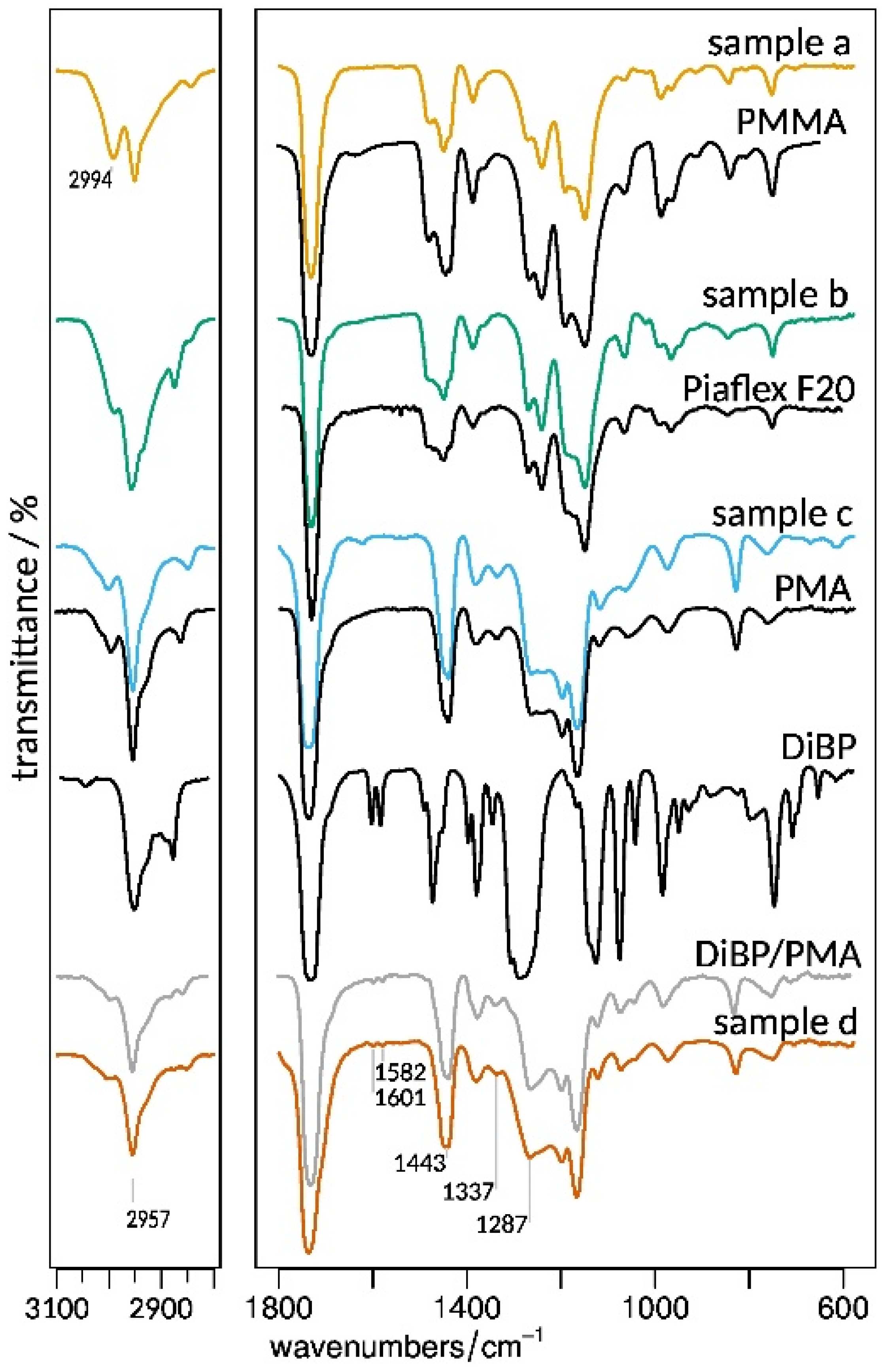
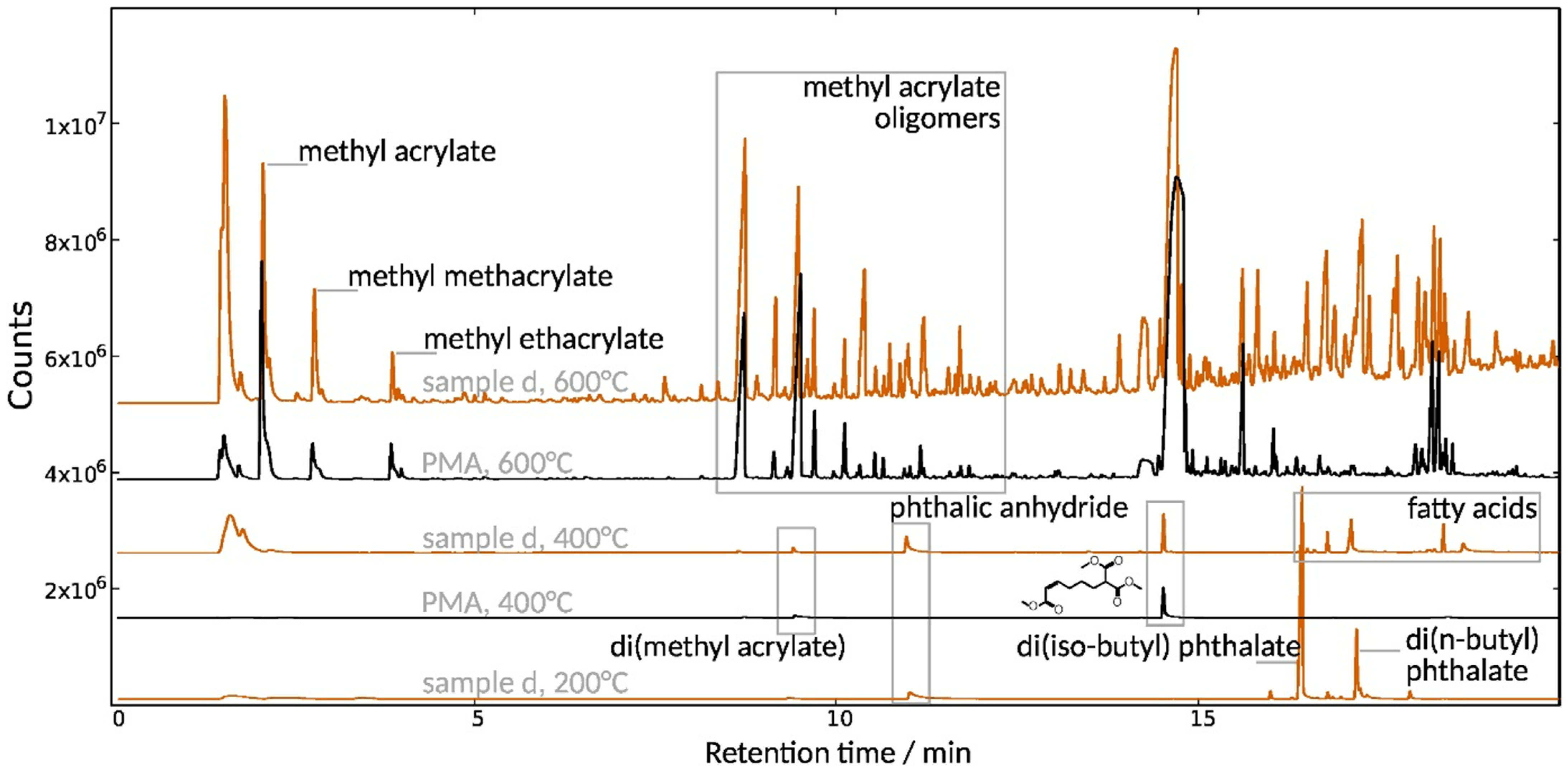
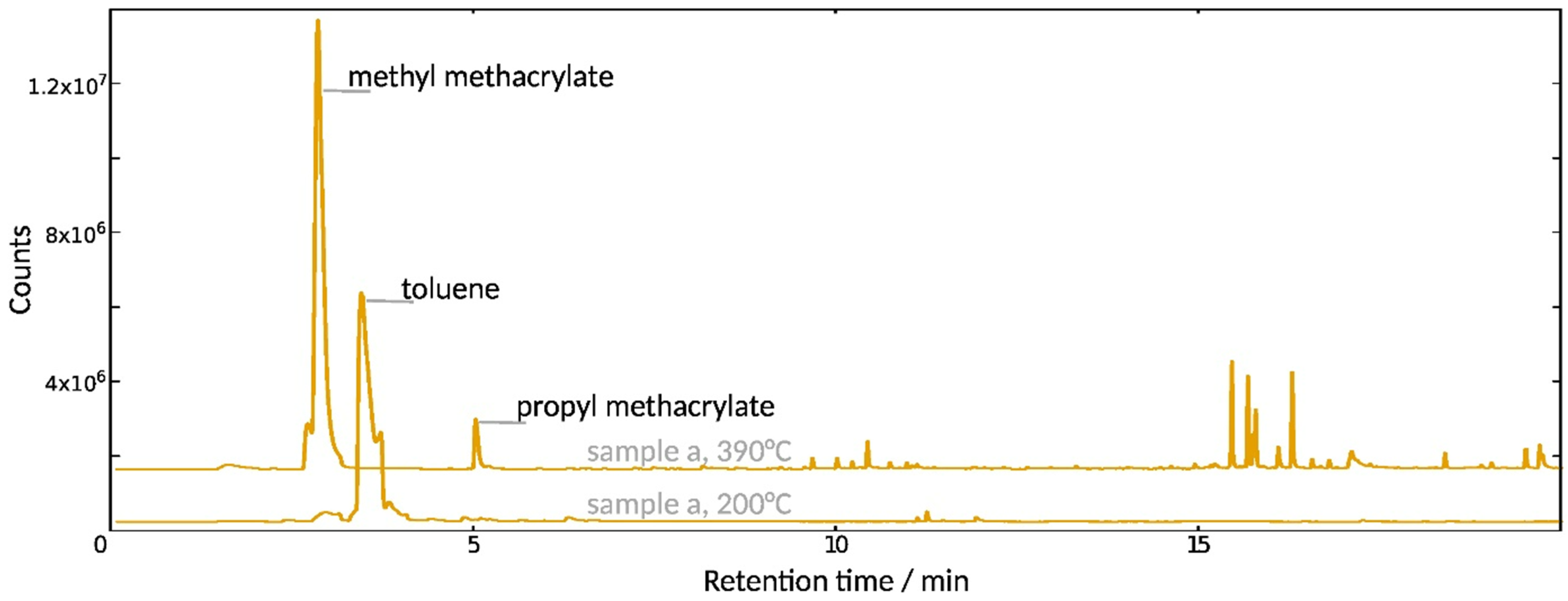
3.2.2. Composition and Molecular Properties
3.2.3. Thermal Behavior
3.2.4. Adhesive Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davison, S. A review of adhesives and consolidants used on glass antiquities. Stud. Conserv. 1984, 29 (Suppl. S1), 191–194. [Google Scholar] [CrossRef]
- Chapman, S.; Mason, D. Literature review: The use of Paraloid B-72 as a surface consolidant for stained glass. J. Am. Inst. Conserv. 2003, 42, 381–392. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerated Aging. Photochemical and Thermal Aspects; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; Available online: https://www.getty.edu/publications/resources/virtuallibrary/0892361255.pdf (accessed on 1 April 2023).
- Cocca, M.; D’Arienzo, L.; D’Orazio, L.; Gentile, G.; Martuscelli, E. Polyacrylates for conservation: Chemico-physical properties and durability of different commercial products. Polym. Test. 2004, 23, 333–342. [Google Scholar] [CrossRef]
- Down, J.L.; Macdonald, M.A.; Tetreault, J.; Williams, R.S. Adhesive testing at the Canadian Conservation Institute-an evaluation of selected poly(vinyl acetate) and acrylic adhesives. Stud. Conserv. 1996, 41, 19–44. [Google Scholar]
- Khallaf, M.K.; El-Midany, A.A.; El-Mofty, S.E. Influence of acrylic coatings on the interfacial, physical, and mechanical properties of stone-based monuments. Prog. Org. Coat. 2011, 72, 592–598. [Google Scholar] [CrossRef]
- Ropret, P.; Zoubek, R.; Kapin, A.S.; Bukovec, P. Effects of ageing on different binders for retouching and on some binder pigment combinations used for restoration of wall paintings. Mater. Charact. 2007, 58, 1148–1159. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, F.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatmsents of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Gómez-Sánchez, E.; Agnini, E.; Bridarolli, A.; Rolland, R.; Kunz, S.; Röhrs, S.; Harrivelle, C.; Casanova, E.; Simon, S.; Calparsoro-Forcada, E. Evaluation of the Solubility and Colour Changes of Artificially and Naturally Aged Adhesives for the Conservation of Ceramics and Glass. Stud. Conserv. 2022, 67, 500–517. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Asquier, M.; Colomban, P.; Milande, V. Raman and infrared analysis of glues used for pottery conservation treatments. J. Raman Sp. 2009, 40, 1641–1644. [Google Scholar] [CrossRef]
- Nel, P.; Lonetti, C.; Lau, D.; Tam, K.; Sloggett, R.S. Analysis of adhesives used on the Melbourne University Cypriot pottery Collection using a portable FTIR-ATR analyzer. Vib. Spectrosc. 2010, 53, 64–70. [Google Scholar] [CrossRef]
- Tennent, N.; Caen, J.; Courtney, P.; Lozano Diz, E. In-situ Raman spectroscopic characterisation of polymers used in past conservation treatments. e-Preserv. Sci. 2009, 6, 107–111. [Google Scholar]
- Noake, E.; Lau, D.; Nel, P. Identification of cellulose nitrate based adhesive repairs in archaeological pottery of the University of Melbourne’s Middle Eastern archaeological pottery collection using portable FTIR-ATR spectroscopy and PCA. Herit. Sci. 2017, 5, 3. [Google Scholar] [CrossRef]
- Lomax, S.Q.; Fisher, S.L. An investigation of the removability of naturally aged synthetic picture varnishes. J. Am. Inst. Conserv. 1990, 29, 181–191. [Google Scholar] [CrossRef]
- Rahfoth, K.; Sterzing, N.; Trappiel, I. Restaurierung der Glasmalereien des Ost- und Westchores im Dom St. Petrus und St. Paulus zu Naumburg a.d. Saale; Unpublished Report; Besucherservice Naumburger Dom: Erfurt, Germany, 2014. [Google Scholar]
- Aman, C. Middle Ages and nineteenth century: The significance of historical interventions in the documentation of and research on stained glass—A short working report from CVMA Potsdam. Folia Hist. Artium Ser. Nowa 2019, 17, 103–115. [Google Scholar] [CrossRef]
- Jacobi, R. Kunststoffe als Grundlage für ein neues Verfahren zur Erhaltung alter Glasmalereien. Angew. Chem. 1940, 53, 452–453. [Google Scholar] [CrossRef]
- Wolff, A. The conservation of mediaeval stained glass according to the Jacobi method of lamination used at Cologne. Stud. Conserv. 1975, 20 (Suppl. S1), 115–120. [Google Scholar] [CrossRef]
- Down, J.L. The effect of modifiers on the stability of a vinyl acetate/ethylene copolymer dispersion. Stud. Conserv. 2016, 61, 26–45. [Google Scholar] [CrossRef]
- Chiantore, O.; Lazzari, M. Photo-oxidative stability of paraloid acrylic protective polymers. Polymer 2001, 42, 17–27. [Google Scholar] [CrossRef]
- Hedlund, H.P.; Johansson, M. Prototypes of Lascaux’s Medium for consolidation: Development of a new custom-made polymer dispersion for use in conservation. Restauro 2005, 111, 432–439. [Google Scholar]
- Torge, M.; Bücker, M.; Feldmann, I. Modellhafte Evaluierung von Restaurierungs- und Konservierungsmaßnahmen an Historischen Glasmalereien mit Starken Schäden Durch Anthropogene Einflüsse, Abschlussbericht; Deutsche Bundesstiftung Umwelt: Osnabrück, Germany, 2011; 185p, Available online: https://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-27312.pdf (accessed on 1 April 2023).
- Marschner, H.; Bertelmann, H.; Tilenschi, C. Untersuchungen zur Beständigkeit mittelalterlicher Fenstergläser anhand von Modellgläsern. In Jahresbericht aus dem Forschungsprogramm Steinzerfall—Steinkonservierung; Snethlage, R., Ed.; Ernst & Sohn: Berlin, Germany, 1995; Volume 3, pp. 221–233. [Google Scholar]
- Michalski, S. Double the life or each five-degree drop, more than double the life for each halving of relative humidity. In ICOM-CC Preprints 13th Triennial Meeting Rio de Janeiro 2002; Vontobel, R., Ed.; James & James: London, UK, 2002; Volume I, pp. 66–72. [Google Scholar]
- DIN 6167:1980-01. Description of Yellowness of Near-White or Near-Colourless Materials. German Institute for Standardisation: Berlin, Germany, 1980.
- Hummel, D.O. Atlas der Polymer- und Kunststoffanalyse, 2nd ed.; VCH: Weinheim, Germany, 1988; Volume 2, Part b/I, p. 451. [Google Scholar]
- Wagner, L. Synthetische Konservierungsmittel in der Deutschen Demokratischen Republik. Ein Überblick über in der DDR Hergestellte und Verwendete Produkte zur Konservierung Bemalter und Gefasster Holzobjekte. Z. Kunsttechnol. Konserv. 2010, 24, 175–192. [Google Scholar]
- Lazzari, M.; Chiantore, O. Thermal-ageing of Paraloid acrylic protective polymers. Polymer 2000, 41, 6447–6455. [Google Scholar] [CrossRef]
- Carlson, J.; Winterthur Museum Garden and Library. ISR00081 Polymethylmethacrylate. In Infrared and Raman Users Group Spectral Database; Price, B.A., Pretzel, B., Quillen, L.S., Eds.; IRUG: Philadelphia, PA, USA, 2007; Available online: https://www.irug.org (accessed on 3 September 2020).
- Hummel, D.O. Atlas of Polymer and Plastics Analysis, 2nd ed.; VCH: Weinheim, Germany, 1985; Volume 1. [Google Scholar]
- Gunawan, L.; Haken, J.K. The mechanisms of thermal degradation of polymethyl acrylate using pyrolysis gas chromatog-raphy mass spectrometry. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 2539–2555. [Google Scholar] [CrossRef]
- Haken, J.K.; Ho, D.K.M.; Houghton, E. Identification of the principal high molecular weight fragments in the thermal degradation of poly(methyl acrylate). J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 1163–1171. [Google Scholar] [CrossRef]
- Hummel, D.O.; Düssel, H.J.; Czybulka, G.; Wenzel, N.; Holl, G. Analytical pyrolysis of copolymers. Spectrochim. Acta Part A Mol. Spectrosc. 1985, 41, 279–290. [Google Scholar] [CrossRef]
- Han, T.U.; Kim, Y.M.; Watanabe, A.; Teramae, N.; Park, Y.K.; Kim, S. Pyrolysis kinetic analysis of poly(methyl methacrylate) using evolved gas analysis-mass spectrometry. Korean J. Chem. Eng. 2017, 34, 1214–1221. [Google Scholar] [CrossRef]
- Ohtani, H.; Takehana, Y.; Tsuge, S. Quantification of end groups in anionically polymerized poly(methyl methacrylate)s by pyrolysis-gas chromatography. Macromolecules 1997, 30, 2542–2545. [Google Scholar] [CrossRef]
- Tsuge, S.; Ohtani, H. Structural characterization of polymeric materials by PyrolysisGC/MS. Polym. Degrad. Stab. 1997, 58, 109–130. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxi-dative degradation of poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Colom, X.; Garca, T.; Suol, J.J.; Saurina, J.; Carrasco, F. Properties of PMMA artificially aged. J. Non-Cryst. Solids 2001, 287, 308–312. [Google Scholar] [CrossRef]
- Kalisch, U. Bericht HAL 78/2017; Unpublished Report; Institut für Diagnostik und Konservierung an Denkmalen in Sachsen und Sachsen-Anhalt e.v. (IDK): Halle/Saale, Germany, 2017. [Google Scholar]
- Horie, V. Materials for Conservation, 2nd ed.; Routledge: London, UK; New York, NY, USA, 2010; pp. 154–161. [Google Scholar]
- Ferucci, S.; Tronchin, L. Resine epossidiche e Paraloid b72 a confronto la ricostruzione dei reperti vitrei, come varia la progettazione dell’intervento e la metodologia applicativa a seconda dell’adesivo, i vantaggi e gli svantaggi. In Volume Degli Atti XVI Congresso Nazionale IGIIC—Lo Stato dell’Arte, Castello del Buonconsiglio, Italy, 25–27 October 2018; Rullo, D., Ed.; Nardini: Firenze, Italy, 2019; Available online: https://www.academia.edu/38544906/ (accessed on 1 April 2023).
- Rauch, I. Evaluierung und Modellhafte Praxiserprobung Eines UV-Strahlungsschutzes für Kunstharze aus Schädigenden Altrestaurierungen auf National Wertvollen Glasmalereien des Naumburger Doms; Deutsche Bundesstiftung Umwelt: Osnabrück, Germany, 2020; p. 5. Available online: www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-34468_01-Hauptbericht.pdf (accessed on 1 April 2023).




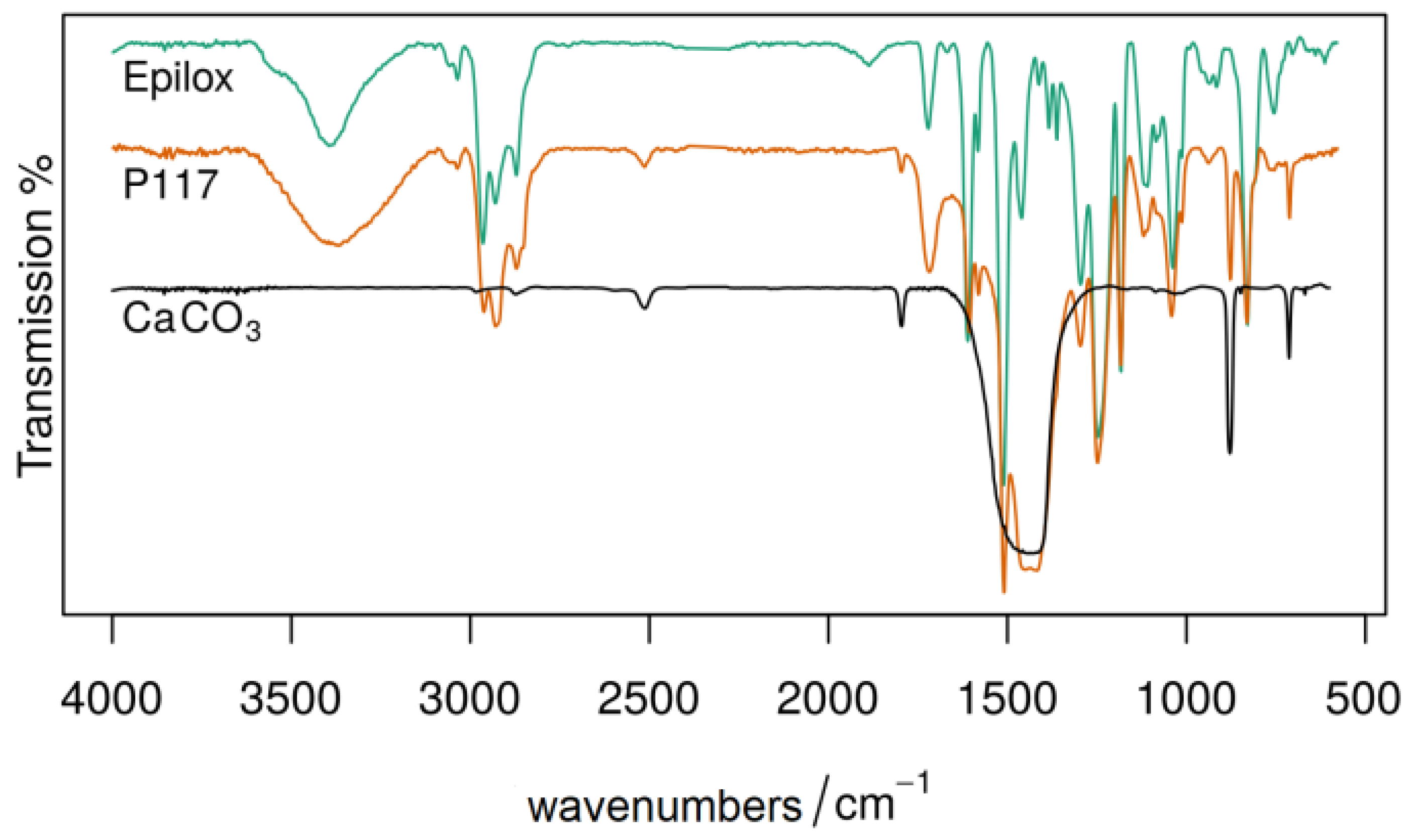

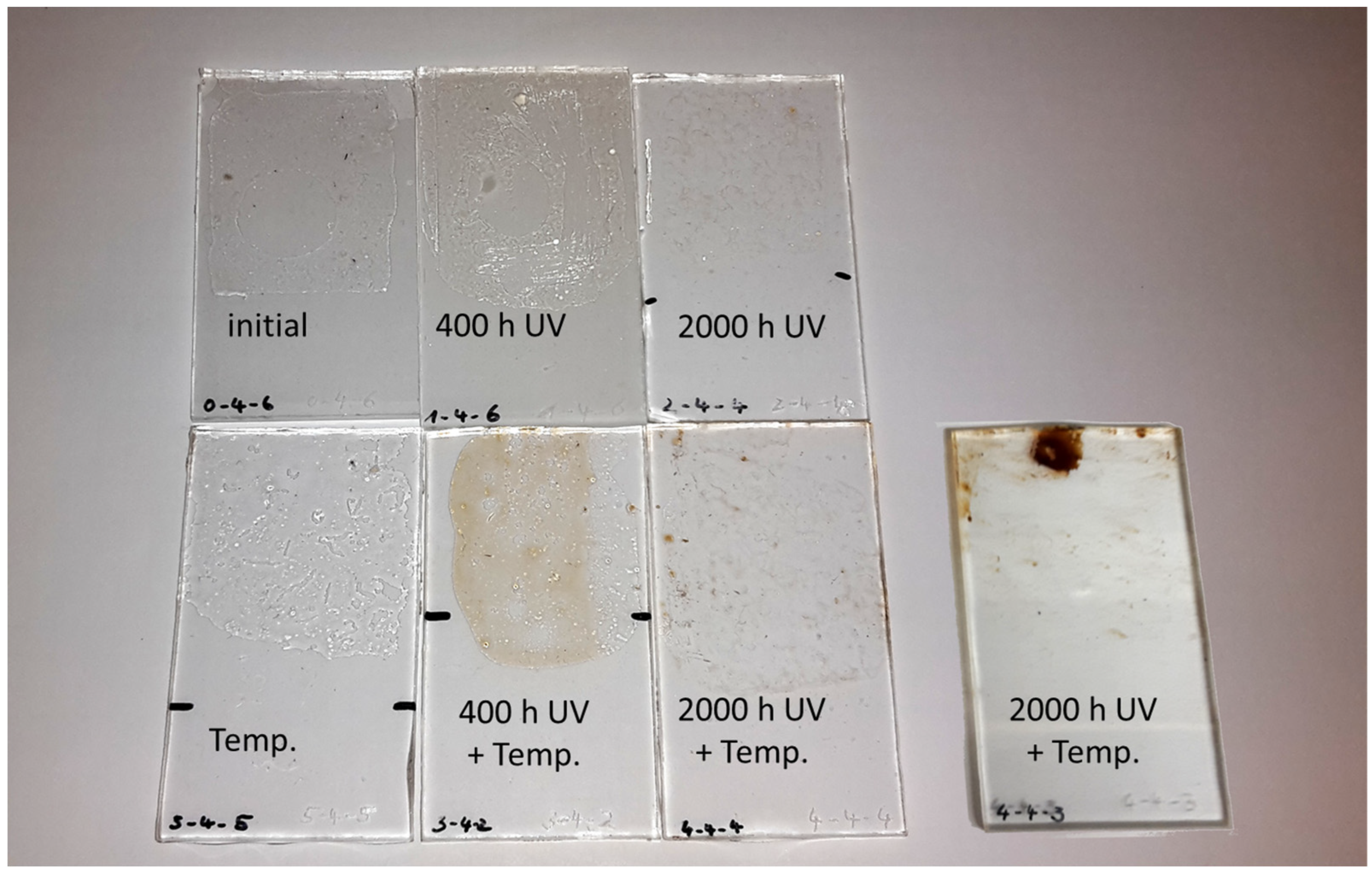
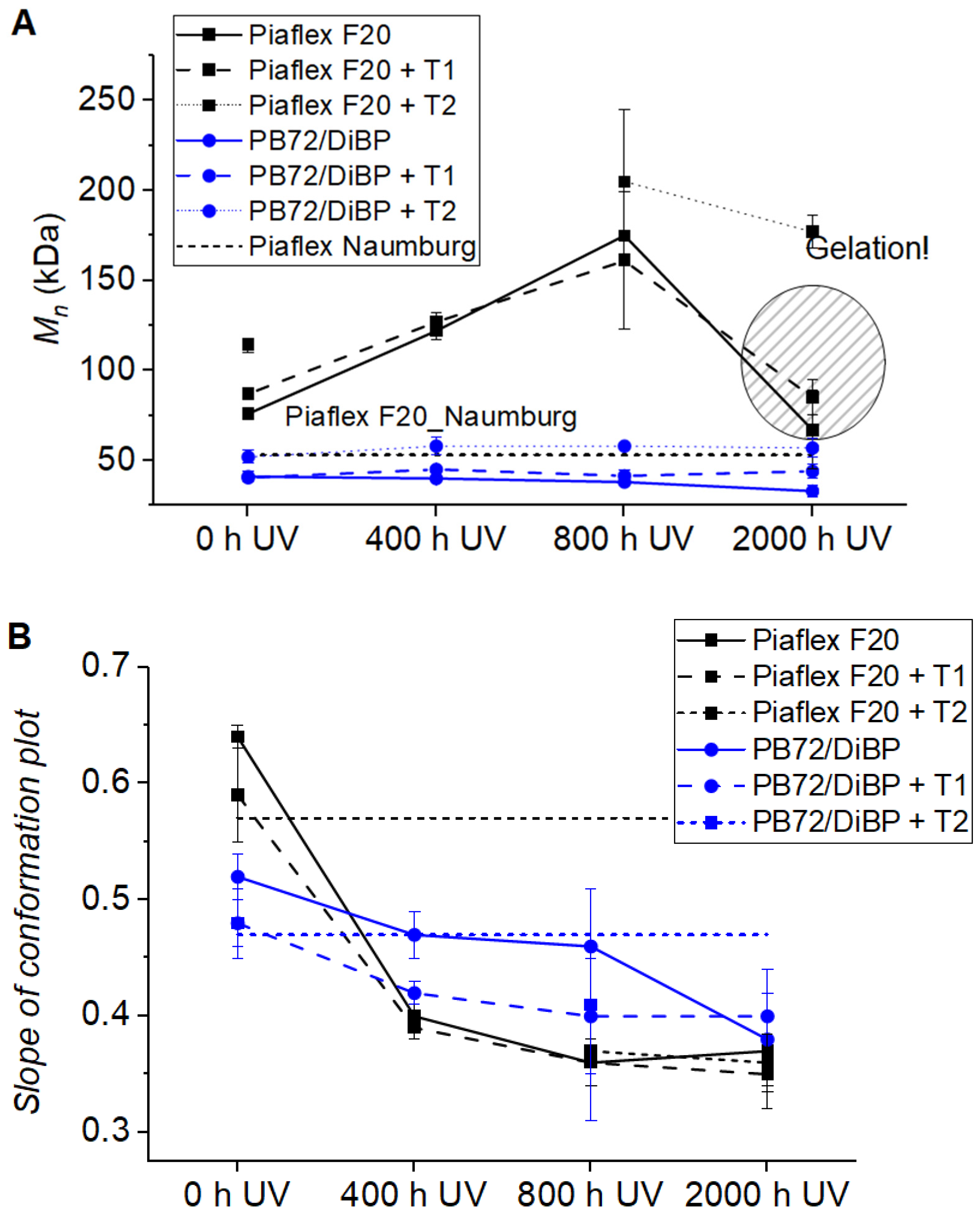


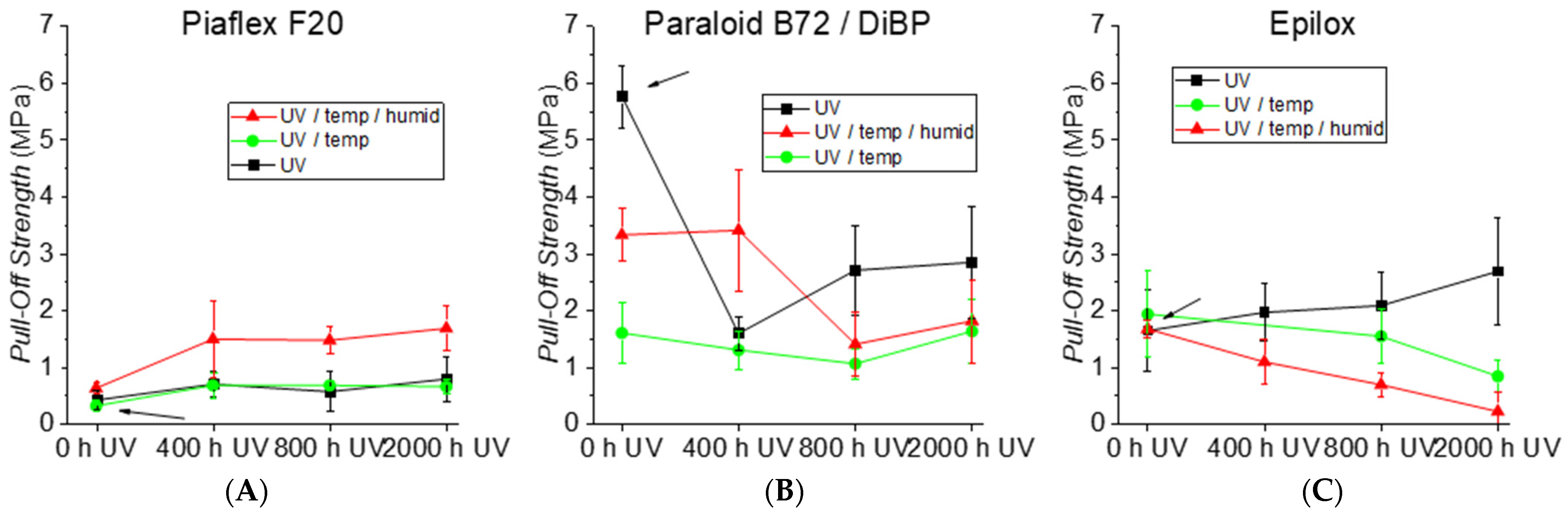
| Polymer | Historical Samples | Reference Material | Literature | Artificial Aging | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Heat | 2nd Heat | Before | Temp | Temp/Humid | UV (2000 h) | UV (2000 h)/Temp | UV (2000 h)/Temp/Humid | |||
| Piaflex F20 | 65–67 | 72–75 | 72 | - | 68 | 76 | 76 | 82 | 79 | 76 |
| PMMA | 63–65 | 80–84 | - | 105 | - | - | - | - | - | - |
| PMA | 16 | 20 | 13 | 6 | - | - | - | - | - | - |
| PMA/DiBP | 7–10 | 14–15 | - | −14 | 16 | - | 30 | 31 | - | |
| Paraloid B72/DiBP | - | - | - | - | 10 | 43 | 46 | 32 | 48 | 46 |
| Paraloid B72 | - | - | 38 | 40 | 23 | 46 | - | 47 | 46 | - |
| Epilox | - | - | - | 70 | 100 | 100 | 71 | 89 | 93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, J.; Kanaki, E.; Fischer, D.; Herm, C. Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows. Polymers 2023, 15, 2595. https://doi.org/10.3390/polym15122595
Brandt J, Kanaki E, Fischer D, Herm C. Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows. Polymers. 2023; 15(12):2595. https://doi.org/10.3390/polym15122595
Chicago/Turabian StyleBrandt, Josef, Elisavet Kanaki, Dieter Fischer, and Christoph Herm. 2023. "Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows" Polymers 15, no. 12: 2595. https://doi.org/10.3390/polym15122595
APA StyleBrandt, J., Kanaki, E., Fischer, D., & Herm, C. (2023). Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows. Polymers, 15(12), 2595. https://doi.org/10.3390/polym15122595







