Developing Transparent and Conductive PolyHEMA Gels Using Deep Eutectic Solvents
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Preparation of PolyHEMA DES Gels
2.3. Preparation of polyHEMA Hydrogels
2.4. Characterization
2.5. Statistical Analysis
3. Results and Discussion
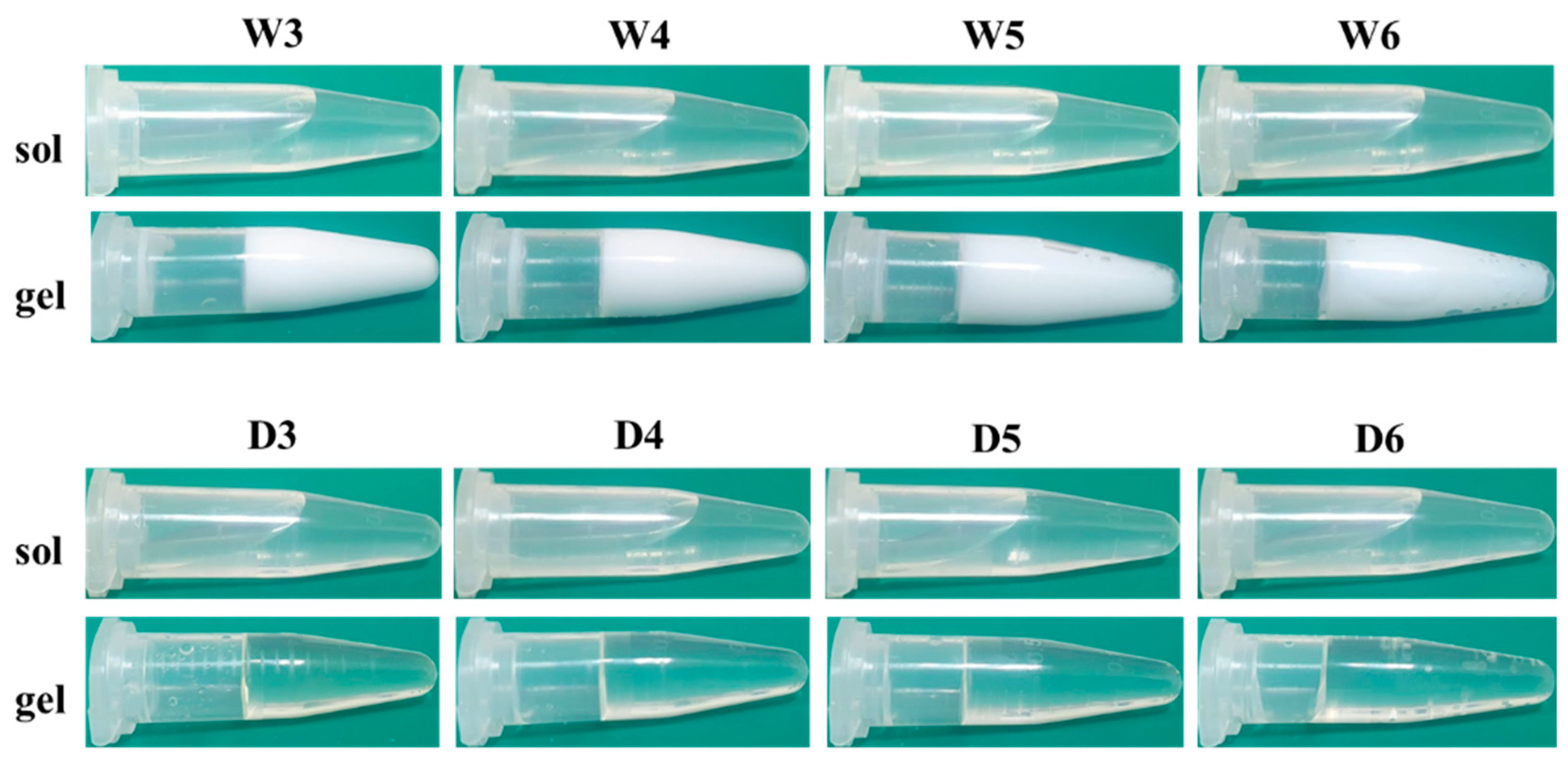
3.1. Transmittance
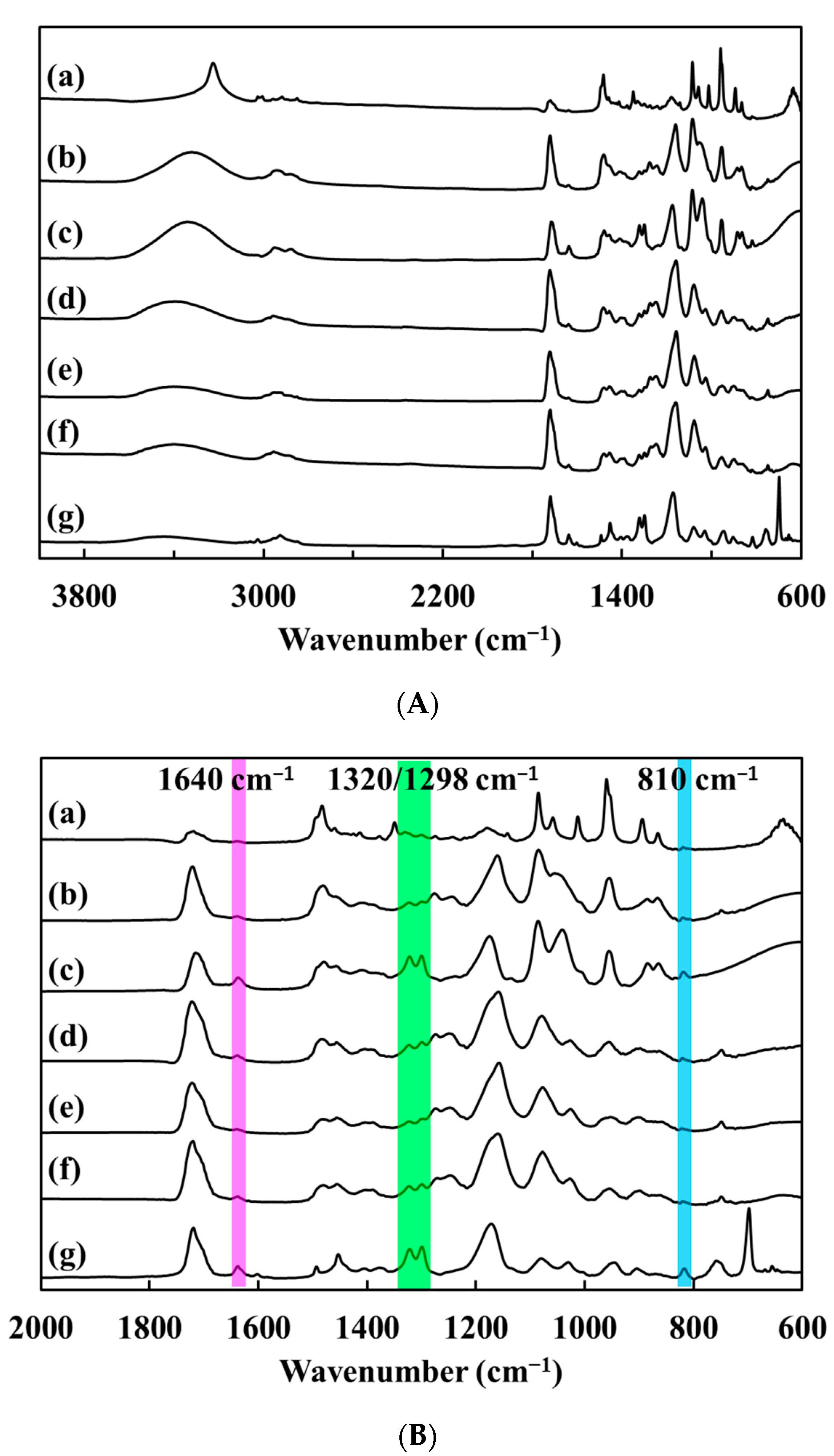
3.2. Study of Polymerization Kinetics by FTIR Analysis
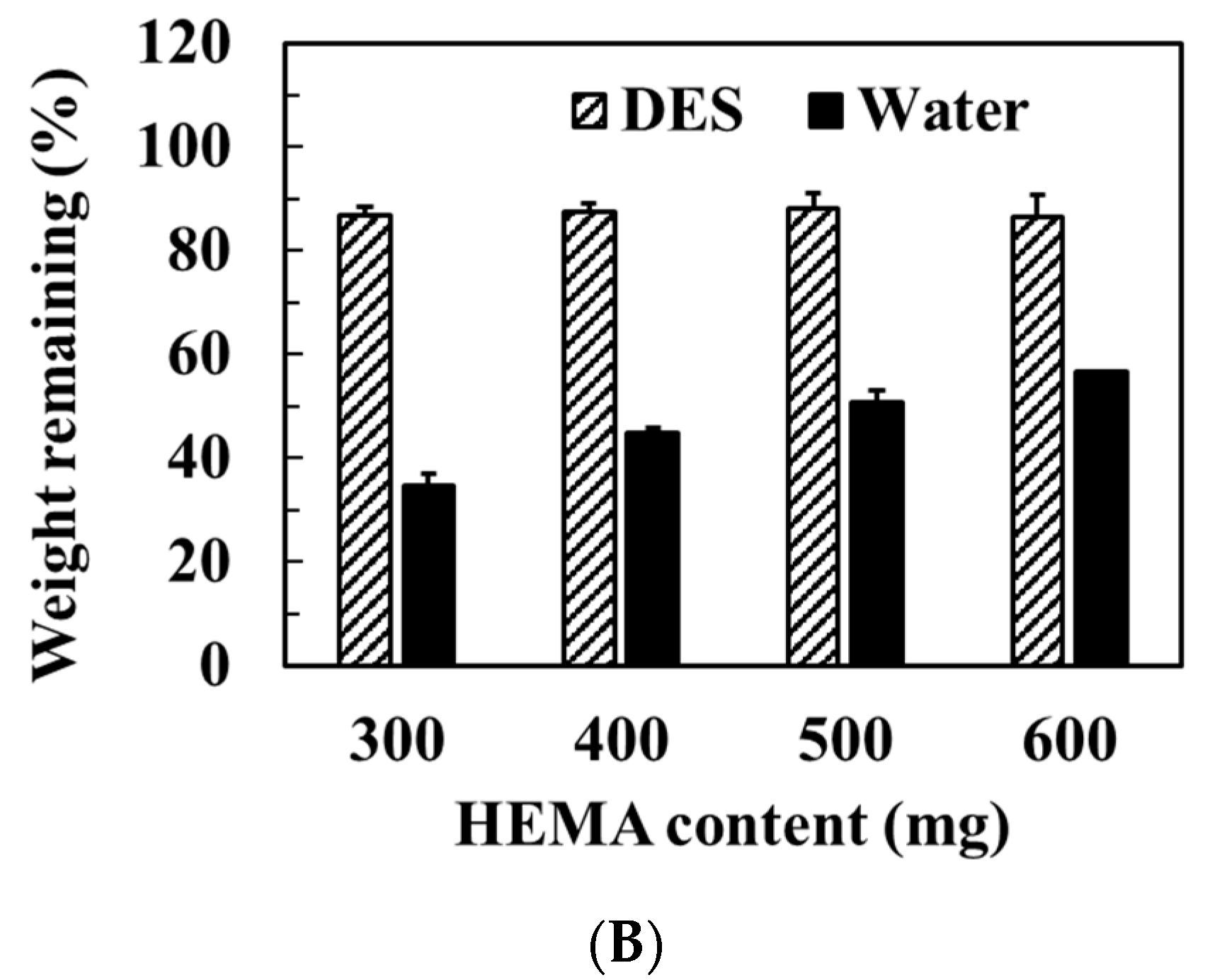
3.3. Swelling Properties
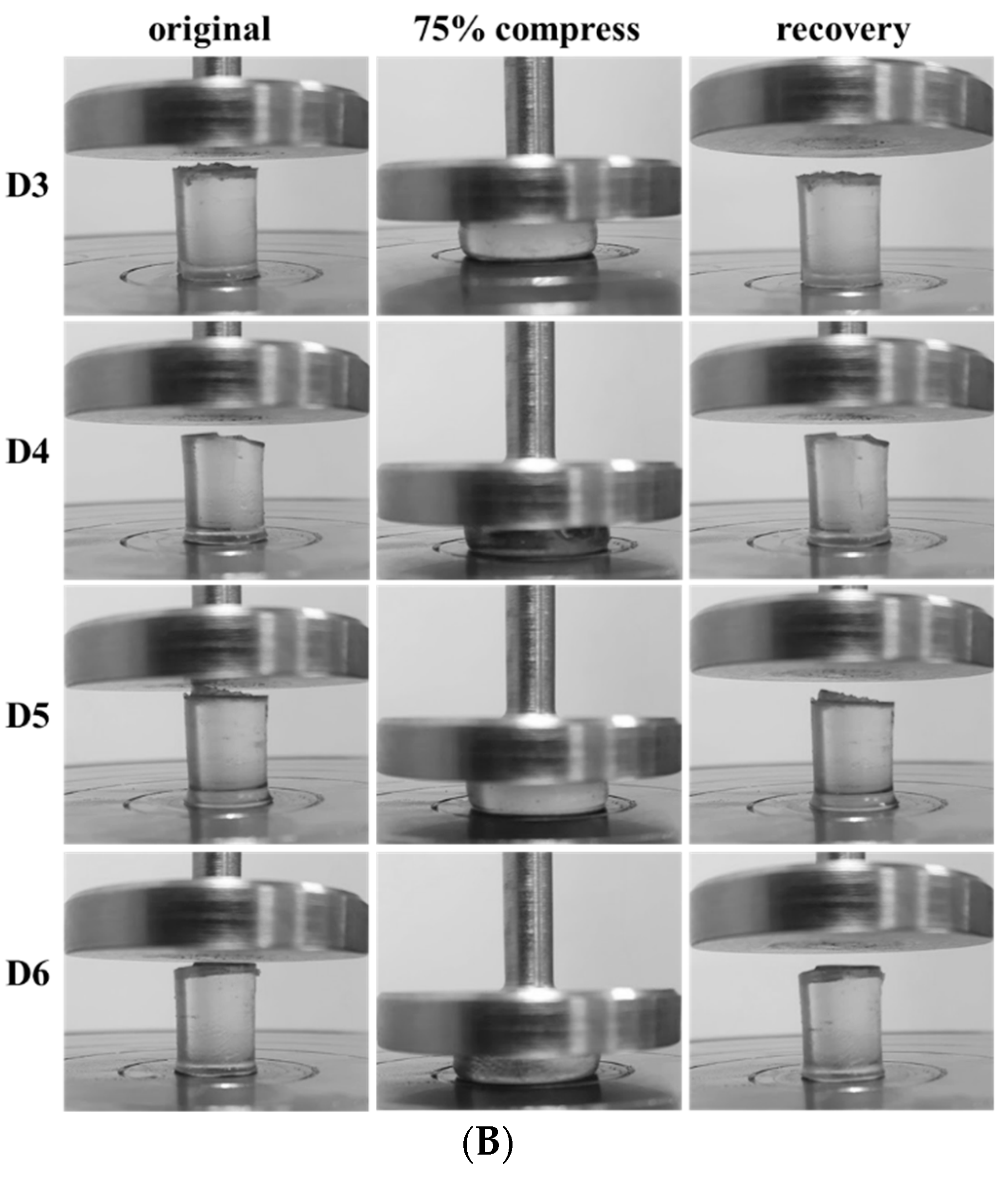
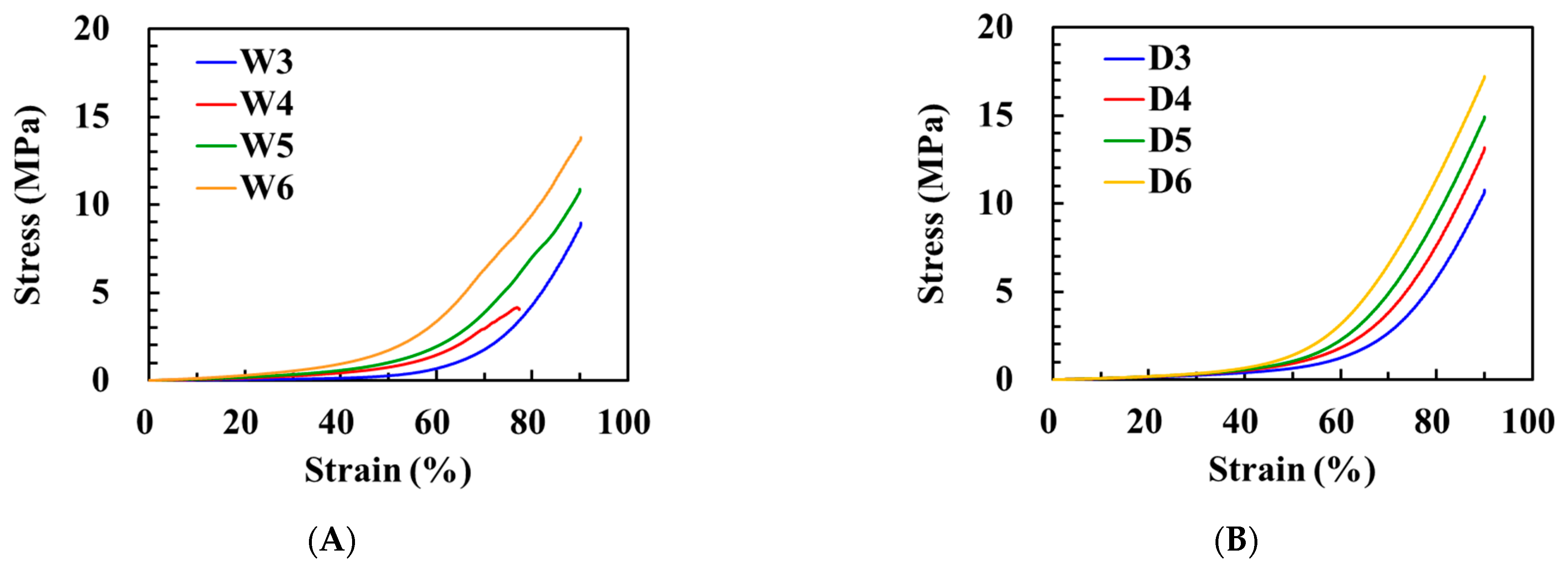
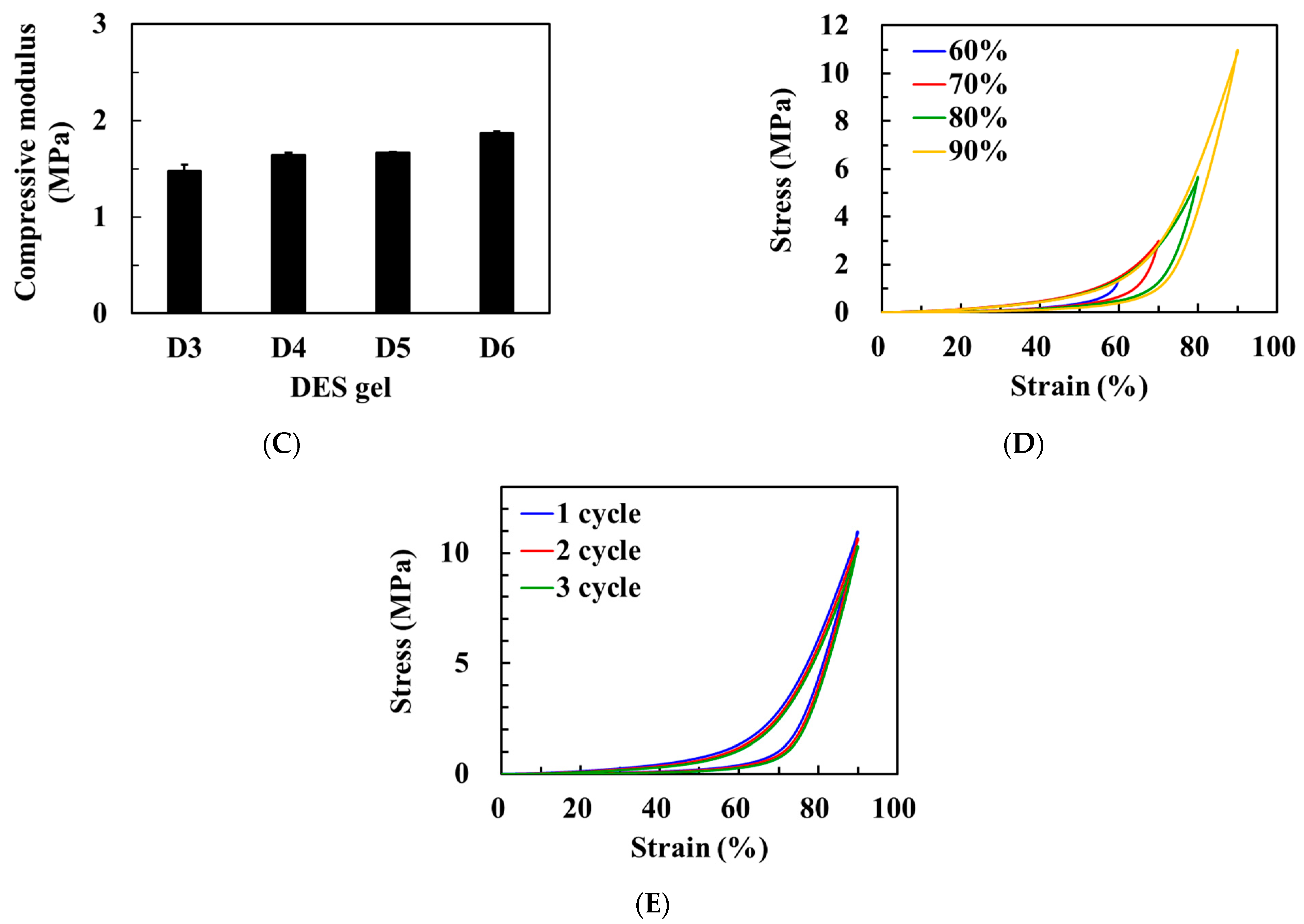
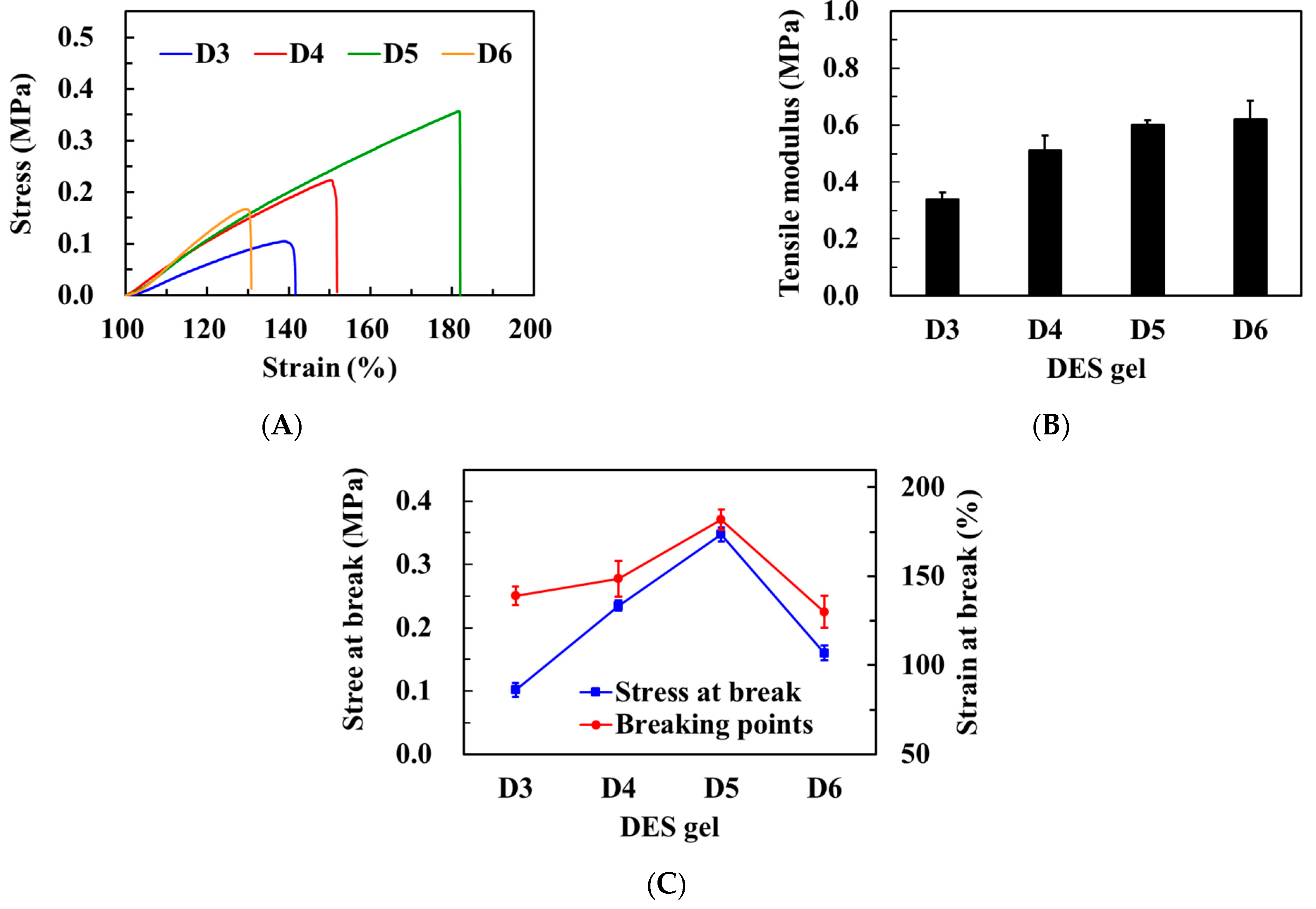
3.4. Mechanical Properties
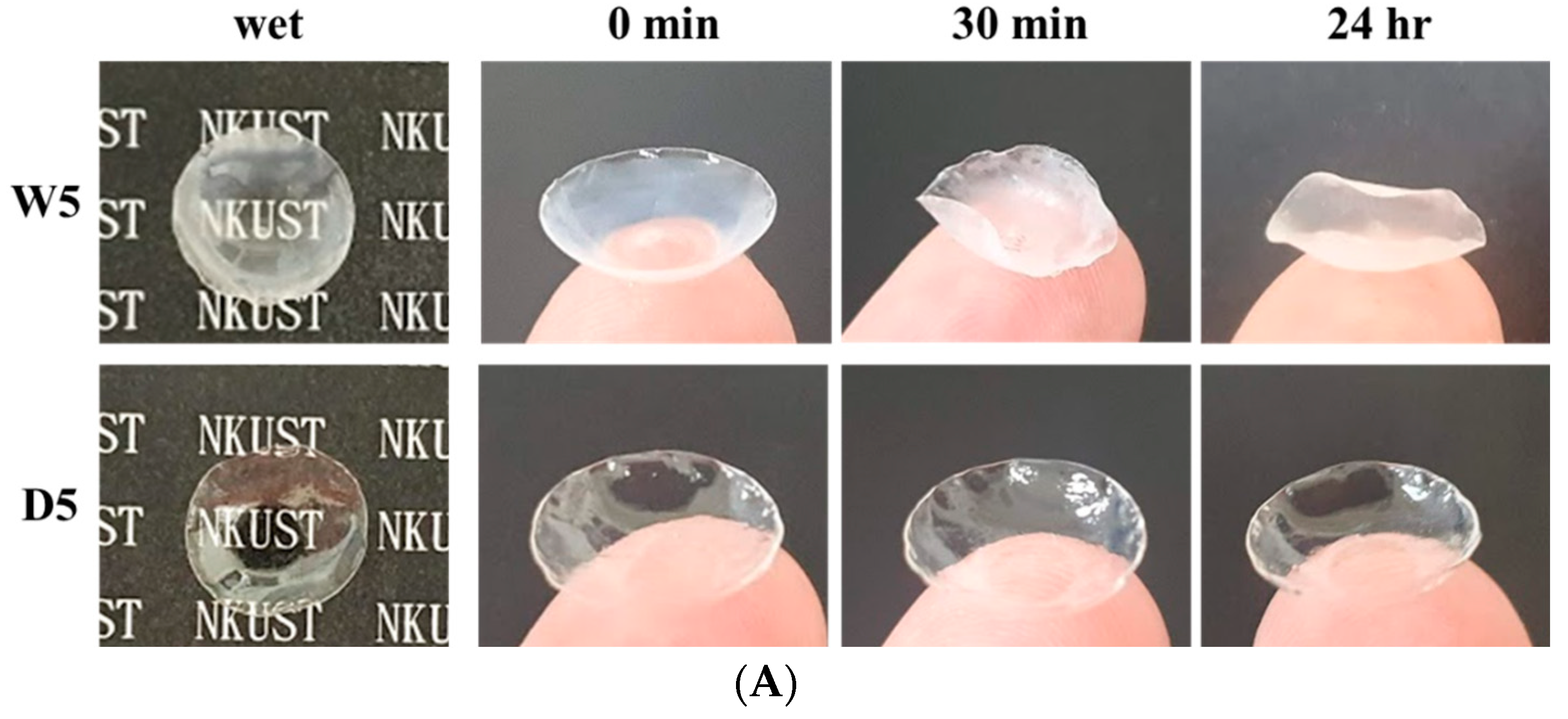
3.5. Dehydration
3.6. Conductivity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Chu, D.; Zheng, M.; Kissel, T.; Agarwal, S. Biocompatible and degradable poly(2-hydroxyethyl methacrylate) based polymers for biomedical applications. Polym. Chem. 2012, 3, 2752–2759. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Sekinea, Y.; Ikeda-Fukazawa, T. Structural changes of water in a hydrogel during dehydration. J. Chem. Phys. 2009, 130, 034501. [Google Scholar] [CrossRef]
- Kudo, K.; Ishida, J.; Syuu, G.; Sekine, Y.; Ikeda-Fukazawa, T. Structural changes of water in poly(vinyl alcohol) hydrogel during dehydration. J. Chem. Phys. 2014, 140, 044909. [Google Scholar] [CrossRef]
- Yu, X.; Qin, Z.; Wu, H.; Lv, H.; Yang, X. Tuning Hydrogel Mechanics by Kinetically Dependent Cross-Linking. Macromolecules 2019, 52, 1249–1256. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D. Emerging Ionic Soft Materials Based on Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124, 8465–8478. [Google Scholar] [CrossRef]
- Lai, C.-W.; Yu, S.-S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing. J. Mater. Chem. C 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Sánchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153. [Google Scholar] [CrossRef]
- Gurkan, B.E.; Maginn, E.J.; Pentzer, E.B. Deep Eutectic Solvents: A New Class of Versatile Liquids. J. Phys. Chem. B 2020, 124, 11313–11315. [Google Scholar] [CrossRef]
- Hu, X.; Liang, R.; Li, J.; Liu, Z.; Sun, G. Mechanically strong hydrogels achieved by designing homogeneous network structure. Mater. Des. 2019, 163, 107547. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Wang, P.; Wang, J.; Xu, B.; Xu, J.; Yuan, J.-G.; Yu, Y.-Y.; Wang, Q. Transparent and tough poly(2-hydroxyethyl methacrylate) hydrogels prepared in water/IL mixtures. New J. Chem. 2020, 44, 4092–4098. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; He, H.; Cheng, C.; Zhu, J.; Xiao, Z.; Zhang, H.; Li, X.; Zheng, J.; Xiao, J. Importance of zwitterionic incorporation into polymethacrylate-based hydrogels for simultaneously improving optical transparency, oxygen permeability, and antifouling properties. J. Mater. Chem. B 2017, 5, 4595–4606. [Google Scholar] [CrossRef]
- Lee, J.H.; Youm, J.S.; Ju, H.T.; Kim, J.C. Influence of N-vinyl-2-pyrrolidone and methacrylic acid on thermal curing process of 2-hydroxyethyl methacrylate hydrogel. J. Appl. Polym. Sci. 2020, 137, 48622. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, M. The pH-induced physical properties of ionic contact lens material. Heliyon 2023, 9, e12996. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Codina, C.; Efron, N. Impact of manufacturing technology and material composition on the mechanical properties of hydrogel contact lenses. Ophthalmic Physiol. Opt. 2004, 24, 551–561. [Google Scholar] [CrossRef]
- Chien, H.-W.; Kuo, C.-J. Preparation, material properties and antimicrobial efficacy of silicone hydrogel by modulating silicone and hydrophilic monomer. J. Biomater. Sci. Polym. Ed. 2019, 30, 1050–1067. [Google Scholar] [CrossRef]
- Chien, H.-W.; Tsai, W.-B.; Jiang, S. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials 2012, 33, 5706–5712. [Google Scholar] [CrossRef]
- Refojo, M.F.; Yasuda, H. Hydrogels from 2-hydroxyethyl methacrylate and propylene glycol monoacrylate. J. Appl. Polym. Sci. 1965, 9, 2425–2435. [Google Scholar] [CrossRef]
- Qin, H.; Panzer, M.J. Chemically cross-linked poly(2-hydroxyethyl methacrylate)-supported deep eutectic solvent gel electrolytes for eco-friendly supercapacitors. ChemElectroChem 2017, 4, 2556. [Google Scholar] [CrossRef]
- Nahar, Y.; Horne, J.; Truong, V.; Bissember, A.C.; Thickett, S.C. Preparation of thermoresponsive hydrogels via polymerizable deep eutectic monomer solvents. Polym. Chem. 2020, 12, 254–264. [Google Scholar] [CrossRef]
- Bednarz, S.; Półćwiartek, K.; Wityk, J.; Strachota, B.; Kredatusová, J.; Beneš, H.; Wesołowska-Piętak, A.; Kowalski, G. Polymerization-crosslinking of renewable itaconic acid in water and in deep eutectic solvents. Eur. Polym. J. 2017, 95, 241. [Google Scholar] [CrossRef]
- Santha Kumar, A.R.S.; Singha, N.K. RAFT polymerization of 2-hydroxyethyl methacrylate in a deep eutectic solvent. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2281–2286. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The Effects of Monomer, Crosslinking Agent, and Filler Concentrations on the Viscoelastic and Swelling Properties of Poly(methacrylic acid) Hydrogels: A Comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef]
- Mukesh, C.; Gupta, R.; Srivastava, D.N.; Nataraj, S.K.; Prasad, K. Preparation of a natural deep eutectic solvent mediated self polymerized highly flexible transparent gel having super capacitive behaviour. RSC Adv. 2016, 6, 28586–28592. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, Stretchable, Compressive Novel Polymer/Graphene Oxide Nanocomposite Hydrogels with Excellent Self-Healing Performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef]
- Kim, E.; Saha, M.; Ehrmann, K. Mechanical Properties of Contact Lens Materials. Eye Contact Lens Sci. Clin. Pract. 2018, 44, S148–S156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Su, J.; Zhang, X.; Wang, J.; Tu, Q. Preparation of a Multifunctional Wound Dressing Based on a Natural Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2020, 8, 14243–14252. [Google Scholar] [CrossRef]
- Mukesh, C.; Upadhyay, K.K.; Devkar, R.V.; Chudasama, N.A.; Raol, G.G.; Prasad, K. Preparation of a Noncytotoxic Hemocompatible Ion Gel by Self-Polymerization of HEMA in a Green Deep Eutectic Solvent. Macromol. Chem. Phys. 2016, 217, 1899–1906. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chen, C.-C.; Hsu, S.-M.; Chuang, H.-S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef] [Green Version]



| Samples | HEMA (mg) | EGDMA (μL) | APS a (μL) | H2O (μL) | DES b (μL) | Swelling (Q) |
|---|---|---|---|---|---|---|
| W3 | 300 | 10 | 20 | 600 | - | 0.102 ± 0.031 |
| W4 | 400 | 10 | 20 | 600 | - | 0.171 ± 0.053 |
| W5 | 500 | 10 | 20 | 600 | - | 0.271 ± 0.075 |
| W6 | 600 | 10 | 20 | 600 | - | 0.442 ± 0.085 |
| D3 | 300 | 10 | 20 | - | 600 | 0.283 ± 0.084 |
| D4 | 400 | 10 | 20 | - | 600 | 0.342 ± 0.059 |
| D5 | 500 | 10 | 20 | - | 600 | 0.374 ± 0.079 |
| D6 | 600 | 10 | 20 | - | 600 | 0.440 ± 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Jiang, Y.-J.; Chien, H.-W. Developing Transparent and Conductive PolyHEMA Gels Using Deep Eutectic Solvents. Polymers 2023, 15, 2605. https://doi.org/10.3390/polym15122605
Chen T-Y, Jiang Y-J, Chien H-W. Developing Transparent and Conductive PolyHEMA Gels Using Deep Eutectic Solvents. Polymers. 2023; 15(12):2605. https://doi.org/10.3390/polym15122605
Chicago/Turabian StyleChen, Tai-Yu, Yi-Jie Jiang, and Hsiu-Wen Chien. 2023. "Developing Transparent and Conductive PolyHEMA Gels Using Deep Eutectic Solvents" Polymers 15, no. 12: 2605. https://doi.org/10.3390/polym15122605





