Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cells
2.1.1. Polymers
2.1.2. Cells
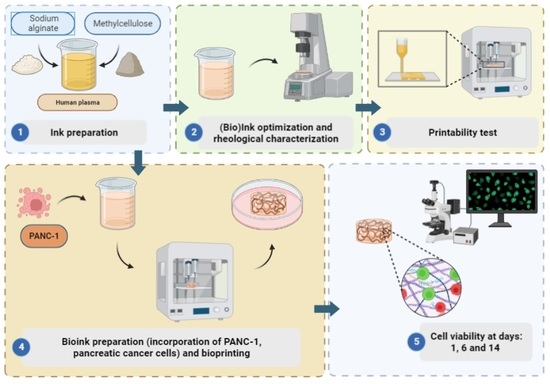
2.2. Inks and Bioink Preparation
2.3. Optimization & Characterization of the Inks
2.4. 3D Bioprinting
2.5. Viability Assay on Cell-Laden Constructs
2.6. Statistics
3. Results
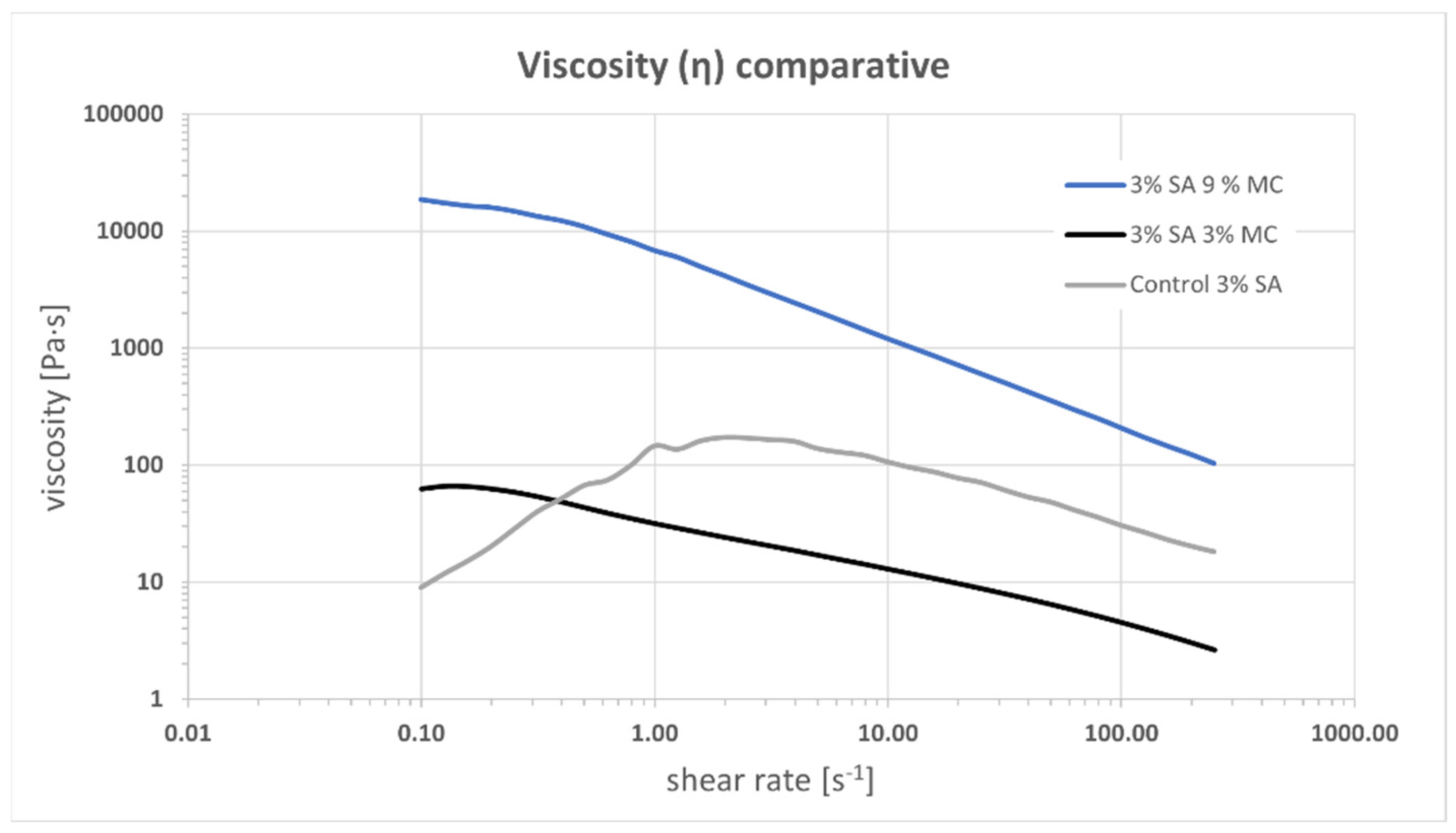
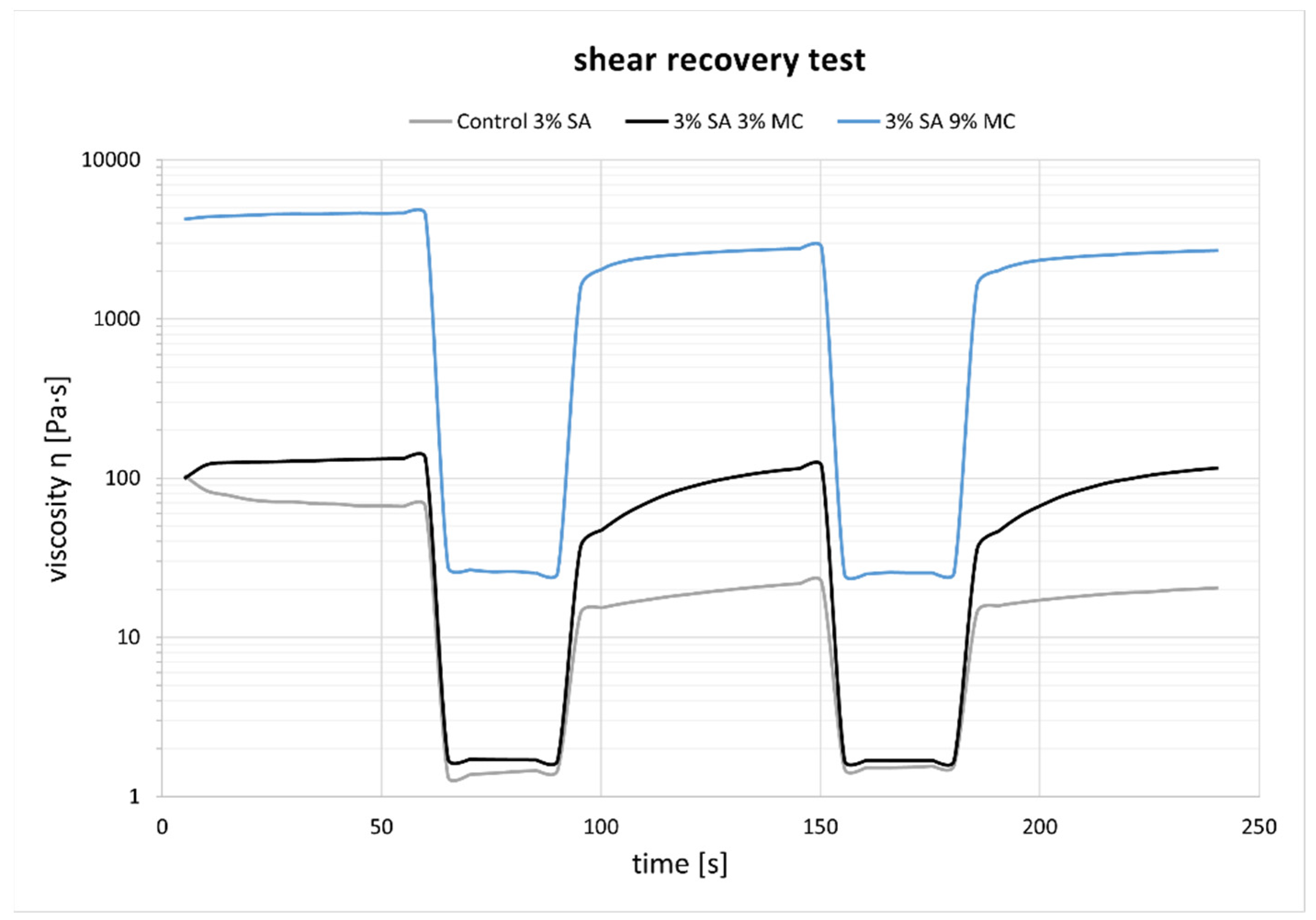
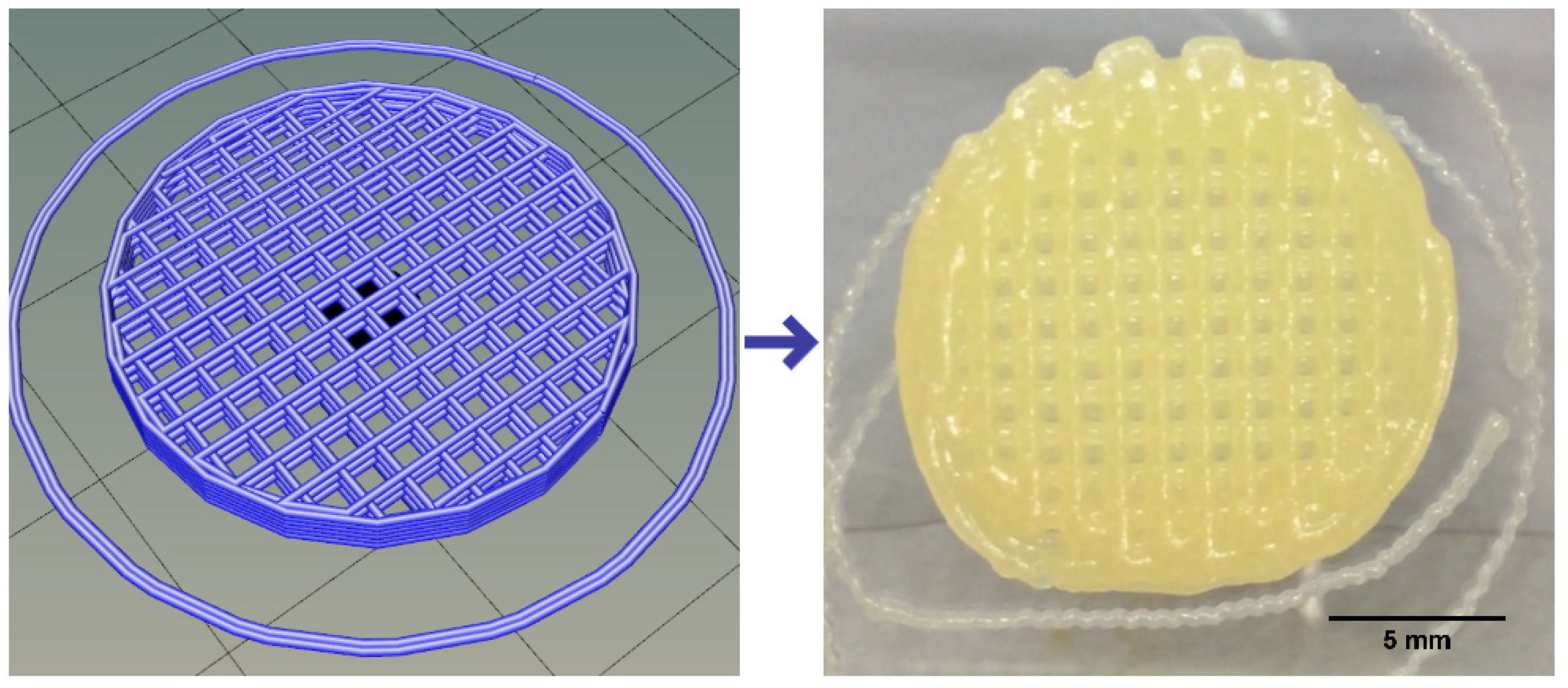
3.1. Ink Selection, Printability and Printing Fidelity
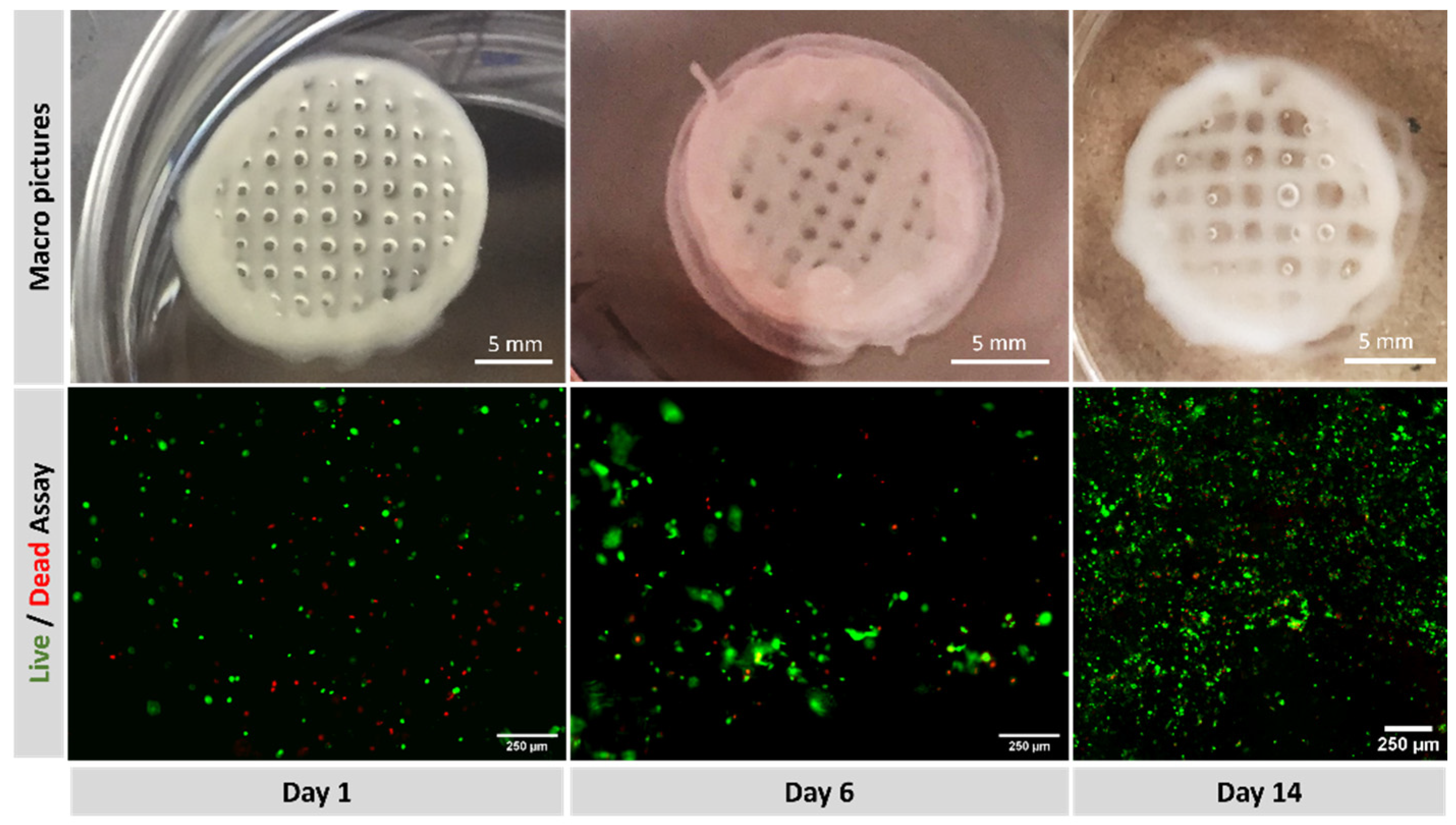
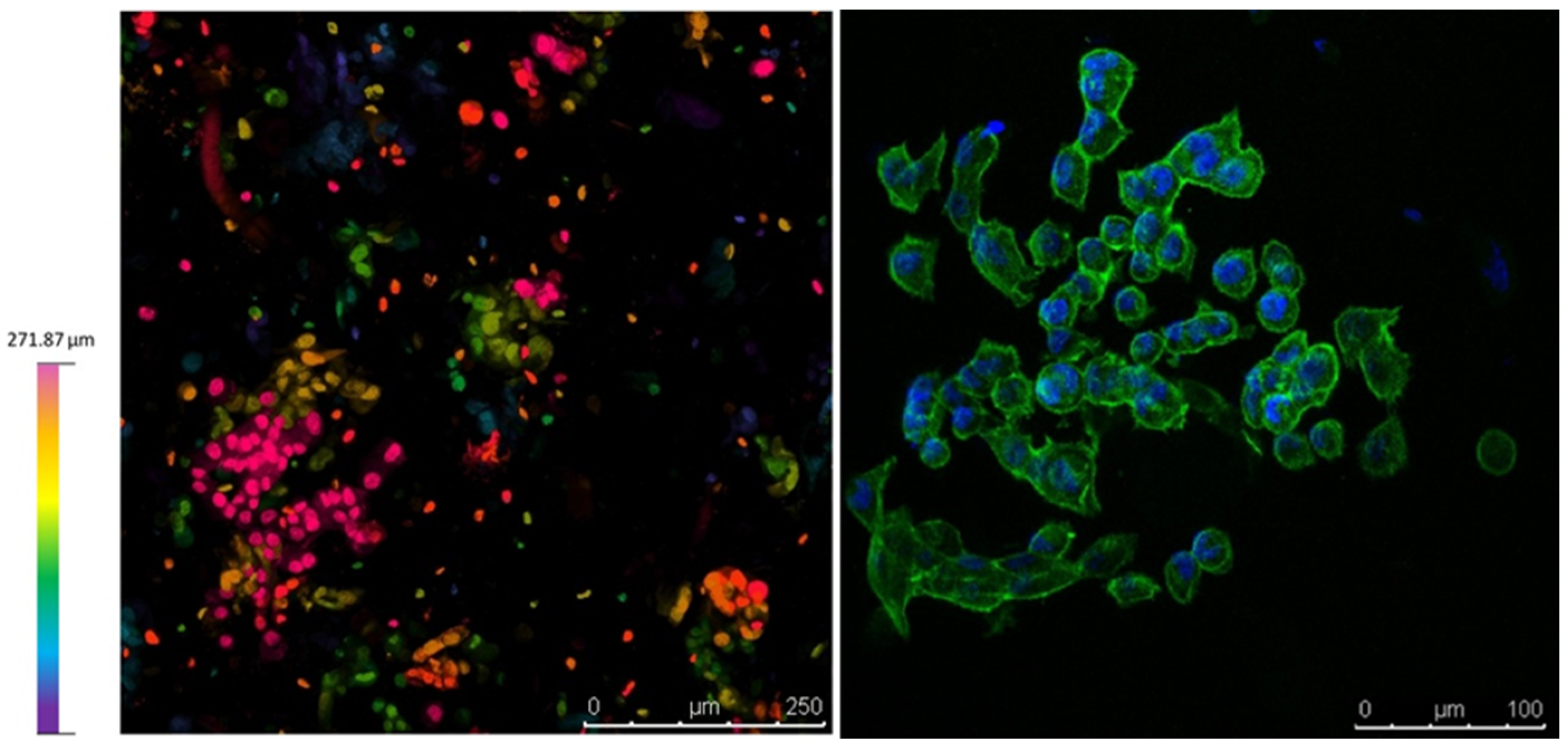
3.2. Viability Assays on Cell-Laden Constructs
4. Discussion
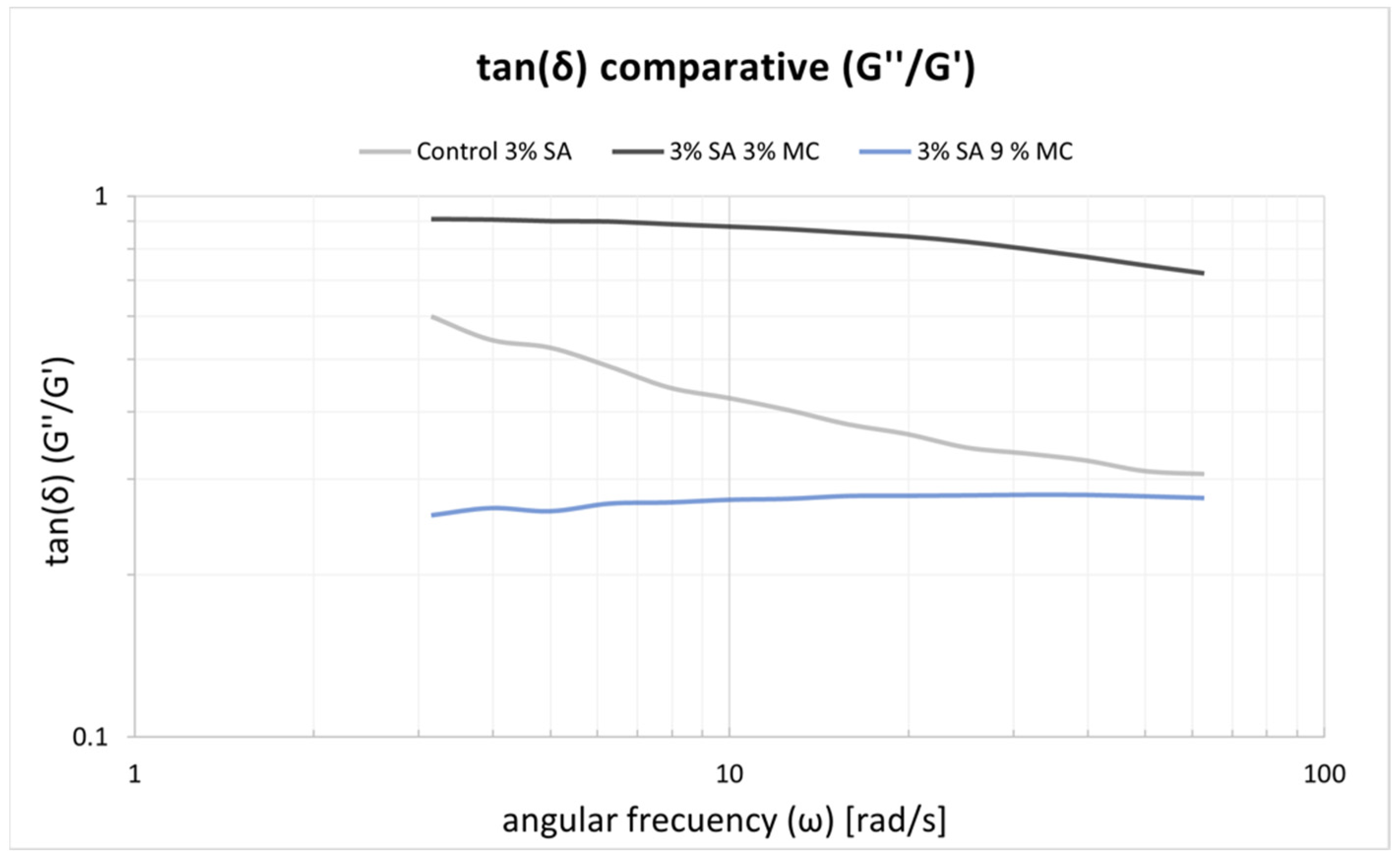
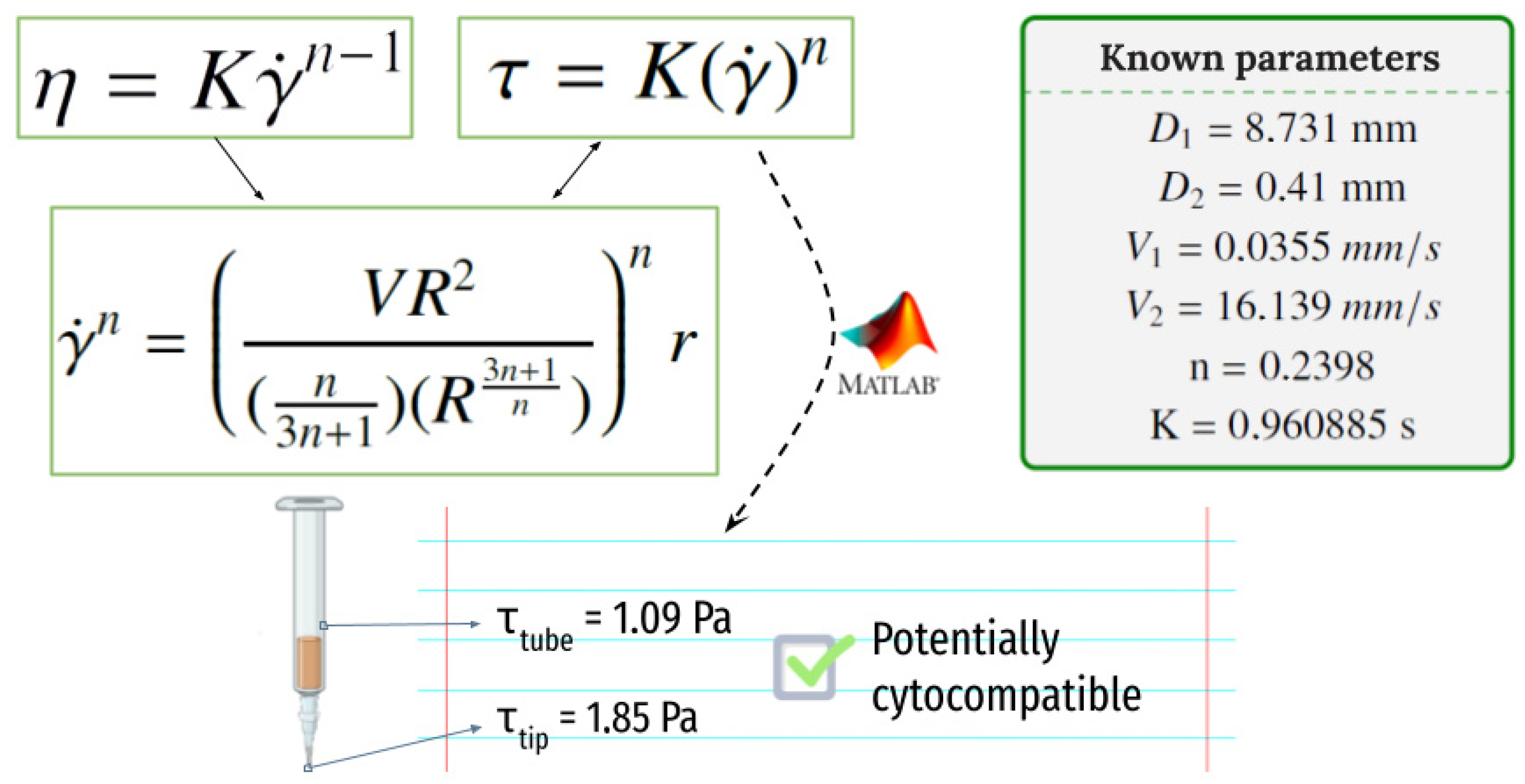
4.1. Selection of Bioink Composition, Printability and Viscoelastic Properties
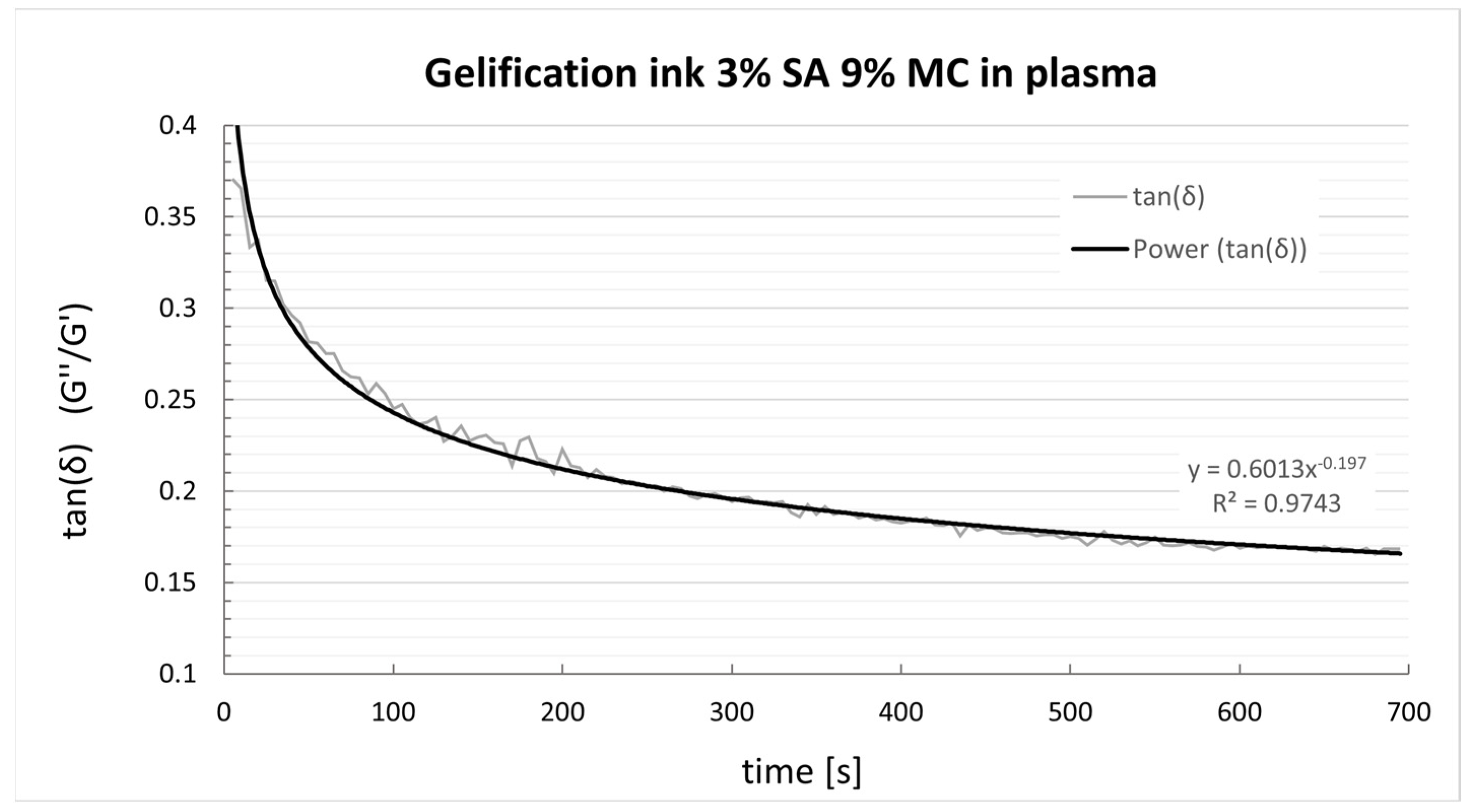
4.2. Crosslinking Behaviour
4.3. PANC-1 Viability on 3D Bioprinted Constructs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N.; et al. Polysaccharide Hydrogel Based 3d Printed Tumor Models for Chemotherapeutic Drug Screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Peela, N.; Truong, D.; Saini, H.; Chu, H.H.; Mashaghi, S.; Ham, S.L.; Singh, S.; Tavana, H.; Mosadegh, B.; Nikkhah, M. Advanced Biomaterials and Microengineering Technologies to Recapitulate the Stepwise Process of Cancer Metastasis. Biomaterials 2017, 133, 176–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef]
- Poggi, A.; Villa, F.; Fernadez, J.L.C.; Costa, D.; Zocchi, M.R.; Benelli, R. Three-Dimensional Culture Models to Study Innate Anti-Tumor Immune Response: Advantages and Disadvantages. Cancers 2021, 13, 3417. [Google Scholar]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an In Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Tian, C.; Tu, Q.; Liu, W.; Wang, J. Recent Advances in Microfluidic Technologies for Organ-on-a-Chip. Trac-Trends Anal. Chem. 2019, 117, 146–156. [Google Scholar] [CrossRef]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveir, J.M.; Reis, R.L.; Correlo, V.M. Emerging Tumor Spheroids Technologies for 3d in Vitro Cancer Modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in Establishment and Analysis of Three-Dimensional Tumor Spheroid-Based Functional Assays for Target Validation and Drug Evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3d Platform to Explore Cell–Ecm Interactions and Drug Resistance of Epithelial Ovarian Cancer Cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Sung, K.E.; Beebe, D.J. Microfluidic 3d Models of Cancer. Adv. Drug Deliv. Rev. 2014, 79–80, 68–78. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Matsusaki, M.; Asano, Y.; Shimoda, H.; Akashi, M. Effects of Angiogenic Factors and 3d-Microenvironments on Vascularization within Sandwich Cultures. Biomaterials 2014, 35, 4739–4748. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3d Bioprinting of Functional Human Skin: Production and In Vivo Analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Cortes, E.D.; Molina, C.M.; Rodriguez-Lorenzo, L.; Cubo-Mateo, N. Generation of Controlled Micrometric Fibers inside Printed Scaffolds Using Standard Fdm 3d Printers. Polymers 2023, 15, 96. [Google Scholar] [CrossRef]
- Hughes, A.M.; Kolb, A.D.; Shupp, A.B.; Shine, K.M.; Bussard, K.M. Printing the Pathway Forward in Bone Metastatic Cancer Research: Applications of 3d Engineered Models and Bioprinted Scaffolds to Recapitulate the Bone–Tumor Niche. Cancers 2021, 13, 507. [Google Scholar]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3d Bioprinting of Tissue/Organ Models. Angew. Chem. Int. Ed. 2016, 55, 4650–4665. [Google Scholar] [CrossRef]
- Sánchez-Salazar, M.G.; Álvarez, M.M.; Santiago, G.T.-D. Advances in 3d Bioprinting for the Biofabrication of Tumor Models. Bioprinting 2021, 21, e00120. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Celada, L.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Chiara, M.D. Three-Dimensional Bioprinted Cancer Models: A Powerful Platform for Investigating Tunneling Nanotube-Like Cell Structures in Complex Microenvironments. Mater. Sci. Eng. C 2021, 128, 112357. [Google Scholar] [CrossRef]
- Sigaux, N.; Pourchet, L.; Breton, P.; Brosset, S.; Louvrier, A.; Marquette, C.A. 3D Bioprinting: Principles, fantasies and prospects. Oral Maxillofac. Surg. 2019, 120, 128–132. [Google Scholar]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 185–212. [Google Scholar]
- de Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in Cell Encapsulation from an Enveloped Cell Perspective. Adv. Drug Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef]
- Dani, S.; Ahlfeld, T.; Albrecht, F.; Duin, S.; Kluger, P.; Lode, A.; Gelinsky, M. Homogeneous and Reproducible Mixing of Highly Viscous Biomaterial Inks and Cell Suspensions to Create Bioinks. Gels 2021, 7, 227. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Guduric, V.; Duin, S.; Akkineni, A.R.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo-Mateo, N.; Dani, S.; Witzleben, M.V.; et al. Methylcellulose-a Versatile Printing Material That Enables Biofabrication of Tissue Equivalents with High Shape Fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef]
- Schütz, K.; Placht, A.-M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-Dimensional Plotting of a Cell-Laden Alginate/Methylcellulose Blend: Towards Biofabrication of Tissue Engineering Constructs with Clinically Relevant Dimensions. J. Tissue Eng. Regen. Med. 2017, 11, 1574–1587. [Google Scholar]
- Rasheed, Z.A.; Matsui, W.; Maitra, A. Pathology of Pancreatic Stroma in Pdac. In Pancreatic Cancer and Tumor Microenvironment; Munshi, H.G., Grippo, P.J., Eds.; Transworld Research Network: Trivandrum, India, 2012. [Google Scholar]
- Gonzalez, I.; Luzuriaga, J.; Valdivieso, A.; Candil, M.; Frutos, J.; Lopez, J.; Hernandez, L.; Rodriguez-Lorenzo, L.; Yaguee, V.; Blanco, J.L.; et al. Low-Intensity Continuous Ultrasound to Inhibit Cancer Cell Migration. Front. Cell Dev. Biol. 2023, 10, 842965. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Synthesis of in-Situ Silica-Alginate Hybrid Hydrogels by a Sol-Gel Route. Carbohydr. Polym. 2020, 250, 116877. [Google Scholar] [CrossRef]
- Chernecky, C.C.; Berger, B.J. Laboratory Tests & Diagnostic Procedures; Elsevier/Saunders: St. Louis, MO, USA, 2013. [Google Scholar]
- Deer, E.L.; Gonzalez-Hernandez, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef]
- Cukjati, D.; Rebersek, S.; Miklavcic, D. A Reliable Method of Determining Wound Healing Rate. Med. Biol. Eng. Comput. 2001, 39, 263–271. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3d Bioprinting of Human Chondrocyte-Laden Nanocellulose Hydrogels for Patient-Specific Auricular Cartilage Regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Li, L. Rheological Study on 3d Printability of Alginate Hydrogel and Effect of Graphene Oxide. Int. J. Bioprint. 2016, 2, 54–66. [Google Scholar] [CrossRef]
- Verma, M. Personalized Medicine and Cancer. J. Pers. Med. 2012, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Bertotti, A.; Leto, S.M.; Vetrano, S. Patient-Derived Tumor Models: A More Suitable Tool for Pre-Clinical Studies in Colorectal Cancer. J. Exp. Clin. Cancer Res. 2021, 40. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Kozinski, J.A.; Chen, X.; Hwang, D.K. Modeling Mechanical Cell Damage in the Bioprinting Process Employing a Conical Needle. J. Mech. Med. Biol. 2015, 15, 1550073. [Google Scholar] [CrossRef]
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human Platelet Lysate-Based Nanocomposite Bioink for Bioprinting Hierarchical Fibrillar Structures. Biofabrication 2019, 12, 015012. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink Properties before, During and after 3d Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Rubiano, A.; Delitto, D.; Han, S.; Gerber, M.; Galitz, C.; Trevino, J.; Thomas, R.M.; Hughes, S.J.; Simmons, C.S. Viscoelastic Properties of Human Pancreatic Tumors and in Vitro Constructs to Mimic Mechanical Properties. Acta Biomater. 2018, 67, 331–340. [Google Scholar] [CrossRef]
- Nicolle, S.; Noguer, L.; Palierne, J.F. Shear Mechanical Properties of the Porcine Pancreas: Experiment and Analytical Modelling. J. Mech. Behav. Biomed. Mater. 2013, 26, 90–97. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3d Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Kulseng, B.; Skjak-Braek, G.; Ryan, L.; Andersson, A.; King, A.; Faxvaag, A.; Espevik, T. Transplantation of Alginate Microcapsules-Generation of Antibodies against Alginates and Encapsulated Porcine Islet-Like Cell Clusters. Transplantation 1999, 67, 978–984. [Google Scholar] [CrossRef]
- Ashworth, J.C.; Thompson, J.L.; James, J.R.; Slater, C.E.; Pijuan-Galitó, S.; Lis-Slimak, K.; Holley, R.J.; Meade, K.A.; Thompson, A.; Arkill, K.P.; et al. Peptide Gels of Fully-Defined Composition and Mechanics for Probing Cell-Cell and Cell-Matrix Interactions In Vitro. Matrix Biol. 2020, 85–86, 15–33. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct Msc Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Aravind, A.; Emma, P.; Christophe, M.; Edwin-Joffrey, C. Rheology, Simulation and Data Analysis toward Bioprinting Cell Viability Awareness. Bioprinting 2021, 21, e00119. [Google Scholar]
- Blaeser, A.; Campos, D.F.D.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3d Bioprinting Is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The Cell in the Ink: Improving Biofabrication by Printing Stem Cells for Skeletal Regenerative Medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Longati, P.; Jia, X.; Eimer, J.; Wagman, A.; Witt, M.-R.; Rehnmark, S.; Verbeke, C.; Toftgård, R.; Löhr, M.; Heuchel, R.L. 3D Pancreatic Carcinoma Spheroids Induce a Matrix-Rich, Chemoresistant Phenotype Offering a Better Model for Drug Testing. BMC Cancer 2013, 13, 95. [Google Scholar] [CrossRef]
- Gorchs, L.; Ahmed, S.; Mayer, C.; Knauf, A.; Moro, C.F.; Svensson, M.; Heuchel, R.; Rangelova, E.; Bergman, P.; Kaipe, H. The Vitamin D Analogue Calcipotriol Promotes an Anti-Tumorigenic Phenotype of Human Pancreatic Cafs but Reduces T Cell Mediated Immunity. Sci. Rep. 2020, 10, 17444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banda Sánchez, C.; Cubo Mateo, N.; Saldaña, L.; Valdivieso, A.; Earl, J.; González Gómez, I.; Rodríguez-Lorenzo, L.M. Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling. Polymers 2023, 15, 3196. https://doi.org/10.3390/polym15153196
Banda Sánchez C, Cubo Mateo N, Saldaña L, Valdivieso A, Earl J, González Gómez I, Rodríguez-Lorenzo LM. Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling. Polymers. 2023; 15(15):3196. https://doi.org/10.3390/polym15153196
Chicago/Turabian StyleBanda Sánchez, Cristina, Nieves Cubo Mateo, Laura Saldaña, Alba Valdivieso, Julie Earl, Itziar González Gómez, and Luis M. Rodríguez-Lorenzo. 2023. "Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling" Polymers 15, no. 15: 3196. https://doi.org/10.3390/polym15153196
APA StyleBanda Sánchez, C., Cubo Mateo, N., Saldaña, L., Valdivieso, A., Earl, J., González Gómez, I., & Rodríguez-Lorenzo, L. M. (2023). Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling. Polymers, 15(15), 3196. https://doi.org/10.3390/polym15153196