Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid
Abstract
:1. Introduction
2. Bioelectricity and Electrical Stimulation
2.1. Bioelectricity in Living Organisms
2.2. The Role of Membrane Potential on Bioelectricity
2.3. Direct Effect of Electrical Stimulation on the Cells
Biochemical Pathways Involved in Signal Transduction of Electrical Stimulation
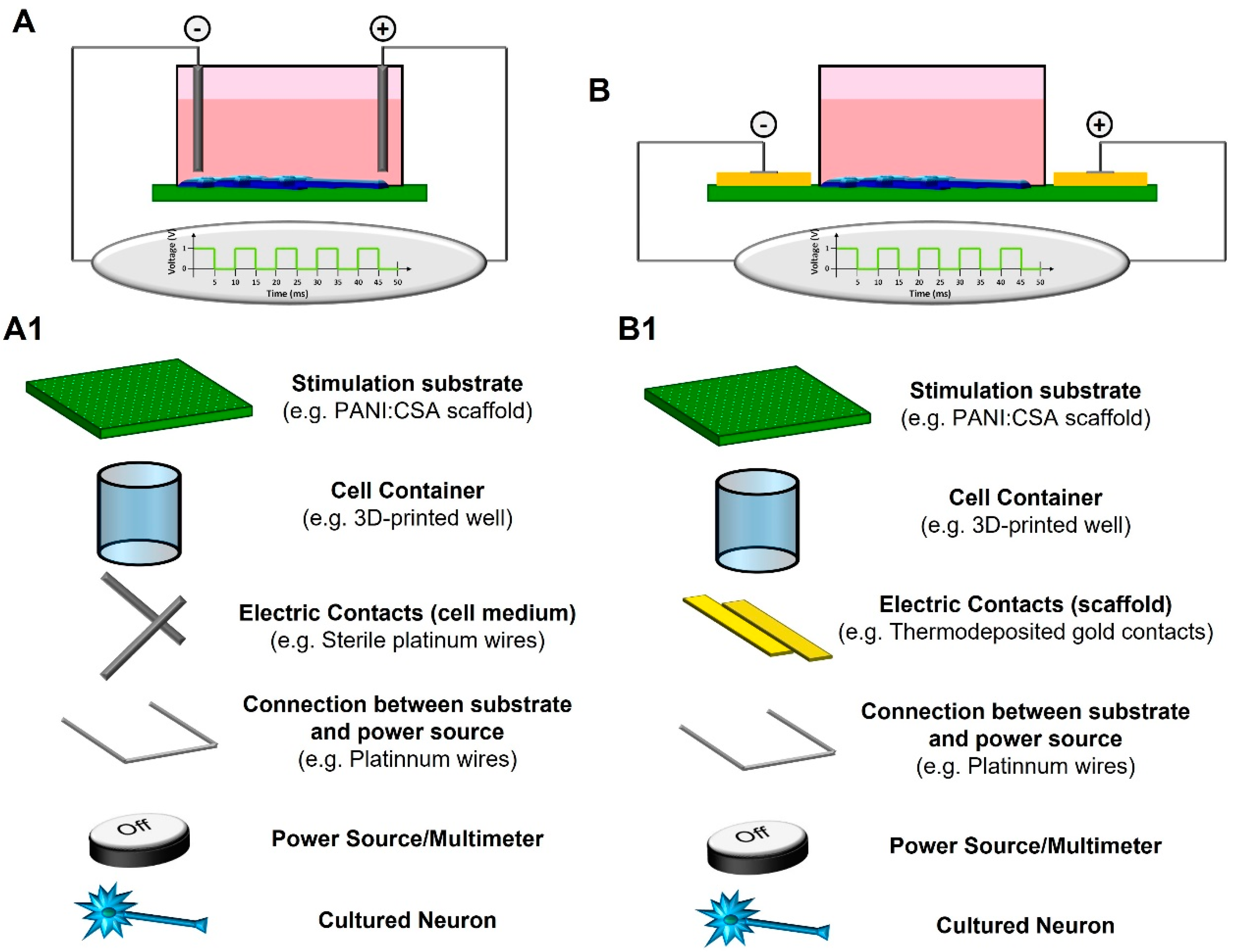
2.4. Methods for Electrical Stimulation—The Importance of Support Materials
| Type | Cells Used | Stimulated Substrate | Types of Electrodes | Electrolyte Solution Used | Stimulation | Power Source Used | Signal Frequency (Hz) | Duration | Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Direct substrate stimulation | mNSCs | Cross-linked poly(acrylonitrile) (PAN) electrospun fibers | Platinum + printed circuit board | (not used) | 100 µA (asymmetric biphasic) | asymmetric biphasic programmable electrical device and a printed circuit board | 100 | 24 h, 1 day after seeding. Cells were allowed to differentiate for more 7 days. | When electrical stimulation was performed, it was observed at the end of the experiment: - Increased cell number; - Increased neurite length; - Increased Tubb3 (qPCR) and Map2/MAP2 (qPCR and IF) and decreased expression of GFAP (IF). | Zhu and colleagues, 2017 [33] |
| Direct substrate stimulation | hNSC | Nanopatterned Titanium coated PUA | Copper wire + PDMS | (not used) | DC (pulsed), maximum 3 µA and 25 V, | programmable digital power supply MK3003P | 1 | 30 min, 2 times a day, 5 days | Enhancement of hNSC differentiation, independently of the type of substrate used; Increased expression of TUJ1 and MAP2 on differentiated neurons | Yang and colleagues, 2017 [42] |
| Direct substrate stimulation | NSCs | PCL-PANI_Gelatin electrospun fibers | 1 platinum and 1 silver electrodes | (not used) | DC (1 V cm−1) | (not referred) | (not referred) | 15, 30 and 60 min. | Increased cell number 1, 3 and 5 days after stimulation. Longer neurite extent (30 µm vs. 22 µm) | Ghasemi-Mobarakeh and colleagues, 2009 [47] |
| Direct substrate stimulation | hNSC (ReN-VM), p3-5 | PANI coated PVV hydrogel | PANI-coated Indium Tin Oxide | (not used) | AC—Charged-Balanced biphasic (15, 35 and 75 mV) | Agilent B2912A precision source/measure unit | 200 | Every 6 h for 1,3,5 and 7 days | Enhanced cell proliferation, that decreased for higher voltage values. Enhanced neurite extension with increasing voltage values. Enhanced neural gene expression with electrical stimulation | Xu and colleagues, 2016 [48] |
| Dual system of conductive stimulating layer and inert cell support | iNPCs | Alginate hydrogel with cells encapsulated on top of (2D) or surrounded by a PPY film (3D). | Silver wire + silver paste | (not used) | AC, 0.4 V cm−1 | (See Oh 2018) | 100 Hz | 1 h of stimulation + 24 h of resting period | Electrical stimulation enhanced the mRNA expression of HBEGF, HSPB1 and the neurotrophins BDNF, GDNF and NT3. 3D structure + electrical stimulation boosted mRNA expression of BDNF and GDNF | Song and colleagues, 2019 [49] |
| Direct substrate stimulation | mNSCs | PPY-coated PAN (no cross-linking) electrospun fibers | Stainless Steel | (not used) | 100 mV cm−1, | AFG3022C, Tektronix, USA | 100 | 4 h of stimulation, total of 7 days. | - Enhanced cell maturation through increased Tau protein expression; - Prevention of neurons growing and differentiating into clusters - Enhanced proliferation of glial cells | Xu and colleagues, 2018 [69] |
| Direct substrate stimulation | PC12 | PLCA-SF-PANI electrospun fibers, monoaxial and hollow co-axial | Similar to Ghasemi-Mobarakeh L et al. 2009 | (not used) | (100 mV cm−1) | (not referred) | (not referred) | 1 h per day, 5 days in total. | Increased neurite-positive cells and respective length. | Zhang and colleagues, 2014 [70] |
| Direct substrate stimulation | PC12 | indium doped tin oxide (ITO) needle coated with PANI | “Wires” | (not used) | AC (100 µA) | TBSI Neural Stimulator ()V1.0.8, Triangle BioSystems, Durham, NC, USA | 1Hz (1 s repeat interval) | 1, 2 and 4 h + 24 h of resting | Cell density higher in the following order: 4 h = 2 h > 1 h > 0 h Increased neurite length with increased duration; Increased protein adsorption with electrical stimulation | Wang and colleagues, 2015 [71] |
| Stimulation of the culture media | Dissociated neurons from Xenopus laevis | Culture media (20% Liebowitz L15 culture medium, 2% penicillin/streptomycin, 1% fetal bovine serum) made in Steinberg’s solution | Ag/AgCl electrodes, indirectly connected to culture through agar bridges. | Steinberg solution for the electrode solution and to prepare the culture media. | DC—50–133 mV mm−1 (low field strength) and 143–200 mV mm−1 (high field strength) | (not referred) | (not referred) | 2–4 h after seeding + 5 h of stimulation | Electrical field induced neurite orientation to the cathode; Addition of CS-6S rich GAGs to culture media enhanced neurite turning, whereas CS-4S rich ones inhibited; | Erskine and colleagues, 1997 [72] |
The Importance of Electroconductive Materials
3. PANI:CSA, a Versatile Electroconductive System Suitable for Neural Tissue Engineering
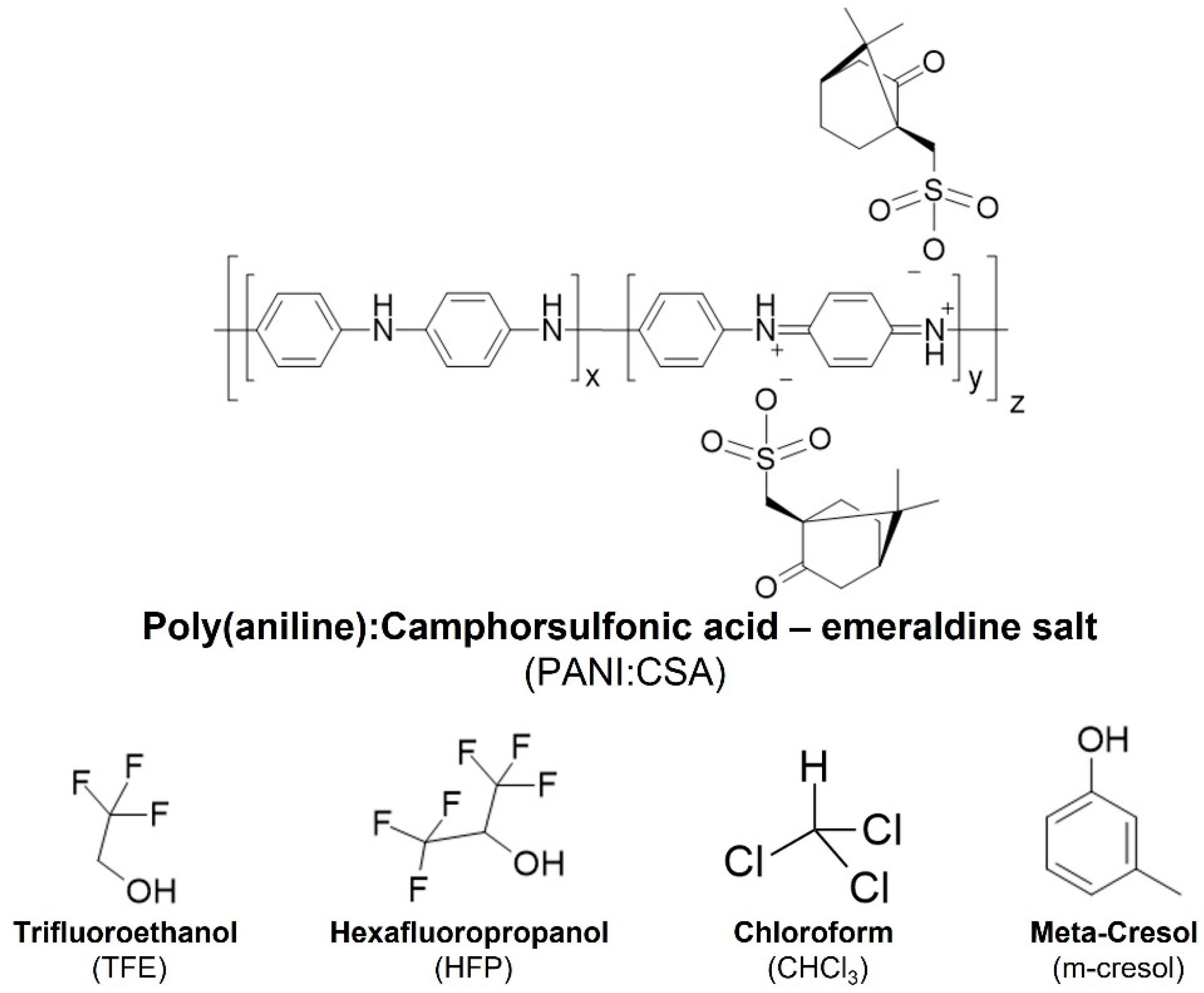
3.1. PANI:CSA–Processing Methods, Solvent Systems and Stability
3.2. Biocompatibility for Mammalian Cells
3.3. PANI:CSA and Blend Preparation for Scaffold Design
3.4. Electrical Stimulation of Neural Cells on PANI:CSA-Based Platforms
3.5. Improving the Bioactivity of the PANI:CSA System
- More contact points for the local adhesion of the cells inducing integrin-binding and clustering, with the consequential increase in integrin-binding domains causing focal adhesion kinase (FAK) activation;
- Actin rearrangement and the modulation of cell contractibility by actomyosin, mediated by the rho-associated protein kinase (ROCK) pathway. The expression of MAP2 levels in hESCs was also important;
- Consequential activation of the mitogen-activated protein kinase (MEK)-ERK pathway, and the enhancement of neuronal differentiation.
4. Challenges, Opportunities and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sankar, T.; Chakravarty, M.; Bescos, A.; Lara, M.; Obuchi, T.; Laxton, A.; McAndrews, M.; Tang-Wai, D.; Workman, C.; Smith, G.; et al. Deep Brain Stimulation Influences Brain Structure in Alzheimer’s Disease. Brain Stimul. 2015, 8, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Mann, A.; Gondard, E.; Tampellini, D.; Milsted, J.; Marillac, D.; Hamani, C.; Kalia, S.; Lozano, A. Chronic deep brain stimulation in an Alzheimer’s disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul. 2018, 11, 435–444. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Khabarova, E.A.; Denisova, N.P.; Dmitriev, A.B.; Slavin, K.V.; Verhagen Metman, L. Deep Brain Stimulation of the Subthalamic Nucleus in Patients with Parkinson Disease with Prior Pallidotomy or Thalamotomy. Brain Sci. 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Raza, C.; Anjum, R.; Shakeel, N.U. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Dao, D.; Wu, Z.; Kandalam, S.; Camacho, F.; Tom, C.; Zhang, W.; Krencik, R.; Rauen, K.; Ullian, E.; et al. Patient-derived iPSCs show premature neural differentiation and neuron type-specific phenotypes relevant to neurodevelopment. Mol. Psychiatr. 2018, 23, 1687–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, R.; Jiménez-Luna, C.; Perales-Adán, J.; Perazzoli, G.; Melguizo, C.; Prados, J. Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol. Ther. 2020, 28, 34–44. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, C.; Chaly, T.; Greene, P.; Breeze, R.; Fahn, S.; Freed, C.; Dhawan, V.; Eidelberg, D. Dopamine Cell Implantation in Parkinson’s Disease: Long-Term Clinical and 18F-FDOPA PET Outcomes. J. Nucl. Med. 2010, 51, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Radoszkiewicz, K.; Hribljan, V.; Isakovic, J.; Mitrecic, D.; Sarnowska, A. Critical points for optimizing long-term culture and neural differentiation capacity of rodent and human neural stem cells to facilitate translation into clinical settings. Exp. Neurol. 2023, 363, 114353. [Google Scholar] [CrossRef] [PubMed]
- Kolagar, T.; Farzaneh, M.; Nikkar, N.; Anbiyaiee, A.; Heydari, E.; Khoshnam, S. Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances, and Limitations. Curr. Stem. Cell Res. Ther. 2020, 14, 102–110. [Google Scholar] [CrossRef]
- Mathews, J.; Levin, M. The body electric 2.0: Recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotech. 2018, 52, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Reguera, G. Biology and biotechnology of microbial pilus nanowires. J. Ind. Microbiol. Biot. 2020, 47, 897–907. [Google Scholar] [CrossRef]
- Pfeffer, C.; Larsen, S.; Song, J.; Dong, M.; Besenbacher, F.; Meyer, R.; Kjeldsen, K.; Schreiber, L.; Gorby, Y.; El-Naggar, M.; et al. Filamentous bacteria transport electrons over centimetre distances. Nature 2012, 491, 218. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous bioelectric signaling networks: Exploiting voltage gradients for control of growth and form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Levin, M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev. Biol. 2018, 433, 177–189. [Google Scholar] [CrossRef]
- Vertkin, I.; Styr, B.; Slomowitz, E.; Ofir, N.; Shapira, I.; Berner, D.; Fedorova, T.; Laviv, T.; Barak-Broner, N.; Greitzer-Antes, D.; et al. GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal networks. Proc. Natl. Acad. Sci. USA 2015, 112, E3291–E3299. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B. Neuron-Astrocyte Signaling in the Development and Plasticity of Neural Circuits. Neurosignals 2008, 16, 278–288. [Google Scholar] [CrossRef]
- Levine, M.; Stevenson, C.G. Regulation of Cell Behavior and Tissue Patterning by Bioelectrical Signals: Challenges and Opportunities for Biomedical Engineering. Annu. Rev. Biomed. Eng. 2012, 14, 295–332. [Google Scholar] [CrossRef]
- Drew, D.; Boudker, O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef]
- Counillon, L.; Bouret, Y.; Marchiq, I.; Pouysségur, J. Na+/H+ antiporter (NHE1) and lactate/H+ symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2465–2480. [Google Scholar] [CrossRef]
- Nguyen, H.; Bursac, N. Ion channel engineering for modulation and de novo generation of electrical excitability. Curr. Opin. Biotechnol. 2019, 58, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, J.; Pu, J.; Collinson, J.; Forrester, J.; McCaig, C. Endogenous bioelectric currents promote differentiation of the mammalian lens. J. Cell Physiol. 2018, 233, 2202–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, J.I.; Pereda, A.E.; Rash, J.E. Electrical Synapses in Mammalian CNS: Past Eras, Present Focus and Future Directions. Biochim. Biophys. Acta 2018, 1860, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Aghayeva, U.; Berghoff, E.G.; Hobert, O. Plasticity of the electrical connectome of C. elegans. Cell 2019, 176, 1174–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Chen, B.; Mailler, R.; Wang, Z.-W. Antidromic-rectifying gap junctions amplify chemical transmission at functionally mixed electrical-chemical synapses. Nat. Commun. 2017, 8, 14818. [Google Scholar] [CrossRef] [Green Version]
- McCaig, C.; Rajnicek, A.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [Green Version]
- Heubach, J.; Graf, E.; Leutheuser, J.; Bock, M.; Balana, B.; Zahanich, I.; Christ, T.; Boxberger, S.; Wettwer, E.; Ravens, U. Electrophysiological properties of human mesenchymal stem cells. J. Physiol. 2004, 554, 659–672. [Google Scholar] [CrossRef]
- Wang, K.; Xue, T.; Tsang, S.; Huizen, R.; Wong, C.; Lai, K.; Ye, Z.; Cheng, L.; Au, K.; Zhang, J.; et al. Electrophysiological Properties of Pluripotent Human and Mouse Embryonic Stem Cells. Stem. Cells 2005, 23, 1526–1534. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Rushing, S.; Kong, C.; Fu, J.; Lieu, D.; Chan, C.; Deng, W.; Li, R. Electrophysiological properties of human induced pluripotent stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C486–C495. [Google Scholar] [CrossRef] [Green Version]
- Sordini, L.; Garrudo, F.; Rodrigues, C.; Linhardt, R.; Cabral, J.; Ferreira, F.; Morgado, J. Effect of Electrical Stimulation Conditions on Neural Stem Cells Differentiation on Cross-Linked PEDOT:PSS Films. Front. Bioeng. Biotechnol. 2021, 9, 591838. [Google Scholar] [CrossRef]
- Freeman, D.; Eddington, D.; Rizzo, J.; Fried, S. Selective Activation of Neuronal Targets With Sinusoidal Electric Stimulation. J. Neurophysiol. 2010, 104, 2778–2791. [Google Scholar] [CrossRef] [Green Version]
- Pires, F.; Ferreira, Q.; Rodrigues, C.; Morgado, J.; Ferreira, F. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Zhu, W.; Ye, T.; Lee, S.-J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L. Enhanced Neural Stem Cell Functions in Conductive Annealed Carbon Nanofibrous Scaffolds with Electrical Stimulation. Nanomedicine 2017, 14, 2485–2494. [Google Scholar] [CrossRef]
- Derhambakhsh, S.; Mohammadi, J.; Shokrgozar, M.; Rabbani, H.; Sadeghi, N.; Nekounam, H.; Mohammadi, S.; Lee, K.-B.; Khakbiz, M. Investigation of electrical stimulation on phenotypic vascular smooth muscle cells differentiation in tissue-engineered small-diameter vascular graft. Tissue Cell 2023, 81, 101996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jin, L.; Li, C.; Kuddannayai, S.; Zhang, Y. The effect of electrical stimulation on cortical cells in 3D nanofibrous scaffolds. RSC Adv. 2018, 8, 11027–11035. [Google Scholar] [CrossRef] [Green Version]
- Garrudo, F.; Mikael, P.; Rodrigues, C.; Udangawa, R.; Paradiso, P.; Chapman, C.; Hoffman, P.; Colaço, R.; Cabral, J.; Morgado, J.; et al. Polyaniline-polycaprolactone fibers for neural applications: Electroconductivity enhanced by pseudo-doping. Mater. Sci. Eng. C 2021, 120, 111680. [Google Scholar] [CrossRef]
- Garrudo, F.F.; Nogueira, D.E.S.E.; Rodrigues, C.A.V.A.; Ferreira, F.A.A.; Paradiso, P.; Colaço, R.; Marques, A.C.; Cabral, J.M.S.M.; Morgado, J.; Linhardt, R.J.; et al. Electrical stimulation of neural-differentiating iPSCs on novel coaxial electroconductive nanofibers. Biomater. Sci. 2021, 9, 5359–5382. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhang, H.; Zhang, Q.; Wang, L.; Li, C.; Dai, B.; Yang, J.; Liu, J.; Sun, D. Electrically-responsive core-shell hybrid microfibers for controlled drug release and cell culture. Acta Biomater. 2017, 55, 434–442. [Google Scholar] [CrossRef]
- Koppes, A.N.; Zaccor, N.W.; Rivet, C.J.; Williams, L.A.; Piselli, J.M.; Gilbert, R.J.; Thompson, D.M. Neurite outgrowth on electrospun PLLA fibers is enhanced by exogenous electrical stimulation. J. Neural Eng. 2014, 11, 046002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Yang, A.; Huang, Z.; Yin, G.; Pu, X.; Jin, J. Enhancement of neurite adhesion. alignment and elongation on conductive polypyrrole-poly(lactide acid) fibers with cell-derived extracellular matrix. Colloids Surf. B 2017, 149, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, S.; Lee, J.; Lee, H.-R.; Chang, G.-E.; Seo, J.; Lee, T.; Cheong, E.; Im, S.G.; Cho, S.W. Electroconductive nanoscale topography for enhanced neuronal differentiation and electrophysiological maturation of human neural stem cells. Nanoscale 2017, 9, 18737–18752. [Google Scholar] [CrossRef] [PubMed]
- Koppes, A.; Seggio, A.; Thompson, D. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J. Neural. Eng. 2011, 8, 046023. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Tan, Z.; Li, J.; Hou, Y.; Wang, X.; Liu, B.; Xing, X.; Rong, L.; He, L. On-demand release of the small-molecule TrkB agonist improves neuron-Schwann cell interactions. J. Control Release 2022, 343, 482–491. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.; Chan, C.-B.; Song, M.; Yu, S.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Jun, S.; Song, J.; Kim, S. Activity-dependent neuronal cell migration induced by electrical stimulation. Med. Biol. Eng. Comput. 2009, 47, 93–99. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef]
- Xu, B.; Bai, T.; Sinclair, A.; Wang, W.; Wu, Q.; Gao, F.; Jia, H.; Jiang, S.; Liu, W. Directed neural stem cell differentiation on polyaniline-coated high strength hydrogels. Mater. Today Chem. 2016, 1, 15–22. [Google Scholar] [CrossRef]
- Song, S.; Amores, D.; Chen, C.; McConnell, K.; Oh, B.; Poon, A.; George, P. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep. 2019, 9, 19565. [Google Scholar] [CrossRef] [Green Version]
- Borah, R.; Ingavle, G.; Sandeman, S.; Kumar, A.; Mikhalovsky, S. Electrically conductive MEH-PPV:PCL electrospun nanofibres for electrical stimulation of rat PC12 pheochromocytoma cells. Biomater. Sci. 2018, 6, 2342–2359. [Google Scholar] [CrossRef]
- Blanquie, O.; Kilb, W.; Sinning, A.; Luhmann, H. Homeostatic interplay between electrical activity and neuronal apoptosis in the developing neocortex. Neuroscience 2017, 358, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Schild, C.; Schwede, F.; Hucho, T. Crosstalk from cAMP to ERK1/2 emerges during postnatal maturation of nociceptive neurons and is maintained during aging. J. Cell Sci. 2017, 130, 2134–2146. [Google Scholar] [PubMed] [Green Version]
- Meissner, A.; Noack, T. Proliferation of human lens epithelial cells (HLE-B3) is inhibited by blocking of voltage-gated calcium channels. Pflügers. Arch. Eur. J. Physiol. 2008, 457, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Wen, Y.; Shi, L.; Zhu, Z.; Ke, G.; Gu, Y. LncRNA KCNQ1OT1 knockdown inhibits viability, migration and epithelial-mesenchymal transition in human lens epithelial cells via miR-26a-5p/ITGAV/TGF-beta/Smad3 axis. Exp. Eye Res. 2020, 200, 108251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, S.; Sun, C.; Liu, H.; Liu, T.; Ma, X. Electrical stimulation enhances the neuronal differentiation of neural stem cells in three-dimensional conductive scaffolds through the voltage-gated calcium ion channel. Brain Res. 2023, 1798, 148163. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.; Cartmell, S. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Pascoal-Faria, P.; Ferreira, P.; Datta, A.; Amado, S.; Moura, C.; Alves, N. Electrical Stimulation Optimization in Bioreactors for Tissue Engineering Applications. Appl. Mech. Mater. 2019, 890, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Bodamyali, T.; Kanczler, J.; Simon, B.; Blake, D.; Stevens, C. Effect of Faradic Products on Direct Current-Stimulated Calvarial Organ Culture Calcium Levels. Biochem. Bioph. Res. Commun. 1999, 264, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Dimitrievski, K.; Siegbahn, P.; Nørskov, J. Comparing Electrochemical and Biological Water Splitting. J. Phys. Chem. C 2007, 111, 18821–18823. [Google Scholar] [CrossRef]
- Meneses, J.; Fernandes, S.; Alves, N.; Pascoal-Faria, P.; Miranda, P. Effects of Scaffold Electrical Properties on Electric Field Delivery in Bioreactors. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico, 1–5 November 2021. [Google Scholar]
- Wang, L.-P.; Wang, W.; Di, L.; Lu, Y.-N.; Wang, J.-Y. Protein adsorption under electrical stimulation of neural probe coated with polyaniline. Colloids Surf. B Biointerfaces 2010, 80, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, W.; Yang, H.; Zhang, P.; Wang, J. Intact polyaniline coating as a conductive guidance is beneficial to repairing sciatic nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Cantù, E.; Tonello, S.; Serpelloni, M.; Lopomo, N.; Sardini, E. A Review on Biomaterials for 3D Conductive Scaffolds for Stimulating and Monitoring Cellular Activities. Appl. Sci. 2019, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.; Dirgantara, T.; Bartolo, P. 3D Printing of Polycaprolactone–Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [Green Version]
- Menzel, V.; Tudela, I. Additive manufacturing of polyaniline-based materials: An opportunity for new designs and applications in energy and biotechnology. Curr. Opin. Chem. Eng. 2022, 35, 100742. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Humpolíček, P.; Radaszkiewicz, K.; Capáková, Z.; Pacherník, J.; Bober, P.; Kašpárková, V.; Rejmontová, P.; Lehocký, M.; Ponížil, P.; Stejskal, J. Polyaniline cryogels: Biocompatibility of novel conducting macroporous material. Sci. Rep. 2018, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wong, C.-W.; Hsu, S. An Injectable. Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing. Chem. Mater. 2020, 32, 10407–10422. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.; Sun, B.; Fang, J.; Zhang, K.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Q.; Wang, J.-Y. Nanostructured Polyaniline Coating on ITO Glass Promotes the Neurite Outgrowth of PC 12 Cells by Electrical Stimulation. Langmuir 2015, 31, 12315–12322. [Google Scholar] [CrossRef]
- Erskine, L.; McCaig, C.D. Integrated interactions between chondroitin sulphate proteoglycans and weak dc electric fields regulate nerve growth cone guidance in vitro. J. Cell Sci. 1997, 110, 1957–1965. [Google Scholar] [CrossRef]
- Wang, H.; Mullins, M.; Cregg, J.; McCarthy, C.; Gilbert, R. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef]
- Arteshi, Y.; Aghanejad, A.; Davaran, S.; Omidi, Y. Biocompatible and electroconductive polyaniline-based biomaterials for electrical stimulation. Eur. Pol. J. 2018, 108, 150–170. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.-M.; Hoffman-Kim, D.; Song, H.-K.; Palmore, G. Quantitative Control of Neuron Adhesion at a Neural Interface Using a Conducting Polymer Composite with Low Electrical Impedance. ACS Appl. Mater. Interfaces 2010, 3, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Alba, N.; Du, Z.; Catt, K.; Kozai, T.; Cui, X. In Vivo Electrochemical Analysis of a PEDOT/MWCNT Neural Electrode Coating. Biosensors 2015, 5, 618–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimard, N.; Gomez, N.; Schmidt, C. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.; Cartmell, S. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mat. Sci. Eng. C 2017, 77, 1302–1315. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.; Cho, J.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef]
- Rocha, I.; Cerqueira, G.; Penteado, F.; de Torresi, S.I.C. Electrical Stimulation and Conductive Polymers as a Powerful Toolbox for Tailoring Cell Behaviour in vitro. Front. Med. Technol. 2021, 3, 670274. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.G.; Pun, M.Y.; Kang, E.T.; Tan, K.L. Polyaniline treated with organic acids: Doping characteristics and stability. Synth. Met. 1995, 73, 209–215. [Google Scholar] [CrossRef]
- Dimitriev, O.P.; Grinko, D.A.; Noskov, Y.V.; Ogurtsov, N.A.; Pud, A.A. PEDOT:PSS films—Effect of organic solvent additives and annealing on the film conductivity. Synth. Met. 2009, 159, 2237–2239. [Google Scholar] [CrossRef]
- Glipa, X.; Bonnet, B.; Mula, B.; Deborah, J.; Rozière, J. Investigation of the conduction properties of phosphoric and sulfuric acid doped polybenzimidazole. J. Mater. Chem. 1999, 9, 3045–3049. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Boccaccini, A.R. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [Google Scholar] [CrossRef]
- Wei, L.; Wang, S.; Shan, M.; Li, Y.; Wang, Y.; Wang, F.; Wang, L.; Mao, J. Conductive fibers for biomedical applications. Bioact. Mater. 2023, 22, 343–364. [Google Scholar] [CrossRef]
- Holland, E.R.; Pomfret, S.J.; Adams, P.N.; Monkman, A.P. Conductivity studies of polyaniline doped with CSA. J. Phys. Condens. Matter. 1996, 8, 2991–3002. [Google Scholar] [CrossRef]
- Focke, W.; Wnek, G. Conduction mechanisms in polyaniline (emeraldine salt). J. Electroanal. Chem. Interfacial Electrochem. 1988, 256, 343–352. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.; Lelkes, P. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Hopkins, A.R.; Rasmussen, P.G. Characterization of solution and solid state properties of undoped and doped polyanilines processed from hexafluoro-2-propanol. Macromolecules 1996, 29, 7838–7846. [Google Scholar] [CrossRef]
- Chaudhari, H.; Kelkar, D. Investigation of Structure and Electrical Conductivity in Doped Polyaniline. Polym. Int. 1997, 42, 380–384. [Google Scholar] [CrossRef]
- Blinova, N.; Stejskal, J.; Trchová, M.; Prokeš, J. Control of polyaniline conductivity and contact angles by partial protonation. Polym. Int. 2008, 57, 66–69. [Google Scholar] [CrossRef]
- Leite, F.; Neto, M.; Paterno, L.; Ballestero, M.; Polikarpov, I.; Mascarenhas, Y.; Herrmann, P.; Mattoso, L.; Oliveira, O. Nanoscale conformational ordering in polyanilines investigated by SAXS and AFM. J. Colloid Interf. Sci. 2007, 316, 376–387. [Google Scholar] [CrossRef]
- Lobov, I.; Davletkildeev, N.; Sokolov, D. AFM study of the supramolecular transformation of polyaniline and polyaniline/carbon nanotubes composite upon doping with dodecylbenzenesulfonic acid in the presence of a solvent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 256, 012017. [Google Scholar] [CrossRef]
- Cao, Y.; Qiu, J.; Smith, P. Effect of solvents and co-solvents on the processibility of polyaniline: I. Solubility and conductivity studies. Synth. Met. 1995, 69, 187–190. [Google Scholar] [CrossRef]
- Fryczkowski, R.; Gorczowska, M.; Fryczkowska, B.; Janicki, J. The effect of solvent on the properties of nanofibres obtained by electrospinning from a mixture of poly(3-hydroxybutyrate) and polyaniline. Synth. Met. 2013, 166, 14–21. [Google Scholar] [CrossRef]
- Xia, Y.; Wiesinger, J.M.; MacDiarmid, A.G. Camphorsulfonic acid fully doped polyaniline emeraldine salt: Conformations in different solvents studied by an ultraviolet/visible/near-infrared spectroscopic method. Chem. Mater. 1995, 7, 443–445. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Wang, Y.; Sun, J.; Zeng, H.; Jin, Z.; Huang, X.; Chen, L. The synergic regulation of conductivity and Seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains. J. Mater. Chem. A 2014, 2, 2634. [Google Scholar] [CrossRef]
- Lenin, R.; Singh, A.; Bera, C. Effect of dopants and morphology on the electrical properties of polyaniline for various applications. J. Mater. Sci. Mater. Electron. 2021, 32, 24710–24725. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Park, S.; Heeger, A.; Lee, C.-W.; Lee, S.-H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Roy, I.; Tice, A.; Chapman, C.; Udangawa, R.; Chakrapani, V.; Plawsky, J.L.; Linhardt, R.J. High Conductivity and High Capacitance Electrospun Fibers for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2020, 12, 19369–19376. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Liu, C.; Liu, G.; Wei, J.; Xu, J.; Jiang, Q.; Liu, X.; Jiang, F. Microstructure control for high-capacitance polyaniline. Electrochim. Acta 2021, 391, 138977. [Google Scholar] [CrossRef]
- Wang, M.; Tremblay, P.-L.; Zhang, T. Optimizing the electrical conductivity of polyacrylonitrile/polyaniline with nickel nanoparticles for the enhanced electrostimulation of Schwann cells proliferation. Bioelectrochemistry 2021, 140, 107750. [Google Scholar] [CrossRef]
- Ayad, M.; Zaki, E. Doping of polyaniline films with organic sulfonic acids in aqueous media and the effect of water on these doped films. Eur. Polym. J. 2008, 44, 3741–3747. [Google Scholar] [CrossRef]
- Hobaica, S. Stability of polyaniline in air and acidic water. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 807–822. [Google Scholar] [CrossRef]
- Bidez, P.; Li, S.; Macdiarmid, A.; Venancio, E.; Wei, Y.; Lelkes, P. Polyaniline, an electroactive polymer. supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [Green Version]
- López-Palacios, J.; Muñoz, E.; Heras, A.; Colina, Á.; Ruiz, V. Study of polyaniline films degradation by thin-layer bidimensional spectroelectrochemistry. Electrochim. Acta 2006, 52, 234–239. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yoneyama, H.; Tamura, H. Oxidative degradation pathway of polyaniline film electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1984, 177, 293–297. [Google Scholar] [CrossRef]
- Di, L.; Wang, L.-P.; Lu, Y.-N.; He, L.; Lin, Z.-X.; Wu, K.-J.; Ren, Q.-S.; Wang, J.-Y. Protein adsorption and peroxidation of rat retinas under stimulation of a neural probe coated with polyaniline. Acta Biomater. 2011, 7, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.; Junkar, I.; Lehocký, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity. embryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef]
- Kašpárková, V.; Humpolíček, P.; Stejskal, J.; Capáková, Z.; Bober, P.; Skopalová, K.; Lehocký, M. Exploring the Critical Factors Limiting Polyaniline Biocompatibility. Polymers 2019, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, M.; Dou, C.; Ma, G.; Wang, Y.; Feng, N.; Wang, W.; Fang, L. Synthesis and biocompatibility assessment of polyaniline nanomaterials. J. Bioact. Compat. Polym. 2019, 34, 16–24. [Google Scholar] [CrossRef]
- Ibarra, L.; Tarres, L.; Bongiovanni, S.; Barbero, C.; Kogan, M.; Rivarola, V.; Bertuzzi, M.; Yslas, E. Assessment of polyaniline nanoparticles toxicity and teratogenicity in aquatic environment using Rhinella arenarum model. Ecotox. Environ. Safe 2015, 114, 84–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Sun, W.; Zhou, B.; Zhu, J.; Peng, C.; Shen, M.; Shi, X. Polyaniline-loaded γ-polyglutamic acid nanogels as a platform for photoacoustic imaging-guided tumor photothermal therapy. Nanoscale 2017, 9, 12746–12754. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.; Muir, E.; Bora, U. Electrospun silk-polyaniline conduits for functional nerve regeneration in rat sciatic nerve injury model. Biomed. Mater. 2017, 12, 045025. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.; Jeong, S.; Lee, T.; Jun, I.; Lee, Y.; Kim, B.; Shin, H. Electroactive Electrospun Polyaniline/Poly[(L-lactide)-co-(ε-caprolactone)] Fibers for Control of Neural Cell Function. Macromol. Biosci. 2012, 12, 402–411. [Google Scholar] [CrossRef]
- Mawad, D.; Stewart, E.; Officer, D.; Romeo, T.; Wagner, P.; Wagner, K.; Wallace, G.A. A Single Component Conducting Polymer Hydrogel as a Scaffold for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2692–2699. [Google Scholar] [CrossRef]
- Guarino, V.; Alvarez-Perez, M.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA Macroporous Hydrogels For Nerve Regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef]
- Low, K.; Chartuprayoon, N.; Echeverria, C.; Li, C.; Bosze, W.; Myung, N.; Nam, J. Polyaniline/poly(ε-caprolactone) composite electrospun nanofiber-based gas sensors: Optimization of sensing properties by dopants and doping concentration. Nanotechnology 2014, 25, 115501. [Google Scholar] [CrossRef] [PubMed]
- Garrudo, F.F.F.; Chapman, C.A.; Hoffman, P.R.; Udangawa, R.W.; Silva, J.C.; Mikael, P.E.; Rodrigues, C.A.V.; Cabral, J.M.S.; Morgado, J.M.F.; Ferreira, F.C.; et al. Polyaniline-Polycaprolactone Blended Nanofibers for Neural Cell Culture. Eur. Polym. J. 2019, 117, 28–37. [Google Scholar] [CrossRef]
- Hong, S.-G.; Kim, H.; Kim, J. Highly Stabilized Lipase in Polyaniline Nanofibers for Surfactant-Mediated Esterification of Ibuprofen. Langmuir 2014, 30, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Dwivedee, B.; Banerjee, U. Tailoring a stable and recyclable nanobiocatalyst by immobilization of surfactant treated Burkholderia cepacia lipase on polyaniline nanofibers for biocatalytic application. Int. J. Biol. Macromol. 2020, 161, 573–586. [Google Scholar] [CrossRef]
- Hanumantharao, S.; Que, C.; Rao, S. Self-Assembly of 3D Nanostructures in Electrospun Polycaprolactone-Polyaniline Fibers and their Application as Scaffolds for Tissue Engineering. Materialia 2019, 6, 100296. [Google Scholar] [CrossRef]
- Licciardello, M.; Ciardelli, G.; Tonda-Turo, C. Biocompatible Electrospun Polycaprolactone-Polyaniline Scaffold Treated with Atmospheric Plasma to Improve Hydrophilicity. Bioengineering 2021, 8, 24. [Google Scholar] [CrossRef]
- Ku, S.; Lee, S.; Park, C. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials 2012, 33, 6098–6104. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhao, R.; Wang, C.; Qiu, F.; Sun, B.; Ji, H.; Qiu, J.; Wang, C. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater. Sci. Eng. C 2017, 72, 106–112. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchová, M.; Kovalcik, A.; Hodan, J.; Hromádková, J.; Prokeš, J. Polyaniline Cryogels Supported with Poly(vinyl alcohol): Soft and Conducting. Macromolecules 2017, 50, 972–978. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, A.; Zhao, Y.; Chen, F.; Ke, M.; Zhang, Q.; Zhang, J.; Shi, X.; He, X.; Chen, Y. An implantable and versatile piezoresistive sensor for the monitoring of human–machine interface interactions and the dynamical process of nerve repair. Nanoscale 2019, 11, 21103–21118. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Y.; Chen, F.; Xiao, A.; Du, Q.; Dong, Q.; Ke, M.; Liang, X.; Zhou, Q.; Chen, Y. Conductive Hydroxyethyl Cellulose/Soy Protein Isolate/Polyaniline Conduits for Enhancing Peripheral Nerve Regeneration via Electrical Stimulation. Front. Bioeng. Biotechnol. 2020, 8, 709. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Park, J.; Lee, S.; Hong, S.; Park, J.; Park, K.-H. Fabrication of Nanocomposites Complexed with Gold Nanoparticles on Polyaniline and Application to Their Nerve Regeneration. Acs Appl. Mater. Inter. 2020, 12, 30750–30760. [Google Scholar] [CrossRef]
- Song, I.; Dityatev, A. Crosstalk between glia. extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Thompson, R.; Pardieck, J.; Smith, L.; Kenny, P.; Crawford, L.; Shoichet, M.; Sakiyama-Elbert, S. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 2018, 162, 208–223. [Google Scholar] [CrossRef]
- Tan, K.; Tann, J.; Sathe, S.; Goh, S.; Ma, D.; Goh, E.; Ma, D.; Goh, E.; Yim, E. Enhanced differentiation of neural progenitor cells into neurons of the mesencephalic dopaminergic subtype on topographical patterns. Biomaterials 2015, 43, 32–43. [Google Scholar] [CrossRef]
- Yang, H.; Lee, B.; Tsui, J.; Macadangdang, J.; Jang, S.; Im, S.; Kim, D. Electroconductive Nanopatterned Substrates for Enhanced Myogenic Differentiation and Maturation. Adv. Healthc. Mater. 2016, 5, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankam, S.; Suryana, M.; Chan, L.; Moe, A.; Teo, B.; Law, J.; Sheetz, M.; Low, H.; Yim, E. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater. 2013, 9, 4535–4545. [Google Scholar] [CrossRef]
- Ankam, S.; Lim, C.; Yim, E. Actomyosin contractility plays a role in MAP2 expression during nanotopography-directed neuronal differentiation of human embryonic stem cells. Biomaterials 2015, 47, 20–28. [Google Scholar] [CrossRef]
- Yang, K.; Park, E.; Lee, J.; Kim, I.; Hong, K.; Park, K.; Cho, S.; Yang, H. Biodegradable Nanotopography Combined with Neurotrophic Signals Enhances Contact Guidance and Neuronal Differentiation of Human Neural Stem Cells. Macromol. Biosci. 2015, 15, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, G.; Song, H.; Mao, H.-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; Zuidema, J.M.; Kearns, K.R.; Maguire, A.B.; Desmond, G.P.; Thompson, D.M.; Gilbert, R.J. The Effect of Electrospun Fiber Diameter on Astrocyte-Mediated Neurite Guidance and Protection. ACS Appl. Bio. Mater. 2019, 2, 104–117. [Google Scholar] [CrossRef]
- Chang, W.; Shah, M.; Lee, P.; Yu, X. Tissue-Engineered Spiral Nerve Guidance Conduit for Peripheral Nerve Regeneration. Acta Biomater. 2018, 73, 302–311. [Google Scholar] [CrossRef]
- Morgado, J. Modulation of the electrical double layer in metals and conducting polymers. Sci. Rep. 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Morgado, J.; Sordini, L.; Ferreira, F. Electronic to ionic transduction of the electric field applied to PEDOT:PSS substrates to the cell cultures on top. Bioelectrochemistry 2022, 145, 108099. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Ho, P.; Ng, S.; Tan, B.; Tan, K. A new water-soluble, self-doping conducting polyaniline from poly (o-aminobenzylphosphonic acid) and its sodium salts: Synthesis and characterization. J. Am. Chem. Soc. 1995, 117, 8517–8523. [Google Scholar] [CrossRef]
- Alva, K.S.; Kumar, J.; Marx, K.A.; Tripathy, S.K. Enzymatic synthesis and characterization of a novel water-soluble polyaniline: Poly (2, 5-diaminobenzenesulfonate). Macromolecules 1997, 30, 4024–4029. [Google Scholar] [CrossRef]
- Wang, S.; Guan, S.; Wang, J.; Liu, H.; Liu, T.; Ma, X.; Cui, Z. Fabrication and characterization of conductive poly (3,4-ethylenedioxythiophene) doped with hyaluronic acid/poly (l-lactic acid) composite film for biomedical application. J. Biosci. Bioeng. 2017, 123, 116–125. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, X.; Liu, Y.; Bayaniahangar, R.; Li, H.; Lu, C.; Xu, N.; Yao, Z.; Qiao, Y.; Zhang, D.; et al. Polyaniline-decorated hyaluronic acid-carbon nanotube hybrid microfiber as a flexible supercapacitor electrode material. Carbon 2020, 159, 65–73. [Google Scholar] [CrossRef]
- Garrudo, F.F.F.; Udangawa, R.N.; Hoffman, P.R.; Sordini, L.; Chapman, C.A.; Mikael, P.E.; Ferreira, F.A.; Silva, J.C.; Rodrigues, C.A.V.; Cabral, J.M.S.; et al. Polybenzimidazole nanofibers for neural stem cell culture. Mater. Today Chem. 2019, 14, 100185. [Google Scholar] [CrossRef]
- Dickhaus, B.; Priefer, R. Determination of polyelectrolyte pKa values using surface-to-air tension measurements. Colloids Surf. Physicochem. Eng. Asp. 2016, 488, 15–19. [Google Scholar] [CrossRef]
- Håkansson, A.; Han, S.; Wang, S.; Lu, J.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X.; Fabiano, S. Effect of (3-glycidyloxypropyl)trimethoxysilane (GOPS) on the electrical properties of PEDOT:PSS films. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Mantione, D.; del Agua, I.; Schaafsma, W.; ElMahmoudy, M.; Uguz, I.; Sanchez-Sanchez, A.; Sardon, H.; Castro, B.; Malliaras, G.; Mecerreyes, D. Low-Temperature Cross-Linking of PEDOT:PSS Films Using Divinylsulfone. ACS Appl. Mater. Interfaces 2017, 9, 18254–18262. [Google Scholar] [CrossRef] [PubMed]
- Alesary, H.; Ismail, H.; Khudhair, A.; Mohammed, M. Effects of Dopant Ions on the Properties of Polyaniline Conducting Polymer. Orient. J. Chem. 2018, 34, 2525–2533. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Chen, J.-T.; Wang, L.-F.; Wu, S.; Zhang, G.; Yu, H.-Q.; Ye, X.; Shi, Q.-S. Conformations and molecular interactions of poly-γ-glutamic acid as a soluble microbial product in aqueous solutions. Sci. Rep. 2017, 7, 12787. [Google Scholar] [CrossRef] [Green Version]
- Thaplyal, P.; Bevilacqua, P.C. Experimental Approaches for Measuring pKa’s in RNA and DNA. Methods Enzymol. 2014, 549, 189–219. [Google Scholar]
- Bhattacharya, S.; Kim, D.; Gopal, S.; Tice, A.; Lang, K.; Dordick, J.; Plawsky, J.; Linhardt, R. Antimicrobial effects of positively charged. conductive electrospun polymer fibers. Mater. Sci. Eng. C 2020, 116, 111247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrudo, F.F.F.; Linhardt, R.J.; Ferreira, F.C.; Morgado, J. Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid. Polymers 2023, 15, 2674. https://doi.org/10.3390/polym15122674
Garrudo FFF, Linhardt RJ, Ferreira FC, Morgado J. Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid. Polymers. 2023; 15(12):2674. https://doi.org/10.3390/polym15122674
Chicago/Turabian StyleGarrudo, Fábio F. F., Robert J. Linhardt, Frederico Castelo Ferreira, and Jorge Morgado. 2023. "Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid" Polymers 15, no. 12: 2674. https://doi.org/10.3390/polym15122674
APA StyleGarrudo, F. F. F., Linhardt, R. J., Ferreira, F. C., & Morgado, J. (2023). Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid. Polymers, 15(12), 2674. https://doi.org/10.3390/polym15122674







