Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review
Abstract
:1. Introduction
2. Spray-Drying Process
Stages of the Spray-Drying Process
3. Wall Materials Used in Spray-Drying
3.1. Polysaccharides
- Starch is a complex polysaccharide composed of amylose and amylopectin, primarily derived from tubers (e.g., potatoes, cassava, and sweet potatoes) and cereals (e.g., corn, sorghum, wheat, rice, rye, oats, and barley) [19]. This carbohydrate is made up of glucose monomers with free hydroxyl groups (-OH) at positions C2, C3, and C6, giving it a highly hydrophilic helical structure [20]. Starch finds applications in various industries such as textiles, chemicals, healthcare, and food, due to its physicochemical properties such as solubility, viscosity, texture, and thermal stability [20].
- Maltodextrin is a polysaccharide derived from the hydrolysis of starch (from corn, rice, wheat, tapioca, sorghum, barley, etc.) with a dextrose equivalent value (DE: the ratio of reducing sugars to total sugars) of less than 20 [21]. Maltodextrin has different characteristics and properties compared to starch, leading to varied applications [22]. It is used as an additive in food products and beverages [23] and as a fat replacer in dairy, meat, and baked goods due to its ability to form gels, its hygroscopicity, solubility, viscosity, and sweetness [24].
- Chitosan is a structural polysaccharide extracted from microorganisms (such as fungi and algae), marine animals (such as crustaceans and mollusks), and insects (such as scorpions and spiders), or obtained through the chemical deacetylation of chitin [25]. Chitosan is highly regarded for its antibacterial, antifungal, and antiviral activity, attributed to its cationic polyelectrolyte character. It also possesses the ability to form gels due to its viscosity, plasticity, and solubility [26]. In recent years, chitosan has found applications in post-harvest pathogen control [27] and the development of biodegradable packaging [28].
- Dextran is a polysaccharide synthesized by microorganisms, particularly lactic acid bacteria, and it possesses various thermal, rheological, viscosity, and solubility properties due to its branching structure [29]. The application of dextran has been primarily explored as a food additive in the formulation of emulsions, nanoparticles, and immobilizers [30]. It is also used as an excipient in the formulation of inhaled drugs (such as rifampicin and budesonide) due to its humectant, stabilizing, and preserving action [31,32].
- Carrageenan is a sulfated polysaccharide extracted from red seaweeds such as Kappaphycus and Eucheuma. It exhibits structural diversity due to the degree of sulfation and can be classified as κ-, ι-, θ-, μ-, ν-, and λ-carrageenan [33]. Carrageenan does not have proven nutritional value, but it finds special application in the food industry due to its gelling, stabilizing, binding, and thickening properties. It is used in products such as jellies, dressings, fat substitutes, and pet food. Additionally, carrageenan has been utilized in experimental medicine, pharmaceuticals, and cosmetics as anti-inflammatory agents, hydrogels, drug carriers, and vehicle for drug delivery [34].
- Gums are water-soluble polysaccharides that do not have a specific classification but are recognized for producing viscous–sticky dispersions at low concentrations. Gums are extracted from algae (such as agars and alginates), microorganisms (such as gellan and xanthan), or higher plants (such as pectin, Arabic, and arabinogalactans) [35]. The gel-forming properties of gums are due to their affinity for water, allowing for rapid hydration and swelling of the structure. The degree of hydration results in various rheological properties that enable their application in construction materials (such as adhesives), food products (such as texture enhancers, stabilizers, and coatings), medical and pharmaceutical products (such as encapsulants), and textile products (such as additives) [36].
3.2. Proteins
- Gluten is a mixture of insoluble, gummy proteins found in cereals such as wheat, rye, and barley. It is obtained by removing starch and soluble material from a dough made with grains [39]. The rheological properties of gluten facilitate the retention of air in the dough, making it particularly useful in processed food products such as breads, pasta, cookies, cakes, and other fermented goods [40].
- Casein is a group of proteins found in milk, which can be divided into four phosphoproteins: αS1, αS2, β, and κ-casein. These proteins organize themselves into micellar networks. Casein can be obtained through milk precipitation at pH 4.6, electrophoresis, or membrane processes [41]. The primarily significance of casein lies in the realm of sports, as it contributes to the nutritional composition of dietary supplements. However, it can also be applied in the formulation of nano and micro materials, food additives, and biodegradable films, as it can form gels when interacting with other polymers [42].
- Gelatin is a water-soluble protein derived from the hydrolysis of collagen, an insoluble product found in animal cartilage, skin, fibers, and tendons. Gelatin is classified as a hydrocolloid due to its high water-holding capacity. Its viscosity is its main property, which allows it to texture, thicken, stabilize emulsions, create foams, and form thermo-reversible gels [43]. Gelatin is free of sugars and fats and is rich in proteins. It is commonly used as an additive in food products such as confectionery, beverages, sweets, and dairy products. It also serves as an excipient in the pharmaceutical industry [44].
- Whey proteins are by-products obtained during the processing of dairy products such as cheese and casein. They can be classified into protein concentrates and protein isolates [45]. Whey proteins can be further categorized into four main proteins: β-lactoglobulin, α-lactalbumin, serum albumin, and immunoglobulin. Apart from their nutritional value, whey proteins possess binding and gelling properties, and they are capable of stabilizing foams and forming emulsions. As a result, they are used in various food products, including supplements, soups, sausages, desserts, and sweets) [46].
3.3. Lipids
- Waxes are soft or sticky substances that form on the surface of plants (e.g., carnauba and candelilla), as well as on the body of animals (e.g., whales and sheep) and insects (e.g., bees). They are composed of long-chain aliphatic compounds that vary depending on their source of production, Waxes may contain fatty acids, primary and secondary alcohols, aldehydes, sterol esters, ketones, triacylglycerols, and triterpenes [49]. Waxes exhibit high hydrophobicity and resistance to hydrolytic degradation, making them suitable for use as protectants, surface polishes, lubricants, and repellents in the food, cosmetic and automotive industries [50].
4. Encapsulation of Bioactive Compounds Using Spray-Drying Processes
4.1. Polysaccharide-Based Wall Materials
- Starch is a carbohydrate that can undergo modifications to interact with hydrophilic and hydrophobic compounds. For example, Ocampo-Salinas et al. [72] stated that substituting the native groups of rice starch with octenyl-succinic anhydride modified its viscosity and thermal properties, enhancing its emulsifying capacity and facilitating the encapsulation process of bioactive compounds from vanilla. García-Gurrola et al. [115] modified starch through phosphorylation, esterification, and acetylation techniques, improving the retention and stability of encapsulated phenolic compounds extracted from red sorghum. This enhancement was attributed to increased hydration and swelling of the capsules. The study by García-Gurrola et al. [115] also demonstrated that starch acetylation increases its hydrophobic nature and improves the retention of lipidic bioactive compounds. Márquez-Gómez [70] reported that the mixture of native starch with modified starches (acetylated starch and maltodextrin) improved the stability and prevented the oxidation of essential orange oil. This improvement was attributed to a reduction in diffusivity and an increase in hydrophobicity through starch acetylation.
- Maltodextrin, a polysaccharide, plays a crucial role in encapsulation, particularly due to its DE level. The study carried out by Laokuldilok and Kanha [116] reports that as the DE decreased (from 30 to 10), the encapsulation efficiency of black rice anthocyanins increased by 30%. The authors observed that the increase in drying temperature also negatively impacted the encapsulation efficiency, but only in the encapsulates with a high DE, which could be attributed to increased oxidative reactions in the polymer. The effect of temperature in spray-drying processes with maltodextrin was also evaluated by Boyano-Orozco et al. [117], who found that the concentration of the wall material significantly affected the encapsulation efficiency when encapsulating phenolic compounds and tannins from rambutan peel. The authors noted that temperatures above 160 °C adversely affected the stability of the bioactive components when using maltodextrin concentrations below 10% w/w. Meanwhile, Balasubramani et al. [118] concluded, after encapsulating garlic oleoresin with maltodextrin, that the concentration of maltodextrin must be in an appropriate ratio to the core material concentration. Specifically, a wall material to core material ratio of 6:1 is required to ensure the highest encapsulation efficiency and component stability.
- Chitosan contains -OH groups at positions C3 and C6, as well as an amino group (-NH2) at position C2, which enables it to form ionic and electrostatic interactions with other molecules [119]. However, to achieve more rigid and resistant matrices, chitosan is often cross-linked with compounds that possess reactive functional groups, such as dialdehydes, glutaraldehyde, or tripolyphosphate [120]. For example, Aranaz et al. [102] encapsulated venlafaxine hydrochloride with chitosan obtained from two sources (blue crab and royal crab) and mixed it with tripolyphosphate. The authors observed that the degree of cross-linking between chitosan and tripolyphosphate varied among different experiments. Specifically, chitosan with higher viscosity exhibited less cross-linking, resulting in lower encapsulation efficiency. The study by Amorim et al. [121] reports that chitosan cross-linking improves with an increase in the inlet temperature during the spray-drying process.
- Dextran’s properties are primarily influenced by the molecular size of its chains. In a study by Wilson et al. [122], proteins were encapsulated with dextrans of two different sizes, 20 and 70 kDa, and it was observed that as the size increased, the available area also increased, resulting in a more rigid and less flexible three-dimensional structure. This improvement in structural properties enhanced the protein encapsulation efficiency. Another influential parameter in dextran encapsulation is temperature, as demonstrated by Wang and Meenach [123]. When encapsulating curcumin, the authors found that the highest encapsulation efficiency was achieved at a low dextran concentration (20%) and a high drying temperature (140 °C). The authors attributed these results to the polydispersity generated in the matrices, suggesting that the combination of low dextran concentration and high temperature contributed to the formation of more uniform and efficient encapsulation matrices.
- Carrageenan’s encapsulation efficiency is influenced by the type of component it encapsulates. Generally, any type of carrageenan is suitable for the encapsulating aqueous extracts. However, the study by Marín-Peñalver et al. [124] demonstrated that the encapsulation of lipid components is deficient. The interaction between carrageenan and lipids is very poor, resulting in incomplete homogenization and the components being left outside the capsules.
- Gums are another type of polysaccharide with gel-forming properties, which are attributed to their chemical structure consisting of -OH groups that may have branching or side substitutions of ester groups (-COO-R) or ether groups (ROR’), giving them a linear, helical, or cyclic conformation [36]. Gums undergo modifications during spray-drying processes, leading to the formation of encapsulates. Correâ-Filho et al. [60] encapsulated β-carotene with gum Arabic and evaluated the encapsulation yield at varying concentrations (5–35%) and temperatures (110–200 °C). The study reports that temperature influenced antioxidant activity only when the percentage of wall material was low, while the highest yield was obtained using intermediate levels of temperature and gum concentration. The morphology was affected by temperature, with lower temperatures resulting in microspheres with higher cavity content and rougher surfaces. Additionally, lower inlet temperatures resulted in smaller particles, which can be attributed to the increased swelling and shrinkage that occurs when water evaporates slowly.
4.2. Protein-Based Wall Materials
4.3. Lipid-Based Wall Materials
4.4. Spray-Drying with Wall Material Mixtures
- The type and concentration of polysaccharides, lipids, and/or proteins have an impact on the encapsulation efficiency and capsule morphology [147].
- Polysaccharides have the greatest influence on the yield within the wall material blends [152].
- Lipids enhance the morphological characteristics of the capsules when combined with polysaccharides and/or proteins [154].
| Wall Material | Core Material | Concentration: Wall Material: Core Material | Conditions (Feed Rate, Inlet Air, Outlet Air) | Particles (Shape/Morphology, Particle Size Distribution) | Process Yield/Encapsulation Efficiency | Encapsulated Compounds | References |
|---|---|---|---|---|---|---|---|
| CH/MD | Tuna fish oil | 15:15:10–40% w/w/w | 0.7 L/h, 180 °C, 85 °C | nr./nr., nr. | nr./81–91% | Anisidine | [156] |
| Lentil-PI/MD | Flaxseed oil | 16.5–19:1% w/w | 3 mL/min, 135 °C, 95 °C | Spherical/Wrinkled, nr. | nr./nr. | Oil and Thiobarbituric acid | [157] |
| Lentil-PI/κ-Carr/MD | Flaxseed oil | 16.5–19:1:5–7.5% w/w/w | 3 mL/min, 135 °C, 95 °C | Spherical/Wrinkled, nr. | nr./85% | Oil and Thiobarbituric acid | [157] |
| Lentil-PI/ι-Carr/MD | Flaxseed oil | 16.5–19:1:5–7.5% w/w/w | 3 mL/min, 135 °C, 95 °C | Spherical/Wrinkled, nr. | nr./83–84% | Oil and Thiobarbituric acid | [157] |
| Kidney bean-PI/κ-Carr | Shrimp oil | 10:0.1:0.1–1% w/w/w | 5 mL/min, 180 °C, 105 °C | Spherical/Wrinkled, 2.5–6.4 μm | nr./43–89% | Fatty acids (C14, C15, C16, C17, C18, C20, C23, C24, SFA, MUFA, and PUFA) | [158] |
| GA/CH/Apple pectin | Satureja khuzistanica Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-spherical/Smooth, 2–5 μm | nr./58% | Phenolic compounds | [96] |
| GA/CH/Apple pectin | Satureja rechingeri Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-spherical/Smooth, 2–5 μm | nr./54% | Phenolic compounds | [96] |
| HCP/Gelatin | Turmeric oleoresin | 30:1:15% w/w/w | 6 mL/min, 170 °C, 80 °C | Spherical/Smooth, 2–20 μm | 40%/72% | Phenolic compounds and curcumin | [152] |
| MD/Gelatin | Turmeric oleoresin | 26:0.6:15% w/w/w | 6 mL/min, 170 °C, 80 °C | Spherical/Smooth, 2–20 μm | 25%/52% | Phenolic compounds and curcumin | [152] |
| Casein/Pectin | Grape (Vitis labrusca) by-product extract | 12.5:12.5:1.41% w/w/w | 10–14 mL/min, 120–160 °C, 68–98 °C | Spherical/Smooth, 10 μm | 3–19%/60–83% | Phenolic compounds and anthocyanins | [159] |
| WPI/Rice-PC | Baltic herring (Clupea harengus membras) oil | 7.5:7.5:15% w/w/w | 17 kg/h, 123–129 °C, 72–78 °C | Non-spherical/Porous, 56 μm | nr./40–50% | Fatty acids (C14, C16, C18, C20, C22, C24, SFA, MUFA, and PUFA) | [160] |
| WPI/MD | Mix (paprika-cinnamon oleoresin) | 2.5–7.5:1:1%w/ratio/ratio | 6 mL/min, 150 °C, 80 °C | Spherical/Porous, 17–19 μm | 40–43%/90–96% | Carotenoids | [80] |
| WP/Mo-Starch | Capsaicin | 1–9:1–9:20 ratio/ratio/%w | nr., 185 °C, 85 °C | Spherical/Wrinkled, 1.2–51.6 μm | 9–64%/50–94% | Capsaicin | [138] |
| WPI/MD | Gurum seed oil | 2:1:1 ratio | 20 mL/min, 180 °C, 80 °C | Spherical/Withered, 3–25 μm | 85%/91% | Oil | [161] |
| GA/MD | Gurum seed oil | 2:1:1 ratio | 20 mL/min, 180 °C, 80 °C | Spherical/Withered, 2–10 μm | 93%/97% | Oil | [161] |
| WPI/GA/MD | Gurum seed oil | 1:1:1:1 ratio | 20 mL/min, 180 °C, 80 °C | Spherical/Withered, 3–10 μm | 90%/93% | Oil | [161] |
| MD/GA | Mamey (Pouteria sapota) pulp | 10:5–10:nr.%/%/nr. | 10 mL/min, 160 °C, 62–81 °C | nr./nr., 3 μm | nr./nr. | Carotenoids | [162] |
| MD/Moringa oleitera gum | Mamey (Pouteria sapota) pulp | 10:5–10:nr.%/%/nr. | 10 mL/min, 160 °C, 62–81 °C | nr./nr., 3 μm | nr./nr. | Carotenoids | [162] |
| MD/GA | Carriot (Daucus carota) pulp | 10:5–10:nr.%/%/nr. | 10 mL/min, 160 °C, 60–87 °C | nr./nr., 3 μm | nr./nr. | Carotenoids | [162] |
| MD/Moringa oleitera gum | Carriot (Daucus carota) pulp | 10:5–10:nr.%/%/nr. | 10 mL/min, 160 °C, 60–87 °C | nr./nr., 3 μm | nr./nr. | Carotenoids | [162] |
| CH/GA/MD | Petai leaf extract | 0–1:75:25:2.5% w/w/w/w | 20 mL/min, nr., 80 °C | Spherical/Collapsed, nr. | nr./nr. | Phenolic compounds | [163] |
| HP-βCD/MD | Grape cane extract | 2.2:10:100% w/w/v | nr., 130 °C, 71 °C | Semi spherical/Smooth, 11 μm | 84%/81% | Phenolic compounds (protocatechuic acid-O-hexoside, protocatechuic acid, ethyl protocatechuate, protocatechuic aldehyde, gallic acid, caftaric acid, ellagic acid pentoside, and hydroxybenzaldehyde), flavonoids (eriodictyol, quercetin-O-glucoside, quercetin-3-O-glucuronide, and astilbin), and stilbenes (resveratrol, stilbenoid tetramer, pallidol, ε-viniferin, stilbene, and restrytisol) | [164] |
| GA/WPI | Basil (Ocimum basilicum L.) essential oil | 2:2:1% w/w | 3 mL/min, 150 °C, nr. | Spherical/Wrinkled, 4.2 μm | 71%/78% | Essential oil | [105] |
| WPI/MD | Basil (Ocimum basilicum L.) essential oil | 2:2:1% w/w | 3 mL/min, 150 °C, nr. | Spherical/Wrinkled, 3.2 μm | 66%/87% | Essential oil | [105] |
| GA/WPI/MD | Basil (Ocimum basilicum L.) essential oil | 1.3:1.3:1% w/w/w | 3 mL/min, 150 °C, nr. | Spherical/Wrinkled, 0.6 μm | 76%/83% | Essential oil | [105] |
| MD/Low methoxylated pectin/Sunflower wax | Flaxseed oil | 3–12:1–2:1–2:1–15% w/w/w/w | 4 mL/min, 135 °C, nr. | Spherical/Wrinkled, 12.9 μm | nr./44–71% | Carotenoids | [154] |
| MD/Mo-Starch | Fish oil | 24:8:8% w/w/w | 40 L/min, 190 °C, 100 °C | Spherical/Wrinkled, 0.3–69.2 μm | nr./69% | Fatty acids (saturated, monounsaturated, and polyunsaturated) | [165] |
| GA/MD | Vitamin A | 7.5:7.5:2% w/w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 20%/96% | Vitamin A | [67] |
| Starch/GA | Vitamin A | 7.5:7.5:2% w/w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 7%/97% | Vitamin A | [67] |
| Starch/MD | Vitamin A | 7.5:7.5:2% w/w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 19%/97% | Vitamin A | [67] |
| Starch/GA/MD | Vitamin A | 5:5:5:2% w/w/w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 20%/98% | Vitamin A | [67] |
| MD/Mo-Starch/WP | Fingered citron extract | 33:33:33:10% w/w/w/w | 17–21 mL/min, 185 °C, 80 °C | Spherical/Irregular, 27.5 μm | 76%/71% | Phenolic compounds | [166] |
| GA/Mo-Starch/WP | Fingered citron extract | 33:33:33:10% w/w/w/w | 17–21 mL/min, 185 °C, 80 °C | Spherical/Irregular, 22.5 μm | 81%/76% | Phenolic compounds | [166] |
| GA/MD/WP | Fingered citron extract | 33:33:33:10% w/w/w/w | 17–21 mL/min, 185 °C, 80 °C | Spherical/Irregular, 14.5 μm | 86%/79 | Phenolic compounds | [166] |
| GA/MD/Mo-Starch | Fingered citron extract | 33:33:33:10% w/w/w/w | 17–21 mL/min, 185 °C, 80 °C | Spherical/Irregular, 22.5 μm | 89%/86% | Phenolic compounds | [166] |
| GA/MD/Mo-Starch/WP | Fingered citron extract | 25:25:25:25:10% w/w/w/w/w | 17–21 mL/min, 185 °C, 80 °C | Spherical/Irregular, 17.5 μm | 78%/84% | Phenolic compounds | [166] |
| Ar-Starch/GA | Blackberry (Rubus fruticosus) pulp | 0.5–2:1:1% w/w/w) | 0.2 kg/h, 100–150 °C, n.r. | Spherical/Withered, 50–120 μm | 29–57/nr. | Ascorbic acid and anthocyanins | [167] |
| MD/GA | Chipilin (Crotalaria longirostrata) extract | 15:2:1% w/w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 47%/90% | Phenolic compounds | [85] |
| MD/Cajanus cajan gum | Chipilin (Crotalaria longirostrata) extract | 15:2:1% w/w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 51%/78% | Phenolic compounds | [85] |
| MD/Cocoa shell pectin | Chipilin (Crotalaria longirostrata) extract | 15:2:1% w/w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 62%/75% | Phenolic compounds | [85] |
| MD/Cajanus cajan protein | Chipilin (Crotalaria longirostrata) extract | 15:2:1% w/w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 61%/93% | Phenolic compounds | [85] |
| MD/SPI | Chipilin (Crotalaria longirostrata) extract | 15:2:1% w/w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 62%/65% | Phenolic compounds | [85] |
| MD/PI | Essential avocado oil | 10:5 w/w ratio | 5 mL/min, 160 °C, 90 °C | Spherical/Aggregates, 0.1–1 μm | nr./62% | Essential oil | [148] |
| OSA-MD | Essential avocado oil | 10:5 w/w ratio | 5 mL/min, 160 °C, 90 °C | Spherical/Aggregates, 0.1–1 μm | nr./45% | Essential oil | [148] |
| OSA-MD/PI | Essential avocado oil | 9:1:5 w/w/w ratio | 5 mL/min, 160 °C, 90 °C | Spherical/Aggregates, 0.1–1 μm | nr./61% | Essential oil | [148] |
| λ-Carr/GA/MD | Propolis extract | 1:0.2:1:1% w/w/w/v | nr., nr., nr. | Spherical/Aggregates, 0.5–6 μm | 45–64%/nr. | Phenolic compounds | [168] |
| Ca-Starch/GA | Lemongrass (Cymbopogon citratus) essential oil | 1–9:1:1–4 ratio | nr., 150–200 °C, nr. | Spherical/Smooth, nr. | nr./43–92% | Essential oil | [169] |
| MD/GA | Horseradish leaf (Armoracia rusticana L.) juice | 20–80:20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 3.8–4.3 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin and sinapic acid | [77] |
| MD/GA | Horseradish root (Armoracia rusticana L.) juice | 20–80:20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 3.6–3.7 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin and sinapic acid | [77] |
| MD/GA | Noni (Morinda citrifolia L.) juice | 5–9:1:5:1% w/w/w | nr., 170 °C, 90 °C | Semi-spherical/Wrinkled, 95–106 μm | nr./nr. | Phenolic compounds | [170] |
| MD/WPC/GG | Rape seed oil | 15.4:3.9:9.5% w/w/w | 77 mL/min, 130 °C, 90 °C | Irregular/Porous, 5–75 μm | 29%/90% | Fatty acids (C14, C16, C18, SFA, MUFA, and PUFA) | [155] |
| MD/WPC/GG | Flax seed oil | 15.4:3.9:9.5% w/w/w | 77 mL/min, 130 °C, 90 °C | Irregular/Porous, 5–75 μm | 30%/88% | Fatty acids (C14, C16, C18, SFA, MUFA, and PUFA) | [155] |
| MD/WPC/GG | Safflower seed oil | 15.4:3.9:9.5% w/w/w | 77 mL/min, 130 °C, 90 °C | Irregular/Porous, 5–75 μm | 30%/82% | Fatty acids (C14, C16, C18, SFA, MUFA, and PUFA) | [155] |
| Gelatin/Chia mucilage | Oregano (Origanum vulgare) essential oil | 1:1:1% w/w/w | nr., 160–180 °C, nr. | Spherical/Aggregates, 38–120 μm | 81–89%/85–96% | Essential oil | [171] |
| Gelatin/GA | Oregano (Origanum vulgare) essential oil | 1:1:1% w/w/w | nr., 160–180 °C, nr. | Spherical/Aggregates, 18–85 μm | 72–88%/89–95% | Essential oil | [171] |
| Casein/MD | Thyme (Thymus vulgaris) essential oil | 4.17:80:20% w/w/w | 7–5 mL/min, 110 °C, 70 °C | Spherical/Irregular, 0.87 μm | nr./89% | Phenolic compounds | [172] |
| OSA-Starch/MD | β-carotene | 1:1–3:1 ratio | 1100 mL/h, 185 °C, nr. | Spherical/Wrinkled, 2–6 μm | nr. | β-carotene | [173] |
| CH/WPI | Garlic bulbs extract | 1:1:nr.% w/w/nr. | nr., 160 °C, nr. | Spherical/Aggregates, 2–10 μm | nr./nr. | Phenolic compounds | [129] |
| Zein/NaCas | Curcumin | 10:10% w/w | 120 L/min, 100 °C, nr. | Spherical/Irregular, 143 nm | nr./90–95% | Curcumin | [149] |
| κ-Carr/MP | Tuna oil | 1:1–50:1 ratio | 2.5 mL/min, 180 °C, 80 °C | Spherical/Smooth, 3–6 μm | 82–97%/91–97% | Oil | [174] |
| λ-Carr/MP | Tuna oil | 1:1–50:1 ratio | 2.5 mL/min, 180 °C, 80 °C | Spherical/Smooth, 3–5 μm | 82–99%/91–98% | Oil | [174] |
| MD/Gelatin | Turmeric (Curcuma longa L.) oleoresin | 12–26:0.6–6:15% w/w/w | 1.4–8.6 mL/min, 124–190 °C, nr. | Spherical/Aggregates, nr. | nr./4–77% | Phenolic compounds | [175] |
| Gelatin/Sodium hexametaphosphate | Anchovy oil | 8:0.5:30% w/w/w | nr., 160 °C, 94 °C | Oval/Rough, 40–60 μm | 96%/100% | Oil | [176] |
| GA/MD | Fish oil | 15:15:15% w/w/w | nr., 118–120 °C, nr. | Spherical/Rugged, 13–105 μm | nr./51–57% | Oil | [177] |
| Casein/Pectin/MD | Fish oil | 15:15:15:15% w/w/w/w | nr., 118–120 °C, nr. | Spherical/Rugged, 11–68 μm | nr./65–68% | Oil | [177] |
| WPC/Hawthorn pectin | Grape seed oil | 1–1.5:1–1.5:1% w/w/w | 40 mL/min, 170 °C, 85 °C | Spherical/Smooth, 1.6–2.6 μm | nr./65–71% | Oil | [178] |
| Ar-Starch/GA | Blackberry (Rubus fruticosus) pulp | 15.4:10.2% w/w | 0.2 kg/h, 100–150 °C, nr. | Spherical/Aggregates, 50.9–119.8 μm | 29–57%/nr. | Phenolic compounds | [179] |
| MD/SP | Lemon by-product aqueous extract | 5:1 ratio | 4 mL/min, 125 °C, 55 °C | Spherical/Smooth, nr. | 58–67%/nr. | Phenolic compounds and flavonoids | [89] |
| MD/ι-Carr | Lemon by-product aqueous extract | 9:1 ratio | 4 mL/min, 125 °C, 55 °C | Spherical/Smooth, nr. | 56–59%/nr. | Phenolic compounds and flavonoids | [89] |
| Gelatin/MD | Fish oil | 7.5:32.5:10% w/w | nr., 180 °C, 80 °C | Spherical/Smooth, nr. | nr./85% | Oil | [180] |
| κ-Carr/MD | Fish oil | 1:38.5:10% w/w | nr., 180 °C, 80 °C | Spherical/Smooth, nr. | nr./67% | Oil | [180] |
| Gelatin/κ-Carr | Fish oil | 7.5:31.5:10% w/w | nr., 180 °C, 80 °C | Spherical/Smooth, nr. | nr./75% | Oil | [180] |
| SC/βCD | Kenaf (Hibiscus cannabinus L.) seed oil | 2:1:1 ratio | 8 g/min, 160 °C, nr. | Spherical/Smooth holes, 37.3 μm | nr./93% | Oil | [181] |
| GA/βCD | Kenaf (Hibiscus cannabinus L.) seed oil | 2:1:1 ratio | 8 g/min, 160 °C, nr. | Spherical/Smooth holes, 30.6 μm | nr./95% | Oil | [181] |
| GA/SC/βCD | Kenaf (Hibiscus cannabinus L.) seed oil | 4:1:1:1 ratio | 8 g/min, 160 °C, nr. | Spherical/Smooth holes, 25.4 μm | nr./90% | Oil | [181] |
| Brea gum/Inulin | Corn oil | 20:10–20:10% w/w/w | nr., 150 °C, 60 °C | Semi-spherical/Dents, 0.8–18 μm | nr./74–92% | Oil | [111] |
| GA/Inulin | Corn oil | 20:10–20:10% w/w/w | nr., 150 °C, 60 °C | Semi-spherical/Dents, 15 μm | nr./87–89% | Oil | [111] |
| MD/Carr | Pouzolzia zeylanica extract | 5–15:0.06–0.1:nr.% w/w/nr. | nr., 180 °C, nr. | nr./nr., 6 μm | nr./nr. | Phenolic compounds, anthocyanins, flavonoids, and tannins | [182] |
| GA/MD | Grape seed oil | 15:15:10% w/w/w | 350 mL/h, 180 °C, 105 °C | Spherical/Collapsed, 27 μm | nr./63% | Fatty acids (C14, C16, C18, C20, SFA, MUFA, and PUFA) and phenolic compounds | [110] |
| MD/GA | Algal (Tetraselmis chuii) biomass | 60:40:1 ratio/ratio/%w | 2.5 mL/min, 150 °C, 60 °C | Spherical/Rough, 3.5–13.7 μm | 22–45%/54–84% | Phenolic compounds, β-carotene, and carotenoids | [99] |
| MD/GA | Peach palm peel extract | 7.6:7.6:nr.% w/w/nr. | 12.6 mL/min, 160 °C, 70 °C | Irregular/Irregular, nr. | 72%/67% | β-carotene | [183] |
| WPI/SC | Conjugated linoleic acid | 1:4:8% w/w/w | nr., 160 °C, 80 °C | Spherical/Irregular, 10–25 μm | nr./96% | Conjugated linoleic acid | [141] |
| SOS-Starch/MD | Chili seed oil | 1–5:1:20–45% w/w/w | nr., 160 °C, 80 °C | Polyhedral/Irregular, 3–20 μm | nr. | Fatty acids (C14, C16, C18, C20, C22, SFA, MUFA, PUFA, and UFA) | [151] |
| R-Starch/Mo-Starch/MD/HP | Orange essential oil | 0–30:0–30:0–30:0–30:15% w/w/w/w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 38–82%/45–96% | D-Limonene | [70] |
| R-Starch/Mo-Starch | Orange essential oil | 0–30:15% w/w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 65–73%/96–99% | D-Limonene | [70] |
| Mo-Starch/MD | Orange essential oil | 0–30:15% w/w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 58%/99% | D-Limonene | [70] |
| MD/HP | Orange essential oil | 0–30:15% w/w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 33%/44% | D-Limonene | [70] |
| R-Starch/HP | Orange essential oil | 0–30:15% w/w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 87%/58% | D-Limonene | [70] |
| MD/GA | Drumstick (Moringa oleifera) oil | 25–75:25–75:30% w/w/w | 10 g/min, 180 °C, 85 °C | Spherical/Smooth, 23–28 μm | nr./83–91 | Oil | [184] |
| MD/WPC | Drumstick (Moringa oleifera) oil | 25–75:25–75:30% w/w/w | 10 g/min, 180 °C, 85 °C | Spherical/Smooth, 11–18 μm | nr./66–73% | Oil | [184] |
| MD/GA | Spent coffee ground extract | 1:1:10 w/w/v ratio | 108 mL/min, 100 °C, nr. | Spherical/Withered, <30 μm | nr./25–80% | Phenolic compounds and flavonoids | [94] |
| MD/Moringa oleitera gum | Tender coconut (Cocos nucifera) water | 10–50:0.5–1.5% w/w | 0.4 kg/h, 100–140 °C, 90–97 °C | Spherical/Irregular, 2.5–15 μm | 9–38%/38–95% | Phenolic compounds | [185] |
| MD-CAP | Vitamin A | 70:30:1% w/w/w | 2 mL/min, 120 °C, 74 °C | Semi-spherical/Dented, 2–4 μm | 80–81%/48–100% | Vitamin A | [186] |
| MD-SC | Vitamin E | 70:30:1% w/w/w | 2 mL/min, 120 °C, 74 °C | Semi-spherical/Dented, 2–4 μm | 77–85%/23–29% | Vitamin E | [186] |
| MD/CAP | Vitamin A | 2.2–6.6:2.2–6.6:6% w/w/w | 1–5 mL/min, 110–130 °C, 55–60 °C | Spherical/Irregular, 3–15 μm | nr./59–63% | Vitamin A | [150] |
| MD/GV | Propolis | 30:0.3:0.123% w/w/w | 8 mL/min, 120 °C, 70–74 °C | Deformed spherical/Smooth, nr. | 60%/81–89% | Phenolic compounds | [91] |
| MD/GA | Propolis | 30:0.3:0.123% w/w/w | 8 mL/min, 120 °C, 70–74 °C | Deformed spherical/Smooth, nr. | 68%/84–93% | Phenolic compounds | [91] |
| C-Zein/-βCD | α-Tocopherol | 2.5:1.85:0.5% w/w/w | 7–9 mL/min, 110–180 °C, nr. | Spherical/Smooth holes, 10 μm | 44–77%/31–42% | α-Tocopherol | [137] |
| Gelatin/GA | Fish oil | 0.5:0.5:2% w/w/w | 6 mL/min, 190 °C, 90 °C | Spherical/Smooth, 2–6 μm | nr./87–94% | Oil | [187] |
| WPC/GA | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 65%/60% | Oil | [153] |
| WPC/C-Starch | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 60%/30% | Oil | [153] |
| WPC/Ma-Starch | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 55%/40% | Oil | [153] |
| WPC/MD | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 56%/93% | Oil | [153] |
| WPC/Glucose | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 56%/95% | Oil | [153] |
| WPC/Sucrose | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 53%/96% | Oil | [153] |
| WPC/Lactose | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 48%/95% | Oil | [153] |
| WPC/Maltose | Pumpkin (Cucurbita spp.) seed oil | 5:5:5% w/w/w | 0.8 L/h, 160 °C, 60 °C | Spherical/Cracked, nr. | 56%/95% | Oil | [153] |
| Pectin/WPC | Folic acid | 0.1–2:0.1–0.3% w/w/w | 450 mL/h, 180 °C, 90 °C | Spherical/Smooth, 2–10 μm | nr./nr. | Folic acid | [188] |
5. Concluding Remarks
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Risch, S.J. Encapsulation: Overview of Uses and Techniques. In Encapsulation and Controlled Release of Food Ingredients; Risch, S.J., Reineccius, G.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 2–7. ISBN 9780841231641. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Han, X.-Q. Encapsulation of Food Fngredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Ahmad, M.; Madni, A.; Usman, M.; Munir, A.; Akhtar, N.; Shoaib Khan, H.M. Pharmaceutical Micro Encapsulation Technology for Development of Controlled Release Drug Delivery Systems. World Acad. Sci. Eng. Technol. 2011, 75, 384–387. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of Cosmetic Active Ingredients for Topical Application—A Review. J. Microencapsul. 2015, 33, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Ko, S. Rationales of Nano- and Microencapsulation for Food Ingredients. In Nano- and Microencapsulation for Foods; Kwak, H.-S., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; Volume 9781118292, pp. 43–64. ISBN 9781118292327. [Google Scholar]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of Health-Promoting Ingredients: Applications in Foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer Science + Business Media: New York, NY, USA, 2010; pp. 31–100. ISBN 9781441910073. [Google Scholar]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products and Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer Science + Business Media: New York, NY, USA, 2010; pp. 3–29. ISBN 9781441910080. [Google Scholar]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Vehring, R.; Snyder, H.; Lechuga-Ballesteros, D. Spray Drying. In Drying Technologies for Biotechnology and Pharmaceutical Applications; Ohtake, S., Izutsu, K., Lechuga-Ballesteros, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; ISBN 9783527341122. [Google Scholar]
- Coimbra, P.P.S.; Cardoso, F.d.S.N.; Gonçalves, É.C.B.d.A. Spray-Drying Wall Materials: Relationship with Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2020, 61, 2809–2826. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C.; Ishwarya, S.P. (Eds.) Selection of Wall Material for Encapsulation by Spray Drying. In Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 77–100. ISBN 9781118863985. [Google Scholar]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 67–89. ISBN 9780123969835. [Google Scholar]
- Yu, Y.; Delbianco, M. Synthetic Polysaccharides. In Recent Trends in Carbohydrate Chemistry; Rauter, A.P., Christensen, B.E., Somsák, L., Kosma, P., Adamo, R., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 333–371. ISBN 9780128174678. [Google Scholar]
- Jindal, N.; Singh Khattar, J. Microbial Polysaccharides in Food Industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Oxford, UK, 2018; pp. 95–123. ISBN 9780128114490. [Google Scholar]
- Jane, J.L. Structural Features of Starch Granules II. In Starch—Chemistry and Technology; BeMiller, J., Whistler, R., Eds.; Elsevier Inc.: Oxford, UK, 2009; pp. 193–236. ISBN 9780127462752. [Google Scholar]
- Omoregie Egharevba, H. Chemical Properties of Starch and Its Application in the Food Industry. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2019; pp. 1–26. ISBN 9781838801168. [Google Scholar]
- Parikh, A.; Agarwal, S.; Raut, K. A Review on Applications of Maltodextrin in Pharmaceutical Industries. Int. J. Pharm. Biol. Sci. 2014, 4, 67–74. [Google Scholar]
- Ishwarya, S.P.; Anandharamakrishnan, C. Nanospray Drying: Principle and Food Processing Applications. In Innovative Food Processing Technolgies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 605–633. ISBN 9780128157824. [Google Scholar]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J.P.H. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.S.; Khedkar, C.D.; Bhatt, S. Fat Replacer. Encycl. Food Health 2016, 2, 589–595. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Funtional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Elsevier Inc.: Oxford, UK, 2016; pp. 3–31. ISBN 9780128027356. [Google Scholar]
- Bautista-Baños, S.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M.L. Quitosano: Un Polisacárido Antimicrobiano Versátil Para Frutas y Hortalizas En Poscosecha—Una Revisión. Rev. Chapingo Ser. Hortic. 2017, 23, 103–121. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in Chitosan as a Primary Biopolymer for Functional Films and Coatings Manufacture for Food and Natural Products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional Polymers Based on Dextran. In Polysaccharides II; Klemm, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 199–291. [Google Scholar]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ahmadi, F.; Emami, J.; Tavakoli, N.; Minaiyan, M.; Mahzouni, P.; Dorkoosh, F. Microencapsulation of Budesonide with Dextran by Spray Drying Technique for Colon-Targeted Delivery: An in Vitro/in Vivo Evaluation in Induced Colitis in Rat. J. Microencapsul. 2011, 28, 62–73. [Google Scholar] [CrossRef]
- Kadota, K.; Yanagawa, Y.; Tachikawa, T.; Deki, Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. Development of Porous Particles Using Dextran as an Excipient for Enhanced Deep Lung Delivery of Rifampicin. Int. J. Pharm. 2018, 555, 280–290. [Google Scholar] [CrossRef]
- Ortiz-Tena, J.G.; Schieder, D.; Sieber, V. Carrageenan and More: Biorefinery Approaches with Special Reference to the Processing of Kappaphycus. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 9, pp. 155–164. ISBN 9783319634975. [Google Scholar]
- Loureido, R.R.; Cornish, M.L.; Neish, I.C. Applications of Carrageenan: With Special Reference to Iota and Kappa Forms as Derived from the Eucheumatoid Seaweeds. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 165–171. ISBN 9783319634975. [Google Scholar]
- BeMiller, J.N. (Ed.) Guar, Locust Bean, Tara, and Cassia Gums. In Carbohydrate Chemistry for Food Scientists; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 241–252. ISBN 9780128120699. [Google Scholar]
- BeMiller, J.N. Gums and Related Polysaccharides. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1513–1533. ISBN 9783540304296. [Google Scholar]
- Blanco, A.; Blanco, G. (Eds.) Proteins. In Medical Biochemistry; Elsevier Inc.: Oxford, UK, 2017; pp. 21–71. ISBN 9780128035504. [Google Scholar]
- Lafarga, T. Potential Applications of Plant-Derived Proteins in the Food Industry. In Novel Proteins for Food, Pharmaceuticals and Agriculture: Sources, Applications and Advances; Hayes, M., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 117–137. ISBN 9781119385301. [Google Scholar]
- Shewry, P. What Is Gluten—Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-Gluten Uses and Industry Needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- O’Kennedy, B.T. Caseins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 13–29. ISBN 9781845697587. [Google Scholar]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 92–115. ISBN 9781845697587. [Google Scholar]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, Source, Extraction and Industrial Applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Boland, M. Whey Protein. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 30–55. ISBN 9781845697587. [Google Scholar]
- Jovanović, S.; Barać, M.; Maćej, O. Whey Proteins-Properties and Possibility of Application. Mljekarstvo 2005, 55, 215–233. [Google Scholar]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The Lipids. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 181–257. ISBN 9780123196521. [Google Scholar]
- Tao, B.Y. Industrial Applications for Plant Oils and Lipids. In Bioprocessing for Value-Added Products from Renewable Resources—New Technologies and Applications; Yang, S.-T., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 611–627. ISBN 9780444521149. [Google Scholar]
- Tinto, W.F.; Elufioye, T.O.; Roach, J. Waxes. In Pharmacognosy: Fundamentals, Applications and Strategy; Badal, S., Delgoda, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 443–455. ISBN 9780128021040. [Google Scholar]
- Vanhercke, T.; Wood, C.C.; Stymne, S.; Singh, S.P.; Green, A.G. Metabolic Engineering of Plant Oils and Waxes for Use as Industrial Feedstocks. Plant Biotechnol. J. 2013, 11, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of Bioactive Compounds: Challenges and Perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Ceja-Medina, L.I.; Ortiz-Basurto, R.I.; Medina-Torres, L.; Calderas, F.; Bernad-Bernad, M.J.; González-Laredo, R.F.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-ávila, M.; Andrade-González, I.; et al. Microencapsulation of Lactobacillus Plantarum by Spray Drying with Mixtures of Aloe Vera Mucilage and Agave Fructans as Wall Materials. J. Food Process Eng. 2020, 43, e13436. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of Gallic Acid by Spray Drying with Aloe Vera Mucilage (Aloe Barbadensis Miller) as Wall Material. Ind. Crop. Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Desai, K.G.; Liu, C.; Park, H.J. Characteristics of Vitamin C Encapsulated Tripolyphosphate-Chitosan Microspheres as Affected by Chitosan Molecular Weight. J. Microencapsul. 2006, 23, 79–90. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, F.; Shi, K.; Wang, J.; Niu, M.; Ma, R. The Effect of Chitosan Molecular Weight on the Characteristics of Spray-Dried Methotrexate-Loaded Chitosan Microspheres for Nasal Administration. Drug Dev. Ind. Pharm. 2009, 35, 379–386. [Google Scholar] [CrossRef]
- Chang, C.; Nickerson, M.T. Encapsulation of Omega 3-6-9 Fatty Acids-Rich Oils Using Protein-Based Emulsions with Spray Drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Subtil, S.F.; Rocha-Selmi, G.A.; Thomazini, M.; Trindade, M.A.; Netto, F.M.; Favaro-Trindade, C.S. Effect of Spray Drying on the Sensory and Physical Properties of Hydrolysed Casein Using Gum Arabic as the Carrier. J. Food Sci. Technol. 2014, 51, 2014–2021. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.A.; Cano-Higuita, D.M.; De Oliveira, R.A.; Telis, V.R.N. Influence of Different Combinations of Wall Materials on the Microencapsulation of Jussara Pulp (Euterpe edulis) by Spray Drying. Food Chem. 2016, 212, 1–9. [Google Scholar] [CrossRef]
- Correâ-Filho, L.C.; Lourenço, M.M.; Moldaõ-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of Walnut Oil by Spray Drying: Effects of Wall Material and Drying Conditions on Physicochemical Properties of Microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Encapsulation of Fruit Flavor Compounds through Interaction with Polysaccharides. Molecules 2021, 26, 4207. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Castro-Rosas, J.; Rangel-Vargas, E.; Navarro-Cortez, R.O.; Cabrera-Canales, Z.E.; Díaz-Batalla, L.; Martínez-Bustos, F.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. A Modified Achira (Canna indica L.) Starch as a Wall Material for the Encapsulation of Hibiscus Sabdariffa Extract Using Spray Drying. Food Res. Int. 2019, 119, 547–553. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, C.; Huang, Z.; Yang, R.; Liu, C. Microcapsules with Slow-Release Characteristics Prepared by Soluble Small Molecular Starch Fractions through the Spray Drying Method. Int. J. Biol. Macromol. 2022, 200, 34–41. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Konopka, I.Z.; Tańska, M.; Brandt, W.; Piłat, B. Effect of Maltodextrin Replacement by Selected Native Starches and Disaccharides on Physicochemical Properties of Pumpkin Oil Capsules Prepared by Spray-Drying. Appl. Sci. 2022, 12, 33. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Duodu, K.G.; Emmambux, M.N. Encapsulation and Antioxidant Activity of Ascorbyl Palmitate with Normal and High Amylose Maize Starch by Spray Drying. Food Hydrocoll. 2019, 86, 124–133. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by Spray-Drying, Using Binary and Ternary Blends of Gum Arabic, Starch and Maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of Vitamin B1 Microparticles by a Spray Drying Process Using Different Biopolymers as Wall Materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; de Fernandes, R.V.B.; Antoniassi, R.; de Faria-Machado, A.F.; de Feitosa, J.P.A.; de Paula, R.C.M. Application of Cashew Tree Gum on the Production and Stability of Spray-Dried Fish Oil. Food Chem. 2017, 221, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Gómez, M.; Galicia-García, T.; Márquez-Meléndez, R.; Ruiz-Gutiérrez, M.; Quintero-Ramos, A. Spray-Dried Microencapsulation of Orange Essential Oil Using Modified Rice Starch as Wall Material. J. Food Process. Preserv. 2017, 42, e13428. [Google Scholar] [CrossRef]
- Sepelevs, I.; Stepanova, V.; Galoburda, R. Encapsulation of Gallic Acid with Acid-Modified Low Dextrose Equivalent Potato Starch Using Spray-and Freeze-Drying Techniques. Polish J. Food Nutr. Sci. 2018, 68, 273–280. [Google Scholar] [CrossRef]
- Ocampo-Salinas, I.O.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Vargas-León, E.A.; Guzmán-Ortiz, F.A.; Calcáneo-Martínez, N.; Falfán-Cortés, R.N. Development of Wall Material for the Microencapsulation of Natural Vanilla Extract by Spray Drying. Cereal Chem. 2020, 97, 555–565. [Google Scholar] [CrossRef]
- Abedi, A.-S.; Rismanchi, M.; Moosavi, M.H.; Khaneghah, A.M.; Mohammadi, A.; Mahmoudzadeh, M. A Mixture of Modified Starch-Maltodextrin for Spray Drying Encapsulation of Nigella Sativa Seeds Oil Containing Thymoquinone. Starch/Staerke 2020, 73, 1900255. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of Spray Drying Microencapsulation of Almond Oil into Taro Starch Spherical Aggregates. LWT Food Sci. Technol. 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Chavez-Salazar, A.; Castellanos-Galeano, F.; Bello-Perez, L.A.; Alvarez-Ramirez, J. Physical and Chemical Stability of L-Ascorbic Acid Microencapsulated into Taro Starch Spherical Aggregates by Spray Drying. Food Hydrocoll. 2018, 83, 143–152. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H.; Flórez-Fernández, N. Spray-Drying Microencapsulation of Tea Extracts Using Green Starch, Alginate or Carrageenan as Carrier Materials. Int. J. Biol. Macromol. 2022, 203, 417–429. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburba, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying Lolita. Foods 2020, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Korma, S.A.; Khan, I.M.; Mohsin, A.; Manzoor, M.F.; Ashraf, W.; Mushtaq, B.S.; Zainab, S.; et al. Spray Dried Nanoemulsions Loaded with Curcumin, Resveratrol, and Borage Seed Oil: The Role of Two Different Modified Starches as Encapsulating Materials. Int. J. Biol. Macromol. 2021, 186, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.B.H.; Nguyen, T.K.H.; Tran, T.T.T.; Nguyen, T.N.L.; Mai, H.C. Microencapsulation of Corn Mint (Mentha arvensis L.) Essential Oil Using Spray-Drying Technology. Food Res. 2022, 6, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Procopio, F.R.; Furtado, G.D.F. Co-Encapsulation of Paprika and Cinnamon Oleoresin by Spray Drying Using Whey Protein Isolate and Maltodextrin as Wall Material: Development, Characterization and Storage Stability. Food Res. Int. 2022, 162, 112164. [Google Scholar] [CrossRef] [PubMed]
- De Ferreira, L.M.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.; de Remígio, M.S.d.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef] [PubMed]
- Agatha, R.; Agatha, R.; Maryati, Y.; Susilowati, A.; Aspiyanto, A.; Devi, A.F.; Mulyani, H.; Budiari, S.; Filailla, E.; Rahmawati, D.; et al. Effect of Type and Concentration of Encapsulating Agents on Physicochemical, Phytochemical, and Antioxidant Properties of Red Dragon Fruit Kombucha Powdered Beverage. J. Kim. Terap. Indones. 2021, 23, 7–15. [Google Scholar]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M.S. Optimization and Encapsulation of Phenolic Compounds Extracted from Maize Waste by Freeze-Drying, Spray-Drying, and Microwave-Drying Using Maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Sivagnanam, S.P.; Park, J.S.; Cho, Y.J.; Chun, B.S. Effect of Wall Materials on the Spray Drying Encapsulation of Brown Seaweed Bioactive Compounds Obtained by Subcritical Water Extraction. Algal Res. 2021, 58, 102381. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; del Ayora-Talavera, T.R.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef]
- Zorzenon, M.R.T.; Formigoni, M.; da Silva, S.B.; Hodas, F.; Piovan, S.; Ciotta, S.R.; Jansen, C.A.; Dacome, A.S.; Pilau, E.J.; Mareze-Costa, C.E.; et al. Spray Drying Encapsulation of Stevia Extract with Maltodextrin and Evaluation of the Physicochemical and Functional Properties of Produced Powders. J. Food Sci. 2020, 85, 3590–3600. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Pineapple Peel Extract by Spray Drying Using Maltodextrin, Inulin, and Arabic Gum as Wall Matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Samardžić, J.; Janković, T. Chokeberry Polyphenols Preservation Using Spray Drying: Effect of Encapsulation Using Maltodextrin and Skimmed Milk on Their Recovery Following in Vitro Digestion. J. Microencapsul. 2019, 36, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakoudi, A.; Tsimidou, M.Z. Properties of Encapsulated Saffron Extracts in Maltodextrin Using the Büchi B-90 Nano Spray-Dryer. Food Chem. 2018, 266, 458–465. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis Encapsulation by Spray Drying: Characterization and Stability. LWT Food Sci. Technol. 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Ghani, A.A.; Adachi, S.; Shiga, H.; Neoh, T.L.; Adachi, S.; Yoshii, H. Effect of Different Dextrose Equivalents of Maltodextrin on Oxidation Stability in Encapsulated Fish Oil by Spray Drying. Biosci. Biotechnol. Biochem. 2017, 81, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the Physical Properties and Conservation of the Antioxidants Content, Employing Inulin and Maltodextrin in the Spray Drying of Blueberry Juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Prudkin-Silva, C.; Martínez, J.H.; Mazzobre, F.; Quiroz-Reyes, C.; San-Juan, E.; San-Martín, E.; Pérez, O.E. High Molecular Weight Chitosan Based Particles for Insulin Encapsulation Obtained via Nanospray Technology. Dry. Technol. 2022, 40, 430–445. [Google Scholar] [CrossRef]
- Fathi, F.; Ebrahimi, S.N.; Pereira, D.M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Enriched Extracts of Two Satureja Species by Spray Drying, Evaluation of the Controlled Release Mechanism and Cytotoxicity. Pharm. Sci. 2022, 28, 145–155. [Google Scholar] [CrossRef]
- Hamad, A.; Suriyarak, S.; Devahastin, S.; Borompichaichartkul, C. A Novel Approach to Develop Spray-Dried Encapsulated Curcumin Powder from Oil-in-Water Emulsions Stabilized by Combined Surfactants and Chitosan. J. Food Sci. 2020, 85, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Mulia, K.; Putri, T.; Krisanti, E.A.; Handayani, N.A. Preparation and Evaluation of Chitosan Biopolymers Encapsulated Iron Gluconate Using Spray Drying Method. AIP Conf. Proc. 2019, 2092, 030005. [Google Scholar] [CrossRef]
- De Bonilla-Ahumada, F.J.; Khandual, S.; Lugo-Cervantes, E. del C. Microencapsulation of Algal Biomass (Tetraselmis chuii) by Spray-Drying Using Different Encapsulation Materials for Better Preservation of Beta-Carotene and Antioxidant Compounds. Algal Res. 2018, 36, 229–238. [Google Scholar] [CrossRef]
- Olivares, E.C.; Olivares-Romero, J.L.; Barrera-Méndez, F. Microencapsulation of Potassium Phosphate in Chitosan and the Effect of Spray Drying Operating Variables on the Particle Size Experimental. J. Mex. Chem. Soc. 2018, 62, 67–74. [Google Scholar]
- Altin, G.; Ozcelik, B. Chitosan Coated Liposome Dispersions Loaded with Cacao Hull Waste Extract: Effect of Spray Drying on Physico-Chemical Stability and in Vitro Bioaccessibility. J. Food Eng. 2018, 223, 91–98. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.R.G.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of Chitosan as a Wall Material for Microencapsulation of Squalene by Spray Drying: Characterization and Oxidative Stability Studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Pinho, L.S.; de Lima, P.M.; Henrique, S.; de Sá, S.H.G.; Chen, D.; Campanella, O.H.; da Rodrigues, C.E.C.; Favaro-Trindade, C.S. Encapsulation of Rich-Carotenoids Extract from Guaran á (Paullinia cupana) Byproduct by a Combination of Spray Drying and Spray Chilling. Foods 2022, 11, 2557. [Google Scholar] [CrossRef]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of Basil Essential Oil: Utilization of Gum Arabic/Whey Protein Isolate/Maltodextrin Combinations for Encapsulation Efficiency and in Vitro Release. J. Food Meas. Charact. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of Non-Dewaxed Propolis by Freeze-Drying and Spray-Drying Using Gum Arabic, Maltodextrin and Inulin as Coating Materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-Ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [Green Version]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Bucurescu, A.; Blaga, A.C.; Estevinho, B.N.; Rocha, F. Microencapsulation of Curcumin by a Spray-Drying Technique Using Gum Arabic as Encapsulating Agent and Release Studies. Food Bioprocess Technol. 2018, 11, 1795–1806. [Google Scholar] [CrossRef]
- Böger, B.R.; Georgetti, S.R.; Kurozawa, L.E. Microencapsulation of Grape Seed Oil by Spray Drying. Food Sci. Technol. 2018, 38, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Castel, V.; Rubiolo, A.C.; Carrara, C.R. Brea Gum as Wall Material in the Microencapsulation of Corn Oil by Spray Drying: Effect of Inulin Addition. Food Res. Int. 2018, 103, 76–83. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Evolution of the Phenolic Compounds Profile of Olive Leaf Extract Encapsulated by Spray-Drying during in Vitro Gastrointestinal Digestion. Food Chem. 2019, 279, 40–48. [Google Scholar] [CrossRef]
- Urzúa, C.; González, E.; Dueik, V.; Bouchon, P.; Giménez, B.; Robert, P. Olive Leaves Extract Encapsulated by Spray-Drying in Vacuum Fried Starch–Gluten Doughs. Food Bioprod. Process. 2017, 106, 171–180. [Google Scholar] [CrossRef]
- Fuentes-Ortega, T.; Martínez-Vargas, S.L.; Sortés-Camargo, S.; Guadarrama-Lezama, A.Y.; Gallardo-Rivera, R.; Baeza-Jimenéz, R.; Pérez-Alonso, C. Effects of the process variables of microencapsulation sesame oil (Sesamum indica L.) by spray drying. Rev. Mex. Ing. Química 2017, 16, 477–490. [Google Scholar]
- García-Gurrola, A.; Rincón, S.; Escobar-Puentes, A.A.; Zepeda, A.; Martínez-Bustos, F. Microencapsulation of Red Sorghum Phenolic Compounds with Esterified Sorghum Starch as Encapsulant Materials by Spray Drying. Food Technol. Biotechnol. 2019, 57, 341–349. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Kanha, N. Microencapsulation of Black Glutinous Rice Anthocyanins Using Maltodextrins Produced from Broken Rice Fraction as Wall Material by Spray Drying and Freeze Drying. J. Food Process. Preserv. 2017, 41, e12877. [Google Scholar] [CrossRef]
- Boyano-Orozco, L.; Gallardo-Velázquez, T.; Meza-Márquez, O.G.; Osorio-Revilla, G. Microencapsulation of Rambutan Peel Extract by Spray Drying. Foods 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, P.; Palaniswamy, P.T.; Visvanathan, R.; Thirupathi, V.; Subbarayan, A.; Prakash Maran, J. Microencapsulation of Garlic Oleoresin Using Maltodextrin as Wall Material by Spray Drying Technology. Int. J. Biol. Macromol. 2015, 72, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Ruiz, G.A.; Zuluaga Corrales, H.F. Chitosan, Chitosan Derivatives and Their Biomedical Applications. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E., Ed.; IntechOpen: London, UK, 2017; pp. 87–106. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with Chitosan by Spray Drying for Industry Applications—A Review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- De Amorim, C.M.; Couto, A.G.; Netz, D.J.A.; de Freitas, R.A.; Bresolin, T.M.B. Antioxidant Idebenone-Loaded Nanoparticles Based on Chitosan and N-Carboxymethylchitosan. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 745–752. [Google Scholar] [CrossRef]
- Wilson, N.E.; Mutukuri, T.T.; Zemlyanov, D.Y.; Taylor, L.S.; Topp, E.M.; Zhou, Q.T. Surface Composition and Formulation Heterogeneity of Protein Solids Produced by Spray Drying. Pharm. Res. 2020, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Meenach, S.A. Optimization of Acetalated Dextran–Based Nanocomposite Microparticles for Deep Lung Delivery of Therapeutics via Spray-Drying. J. Pharm. Sci. 2017, 106, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, D.; Alemán, A.; Montero, M.P.; Gómez-Guillén, M.C. Entrapment of Natural Compounds in Spray-Dried and Heat-Dried Iota-Carrageenan Matrices as Functional Ingredients in Surimi Gels. Food Funct. 2021, 12, 2137–2147. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Wang, X.; Prakash, S.; Zhao, Y.; Han, J.; Wang, Z. Influences of Different Carbohydrates as Wall Material on Powder Characteristics, Encapsulation Efficiency, Stability and Degradation Kinetics of Microencapsulated Lutein by Spray Drying. Int. J. Food Sci. Technol. 2020, 55, 2872–2882. [Google Scholar] [CrossRef]
- Santiago, L.G.; Castro, G.R. Novel Technologies for the Encapsulation of Bioactive Food Compounds. Curr. Opin. Food Sci. 2016, 7, 78–85. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman and Company 41: New York, NY, USA, 2007; ISBN 9780716787242. [Google Scholar]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.; Zapata Noreña, C.P. Encapsulation of Garlic Extract Using Complex Coacervation with Whey Protein Isolate and Chitosan as Wall Materials Followed by Spray Drying. Food Hydrocoll. 2019, 89, 360–369. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Lević, S.M.; Pavlović, V.B.; Marković, S.B.; Pjanović, R.V.; Đorđević, V.B.; Nedović, V.; Bugarski, B.M. Freeze vs. Spray Drying for Dry Wild Thyme (Thymus serpyllum L.) Extract Formulations: The Impact of Gelatin as a Coating Material. Molecules 2021, 26, 3933. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Vyas, H.K.N.; Sanderson-Smith, M.L.; Sencadas, V. Development and Optimization of Ciprofloxacin-Loaded Gelatin Microparticles by Single-Step Spray-Drying Technique. Powder Technol. 2018, 330, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, U.; Gill, H.; Chandrapala, J.; Shahid, F. Influence of Spray Drying on Encapsulation Efficiencies and Structure of Casein Micelles Loaded with Anthraquinones Extracted from Aloe Vera Plant. Appl. Sci. 2023, 13, 110. [Google Scholar] [CrossRef]
- Nogueira, M.H.; Tavares, G.M.; Casanova, F.; Silva, C.R.J.; Rocha, J.C.G.; Stringheta, P.C.; Stephani, R.; Perrone, Í.T.; de Carvalho, A.F. Cross-Linked Casein Micelle Used as Encapsulating Agent for Jaboticaba (Plinia jaboticaba) Phenolic Compounds by Spray Drying. Int. J. Dairy Technol. 2020, 73, 765–770. [Google Scholar] [CrossRef]
- Tan, S.; Ebrahimi, A.; Langrish, T. Controlled Release of Caffeine from Tablets of Spray-Dried Casein Gels. Food Hydrocoll. 2019, 88, 13–20. [Google Scholar] [CrossRef]
- Tan, S.; Hadinoto, K.; Ebrahimi, A.; Langrish, T. Fabrication of Novel Casein Gel with Controlled Release Property via Acidification, Spray Drying and Tableting Approach. Colloids Surfaces B Biointerfaces 2019, 177, 329–337. [Google Scholar] [CrossRef]
- Khanji, A.N.; Michaux, F.; Petit, J.; Salameh, D.; Rizk, T.; Jasniewski, J.; Banon, S. Structure, Gelation, and Antioxidant Properties of Curcumin-Doped Casein Micelle Powder Produced by Spray-Drying. Food Funct. 2018, 9, 971–981. [Google Scholar] [CrossRef]
- Saldanha Do Carmo, C.; Maia, C.; Poejo, J.; Lychko, I.; Gamito, P.; Nogueira, I.; Bronze, M.R.; Serra, A.T.; Duarte, C.M.M. Microencapsulation of α-Tocopherol with Zein and β-Cyclodextrin Using Spray Drying for Colour Stability and Shelf-Life Improvement of Fruit Beverages. RSC Adv. 2017, 7, 32065–32075. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zheng, L.; Liang, S.; Lu, Y.; Zheng, J.; Zhang, G.; Li, W.; Jiang, H. Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods 2022, 11, 612. [Google Scholar] [CrossRef]
- Shi, X.; Lee, Y. Encapsulation of Tributyrin with Whey Protein Isolate (WPI) by Spray-Drying with a Three-Fluid Nozzle. J. Food Eng. 2020, 281, 109992. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Wai, M.; Zeng, X.; Brennan, M.; Brennan, C.S. Preparation and Characterization of Whey Protein Isolate-Chlorophyll Microcapsules by Spray Drying: Effect of WPI Ratios on the Physicochemical and Antioxidant Properties. J. Food Eng. 2020, 267, 109729. [Google Scholar] [CrossRef]
- Zhuang, F.; Li, X.; Hu, J.; Liu, X.; Zhang, S.; Tang, C.; Zhou, P. Effects of Casein Micellar Structure on the Stability of Milk Protein-Based Conjugated Linoleic Acid Microcapsules. Food Chem. 2018, 269, 327–334. [Google Scholar] [CrossRef]
- Jansen-alves, C.; Fernandes, K.F.; Crizel-Cardozo, M.M.; Krumreich, F.D.; Borges, C.D.; Zambiazi, R.C. Microencapsulation of Propolis in Protein Matrix Using Spray Drying for Application in Food Systems. Food Bioprocess Technol. 2018, 11, 1422–1436. [Google Scholar] [CrossRef]
- Fu, N.; You, Y.J.; Quek, S.Y.; Wu, W.D.; Chen, X.D. Interplaying Effects of Wall and Core Materials on the Property and Functionality of Microparticles for Co-Encapsulation of Vitamin E with Coenzyme Q10. Food Bioprocess Technol. 2020, 13, 705–721. [Google Scholar] [CrossRef]
- Khalilvandi-Behroozyar, H.; Banadaky, M.D.; Ghaffarzadeh, M. Investigating the Effects of Varying Wall Materials and Oil Loading Levels on Stability and Nutritional Values of Spray Dried Fish Oil. Vet. Res. Forum 2020, 11, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.; Shimpi, S.; Paradkar, A. Preparation and Characterization of Etoricoxib Solid Dispersions Using Lipid Carriers by Spray Drying Technique. AAPS PharmSciTech 2005, 6, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Salminen, H.; Ankenbrand, J.; Zeeb, B.; Badolato Bönisch, G.; Schäfer, C.; Kohlus, R.; Weiss, J. Influence of Spray Drying on the Stability of Food-Grade Solid Lipid Nanoparticles. Food Res. Int. 2019, 119, 741–750. [Google Scholar] [CrossRef]
- Polekkad, A.; Franklin, M.E.E.; Pushpadass, H.A.; Battula, S.N.; Rao, S.B.N.; Pal, D.T. Microencapsulation of Zinc by Spray-Drying: Characterisation and Fortification; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 381, ISBN 0000000266369. [Google Scholar]
- Sotelo-Bautista, M.; Bello-Perez, L.A.; Gonzalez-Soto, R.A.; Yañez-Fernandez, J.; Alvarez-Ramirez, J. OSA-Maltodextrin as Wall Material for Encapsulation of Essential Avocado Oil by Spray Drying. J. Dispers. Sci. Technol. 2020, 41, 235–242. [Google Scholar] [CrossRef]
- Rodriguez, N.J.; Hu, Q.; Luo, Y. Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules 2019, 24, 4061. [Google Scholar] [CrossRef] [Green Version]
- Gangurde, A.B.; Amin, P.D. Microencapsulation by Spray Drying of Vitamin A Palmitate from Oil to Powder and Its Application in Topical Delivery System. J. Encapsul. Adsorpt. Sci. 2017, 7, 10–39. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, B.; Wen, X.; Li, M.; Wang, K.; Ni, Y. Quality Analysis and Microencapsulation of Chili Seed Oil by Spray Drying with Starch Sodium Octenylsuccinate and Maltodextrin. Powder Technol. 2017, 312, 294–298. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Ferreira, S.; Nicoletti, V.R. Turmeric Oleoresin Encapsulated by Spray Drying in Maltodextrin/Gelatin and Starch/Gelatin Blends: Storage Stability and Water Sorption. Acta Sci. Technol. 2022, 44, e56950. [Google Scholar] [CrossRef]
- Le, T.H.; Tran, T.M.V.; Ton, N.M.N.; Tran, T.T.T.; Huynh, T.V.; Nguyen, T.N.; Quang, S.P.; Le, V.V.M. Combination of Whey Protein and Carbohydrate for Microencapsulation of Pumpkin (Cucurbita spp.) Seed Oil by Spray-Drying. Int. Food Res. J. 2017, 24, 1227–1232. [Google Scholar]
- Elik, A. A Comparative Study of Encapsulation of Carotenoid Enriched-Flaxseed Oil and Flaxseed Oil by Spray Freeze-Drying and Spray Drying Techniques. LWT Food Sci. Technol. 2021, 143, 111153. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tanska, M.; Brandt, W.; Czaplicki, S. Impact of the Encapsulation Process by Spray- And Freeze-Drying on the Properties and Composition of Powders Obtained from Cold-Pressed Seed Oils with Various Unsaturated Fatty Acids. Polish J. Food Nutr. Sci. 2020, 70, 241–252. [Google Scholar] [CrossRef]
- Kanwal, S.; Hussain, A.; Nadeem, M.; Abbas, F.; Akram, M.; Inayat, M.; Sughra, F. Development of Chitosan Based Microencapsulated Spray Dried Powder of Tuna Fish Oil: Oil Load Impact and Oxidative Stability. Braz. J. Biol. 2021, 84, e254010. [Google Scholar] [CrossRef]
- Wang, Y.; Ghosh, S.; Nickerson, M.T. Microencapsulation of Flaxseed Oil by Lentil Protein Isolate-κ-Carrageenan and-ι-Carrageenan Based Wall Materials through Spray and Freeze Drying. Molecules 2022, 27, 3195. [Google Scholar] [CrossRef]
- Gulzar, S.; Balange, A.K.; Nagarajarao, R.C.; Zhao, Q.; Benjakul, S. Microcapsules of Shrimp Oil Using Kidney Bean Protein Isolate and κ-Carrageenan as Wall Materials with the Aid of Ultrasonication or High-Pressure Microfluidization: Characteristics and Oxidative Stability. Foods 2022, 11, 1431. [Google Scholar] [CrossRef]
- Bassetto Carra, J.; Luís Nascimento de Matos, R.; Paula Novelli, A.; Oliveira do Couto, R.; Yamashita, F.; Alessandro dos Santos Ribeiro, M.; César Meurer, E.; Aparecido Verri Junior, W.; Casagrande, R.; Regina Georgetti, S.; et al. Spray-Drying of Casein/Pectin Bioconjugate Microcapsules Containing Grape (Vitis labrusca) by-Product Extract. Food Chem. 2022, 368, 130817. [Google Scholar] [CrossRef]
- Damerau, A.; Ogrodowska, D.; Banaszczyk, P.; Dajnowiec, F.; Tanska, M.; Linderborg, K.M. Baltic Herring (Clupea harengus Membras) Oil Encapsulation by Spray Drying Using a Rice and Whey Protein Blend as a Coating Material. J. Food Eng. 2022, 314, 110769. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of Maltodextrin Combination with Gum Arabic and Whey Protein Isolate on the Microencapsulation of Gurum Seed Oil Using a Spray-Drying Method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Antioxidant Activities of Spray-Dried Carotenoids Using Maltodextrin-Arabic Gum as Wall Materials. Bull. Natl. Res. Cent. 2021, 45, 58. [Google Scholar] [CrossRef]
- Buanasari; Sugiyo, W.; Rustaman, H. Preparation and Evaluation of Plant Extract Microcapsules Using Chitosan. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012063. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of Phenolic Compounds from a Grape Cane Pilot-Plant Extract in Hydroxypropyl Beta-Cyclodextrin and Maltodextrin by Spray Drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef]
- Ramos, F.D.M.; Júnior, V.S.; Prata, A.S. Impact of Vacuum Spray Drying on Encapsulation of Fish Oil: Oxidative Stability and Encapsulation Efficiency. Food Res. Int. 2021, 143, 110283. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of Fingered Citron Extract with Gum Arabic, Modified Starch, Whey Protein, and Maltodextrin Using Spray Drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Martin, L.G.P.; Fakhouri, F.M.; de Oliveira, R.A. Influence of Spray Drying on Bioactive Compounds of Blackberry Pulp Microencapsulated with Arrowroot Starch and Gum Arabic Mixture. J. Microencapsul. 2020, 37, 65–76. [Google Scholar] [CrossRef]
- Sari, D.K.; Lestari, R.S.D.; Khinanta, P.; Sahlan, M. Encapsulation Bioactive Compund Propolis with Carrageenan–Gum Arabic by Spray Drying. J. Integr. Proses 2020, 9, 8–11. [Google Scholar] [CrossRef]
- Boonsom, T.; Dumkliang, E. Response Surface Optimization on Microencapsulation of Lemongrass Essential Oil Using Spray Drying. Key Eng. Mater. 2020, 859, 271–276. [Google Scholar] [CrossRef]
- Zhang, C.; Khoo, S.L.A.; Swedlund, P.; Ogawa, Y.; Shan, Y.; Quek, S.Y. Fabrication of Spray-Dried Microcapsules|containing Noni Juice Using Blends of Maltodextrin and Gum Acacia: Physicochemical Properties of Powders and Bioaccessibility of Bioactives during in Vitro Digestion. Foods 2020, 9, 1316. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of Oregano Essential Oil (Origanum vulgare) by Complex Coacervation between Gelatin and Chia Mucilage and Its Properties after Spray Drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Radünz, M.; dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating Multilayer Emulsions by Using OSA Starch and Chitosan Suitable for Spray Drying: Application in the Encapsulation of β-Carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Bakry, A.M.; Huang, J.; Zhai, Y.; Huang, Q. Myofibrillar Protein with κ- or λ-Carrageenans as Novel Shell Materials for Microencapsulation of Tuna Oil through Complex Coacervation. Food Hydrocoll. 2019, 96, 43–53. [Google Scholar] [CrossRef]
- Ferreira, S.; Piovanni, G.M.O.; Malacrida, C.R.; Nicoletti, V.R. Influence of Emulsification Methods and Spray Drying Parameters on the Microencapsulation of Turmeric Oleoresin. Emirates J. Food Agric. 2019, 31, 491–500. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Mathesh, M.; Yang, W.; Barrow, C.J. Anchovy Oil Microcapsule Powders Prepared Using Two-Step Complex Coacervation between Gelatin and Sodium Hexametaphosphate Followed by Spray Drying. Powder Technol. 2019, 358, 68–78. [Google Scholar] [CrossRef]
- Dos Vaucher, A.C.S.; Dias, P.C.M.; Coimbra, P.T.; dos Costa, I.S.M.; Marreto, R.N.; Dellamora-Ortiz, G.M.; De Freitas, O.; Ramos, M.F.S. Microencapsulation of Fish Oil by Casein-Pectin Complexes and Gum Arabic Microparticles: Oxidative Stabilisation. J. Microencapsul. 2019, 36, 459–473. [Google Scholar] [CrossRef]
- Cuevas-Bernardino, J.C.; Pérez-Alonso, C.; Nieto-Ángel, R.; Aguirre-Mandujano, E. Microencapsulation of Grape Seed Oil by Spray Drying Using Whey Protein and Hawthorn Pectin. Ing. Agrícola Y Biosist. 2019, 11, 127–145. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Pereira Martin, L.G.; Matta Fakhouri, F.; de Oliveira, R.A. Microencapsulation of Blackberry Pulp with Arrowroot Starch and Gum Arabic Mixture by Spray Drying. J. Microencapsul. 2018, 35, 482–493. [Google Scholar] [CrossRef]
- Shabanpour, B.; Mehrad, B.; Pourashouri, P.; Jafari, S.M. The Effect of Wall Material and Encapsulation Method on Physicochemical Properties Micro-Encapsulated Fish Oil. J. Res. Innov. Food Sci. Technol. 2018, 7, 13–28. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of Refined Kenaf (Hibiscus cannabinus L.) Seed Oil by Spray Drying Using β-Cyclodextrin/Gum Arabic/Sodium Caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Nguyen, T.D. Optimization of Maltodextrin and Carrageenan Gum Concentration Added in Spray Drying Process of Pouzolzia Zeylanica Extract by Response Surface Methodology. J. Agric. Dev. 2018, 17, 77–85. [Google Scholar] [CrossRef]
- Ordoñez-Santos, L.E.; Martínez-Girón, J.; Villamizar-Vargas, R.H. Encapsulation of β-Carotene Extracted from Peach Palm Residues: A Stability Study Using Two Spray-Dried Processes. DYNA 2018, 85, 128–134. [Google Scholar] [CrossRef]
- Premi, M.; Sharma, H.K. Effect of Different Combinations of Maltodextrin, Gum Arabic and Whey Protein Concentrate on the Encapsulation Behavior and Oxidative Stability of Spray Dried Drumstick (Moringa oleifera) Oil. Int. J. Biol. Macromol. 2017, 105, 1232–1240. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Sellamuthu, P.S.; Perumal, A.B. Microencapsulation of Tender Coconut Water by Spray Drying: Effect of Moringa Oleifera Gum, Maltodextrin Concentrations, and Inlet Temperature on Powder Qualities. Food Bioprocess Technol. 2017, 10, 1668–1684. [Google Scholar] [CrossRef]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribez-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and E as Spray-Dried Additives for the Feed Industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Li, Z.; Zhang, T.; Wei, Y.; Xue, Y.; Xue, C. Influence of Encapsulation Techniques on the Structure, Physical Properties, and Thermal Stability of Fish Oil Microcapsules by Spray Drying. J. Food Process Eng. 2017, 40, e12576. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.; Maghsoudlou, Y. Evaluation of Folic Acid Release from Spray Dried Powder Particles of Pectin-Whey Protein Nano-Capsules. Int. J. Biol. Macromol. 2017, 95, 238–247. [Google Scholar] [CrossRef]
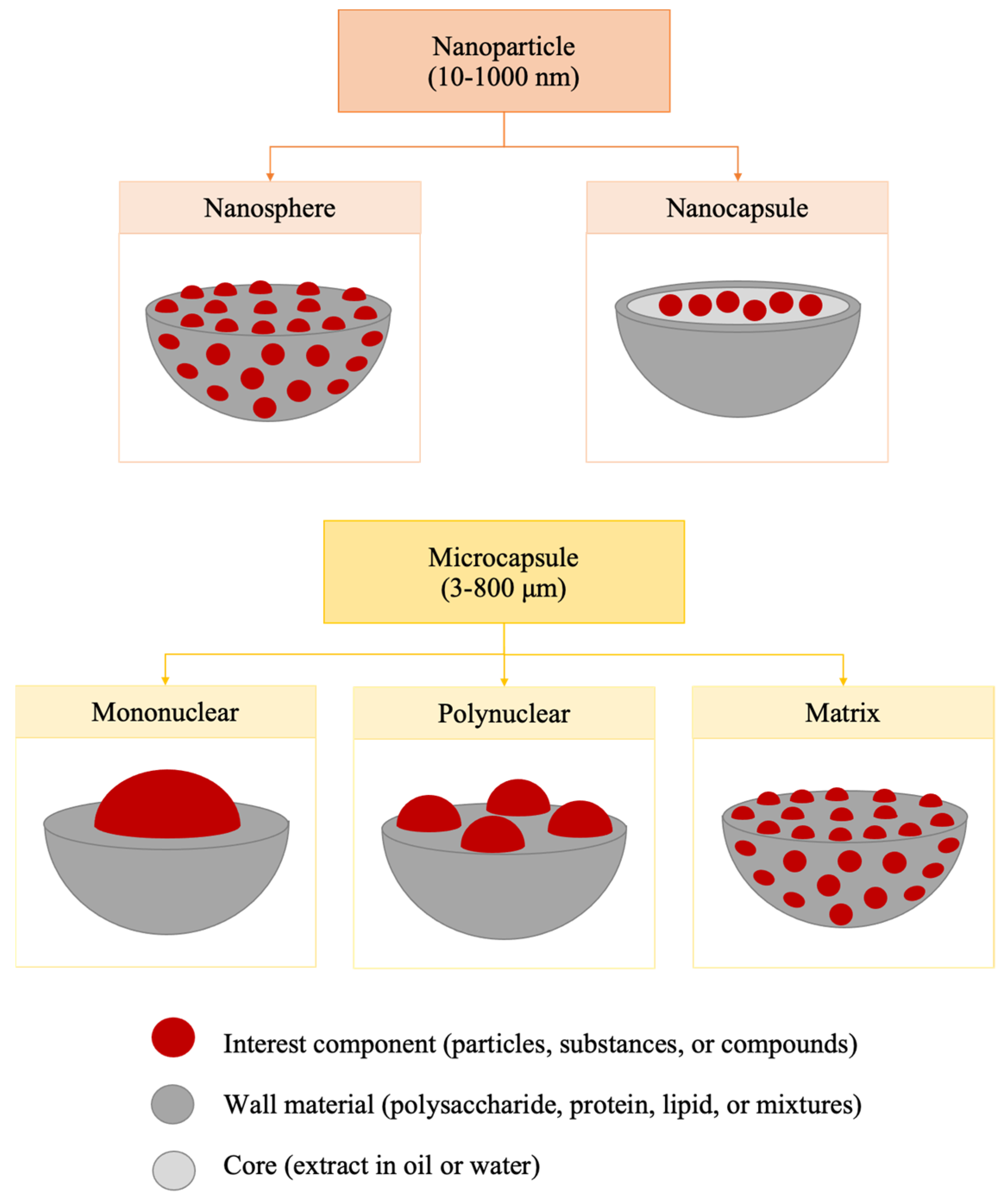


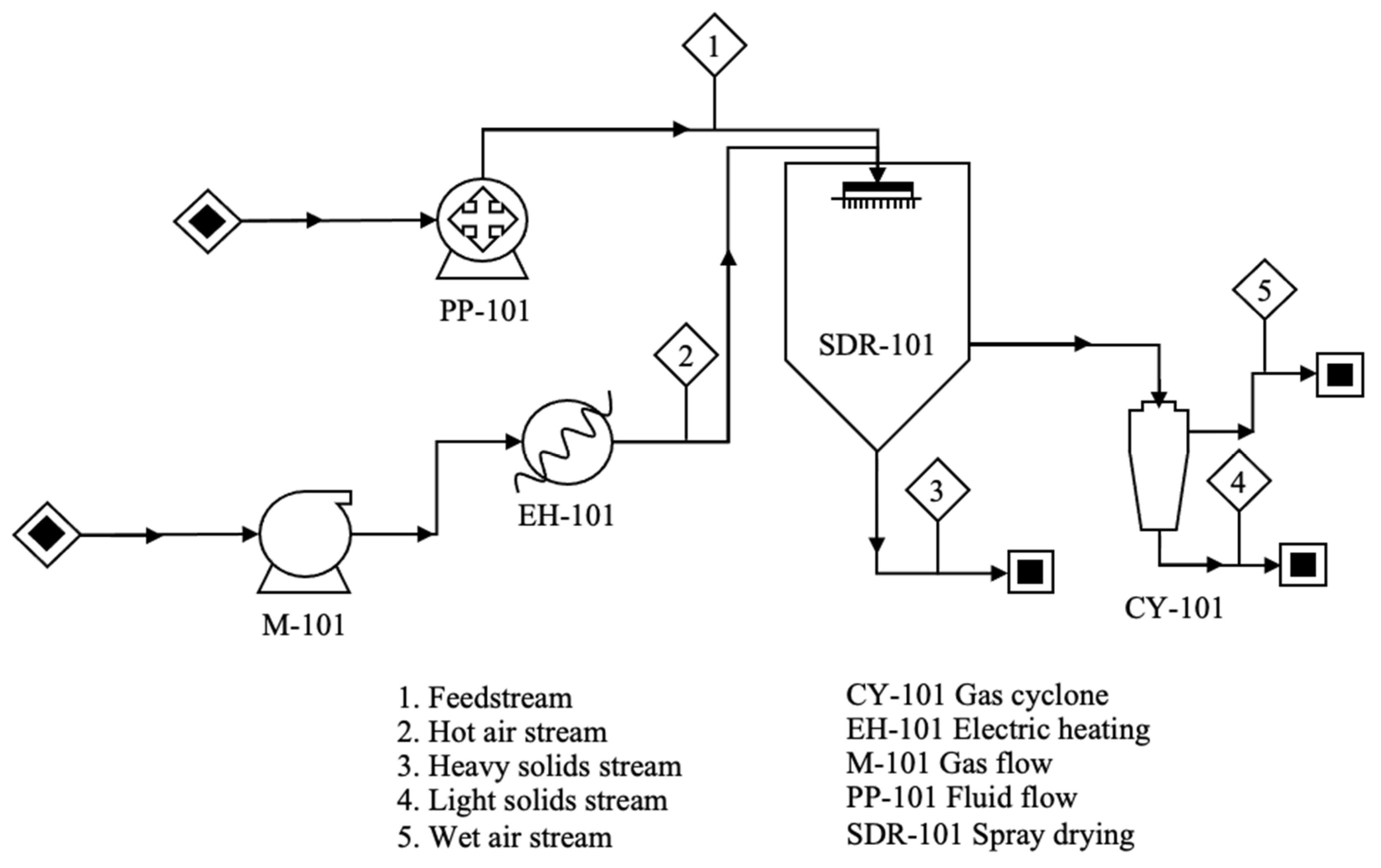
| Wall Material | Core Material | Concentration (Wall Material: Core Material) | Conditions (Feed Rate, Inlet Air, Outlet Air) | Particles (Shape/Morphology, Particle Size Distribution) | Process Yield/Encapsulation Efficiency | Encapsulated Compounds | References |
|---|---|---|---|---|---|---|---|
| A-Starch | Hibiscus sabdariffa extract | 10–15:nr.% w/nr. | 4 mL/min, 100–140 °C, 56–84 °C | Ovoid/Collapsed, 10 μm | nr./15–69% | Antimicrobial compounds | [63] |
| C-Starch | Corn oil | 2:1% w/w | nr., 180 °C, 115 °C | Semispherical/Shrunken, 12–38 μm | 64%/89% | Oil | [64] |
| M-Starch | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 51.6–77.3 μm | nr./42% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| M-Starch | Ascorbyl palmitate | 3:1 ratio | 6.3 mL/min, 185 °C, 70 °C | nr./nr., nr. | nr. | Ascorbyl palmitate | [66] |
| Mo-Starch | Vitamin A | 15:2% w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 17%/89% | Vitamin A | [67] |
| Mo-Starch | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.1–3.6 μm | 25%/95% | Vitamin B1 | [68] |
| Mo-Starch | Fish oil | 15:nr.% w/nr. | 0.7 L/h, 180 °C, nr. | Spherical/Smooth holes, 10–26 μm | nr./76% | Oil | [69] |
| Mo-Starch | Orange essential oil | 30:15% w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 73%/99% | D-Limonene | [70] |
| P-Starch | Gallic acid | 20:0.1–10% w/w | nr., 160 °C, 75 °C | Spherical/Withered, 1–15 μm | nr./70–84% | Gallic acid | [71] |
| R-Starch | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 41.1–67.7 μm | nr./35% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| R-Starch | Vanilla extract | 10–15:nr.% w/nr. | 4 mL/min, 100–140 °C, 70–94 °C | Polyhedral/Irregular, 2–7 μm | 20–44%/15–69% | Vanillin | [72] |
| R-Starch | Orange essential oil | 30:15% w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 73%/37% | D-Limonene | [70] |
| SOS-Starch | Nigella sativa seeds oil | 18.75:6.25:1% w/w/w | nr., 140 °C, 95–98 °C | Spherical/Irregular, 1–30 μm | nr./80–90% | Volatile compounds | [73] |
| T-Starch | Almond oil | 20:10–20% w/w | 10.6 g/min, 145 °C, nr. | Spherical/Aggregates, 1.6–31.1 μm | 56%/38–45% | Peroxides | [74] |
| T-Starch | L-ascorbic acid | 30:10% w/w | 19.5 g/min, 145 °C, 80 °C | Spherical/Aggregates, 2–10 μm | nr. | L-ascorbic acid | [75] |
| W-Starch | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 41.4–70.9 μm | nr./68% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| Starch | Tea (Camelia sinensis L.) leaves extract | 1.5:1% w/w | 4 mL/min, 115 °C, 65 °C | Ovoid/Smooth, 10–50 μm | 59/nr. | Phenolic compounds | [76] |
| Starch | Horseradish leaf (Armoracia rusticana L.) juice | 20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 9.5–14.1 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin and sinapic acid | [77] |
| HCP | Borage seed oil | 10–30:13% w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Dented, 4–15 μm | nr./89% | Oil | [78] |
| HCP | Borage seed oil/Curcumin | 10–30:13:0.6% w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Dented, 4–15 μm | nr./91% | Curcumin | [78] |
| HCP | Borage seed oil/Resveratrol | 10–30:13:0.4% w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Dented, 4–15 μm | nr./88% | Resveratrol | [78] |
| HCP | Borage seed oil/Curcumin/Resveratrol | 10–30:13:0.6:0.4% w/w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Dented, 4–15 μm | nr./93% | Curcumin and resveratrol | [78] |
| C-MD | Corn mint (Mentha arvensis L.) essential oil | 20–30:0.5–2% w/w | 4–10 mL/min, 130–150 °C, nr. | nr./nr., nr. | 69%/99% | Menthol, menthone, menthyl acetate, isomenthone, caryphyllene, eucalyptol, α-terpineol, δ-cadinene, neoisomenthol, pulegone, β-bourbonene, and nerolidol | [79] |
| MD | Mix (paprika-cinnamon oleoresin) | 10:1:1%w/ratio/ratio | 6 mL/min, 150 °C, 80 °C | Spherical/Porous, 33 μm | 34%/65% | Carotenoids | [80] |
| MD | Tucuma coproduct (Astrocaryum vulgare Mart.) almonds extract | 5:5% w/w | 7.5 mL/min, 100 °C, nr. | Spherical/Wrinkled, 2–8 μm | nr./81–96% | Phenolic compounds, flavonoids, and tannins | [81] |
| MD | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 58.0–103.0 μm | nr./70% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| MD | Red dragón fruit (Hylocereus polyrhizus) juice | 5–15:nr.% w/nr. | 500 mL/h, 170 °C, 70 °C | nr./nr., nr. | 53–74%/nr. | Phenolic compounds | [82] |
| MD | Cornsilk extract | 10:90% w/w | nr., nr., nr. | Spherical/Irregular, nr. | 76–87%/99% | Phenolic compounds (gallic acid, protocateuic acid, protocatechuic aldehyde, catechin, caffeic acid, vanillin, p-cumaric acid, ferulic acid, 4-hydroxy benzoic acid, salicylic acid, and ellagic acid) and flavonoids | [83] |
| MD | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 9.3 μm | nr./33% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| MD | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.1 μm | 50%/65% | Vitamin B1 | [68] |
| MD | Vitamin A | 15:2% w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 39%/95% | Vitamin A | [67] |
| MD | Chipilin (Crotalaria longirostrata) extract | 17:1% w/w | 3 mL/min, 120 °C, 60 °C | Amorphous/Irregular, 3–8 μm | 64%/86% | Phenolic compounds | [85] |
| MD | Stevia extract | 2:1 w/w ratio | nr., 130 °C, 87 °C | Spherical/Aggregates, <20 μm | nr./77–88% | Steviol glycoside, stevioside, rebaudioside A, rebaudioside C, and phenolic compounds | [86] |
| MD | Pineapple (Ananas comosus) peel extract | 5:nr.% w/nr. | 3.7 mL/min, 150–190 °C, 80 °C | Spherical/Aggregates, 2–13 μm | nr./nr. | Phenolic compounds | [87] |
| MD | Horseradish root (Armoracia rusticana L.) juice | 20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 3.7–4.0 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin, and sinapic acid | [77] |
| MD | Chokeberry extract | 2:10 w/v ratio | nr., nr., nr. | Spherical/Aggregates, 4–10 μm | nr./97% | Phenolic compounds, anthocyanins, and cyanidin-3-glucoside | [88] |
| MD | Lemon by-product aqueous extract | 30–35:30% w/v | 4 mL/min, 125 °C, 55 °C | Spherical/Smooth, nr. | 56–58%/nr. | Phenolic compounds and flavonoids | [89] |
| MD | Greek saffron extract | 5–20:1 w/w ratio | nr., 100 °C, nr. | Spherical/Aggregates, 2–5 μm | 71–87%/55–80% | Crocins and picrocrocin | [90] |
| MD | Orange essential oil | 30:15% w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 29%/72% | D-Limonene | [70] |
| MD | Propolis | 30:0.123% w/w/w | 8 mL/min, 120 °C, 70–74 °C | Deformed spherical/Smooth, nr. | 68%/86–98% | Phenolic compounds | [91] |
| MD | Fish oil | 34.2:24% w/w | 25 mL/min, 140 °C, 70–95 °C | Spherical/Wrinkled, 1 μm | nr./74–90% | Oil | [92] |
| MD | Blueberry (Vaccinium corymbosum) juice | 30:70% w/w | 7 mL/min, 180 °C, 70 °C | Spherical/Irregular, nr. | nr./nr. | Resveratrol and quercetin 3-D-galactoside | [93] |
| MD | Spent coffee ground extract | 2:10 w/v ratio | 108 mL/min, 100 °C, nr. | Spherical/Withered, <30 μm | nr./50–85% | Phenolic compounds and flavonoids | [94] |
| Mo-CH | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.6 μm | 42%/95% | Vitamin B1 | [68] |
| CH | Insulin | 1:0.2% w/w | 3 mL/min, 120 °C, 50–55 °C | Spherical/Irregular, <1.2 μm | nr./nr. | Insulin | [95] |
| CH | Satureja khuzistanica Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Spherical/Smooth, 2 μm | nr./59% | Phenolic compounds | [96] |
| CH | Satureja rechingeri Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Spherical/Smooth, 2 μm | nr./43% | Phenolic compounds | [96] |
| CH | Curcumin | 0.25–0.5:1.7% w/w | 4 mL/min, 180 °C, nr. | Spherical/Irregular, 1–5 μm | 92–99%/51–72% | Curcumin | [97] |
| CH | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.1–0.8 μm | 35%/95% | Vitamin B1 | [68] |
| CH | Iron gluconate | 3:1 ratio | nr., 160 °C, nr. | Spherical/Wrinkled, 1–10 μm | 28–43%/24–38% | Iron gluconate | [98] |
| CH | Algal (Tetraselmis chuii) biomass | 3:1% w/w | 2.5 mL/min, 150 °C, 60 °C | Spherical/Rough, 1–15.2 μm | 22–45%/53–73% | Phenolic compounds, β-carotene, and carotenoids | [99] |
| CH | Potassium phosphate | 25:5–50% w/w | 1.4–3.6 mL/min, 120–150 °C, nr. | Semi-spherical/collapsed, 1–2 μm | nr./88–92% | Potassium phosphate | [100] |
| CH | Cacao hull waste extract | 0.4:0.1–0.9% w/w | 2.5 cm3/min, 170 °C, 75–80 °C | Semi-spherical/Dented, 156–400 nm | nr./19–88% | Phenolic compounds and flavonoids | [101] |
| CH | Venlafaxine hydrochloride | 0.5:30% w/w | 32 m3/h, 160 °C, nr. | Spherical/Irregular, 3–10 μm | 44–74%/37–94% | Venlafaxine hydrochloride | [102] |
| CH | Squalene | 1:0.3–1 ratio | nr., 160 °C, 90 °C | Spherical/Smooth, 6.8 μm | nr./26% | Squalene | [103] |
| GA | Satureja khuzistanica Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-cubes/Rough, 2.6 μm | nr./50% | Phenolic compounds | [96] |
| GA | Satureja rechingeri Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-cubes/Rough, 2.6 μm | nr./38% | Phenolic compounds | [96] |
| GA | Guaraná (Paullinia cupana) peel extract | 20:20–33% w/w | 10 mL/min, 140 °C, nr. | Spherical/Irregular, 10–16 μm | nr./82–100% | Carotenoids | [104] |
| GA | Red dragón fruit (Hylocereus polyrhizus) juice | 5–15:nr.% w/nr. | 500 mL/h, 170 °C, 70 °C | nr./nr., nr. | 80–91%/nr. | Phenolic compounds | [82] |
| GA | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 34.4 μm | nr./39% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| GA | Basil (Ocimum basilicum L.) essential oil | 4:1% w/w | 3 mL/min, 150 °C, nr. | Spherical/Wrinkled, 0.5 μm | 66%/82% | Essential oil | [105] |
| GA | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.1–0.6 μm | 38%/95% | Vitamin B1 | [68] |
| GA | Vitamin A | 15:2% w/w | 4 mL/min, 150 °C, 80 °C | Spherical/Irregular, 0.1–0.2 μm | 35%/88% | Vitamin A | [67] |
| GA | Pineapple (Ananas comosus) peel extract | 5:nr.% w/nr. | 3.7 mL/min, 150–190 °C, 80 °C | Spherical/Aggregates, 4–8 μm | nr./nr. | Phenolic compounds | [87] |
| GA | Non-dewaxed propolis extract | 4:1 w/w ratio | 8 mL/min, 120 °C, 68 °C | Spherical/Withered, 0.6 μm | nr./46% | Bioflavonoids, pinocembrin, galangin, chrysin and phenolic compounds | [106] |
| GA | L-ascorbic acid | 1–4:1 ratio | 2–7 mL/min, 140 °C, 86 °C | Spherical/Aggregates, 3–10 μm | 67–83%/82–98% | L-ascorbic acid | [107] |
| GA | Tomato (Solanum lycopersicum L.) pomace extract | 9.4–30.6:15% w/w | 3.7 mL/min, 110–200 °C, nr. | Spherical/Smooth holes, 0.7–27 μm | 15–44%/3–21% | Lycopene | [108] |
| GA | Non-dewaxed propolis extract | 4:1 w/w ratio | 0.5 mL/min, 80 °C, 50 °C | Spherical/Withered, 0.5 μm | nr./21% | Bioflavonoids, pinocembrin, galangin, chrysin and phenolic compounds | [106] |
| GA | Curcumin | 10–20:0.1% w/w | 4 mL/min, 150 °C, 88 °C | Spherical/Rough, 0.2–0.3 μm | 29–42%/nr. | Curcumin | [109] |
| GA | Grape seed oil | 30:10% w/w | 350 mL/h, 180 °C, 105 °C | Spherical/Collapsed, 27 μm | nr./68% | Fatty acids (C14, C16, C18, C20, SFA, MUFA, and PUFA) and phenolic compounds | [110] |
| GA | Corn oil | 20:20% w/w | nr., 150 °C, 60 °C | Semi-spherical/Dents, 0.8–10 μm | nr./89% | Oil | [111] |
| GA | Fish oil | 15:nr.% w/nr. | 0.7 L/h, 180 °C, nr. | Spherical/Smooth holes, 9–22 μm | nr./60% | Oil | [69] |
| GA | Spent coffee ground extract | 2:10 w/v ratio | 108 mL/min, 100 °C, nr. | Spherical/Withered, <30 μm | nr./35–80% | Phenolic compounds and flavonoids | [94] |
| SA | Tea (Camelia sinensis L.) leaves extract | 1.5:1% w/w | 4 mL/min, 115 °C, 65 °C | Spherical/Dented, 1–5 μm | 55/nr. | Phenolic compounds | [76] |
| SA | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.6–1.0 μm | 42%/95% | Vitamin B1 | [68] |
| SA | L-ascorbic acid | 1–4:1 ratio | 2–7 mL/min, 140 °C, 86 °C | Spherical/Aggregates, 5–14 μm | 35–76%/92–99% | L-ascorbic acid | [107] |
| SA | Olive (Olea europaea L.) leaves extract | 0.35–2.15:1 ratio | 3 mL/min, 135–195 °C, 70–90 °C | Spherical/Smooth holes, 0.25–20 μm | 35–57%/54–69% | Oleuropein | [112] |
| SC | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 13 μm | nr./63% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| λ-Carr | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.5–1.2 μm | 32%/95% | Vitamin B1 | [68] |
| Carr | Tea (Camelia sinensis L.) leaves extract | 1.5:1% w/w | 4 mL/min, 115 °C, 65 °C | Spherical/Rough, 0.5–4 μm | 46/nr. | Phenolic compounds | [76] |
| Inulin | Red dragón fruit (Hylocereus polyrhizus) juice | 5–15:nr.% w/nr. | 500 mL/h, 170 °C, 70 °C | nr./nr., nr. | 32–46%/nr. | Phenolic compounds | [82] |
| Inulin | Pineapple (Ananas comosus) peel extract | 5:nr.% w/nr. | 3.7 mL/min, 150–190 °C, 80 °C | Spherical/Aggregates, 1–18 μm | nr./nr. | Phenolic compounds | [87] |
| Inulin | Tomato (Solanum lycopersicum L.) pomace extract | 9.4–30.6:15% w/w | 3.7 mL/min, 110–200 °C, nr. | Spherical/Smooth, 0.6–22 μm | 35–50%/7–25% | Lycopene | [108] |
| Inulin | Blueberry (Vaccinium corymbosum) juice | 30:70% w/w | 7 mL/min, 180 °C, 70 °C | Spherical/Irregular, nr. | nr./nr. | Resveratrol and quercetin 3-D-galactoside | [93] |
| Inulin | Olive (O. europaea L.) leaves extract | 0.34–2.15:1 ratio | nr., 135–184 °C, nr. | nr./nr., nr. | 64%/87% | Phenolic compounds | [113] |
| Lactose | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 58.3–104.0 μm | nr./71% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| Lactose | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 127 μm | nr./30% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| Trehalose | Pumpkin oil | 15.4:10.2% w/w | 77 mL/min, 130 °C, 90 °C | Spherical/Withered, 85.2–176.0 μm | nr./59% | α-Tocopherol, γ-tocopherol, squalene, spinasterol, β-sitosterol, stigmastatrienol, stigmasterol, and stigmastadienol | [65] |
| Dextrin | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 43.4 μm | nr./1% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| Xanthan | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.1–0.7 μm | 17%/95% | Vitamin B1 | [68] |
| Skimmed milk | Chokeberry extract | 2:10 w/v ratio | nr., nr., nr. | Spherical/Aggregates, 8–14 μm | nr./74–79% | Phenolic compounds, anthocyanins, and cyanidin-3-glucoside | [88] |
| Apple pectin | Satureja khuzistanica Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-cubes/Porous, 3 μm | nr./51% | Phenolic compounds | [96] |
| Apple pectin | Satureja rechingeri Jamzad extract | 10:1% w/w | 3.5 mL/min, 115 °C, nr. | Semi-cubes/Porous, 3 μm | nr./38% | Phenolic compounds | [96] |
| Apple pectin | Vitamin B1 | 1:0.125% w/w | 4 mL/min, 120 °C, 50–67 °C | Spherical/Irregular, 0.5–1.3 μm | 44%/95% | Vitamin B1 | [68] |
| PG2000 | Borage seed oil | 10–20:13% w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Wrinkled, 4–20 μm | nr./87% | Oil | [78] |
| PG2000 | Borage seed oil/Curcumin | 10–20:13:0.6% w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Wrinkled, 4–20 μm | nr./89% | Curcumin | [78] |
| PG2000 | Borage seed oil/Resveratrol | 10–20:13:0.4% w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Wrinkled, 4–20 μm | nr./86% | Resveratrol | [78] |
| PG2000 | Borage seed oil/Curcumin/Resveratrol | 10–20:13:0.6:0.4% w/w/w/w | 10 mL/min, 170 °C, 80 °C | Asymmetrical/Wrinkled, 4–20 μm | nr./89% | Curcumin and resveratrol | [78] |
| Brea gum | Corn oil | 5–20:10% w/w | nr., 150 °C, 60 °C | Semi-spherical/Dents, 0.8–26 μm | nr./34–76% | Oil | [111] |
| Cashew tree gum | Fish oil | 15:nr.% w/nr. | 0.7 L/h, 180 °C, nr. | Spherical/Smooth holes, 30–63 μm | nr./76% | Oil | [69] |
| Mesquite gum | Sesame (Sesamum indica L.) oil | 1–3:1 ratio | nr., 120–160 °C, nr. | Spherical/Wrinkled, nr. | nr./70–90% | Oil | [114] |
| Wall Material | Core Material | Concentration (Wall Material: Core Material) | Conditions (Feed Rate, Inlet Air, Outlet Air) | Particles (Shape/Morphology, Particle Size Distribution) | Process Yield/Encapsulation Efficiency | Encapsulated Compounds | References |
|---|---|---|---|---|---|---|---|
| Gelatin | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 15.8 μm | nr./87% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| Gelatin | Thyme (Thymus serpyllum L.) extract | 5:100% w/v | nr., 140 °C, 72–75 °C | Semi-spherical/Wrinkled, 130–223 μm | nr./1–44% | Phenolic compounds, flavonoids, and sugars | [130] |
| Gelatin | Algal (Tetraselmis chuii) biomass | 2:1% w/w | 2.5 mL/min, 150 °C, 60 °C | Spherical/Rough, 1.5–15.3 μm | 22–45%/46–78% | Phenolic compounds, β-carotene, and carotenoids | [99] |
| Gelatin | Ciprofloxacin | 0.5–2:1–5% w/w | 90–308 mL/h, 115–140 °C, nr. | Spherical/Smooth, 2.6–3.7 μm | nr./95–100% | Ciprofloxacin | [131] |
| Casein | Aloe vera (Aloe barbadensis) leaves | 4:1% w/w | nr., 160 °C, 70 °C | Spherical/Smooth, 2 μm | nr./nr. | Anthraquinone | [132] |
| Casein | Jaboticaba (Plinia jaboticaba) skin extract | 1:5 ratio | 1 L/h, 190 °C, 81 °C | Spherical/Wrinkled, 3–25 μm | nr./nr. | Phenolic compounds | [133] |
| Casein | Caffeine | 0–1:0–1 ratio | 8 mL/min, 100–190 °C, nr. | Irregular/Smooth, 10 μm | 20–65%/nr. | Caffeine | [134] |
| Casein | Ascorbic acid | 0.62:0.1% w/w | 8 mL/min, 100–150 °C, nr. | Spherical/Wrinkled, 3–27 μm | 60–80%/20–70% | Ascorbic acid | [135] |
| Casein | Curcumin | 15.5:1% w/w | 46.5 mL/min, 180 °C, 90 °C | Spherical/Wrinkled, 33 μm | nr./nr. | Curcumin | [136] |
| SPI | Horseradish leaf (Armoracia rusticana L.) juice | 20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 7.5–14.0 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin and sinapic acid | [77] |
| SPI | Horseradish root (Armoracia rusticana L.) juice | 20–80:20–80 ratio | 0.33 L/h, 120 °C, 80 °C | nr./nr., 6.5–6.9 μm | nr./nr. | Phenolic compounds, rutin, epicatechin, catechin and sinapic acid | [77] |
| C-Zein | α-Tocopherol | 1:6 ratio | 7–9 mL/min, 110–180 °C, nr. | Irregular/Porous, 0.5–5 μm | 44–77%/31–42% | α-Tocopherol | [137] |
| WP | Capsaicin | 10:20% w/w | nr., 185 °C, 85 °C | Spherical/Wrinkled, 0.8–8.1 μm | 68%/95% | Capsaicin | [138] |
| WP | Brown seaweed (Saccharina japonica) extract | 10:100% w/v | 30 mL/min, 230 °C, 105 °C | Spherical/Wrinkled, 143 μm | nr./87% | Phenolic acids (gallic acid, chlorogenic acid, gentisic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and syringic acid) | [84] |
| WPI | Tributyrin | 3–5:1 ratio | nr., 160 °C, nr. | Spherical/Smooth, 7–11 μm | nr./24–35% | Tributyrin | [139] |
| WPI | Fresh kale (Brassica oleracea L.) leaves extract | 5–15:40% w/v | 12 mL/min, 215 °C, 65 °C | Spherical/Wrinkled, 80 μm | nr./99% | Chlorophyll | [140] |
| WPI | Mix (paprika-cinnamon oleoresin) | 10:1:1%w/ratio/ratio | 6 mL/min, 150 °C, 80 °C | Spherical/Porous, 15 μm | 43%/84% | Carotenoids | [80] |
| Milk-PC | Conjugated linoleic acid | 1:8% w/w | nr., 160 °C, 80 °C | Spherical/Irregular, 10–25 μm | nr./84% | Conjugated linoleic acid | [141] |
| Rice protein | Propolis extract | 1:1% v/v | 0.4 m3/min, 120 °C, 72 °C | Spherical/Rough, nr. | 28%/90% | Propolis | [142] |
| Pea protein | Propolis extract | 1:1% v/v | 0.4 m3/min, 120 °C, 72 °C | Spherical/Rough, nr. | 41%/90% | Propolis | [142] |
| Soy protein | Propolis extract | 1:1% v/v | 0.4 m3/min, 120 °C, 72 °C | Spherical/Rough, nr. | 20%/70% | Propolis | [142] |
| Ovalbumin | Propolis extract | 1:1% v/v | 0.4 m3/min, 120 °C, 72 °C | Spherical/Rough, nr. | 53%/73% | Propolis | [142] |
| HP | Orange essential oil | 30% w/w | 3.75 mL/min, 180 °C, 85 °C | Spherical/Rough, 30–40 μm | 36%/89% | D-limonene | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. https://doi.org/10.3390/polym15122659
Díaz-Montes E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers. 2023; 15(12):2659. https://doi.org/10.3390/polym15122659
Chicago/Turabian StyleDíaz-Montes, Elsa. 2023. "Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review" Polymers 15, no. 12: 2659. https://doi.org/10.3390/polym15122659






