Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of LFP/C Composites
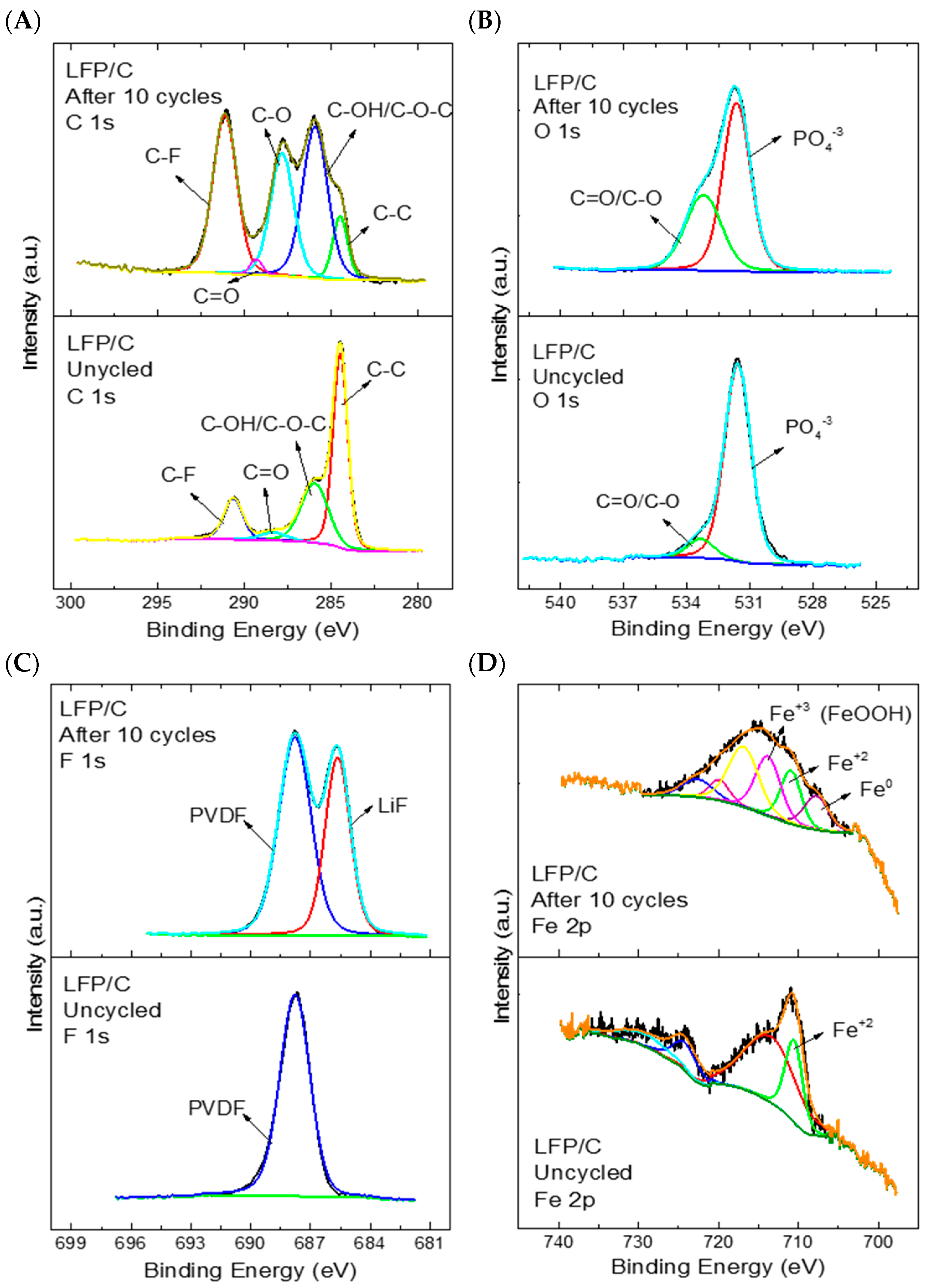
3.2. X-ray Photoelectron Characterisation of LFP/C Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, L.; Sun, F.; Wang, Z. An Overview on Thermal Safety Issues of Lithium-ion Batteries for Electric Vehicle Application. IEEE Access 2018, 6, 23848–23863. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Rugolo, J.; Aziz, M.J. Electricity storage for intermittent renewable sources. Energy Environ. Sci. 2012, 5, 7151. [Google Scholar] [CrossRef]
- Ayodele, T.; Ogunjuyigbe, A. Mitigation of wind power intermittency: Storage technology approach. Renew. Sustain. Energy Rev. 2015, 44, 447–456. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Saji, V.S.; Kim, Y.-S.; Kim, T.-H.; Cho, J.; Song, H.-K. One-dimensional (1D) nanostructured and nanocomposited LiFePO4: Its perspective advantages for cathode materials of lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 19226–19237. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Shi, S.; Wang, Z.; Huang, X.; Chen, L. First-principles study of Li ion diffusion in LiFePO4. Phys. Rev. B 2004, 69, 104303–1043035. [Google Scholar] [CrossRef]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle Size Dependence of the Ionic Diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef]
- Satyavani, T.; Kiran, B.R.; Kumar, V.R.; Kumar, A.S.; Naidu, S. Effect of particle size on dc conductivity, activation energy and diffusion coefficient of lithium iron phosphate in Li-ion cells. Eng. Sci. Technol. Int. J. 2016, 19, 40–44. [Google Scholar] [CrossRef]
- Velásquez, E.A.; Silva, D.P.B.; Falqueto, J.B.; Mejía-López, J.; Bocchi, N.; del Rio, R.; Mazo-Zuluaga, J.; Rocha-Filho, R.C.; Biaggio, S.R. Understanding the loss of electrochemical activity of nanosized LiMn2O4 particles: A combined experimental and ab initio DFT study. J. Mater. Chem. A 2018, 6, 14967–14974. [Google Scholar] [CrossRef]
- Satyavani, T.; Kumar, A.S.; Rao, P.S. Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: A review. Eng. Sci. Technol. Int. J. 2016, 19, 178–188. [Google Scholar] [CrossRef]
- Yang, G.; Ji, H.; Liu, H.; Huo, K.; Fu, J.; Chu, P.K. Fast Preparation of LiFePO4 Nanoparticles for Lithium Batteries by Microwave-Assisted Hydrothermal Method. J. Nanosci. Nanotechnol. 2010, 10, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H. A Simple and Low-cost Synthesis Strategy of LiFePO4 Nanoparticles as Cathode Materials for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 210331. [Google Scholar] [CrossRef]
- Smecellato, P.C.; Davoglio, R.A.; Biaggio, S.R.; Bocchi, N.; Rocha-Filho, R.C. Alternative route for LiFePO4 synthesis: Carbothermal reduction combined with microwave-assisted solid-state reaction. Mater. Res. Bull. 2017, 86, 209–214. [Google Scholar] [CrossRef]
- Xi, Y.; Lu, Y. Toward Uniform In Situ Carbon Coating on Nano-LiFePO4 via a Solid-State Reaction. Ind. Eng. Chem. Res. 2020, 59, 13549–13555. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Liu, F.; Yang, J.; Feng, T. Variation of carbon coatings on the electrochemical performance of LiFePO4 cathodes for lithium ionic batteries. RSC Adv. 2017, 7, 44296–44302. [Google Scholar] [CrossRef]
- Heng, S.; Shi, Q.; Zheng, X.; Wang, Y.; Qu, Q.; Liu, G.; Battaglia, V.S.; Zheng, H. An organic-skinned secondary coating for carbon-coated LiFePO4 cathode of high electrochemical performances. Electrochim. Acta 2017, 258, 1244–1253. [Google Scholar] [CrossRef]
- Kanagaraj, A.B.; Chaturvedi, P.; An, B.H.; AlDahmani, S.; Fadaq, H.; Choi, D.S. Electrochemical performance of freestanding LiFePO4/MWCNT composite electrodes and its ex situ studies. Ionics 2020, 26, 115–125. [Google Scholar] [CrossRef]
- Wu, X.-L.; Guo, Y.-G.; Su, J.; Xiong, J.-W.; Zhang, Y.-L.; Wan, L.-J. Carbon-Nanotube-Decorated Nano-LiFePO4@C Cathode Material with Superior High-Rate and Low-Temperature Performances for Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 1155–1160. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Kanagaraj, A.B.; Al Shibli, H.; Alkindi, T.S.; Susantyoko, R.A.; An, B.H.; AlMheiri, S.; AlDahmani, S.; Fadaq, H.; Choi, D.S. Hydrothermal synthesis of LiFePO4 micro-particles for fabrication of cathode materials based on LiFePO4/carbon nanotubes nanocomposites for Li-ion batteries. Ionics 2018, 24, 3685–3690. [Google Scholar] [CrossRef]
- Meng, Y.; Xia, J.; Wang, L.; Wang, G.; Zhu, F.; Zhang, Y. A comparative study on LiFePO4/C by in-situ coating with different carbon sources for high-performance lithium batteries. Electrochim. Acta 2018, 261, 96–103. [Google Scholar] [CrossRef]
- Cao, H.; Wen, L.; Guo, Z.-Q.; Piao, N.; Hu, G.-J.; Wu, M.-J.; Li, F. Application and prospects for using carbon materials to modify lithium iron phosphate materials used at low temperatures. New Carbon Mater. 2022, 37, 46–58. [Google Scholar] [CrossRef]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Comparison of Microwave Assisted Solvothermal and Hydrothermal Syntheses of LiFePO4/C Nanocomposite Cathodes for Lithium Ion Batteries. J. Phys. Chem. C 2008, 112, 14665–14671. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Etale, A.; Wang, J.; Berglund, L.A.; Li, Y.; Cai, Y.; Chen, C.; Cranston, E.D.; Johns, M.A.; Fang, Z.; et al. Current international research into cellulose as a functional nanomaterial for advanced applications. J. Mater. Sci. 2022, 57, 5697–5767. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A Review of Properties of Nanocellulose, Its Synthesis, and Potential in Biomedical Applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, M.; Liu, H.; Shang, S.; Wang, D.; Liimatainen, H. Facile synthesis of palladium and gold nanoparticles by using dialdehyde nanocellulose as template and reducing agent. Carbohydr. Polym. 2018, 186, 132–139. [Google Scholar] [CrossRef]
- Johnson, L.; Thielemans, W.; Walsh, D.A. Synthesis of carbon-supported Pt nanoparticle electrocatalysts using nanocrystalline cellulose as reducing agent. Green Chem. 2011, 13, 1686–1693. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.; Kim, J.M.; Guccini, V.; Hwang, T.; Jeon, Y.; Salazar-Alvarez, G.; Piao, Y. Facile preparation of cellulose nanofiber derived carbon and reduced graphene oxide co-supported LiFePO4 nanocomposite as enhanced cathode material for lithium-ion battery. Electrochim. Acta 2020, 354, 136707. [Google Scholar] [CrossRef]
- Alsamet, M.A.; Burgaz, E. Synthesis and characterization of nano-sized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods. Electrochim. Acta 2021, 367, 137530. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.; Junke, O.; Guo, Y.; Xiao, D. X-ray diffraction study of LiFePO4 synthesized by hydrothermal method. RSC Adv. 2013, 3, 14652–14660. [Google Scholar] [CrossRef]
- Chen, L.; Feng, W.; Pu, Z.; Wang, X.; Song, C. Impact of pH on preparation of LiFePO4@C cathode materials by a sol-gel route assisted by biomineralization. Ionics 2019, 25, 5625–5632. [Google Scholar] [CrossRef]
- Mos, Y.M.; Vermeulen, A.C.; Buisman, C.J.N.; Weijma, J. X-Ray Diffraction of Iron Containing Samples: The Importance of a Suitable Configuration. Geomicrobiol. J. 2018, 35, 511–517. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, K.; Liang, F.; Dai, Y.; Yao, Y. Optimized solvothermal synthesis of LiFePO4 cathode material for enhanced high-rate and low temperature electrochemical performances. Electrochim. Acta 2017, 258, 1149–1159. [Google Scholar] [CrossRef]
- Wang, J.; Niu, Y.; Fu, Y.; Yang, Y.; Hojamberdiev, M. Urea and Ethylene Glycol-Assisted Solvothermal Synthesis of Spheroidal LiFePO4 /C Nanoparticles as a Cathode Material for Lithium-ion Batteries. Chemistryselect 2018, 3, 5471–5479. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Markevich, E.; Sharabi, R.; Haik, O.; Borgel, V.; Salitra, G.; Aurbach, D.; Semrau, G.; Schmidt, M.; Schall, N.; Stinner, C. Raman spectroscopy of carbon-coated LiCoPO4 and LiFePO4 olivines. J. Power Sources 2011, 196, 6433–6439. [Google Scholar] [CrossRef]
- Burba, C.M.; Palmer, J.M.; Holinsworth, B.S. Laser-induced phase changes in olivine FePO4: A warning on characterizing LiFePO4-based cathodes with Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 225–228. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Xu, J.; Xia, Z.; Sun, J.; Asp, L.E.; Palermo, V. Electrophoretic coating of LiFePO4/Graphene oxide on carbon fibers as cathode electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2021, 208, 108768. [Google Scholar] [CrossRef]
- Nagpure, S.C.; Bhushan, B.; Babu, S. Raman and NMR studies of aged LiFePO4 cathode. Appl. Surf. Sci. 2012, 259, 49–54. [Google Scholar] [CrossRef]
- Bellani, C.F.; Pollet, E.; Hebraud, A.; Pereira, F.V.; Schlatter, G.; Avérous, L.; Bretas, R.E.S.; Branciforti, M.C. Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J. Appl. Polym. Sci. 2016, 133, 43445. [Google Scholar] [CrossRef]
- Sultana, T.; Sultana, S.; Nur, H.P.; Khan, W. Studies on Mechanical, Thermal and Morphological Properties of Betel Nut Husk Nano Cellulose Reinforced Biodegradable Polymer Composites. J. Compos. Sci. 2020, 4, 83. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Acc. Chem. Res. 2018, 51, 3154–3165. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Gao, X.; Qu, Q.; Zhu, G.; Gao, T.; Qian, F.; Shi, Q.; Zheng, H. Piperidinium-based ionic liquid electrolyte with linear solvent and LiODFB for LiFePO4/Li cells at room and high temperature. RSC Adv. 2017, 7, 50135–50142. [Google Scholar] [CrossRef]
- Yan, Y.; Xue, F.; Muhammad, F.; Yu, L.; Xu, F.; Jiao, B.; Shiau, Y.; Li, D. Application of iron-loaded activated carbon electrodes for electrokinetic remediation of chromium-contaminated soil in a three-dimensional electrode system. Sci. Rep. 2018, 8, 5753. [Google Scholar] [CrossRef]
- Huynh, L.T.N.; Nguyen, H.H.A.; Tran, T.T.D.; Nguyen, T.T.T.; La, T.H.; Tran, V.M.; Le, M.L.P. Electrode Composite LiFePO4@Carbon: Structure and Electrochemical Performances. J. Nanomater. 2019, 2019, 2464920. [Google Scholar] [CrossRef]
- Baboo, J.P.; Yatoo, M.A.; Dent, M.; Najafabadi, E.H.; Lekakou, C.; Slade, R.; Hinder, S.J.; Watts, J.F. Exploring Different Binders for a LiFePO4 Battery, Battery Testing, Modeling and Simulations. Energies 2022, 15, 2332. [Google Scholar] [CrossRef]
- Bai, P.; Ji, X.; Zhang, J.; Zhang, W.; Hou, S.; Su, H.; Li, M.; Deng, T.; Cao, L.; Liu, S.; et al. Formation of LiF-rich Cathode-Electrolyte Interphase by Electrolyte Reduction. Angew. Chem. 2022, 134, e202202731. [Google Scholar] [CrossRef]
- Castro, L.; Dedryvère, R.; Ledeuil, J.-B.; Bréger, J.; Tessier, C.; Gonbeau, D. Aging Mechanisms of LiFePO4// Graphite Cells Studied by XPS: Redox Reaction and Electrode/Electrolyte Interfaces. J. Electrochem. Soc. 2012, 159, A357–A363. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive electrochemical synthesis of nickel iron sulphides on nickel foam: Super active and ultra-durable electrocatalysts for alkaline electrolyte membrane water electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
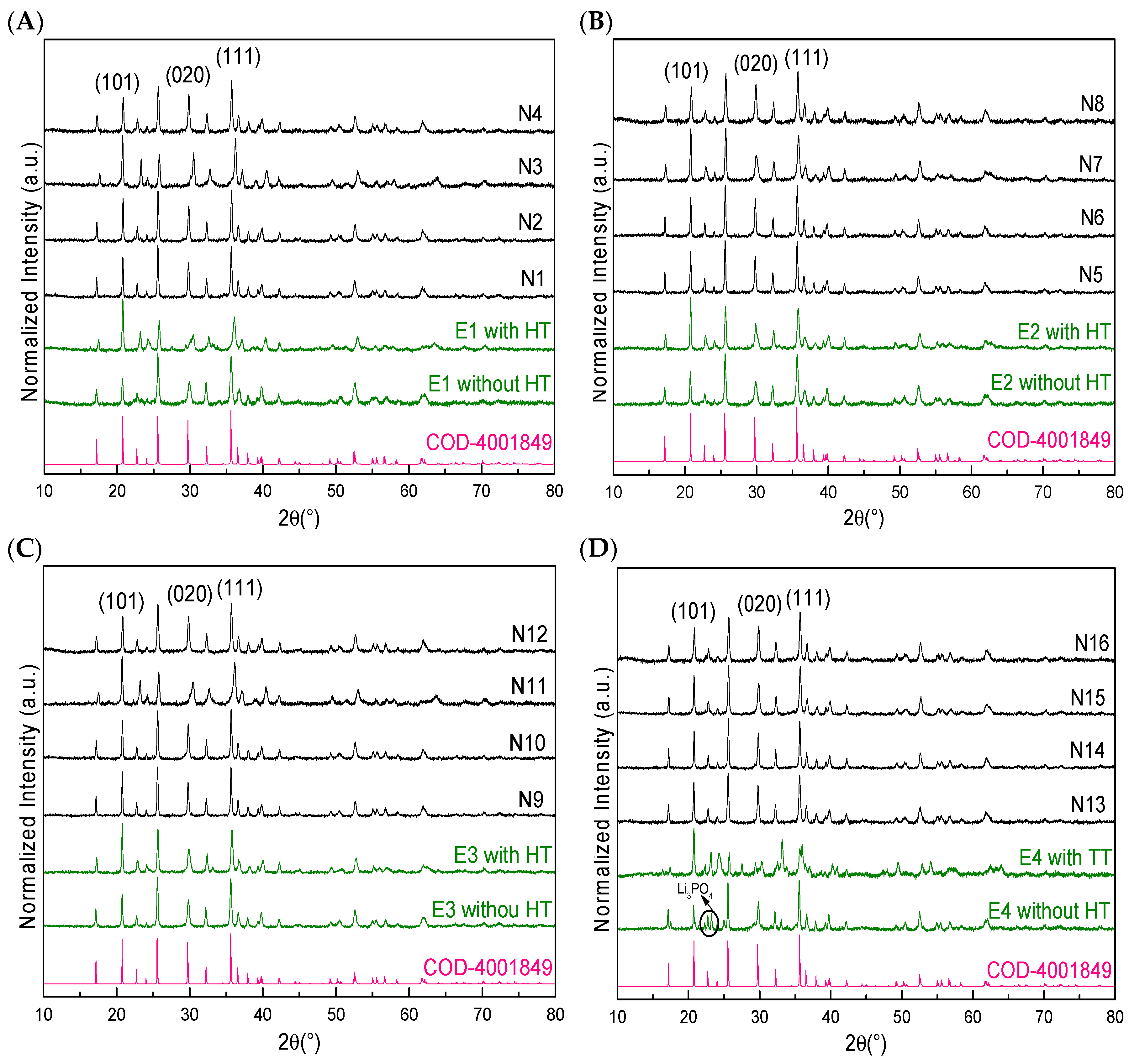
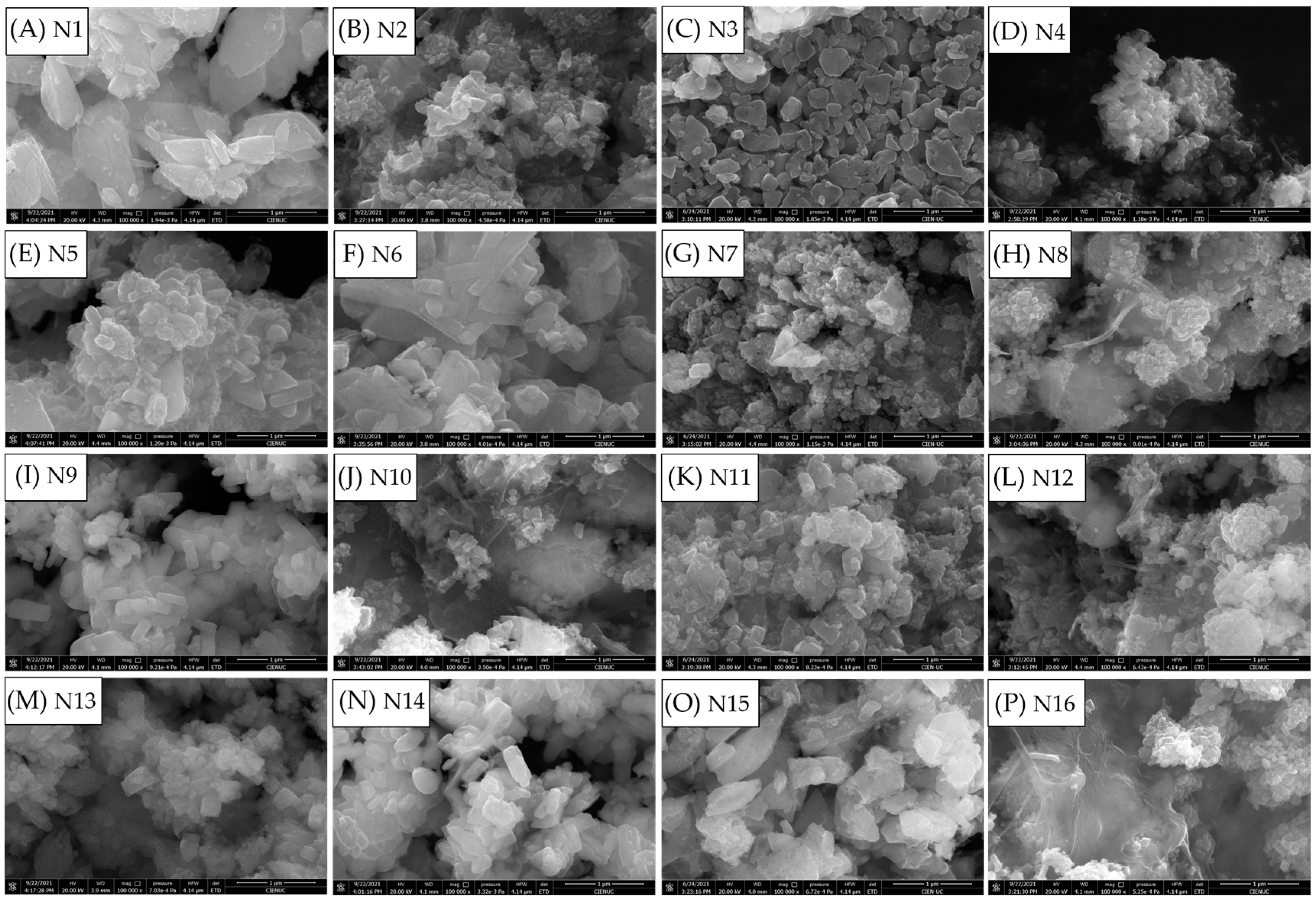
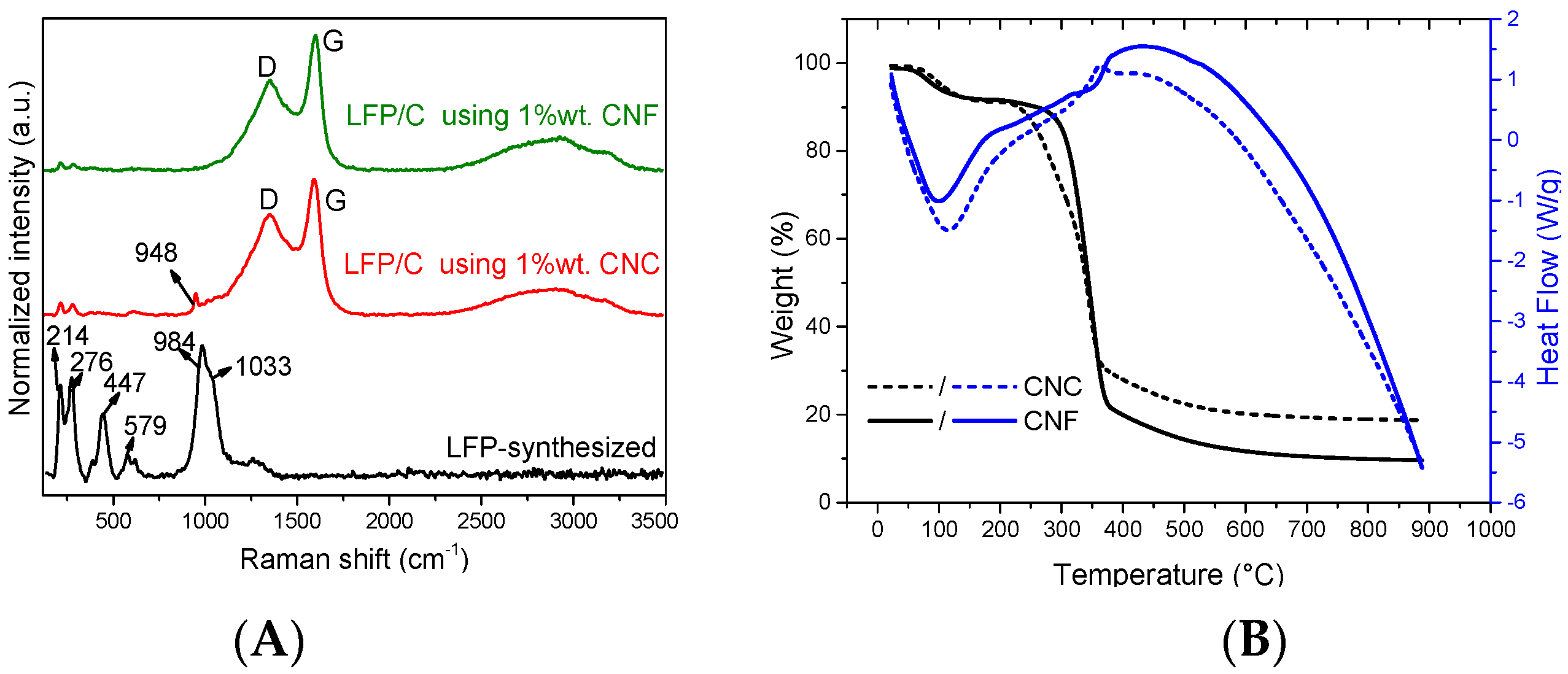


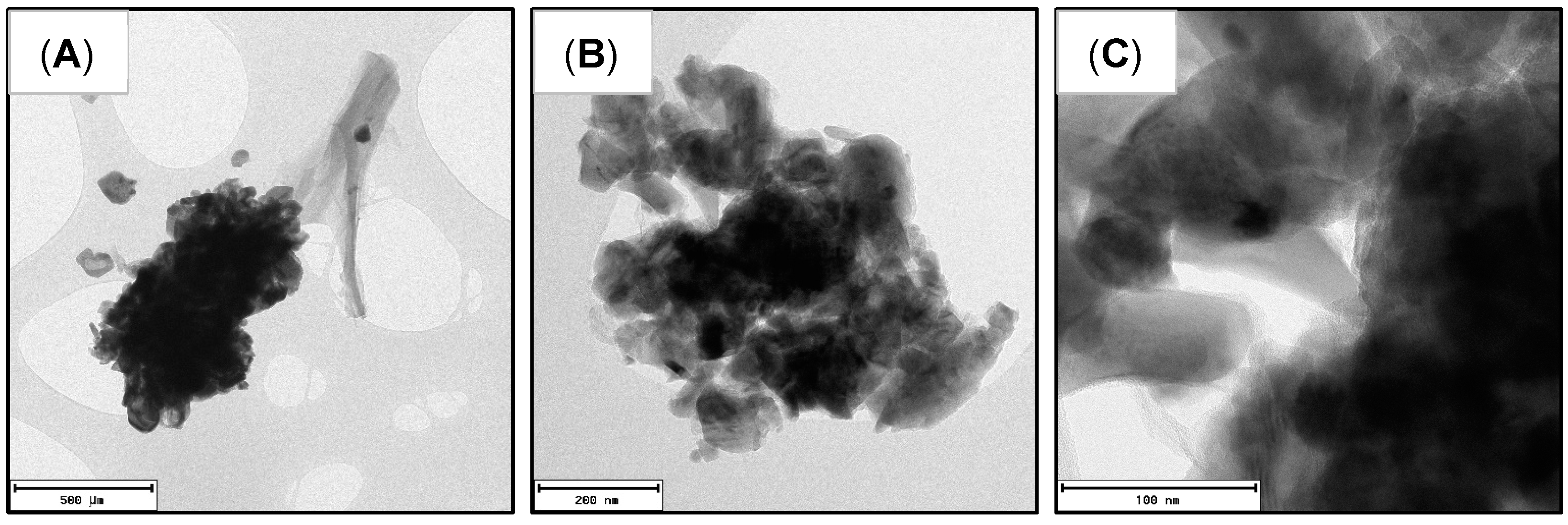

| Sample | Type NC | %wt. NC | Temperature (°C) | Time (min) |
|---|---|---|---|---|
| N1 | CNC | 0.15 | 150 | 15 |
| N2 | CNC | 1 | 150 | 15 |
| N3 | CNF | 0.15 | 150 | 15 |
| N4 | CNF | 1 | 150 | 15 |
| N5 | CNC | 0.15 | 150 | 30 |
| N6 | CNC | 1 | 150 | 30 |
| N7 | CNF | 0.15 | 150 | 30 |
| N8 | CNF | 1 | 150 | 30 |
| N9 | CNC | 0.15 | 205 | 15 |
| N10 | CNC | 1 | 205 | 15 |
| N11 | CNF | 0.15 | 205 | 15 |
| N12 | CNF | 1 | 205 | 15 |
| N13 | CNC | 0.15 | 205 | 30 |
| N14 | CNC | 1 | 205 | 30 |
| N15 | CNF | 0.15 | 205 | 30 |
| N16 | CNF | 1 | 205 | 30 |
| Sample | Type of NC | % wt. of NC Used in Vial Synthesis | % wt. Carbon Obtained in LFP/C Powder |
|---|---|---|---|
| N6 | CNC | 1 | 6.8 |
| N8 | CNF | 1 | 12.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroff, M.; Hevia, S.A.; O’Shea, J.N.; Muro, I.G.d.; Palomares, V.; Rojo, T.; del Río, R. Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source. Polymers 2023, 15, 2628. https://doi.org/10.3390/polym15122628
Kroff M, Hevia SA, O’Shea JN, Muro IGd, Palomares V, Rojo T, del Río R. Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source. Polymers. 2023; 15(12):2628. https://doi.org/10.3390/polym15122628
Chicago/Turabian StyleKroff, Macarena, Samuel A. Hevia, James N. O’Shea, Izaskun Gil de Muro, Verónica Palomares, Teófilo Rojo, and Rodrigo del Río. 2023. "Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source" Polymers 15, no. 12: 2628. https://doi.org/10.3390/polym15122628
APA StyleKroff, M., Hevia, S. A., O’Shea, J. N., Muro, I. G. d., Palomares, V., Rojo, T., & del Río, R. (2023). Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source. Polymers, 15(12), 2628. https://doi.org/10.3390/polym15122628








