Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review
Abstract
:1. Introduction
1.1. Nanocelluloses (NCs)
1.2. CQDs
1.3. P-CQDs
1.4. Applications of CQDs
1.4.1. Industrial Field
1.4.2. Medicinal Field
Bacterial Field
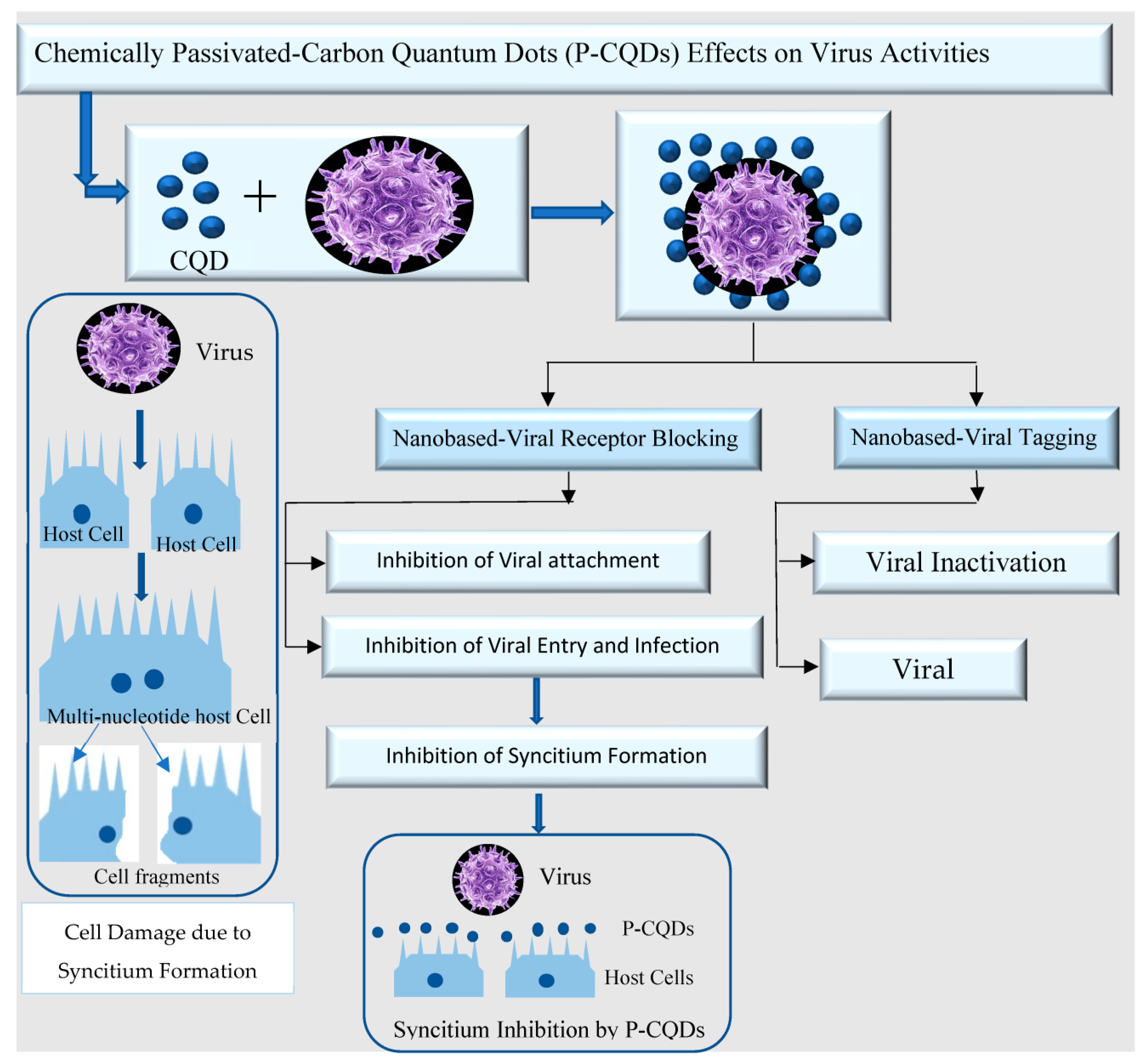
Viral Field
2. Material and Methods
2.1. Synthesis of CQDs
Synthesis of CQDs from Natural Resources
Synthesis of Microcrystalline Cellulose (MCC)
Synthesis of Nanocrystalline Cellulose (NCC)
- I.
- Ordinary Synthesis Methods
- II.
- Cryogenic Synthesis Methods
- III.
- Removal of NCCs’ sulfate groups
Converting MCC into NCC
2.2. Synthesis of CQDs from Synthetic Resources
2.3. Syntheses of P-CQDs
2.3.1. Characterization of Nanocelluloses
2.3.2. Characterization of CQDs
2.3.3. Evaluation of Viral Therapeutic Efficacy of P-CQDs
3. Results and Discussion
3.1. Viral Therapy of P-CQDs on NoVs
3.1.1. Absolute Efficacy
3.1.2. Comparative Efficacy for Other Carbon Nanomaterials (CNMs)
3.2. Bacterial Therapy Efficacy of P-CQDs
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Definition | Symbol | Definition |
| CNM | Carbon nanomaterials | NCC | Nanocrystalline cellulose |
| CNT | Carbon nanotubes | n−-CQD | Negative carbon quantum dot |
| COD | Carbon quantum dots | NMR | Nuclear magnetic resonance |
| DP | Degree of polymerization | NoVs | Noroviruses |
| DSC | Differential scanning calorimetry | NuPAGE LDS | Gel electrophoresis contains lithium dodecyl sulfate |
| DTA | Differential thermal analysis | Ox-MWCNTs | Oxidized multiwalled carbon nanotubes |
| ECL | Electroluminescence | Ox-SWCNTs | Oxidized single walled–carbon nanotubes (Ox-SWCNTs) |
| ECM | Extracellular matrix (ECM) | PBS | Phosphate-buffered saline |
| EDA | 2,2′-ethylenedioxy-bis-ethylamine | PBST | Phosphate buffered saline with tween 20 |
| ELISA | Enzyme-linked immunosorbent assay | P-CQD | Passivated-carbon quantum dot |
| EPA | 3-ethoxypropylamine | p+-CQDs | Positive carbon quantum dot |
| EPS | Extracellular polymeric substances (EPS) | PL | Photoluminescence |
| FGY | Fluorescence quantum yield | PS | Particle size |
| FTIR | Fourier transform infrared spectroscopy | QCE | Quantum confinement effect |
| GI.1, GII.4 | Types of VLPs | ROS | Reactive oxygen species (ROS |
| GelCode | Blue stain for protein | SCS | Surficial charge status |
| HBGA | Histo-blood group antigens | SDS-PAGE | Gel electrophoresis containing sodium dodecyl sulphate polyacrylamide |
| HRP | Horseradish peroxidase | SEM | Scanning electron microscope |
| KDa | Kilodaltons, a molecular weight unit | TEM | Transmission electron microscope |
| LCD | Liquid crystal displays | TG | Terminal group of the surface molecule |
| LED | Light-emitting diodes | TGA | Thermogravimetric analysis |
| MCC | Microcrystalline cellulose | TMB | 3,3′,5,5′-tetramethylbenzidine |
| MDR | Multidrug resistant | UV | Ultraviolet |
| MeOH | Methanol | VCN | Viable cell number |
| MIC | Minimum inhibitory concentration | VLP | Virus-like particles |
| MOPS SDS | Running buffer: 3-(N-morpholino) propanesulfonic acid | WB | Western blotting |
| MW | Molecular weight of the surface molecule | XRD | X-ray diffrection |
References
- Hindi, S.S.; Abohassan, R.A. Cellulosic microfibril and its embedding matrix within plant cell wall. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 2727–2734. [Google Scholar]
- Hindi, S.S. Microcrystalline cellulose: The inexhaustible treasure for pharmaceutical industry. Nanosci. Nanotechnol. Res. 2017, 4, 22–31. [Google Scholar]
- Hindi, S.S. Suitability of date palm leaflets for sulphated cellulose nanocrystals synthesis. Nanosci. Nanotechnol. Res. 2017, 4, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Hindi, S.S. Differentiation and synonyms standardization of amorphous and crystalline cellulosic products. Nanosci. Nanotechnol. Res. 2017, 4, 73–85. [Google Scholar]
- Hindi, S.S. Nanocrystalline cellulose: Synthesis from pruning waste of Zizyphus spina christi and characterization. Nanosci. Nanotechnol. Res. 2017, 4, 106–114. [Google Scholar]
- Hindi, S.S.; Raefat, A.; Abouhassan, R.A. Method for Making Nanoocrystalline Cellulose. U.S. Patent No. 10,144,786B2, 4 December 2018. [Google Scholar]
- Hindi, S.S. Nanocrystalline Cellulose. U.S. Patent No. 11,161,918, 2 November 2021. [Google Scholar]
- Hindi, S.S. Urchin-Shaped Nanocrystalline Material. U.S. Patent No. 11,242,410, 8 February 2022. [Google Scholar]
- Hindi, S.S. Sulfate-Grafted Nanocrystalline Cellulose. U.S. Patent No. 11,242,411, 8 February 2022. [Google Scholar]
- Hindi, S.S. A Method for Converting Micro- to Nanocrystalline Cellulose. U.S. Patent No. 10,808,045, 20 October 2020. [Google Scholar]
- Marpongahtun, M.; Gea, S.; Muis, Y.; Andriayani, A.; Novita, T.; Piliang, A.F. Synthesis of carbon nanodots from cellulose nanocrystals oil palm empty fruit by pyrolysis. J. Phys. Conf. Ser. 2018, 1120, 012071. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Johansson, L.-S.; Seppälä, J. Manipulation of cellulose nanocrystal surface sulfate groups toward biomimetic nanostructures in aqueous media. Carbohydr. Polym. 2015, 126, 23–31. [Google Scholar] [CrossRef]
- Dong, X.; Overton, C.M.; Tang, Y.; Darby, J.P.; Sun, Y.-P.; Yang, L. Visible light-activated carbon dots for inhibiting biofilm formation and inactivating biofilm-associated bacterial cells. Front. Bioeng. Biotechnol. 2021, 9, 786077. [Google Scholar] [CrossRef]
- Dong, S.; Dong, S.; Yuan, Z.; Zhang, L.; Lin, Y.; Lu, C. Rapid screening of oxygen-states in carbon quantum dots by chemiluminescence probe. Anal. Chem. 2017, 89, 12520–12526. [Google Scholar] [CrossRef]
- Dong, X.; Moyer, M.M.; Yang, F.; Sun, Y.P.; Yang, L. Carbon dots’ antiviral functions against noroviruses. Sci. Rep. 2017, 7, 519. [Google Scholar] [CrossRef]
- Mogharbel, A.T.; Abu-Melha, S.; Hameed, A.; Attar, R.M.S.; Alrefaei, A.F.; Almahri, A.; El-Metwaly, N. Anticancer and microbicide action of carbon quantum dots derived from microcrystalline cellulose: Hydrothermal versus infrared assisted techniques. Arab. J. Chem. 2023, 15, 104419. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, Y.; Li, Z.; Zhou, H.; Mu, Q.; Yan, B. Functionalized carbon nanotubes specifically bind to alpha-chymotrypsin’s catalytic site and regulate its enzymatic function. Nano Lett. 2009, 9, 2280–2284. [Google Scholar] [CrossRef] [Green Version]
- Nepal, D.; Geckeler, K.E. pH-sensitive dispersion and debundling of single-walled carbon nanotubes: Lysozyme as a tool. Small 2006, 2, 406–412. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Calvaresi, M.; Bottoni, A.; Zerbetto, F. Thermodynamics of binding between proteins and carbon nanoparticles: The case of C-60@Lysozyme. J. Phys. Chem. C 2015, 119, 28077–28082. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the Hiv-1 protease by fullerene Derivatives—Model-building studies and experimental-verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. Rsc. Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; LeCroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-light-activated bactericidal functions of carbon “quantum” dots. ACS Appl. Mater. Interfaces. 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [Green Version]
- LeCroy, G.E.; Yang, S.-T.; Yang, F.; Liu, Y.; Fernando, K.A.S.; Bunker, C.B.; Hu, Y.; Luo, P.G.; Sun, Y.-P. Functionalized carbon nanoparticles: Syntheses and applications in optical bioimaging and energy conversion. Coordin. Chem. Rev. 2016, 320, 66–81. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Sonkar, S.K.; Yang, F.; Veca, L.M.; Wang, P.; Tackett, K.N.; Yu, J.J.; Vasile, E.; Qian, H.; Liu, Y. Toward structurally defined carbon dots as ultracompact fluorescent probes. ACS Nano. 2014, 8, 4522–4529. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon dots as potent antimicrobial Agents. Theranostics 2020, 10, 671–680. [Google Scholar] [CrossRef]
- Jhonsi, M.A. Carbon Quantum Dots for Bioimaging: State of the Art in Nano-Bioimaging, State of the Art in Nano-Bioimaging; Ghamsari, M.S., Ed.; IntechOpen: London, UK, 2018; pp. 35–53. [Google Scholar]
- Yang, F.; LeCroy, G.E.; Wang, P.; Liang, W.; Chen, J.; Fernando, K.S. Functionalization of carbon nanoparticles and defunctionalization toward structural and mechanistic elucidation of carbon quantum dots. J. Phys. Chem. C 2016, 120, 25604–25611. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Shiral Fernando, K.A.; LeCroy, G.E.; Maimaiti, H.; Harruff-Miller, B.A.; Lewis, W.K.; Bunker, C.E.; Hou, Z.L.; Sun, Y.P. Enhanced fluorescence properties of carbon dots in polymer films. J. Mater. Chem. C 2016, 4, 6967–6974. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Wang, Z.; Wu, F.-G. Carbon dots for killing microorganisms: An update since 2019. J. Pharm. 2022, 15, 1236. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Mahat, N.A.; Shamsudin, S.A.; Jullok, N.; Ma’Radzi, A.H. Carbon quantum dots embedded polysulfone membranes for antibacterial performance in the process of forward osmosis. Desalination 2020, 493, 114618. [Google Scholar] [CrossRef]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoost, M. Novel antifouling and antibacterial polyethersulfone membrane prepared by embedding nitrogen-doped carbon dots for efficient salt and dye rejection. Mater. Sci. Eng. C 2020, 111, 110787. [Google Scholar] [CrossRef]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Tang, W.; Li, P.; Zhang, G.; Yang, X.; Yu, M.; Lu, H.; Xing, X. Antibacterial carbon dots derived from polyethylene glycol/polyethyleneimine with potent anti-friction performance as water-based lubrication additives. J. Appl. Polym. Sci. 2021, 138, e50620. [Google Scholar] [CrossRef]
- Geng, B.; Li, P.; Fang, F.; Shi, W.; Glowacki, J.; Pan, D.; Shen, L. Antibacterial and osteogenic carbon quantum dots for regeneration of bone defects infected with multidrug-resistant bacteria. Carbon 2021, 184, 375–385. [Google Scholar] [CrossRef]
- Mazumdar, A.; Haddad, Y.; Milosavljevic, V.; Michalkova, H.; Guran, R.; Bhowmick, S.; Moulick, A. Peptide-carbon quantum dots conjugate, derived from human retinoic acid receptor responder protein 2, against antibiotic-resistant Gram positive and Gram negative pathogenic bacteria. Nanomaterials 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Quan, T.; Yang, L.; Deng, L.; Kang, X.; Gao, M.; Xia, Z.; Li, X.; Gao, D. N,Cl-codoped carbon dots from Impatiens balsamina L. stems and a deep eutectic solvent and their applications for Gram-positive bacteria identification, antibacterial activity, cell imaging, and ClO− sensing. ACS Omega 2021, 6, 29022–29036. [Google Scholar] [CrossRef]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-derived carbon dots for toxic metal ion detection and as antibacterial and anticancer agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Wang, H.; Wang, B.; Huang, H.; Liu, Y.; Kang, Z. N-doped carbon dots derived from leaves with low toxicity via damaging cytomembrane for broad-spectrum antibacterial activity. Mater. Today Commun. 2020, 24, 101222. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Priya, S.S.; Geetha, P.; Rameshkumar, P.; Kannan, K.; Hegde, T.A.; Vinitha, G. Fluorescent carbon quantum dots from Ananas comosus waste peels: A promising material for NLO behaviour, antibacterial, and antioxidant activities. Inorg. Chem. Commun. 2021, 124, 108397. [Google Scholar] [CrossRef]
- Genc, M.T.; Yanalak, G.; Aksoy, I.; Aslan, E.; Patır, I.H. Green carbon dots (GCDs) for photocatalytic hydrogen evolution and antibacterial applications. ChemistrySelect 2021, 6, 7317–7322. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.T.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Eskalen, H.; Çeşme, M.; Kerli, S.; Özğan, Ş. Green synthesis of water-soluble fluorescent carbon dots from rosemary leaves: Applications in food storage capacity, fingerprint detection, and antibacterial activity. J. Chem. Res. 2021, 45, 428–435. [Google Scholar] [CrossRef]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible carbon quantum dots derived from sugarcane industrial wastes for effective nonlinear optical behaviour and antimicrobial activity applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef]
- Qing, W.; Chen, K.; Yang, Y.; Wang, Y.; Liu, X. Cu2+-doped carbon dots as fluorescence probe for specific recognition of Cr (VI) and its antimicrobial activity. Microchem. J. 2020, 152, 104262. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ren, S.; Hu, Z.; Wang, Y. One-pot synthesis of Forsythia@carbon quantum dots with natural anti-wood rot fungus activity. Mater. Des. 2021, 206, 109800. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Ma, Y.; Wang, B.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Selective inactivation of Gram-negative bacteria by carbon dots derived from natural biomass: Artemisia argyi leaves. J. Mater. Chem. B 2020, 8, 2666–2672. [Google Scholar] [CrossRef]
- Li, C.; Lin, F.; Sun, W.; Wu, F.G.; Yang, H.; Lv, R.; Zhu, Y.X.; Jia, H.R.; Wang, C.; Gao, G.; et al. Self-assembled rose bengalexo polysaccharide nanoparticles for improved photodynamic inactivation of bacteria by enhancing singlet oxygen generation directly in the solution. ACS Appl. Mater. Interfaces 2018, 10, 16715–16722. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.W.; Wu, F.G. Improving the phototherapeutic efficiencies of molecular and nanoscale materials by targeting mitochondria. Molecules 2018, 23, 3016. [Google Scholar] [CrossRef] [Green Version]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial effects of carbon quantum dots@hematite nanostructures deposited on titanium against Gram-positive and Gram-negative bacteria. J. Potoch. Photobio. A 2019, 379, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cell Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [Green Version]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. The Extracellular Bastions of Bacteria—A Biofilm Way of Life. Nat. Educ. Knowl. 2013, 44, 22. [Google Scholar]
- Donlan, R.M. Biofilm Formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Abbas, K.; Yang, Y.; Li, Z.; Tedesco, A.C.; Bi, H. Photodynamic anti-bacteria by carbon dots and their nano-composites. J. Pharm. 2022, 15, 487. [Google Scholar] [CrossRef]
- Joo, K.I.; Lei, Y.; Lee, C.L.; Lo, J.; Xie, J.; Hamm-Alvarez, S.F.; Wang, P. Site-specific labelling of enveloped viruses with quantum dots for single virus tracking. ACS Nano 2008, 2, 1553–1562. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.P.; Ando, T.; Fankhauser, R.L.; Beard, R.S.; Glass, R.I.; Monroe, S.S. Norovirus classification and proposed strain nomenclature. Virol. J. 2006, 346, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Bok, K.; Parra, G.I.; Mitra, T.; Abente, E.; Shaver, C.K.; Boon, D.; Engle, R.; Yu, C.; Kapikian, A.Z.; Sosnovtsev, S.V.; et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc. Natl. Acad. Sci. USA 2011, 108, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.M.; Hall, A.J.; Vinje, J.; Parashar, U.D. Noroviruses: A comprehensive review. J. Clin. Virol. 2019, 44, 1–8. [Google Scholar] [CrossRef]
- Bozkurt, H.; D’Souza, D.H.; Davidson, P.M. Thermal inactivation of human norovirus surrogates in spinach and measurement of its uncertainty. J. Food Prot. 2014, 77, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Vimont, A.; Fliss, I.; Jean, J. Efficacy and mechanisms of murine norovirus inhibition by pulsed-light technology. Appl. Environ. Microbiol. 2015, 81, 2950–2957. [Google Scholar] [CrossRef] [Green Version]
- Todd, K.V.; Tripp, R.A. Human norovirus: Experimental models of infection. Viruses 2019, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Yuen, Y.; Hsiao, H.M.; Jaykus, L.A.; Moe, C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl. Environ. Microbiol. 2010, 76, 394–399. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral activity of gold/copper sulphide core/shell nanoparticles against Human norovirus virus-like particles. PLoS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef]
- Gerrity, D.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J. Environ. Sci. Health A 2008, 43, 1261–1270. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colourful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Vega, E.; Barclay, L.; Gregoricus, N.; Shirley, S.H.; Lee, D.; Vinjé, J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States. 2009 to 2013. J. Clin. Microbiol. 2014, 52, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Reeck, A.; Kavanagh, O.; Estes, M.K.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; Atmar, R.L. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 2010, 202, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Farkas, T.; Marionneau, S.; Zhong, W.; Ruvoën-Clouet, N.; Morrow, A.L.; Altaye, M.; Pickering, L.K.; Newburg, D.S.; LePendu, J.; et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: Identification of 4 distinct strain specific patterns. Int. J. Infect. Dis. 2003, 188, 19–31. [Google Scholar] [CrossRef]
- Huang, P.; Farkas, T.; Zhong, W.; Tan, M.; Thornton, S.; Morrow, A.L.; Jiang, X. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. Virol. J. 2005, 79, 6714–6722. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Tan, M.; Chhabra, M.; Dai, Y.C.; Meller, J.; Jiang, X. Inhibition of histo-blood group antigen binding as a novel strategy to block norovirus infections. PLoS ONE 2013, 8, e69379. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Leuthold, M.M.; Hansman, G.S. Human noroviruses’ fondness for histo-blood group antigens. Virol. J. 2015, 89, 2024–2040. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Lu, Y.; Liu, C.; Yang, W. Carbon quantum dots displaying dual wavelength photoluminescence and electrochemiluminescence prepared by high-energy ball milling. Carbon 2015, 94, 472–478. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Wu, M.; Yue, W.; Wu, W.; Hu, C.; Wang, X.; Zheng, J.-T.; Li, Z.; Jiang, B.; Qiu., J. Preparation of functionalized water-soluble photoluminescent carbon quantum dots from petroleum coke. Carbon 2014, 78, 480–489. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Xiao, A.; Lu, H.; Liu, Z.; Chen, J.-Q. Determination of metronidazole by a flow injection chemiluminescence method using ZnO-doped carbon quantum dots. New Carbon Mater. 2014, 29, 216–224. [Google Scholar] [CrossRef]
- Li, M.; Hu, C.; Yu, C.; Wang, S.; Zhang, P.; Qiu, J. Organic amine-grafted carbon quantum dots with tailored surface and enhanced photoluminescence properties. Carbon 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, L.; Liu, Y.; Meng, X.; Xu, H.; Xu, Y.Q.; Liu, B.; Fang, X.; Li, H.; Ding, T. Supramolecular interactions via hydrogen bonding contributing to citric-acid derived carbon dots with high quantum yield and sensitive photoluminescence. RSC Adv. 2017, 7, 20345–20353. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Long, Y.; Zhang, L.; Gao, M.; Bai, W. Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon 2011, 49, 3134–3140. [Google Scholar] [CrossRef]
- Wang, J.; Xin, X.; Lin, Z. Cu2ZnSnS4 nanocrystals and graphene quantum dots for photovoltaics. Nanoscale 2011, 3, 3040–3048. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Yu, H.; Kang, Z.; Lee, S.T. Synthesis of fluorescent carbon nanoparticles directly from active carbon via a one-step ultrasonic treatment. Mater. Res. Bull. 2011, 46, 147–151. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, F.; Lagally, M.G.; Denes, F.S. New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir 2009, 26, 1991–1995. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Georgakilas, V.; Giannelis, E.P. Photoluminescent carbogenic dots. Chem. Mater. 2008, 20, 4539–4541. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Liu, S.; Koynov, K.; Knoll, W.; Li, Q. An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers. Angew. Chem. 2009, 121, 4668–4671. [Google Scholar] [CrossRef]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar] [CrossRef]
- Hamilton, I.P.; Li, B.; Yan, X.; Li, L.S. Alignment of colloidal graphene quantum dots on polar surfaces. Nano Lett. 2011, 11, 1524–1529. [Google Scholar] [CrossRef]
- Mueller, M.L.; Yan, X.; Dragnea, B.; Li, L.S. Slow hot-carrier relaxation in colloidal graphene quantum dots. Nano Lett. 2011, 11, 56–60. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef]
- Lu, J.; Yeo, P.S.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Colón, L.A. Hidden properties of carbon dots revealed after HPLC fractionation. J. Phys. Chem. Lett. 2012, 4, 239–243. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef]
- Zheng, X.T.; Than, A.; Ananthanaraya, A.; Kim, D.H.; Chen, P. Graphene quantum dots as universal fluorophores and their use in revealing regulated trafficking of insulin receptors in adipocytes. ACS Nano 2013, 7, 6278–6286. [Google Scholar] [CrossRef]
- Shan, F.; Fu, L.; Chen, X.; Xie, X.; Liao, C.; Zhu, Y.; Xia, H.; Zhang, J.; Yan, L.; Wang, Z.; et al. Waste-to-wealth: Functional biomass carbon dots based on bee pollen waste and application. Chin. Chem. Lett. 2022, 33, 2942–2948. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef] [Green Version]
- Vandarkuzhali, S.A.A.; Jeyalakshmi, V.; Sivaraman, G.; Singaravadivel, S.; Krishnamurthy, K.R.; Viswanathan, B. Highly fluorescent carbon dots from pseudo-stem of banana plant: Applications as nanosensor and bio-imaging agents. Sens. Actuators B Chem. 2017, 252, 894–900. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasaa, S.K. Synthesis of fluorescent carbon dots using Daucus carota subsp. Sativus roots for mitomycin drug delivery. Optik 2018, 158, 893–900. [Google Scholar]
- Amin, N.; Afkhami, A.; Hosseinzadeh, L.; Madrakian, T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta 2018, 1030, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kasibabu, B.S.B.; D’souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from carica papaya juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater. Sci. Eng. C 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, Y.; Li, M.; Xing, M.; Wu, Q. Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng. C 2017, 79, 473–480. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Li, X.; Liu, R.; Lin, L.; Zhao, S. Green preparation of S and N Co-doped carbon dots from water chestnut and onion as well as their use as an off-on fluorescent probe for the quantification and imaging of coenzyme A. ACS. Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators. B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Yang, M.; Meng, X.; Li, B.; Ge, S.; Lu, Y. N, S co-doped carbon dots with high quantum yield: Tunable fluorescence in liquid/solid and extensible applications. J. Nanopart. Res. 2017, 19, 217. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, Y.; Zhao, J.; Miao, M.; Cao, S.; Fang, J.; Shi, L. Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging. RSC Adv. 2015, 5, 31250–31254. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.J.; Zhou, D.L.; Bao, N.; Xu, Y.; Wang, A.J.; Feng, J.J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC. Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C 2013, 33, 2914–2917. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Sethuraman, M.G.; Lee, Y.R. Efficient synthesis of highly fluorescent nitrogen-doped carbon dots for cell imaging using unripe fruit extract of Prunus mume. Appl. Surf. Sci. 2016, 384, 432–441. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.-W.; Meng, X.; Lee, C.-S.; Wang, P.; Zhang, W. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, R.B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Tran, T.S.; Tung, T.T.; Losic, D.; Kim, T. Water soluble fluorescent carbon nanodots from biosource for cells imaging. J. Nanomater. 2017, 2017, 7029731. [Google Scholar] [CrossRef] [Green Version]
- Hoan, B.T.; Tam, P.D.; Pham, V.H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotechnol. 2019, 2019, 2852816. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Banerjee, S.L.; Khamrai, M.; Samanta, S.; Singh, S.; Kundu, P.P.; Anup, K.; Ghosh, A.K. Hydrothermal synthesis of gelatine quantum dots for high-performance biological imaging applications. Photochem. Photobiol. B 2020, 212, 112014. [Google Scholar] [CrossRef]
- Yu, J.; Song, N.; Zhang, Y.-K.; Zhong, S.-X.; Wang, A.-J.; Chen, J. Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens. Actuators B Chem. 2015, 214, 29–35. [Google Scholar] [CrossRef]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef] [Green Version]
- Balajia, M.; Jegatheeswarana, S.; Nithyaa, P.; Boomib, P.; Selvamc, S.; Sundrarajana, M. Photoluminescent reduced graphene oxide quantum dots from latex of Calotropis gigantea for metal sensing, radical scavenging, cytotoxicity, and bioimaging in Artemia salina: A greener route. J. Photochem. Photobiol. 2018, 178, 371–379. [Google Scholar]
- Gu, D.; Shang, S.; Yu, Q.; Shen, J. Green synthesis of nitrogen-doped carbon dots from lotus root for Hg (II) ions detection and cell imaging. Appl. Surf. Sci. 2016, 390, 38–42. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots from Mangifera indica: Application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Jesny, S.; Jose, A.R.; Kumar, K.G. Fluorescence determination of glutathione using tissue paper-derived carbon dots as fluorophores. Anal. Sci. 2017, 33, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Kim, C.H.; Lee, Y.C.; Huh, Y.S.; Lee, J. Photoluminescent green carbon nanodots from food-waste-derived sources: Large-scale synthesis, properties, and biomedical applications. ACS. Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosen. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef]
- Singh, A.; Eftekhari, E.; Scott, J.; Kaur, J.; Yambem, S.; Leusch, F.; Wellings, R.; Gould, T.; Ostrikov, K.; Sonar, P.; et al. Carbon dots derived from human hair for ppb level chloroform sensing in water. Sustain. Mater. Technol. 2020, 25, e00159. [Google Scholar] [CrossRef]
- Janus, L.; Piątkowski, M.; Radwan-Praglowska, J.; Bogdal, D.; Matysek, D. Chitosan-based carbon quantum dots for biomedical applications: Synthesis and characterization. Nanomaterials 2019, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Koshizawa, T. Degradation of wood cellulose and cotton linters in phosphoric acid. Japan TAPPI J. 1960, 14, 455–458. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Sucaldito, M.R.; Camacho, D.H. Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. Carbohydr. Polym. 2017, 169, 315–323. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Yue, X.; Dai, L.; Ni, Y. Isolation and characterization of microcrystalline cellulose from bamboo pulp through extremely low acid hydrolysis. J. Wood Chem. Technol. 2019, 39, 242–254. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Kupiainen, L.; Ahola, J.; Tanskanen, J. Kinetics of formic acid-catalyzed cellulose hydrolysis. BioResources 2014, 9, 2645–2658. [Google Scholar] [CrossRef]
- Zambrano, L.-F.; Villasana, Y.; Bejarano, M.L.; Luciani, C.; Niebieskikwiat, D.; Cueva, D.F.; Aguilera, D.; Orejuela, L.M. Optimization of Microcrystalline Cellulose Isolation from Cocoa Pod Husk via Mild Oxalic Acid Hydrolysis: A Response Surface Methodology Approach. Available online: https://ssrn.com/abstract=4307097 (accessed on 17 March 2023).
- Nurhadi, B.; Angeline, A.; Sukri, N.; Masruchin, N.; Arifin, H.R.; Saputra, R.A. Characteristics of microcrystalline cellulose from nata de coco: Hydrochloric acid versus maleic acid hydrolysis. J. Appl. Polym. Sci. 2021, 139, 51576. [Google Scholar] [CrossRef]
- Trusovs, S. Microcrystalline Cellulose. U.S. Patent 6392034 B1, 21 May 2002. [Google Scholar]
- Nguyen, X.T. Process for Preparing Microcrystalline Cellulose. U.S. Patent 7005514 B2, 28 February 2006. [Google Scholar]
- Kassaye, S.; Pant, K.K.; Sapna, J. Hydrolysis of cellulosic bamboo biomass into reducing sugars via a combined alkaline solution and ionic liquid pretreament steps. Renew. Energy 2017, 104, 177–184. [Google Scholar] [CrossRef]
- DeLong, E.A. Method of Producing Level off DP Microcrystalline Cellulose and Glucose from Lignocellulosic Material. U.S. Patent 4,645,541, 19 July 1989. [Google Scholar]
- Prosvirnikov, D.B.; Safin, R.G.; Zakirov, S.R. Microcrystalline cellulose based on cellulose containing raw material modified by steam explosion treatment. In Solid State Phenomena; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2018; Volume 284. [Google Scholar]
- Prosvirnikov, D.B.; Timerbaev, N.F.; Safin., R.G. Microcrystalline cellulose from lignocellulosic material activated by steam explosion treatment and mathematical modeling of the processes accompanying its preparation. Mater. Sci. Forum 2019, 945, 911–918. [Google Scholar]
- Ha, E.Y.; Landi, C.D. Method for Producing Microcrystalline Cellulose. U.S. Patent 5,769,934, 23 June 1998. [Google Scholar]
- Hanna, M.; Biby, G.; Miladinov, V. Production of Microcrystalline Cellulose by Reactive Extrusion. U.S. Patent 6,228,213, 8 May 2001. [Google Scholar]
- Merci, A.; Urbano, M.V.E.; Grossmann, C.A.; Tischer, S.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Stupińska, H.; Iller, E.; Zimek, Z.; Wawro, D.; Ciechańska, D.; Kopania, E.; Palenik, J.; Milczarek, S.; Steplewski, W.; Krzyzanowska, G. An environment-friendly method to prepare microcrystalline cellulose. Fibres Text. East. Eur. 2007, 5–6, 167–172. [Google Scholar]
- Pranger, L.; Rina, T. Biobased nanocomposites prepared by in situ polymerization of furfuryl alcohol with cellulose whiskers or montmorillonite clay. Macromolecules 2008, 41, 8682–8687. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Usuda, M. Hydrolysis of cellulose in concentrated phosphoric acid: Effect of functional groups on the rate of hydrolysis. Kogyo Kagaku Zasshi. 1967, 70, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Li, B.; Xu, W.; Kronlund, D.; Määttänen, A.; Liu, J.; Smått, J.H.; Peltonen, J.; Willför, S.; Mu, X.; Xu, C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr. Polym. 2015, 133, 605–612. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.M.E. Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhu, J.Y.; Carlos, B.; Kitin, P.B.; Elder, T.J. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Biores. Technol. 2009, 100, 2259–2264. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009, 11, 1808–1814. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of carboxylated cellulose nanocrystals resulting from TEMPO-mediated oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly (ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chemist. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Kaushik, A.; Mandeep, S.; Gaurav, V. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010, 82, 337–345. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Wang, H.; Zeng., G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 511, 296–306. [Google Scholar] [CrossRef]
- Chen, D.; Zhuang, X.; Zhai, J.; Zheng, Y.; Lu, H.; Chen, L. Preparation of highly sensitive Pt nanoparticles-carbon quantum dots/ionic liquid functionalized graphene oxide nanocomposites and application for H2O2 detection. Sens. Actuators B 2018, 255, 1500–1506. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. 2013, 125, 7954–7958. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Luo, H.; Gao, Y. One-step preparation of nitrogen-doped and surface-passivated carbon quantum dots with high quantum yield and excellent optical properties. RSC Adv. 2014, 4, 7648. [Google Scholar] [CrossRef]
- Yu, C.; Xuan, T.; Chen, Y.; Zhao, Z.; Liu, X.; Lian, G.; Li, H. Gadolinium-doped carbon dots with high quantum yield as an effective fluorescence and magnetic resonance bimodal imaging probe. J. Alloy. Compd. 2016, 688, 611–619. [Google Scholar] [CrossRef]
- Niu, W.-J.; Li, Y.; Zhu, R.-H.; Shan, D.; Fan, Y.-R.; Zhang, X.-J. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuators B 2015, 218, 229–236. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolour patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Gao, Z.; Lin, Z.Z.; Chen, X.; Lai, Z.; Huang, Z. Carbon dots-based fluorescent probe for trace Hg2+ detection in water sample. Sens. Actuators B 2016, 222, 965–971. [Google Scholar] [CrossRef]
- Su, H. Facile synthesis of N–rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen., J. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem. Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Li, M.; Yu, C.; Hu, C.; Yang, W.; Zhao, C.; Wang, S.; Zhang, M.; Zhao, J.; Wang, X.; Qiu, J. Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield. Chem. Eng. J. 2017, 320, 570–575. [Google Scholar] [CrossRef]
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Xu, S.; Wan, J.; Wu, P. Facile synthesis of N-rich carbon quantum dots by spontaneous polymerization and incision of solvents as efficient bioimaging probes and advanced electrocatalysts for oxygen reduction reaction. Nanoscale 2016, 8, 2219–2226. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.-W.; Chi, Y.; Lin, X.; Chen, G. Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Niu, J.; Gao, H. Synthesis and drug detection performance of nitrogen-doped carbon dots. J. Lumin. 2014, 149, 159–162. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.; Wang, Y. High quantum yield green-emitting carbon dots for Fe (III) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Cai, J.; Fang, Q.; You, X.; Chi, Y. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots: A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhan, Q.; Dai, L.; Sowards, L.; Pender, M.; Naik, R.R. Direct measurements of interactions between polypeptides and carbon nanotubes. J. Phys. Chem. B 2006, 110, 12621–12625. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.P.; Nafisi, P.M.; Gardner, A.; Mackay, J.L.; Kundu, K.; Kumar, S.; Murthy, N.A. A fluorescent peroxidase probe increases the sensitivity of commercial ELISAs by two orders of magnitude. Chem. Commun. 2013, 49, 10379–10381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Rabe, D.I.; Al-Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 23, 2655–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Ge, L.; Abu Rabe, D.I.; Mohammed, O.O.; Wang, P.; Tang, Y.; Kathariou, S.; Yang, L.; Sun, Y.-P. Photoexcited state properties and antibacterial activities of carbon dots relevant to mechanistic features and implications. Carbon 2020, 170, 137–145. [Google Scholar] [CrossRef]
- Hutson, A.M.; Atmar, R.L.; Marcus, D.M.; Estes, M.K. Norwalk virus–like particle hemagglutination by binding to h histo–blood group antigens. Virol. J. 2003, 77, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Norovirus and its histo-blood group antigen receptors: An answer to a historical puzzle. Trends Microbi. 2005, 13, 285–293. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Norovirus-host interaction: Implications for disease control and prevention. Expert. Rev. Mol. Med. 2007, 9, 1–22. [Google Scholar] [CrossRef]
- Harrington, P.R.; Lindesmith, L.; Yount, B.; Moe, C.L.; Baric, R.S. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. Virol. J. 2002, 76, 12335–12343. [Google Scholar] [CrossRef] [Green Version]
- LoBue, A.D.; Lindesmith, L.; Yount, B.; Harrington, P.R.; Thompson, J.M.; Johnston, R.E.; Moe, C.L.; Baric, R.S. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 2006, 24, 5220–5234. [Google Scholar] [CrossRef]
- Hale, A.; Mattick, K.; Lewis, D.; Estes, M.; Jiang, X.; Green, J.; Eglin, R.; Brown, D. Distinct epidemiological patterns of Norwalk-like virus infection. J. Med. Virol. 2000, 62, 99–103. [Google Scholar] [CrossRef]
- Hardy, M.E.; Tanaka, T.N.; Kitamoto, N.; White, L.J.; Ball, J.M.; Jiang, X.; Estes, M.K. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virol. J. 1996, 217, 252–261. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, D.H.; Su, X.W.; Roach, A.; Harte, F. High-pressure homogenization for the inactivation of human enteric virus surrogates. J. Food Prot. 2009, 72, 2418–2422. [Google Scholar] [CrossRef]
- Prasad, B.V.; Hardy, M.E.; Dokland, T.; Bella, J.; Rossmann, M.G.; Estes, M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science 1999, 286, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tan, M.; Xia, M.; Hao, N.; Zhang, X.C.; Huang, P.; Jiang, X.; Li, X.; Rao, Z. Crystallography of a Lewis-binding Norovirus, elucidation of strain-specificity to the polymorphic human histoblood group antigens. PLoS Pathog. 2011, 7, e1002152. [Google Scholar] [CrossRef]
- Tan, M.; Xia, M.; Chen, Y.; Bu, W.; Hegde, R.S.; Meller, J.; Li, X.; Jiang, X. Conservation of carbohydrate binding interfaces—Evidence of human HBGA selection in Norovirus evolution. PLoS ONE 2009, 4, e5058. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef]
- Molaei, J.M. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460. [Google Scholar] [CrossRef]







| Property of the CQDs | 2 HBGA’s Type | Concentration µg.mL−1 | VLS Strains | P-CQDs | |
|---|---|---|---|---|---|
| EDA | EPA | ||||
| Inhibitory rate of CQDs on NoVs, % | 8 | 90 | 8 | ||
| 16 | 92 | 24 | |||
| 32 | 88 | 26 | |||
| Inhibition of HBGA binding, % | A | 2 | GI.1 | 93.6 | 53.3 |
| GII.2 | 88 | 61.2 | |||
| 5 | GI.1 | 100 | 93 | ||
| GII.2 | 100 | 100 | |||
| B | 2 | GI.1 | 74.5 | 36.4 | |
| GII.2 | 78.2 | 38.2 | |||
| 5 | GI.1 | 99.1 | 75.5 | ||
| GII.2 | 100 | 81.8 | |||
| O | 2 | GI.1 | 79.1 | 54.4 | |
| GII.2 | 59.5 | 38.9 | |||
| 5 | GI.1 | 100 | 77.2 | ||
| GII.2 | 100 | 79.3 | |||
| Property of the CQDs | 1 AT Hour | 2 Conc. µg.mL−1 | P-CQDs | CQDs | Reference | |||
|---|---|---|---|---|---|---|---|---|
| EDA | EPA | 3 HT | 4 IR | |||||
| Particle size, nm | 4–5 | 4–5 | [200] | |||||
| Molecular weight | 148 | 103 | ||||||
| Surficial terminal group | -NH2 | -CH3 | ||||||
| Fluorescence quantum yield, % | ~20 | ~20 | ||||||
| Viable cell number of bacteria, CFU/mL | 0 | 11 × 106 | 11 × 106 | |||||
| 0.1 | 9 × 103 | 5 × 106 | ||||||
| 0.2 | 0.4 × 103 | 1.5 × 106 | ||||||
| Inhibitory effect OF ADE-CQDs on a bacterial biofilm formation, % | 10 | 95.86 | [13] | |||||
| 1 | 20 | 100 | ||||||
| 30 | 100 | |||||||
| 2 | 10 | 72.2 | ||||||
| 20 | 96 | |||||||
| 30 | 100 | |||||||
| 3 | 10 | 34.25 | ||||||
| 20 | 41 | |||||||
| 30 | 50 | |||||||
| Minimum inhibitory concentration, µg/mL | 5 Gram+-bacterium | 250 | 350 | [16] | ||||
| 6 Gram−-bacterium | 100 | 300 | ||||||
| 7 Unicellular fungi | 350 | 400 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindi, S.S.; Sabir, J.S.M.; Dawoud, U.M.; Ismail, I.M.; Asiry, K.A.; Mirdad, Z.M.; Abo-Elyousr, K.A.; Shiboob, M.H.; Gabal, M.A.; Albureikan, M.O.I.; et al. Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review. Polymers 2023, 15, 2660. https://doi.org/10.3390/polym15122660
Hindi SS, Sabir JSM, Dawoud UM, Ismail IM, Asiry KA, Mirdad ZM, Abo-Elyousr KA, Shiboob MH, Gabal MA, Albureikan MOI, et al. Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review. Polymers. 2023; 15(12):2660. https://doi.org/10.3390/polym15122660
Chicago/Turabian StyleHindi, Sherif S., Jamal S. M. Sabir, Uthman M. Dawoud, Iqbal M. Ismail, Khalid A. Asiry, Zohair M. Mirdad, Kamal A. Abo-Elyousr, Mohamed H. Shiboob, Mohamed A. Gabal, Mona Othman I. Albureikan, and et al. 2023. "Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review" Polymers 15, no. 12: 2660. https://doi.org/10.3390/polym15122660
APA StyleHindi, S. S., Sabir, J. S. M., Dawoud, U. M., Ismail, I. M., Asiry, K. A., Mirdad, Z. M., Abo-Elyousr, K. A., Shiboob, M. H., Gabal, M. A., Albureikan, M. O. I., Alanazi, R. A., & Ibrahim, O. H. M. (2023). Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review. Polymers, 15(12), 2660. https://doi.org/10.3390/polym15122660








