Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
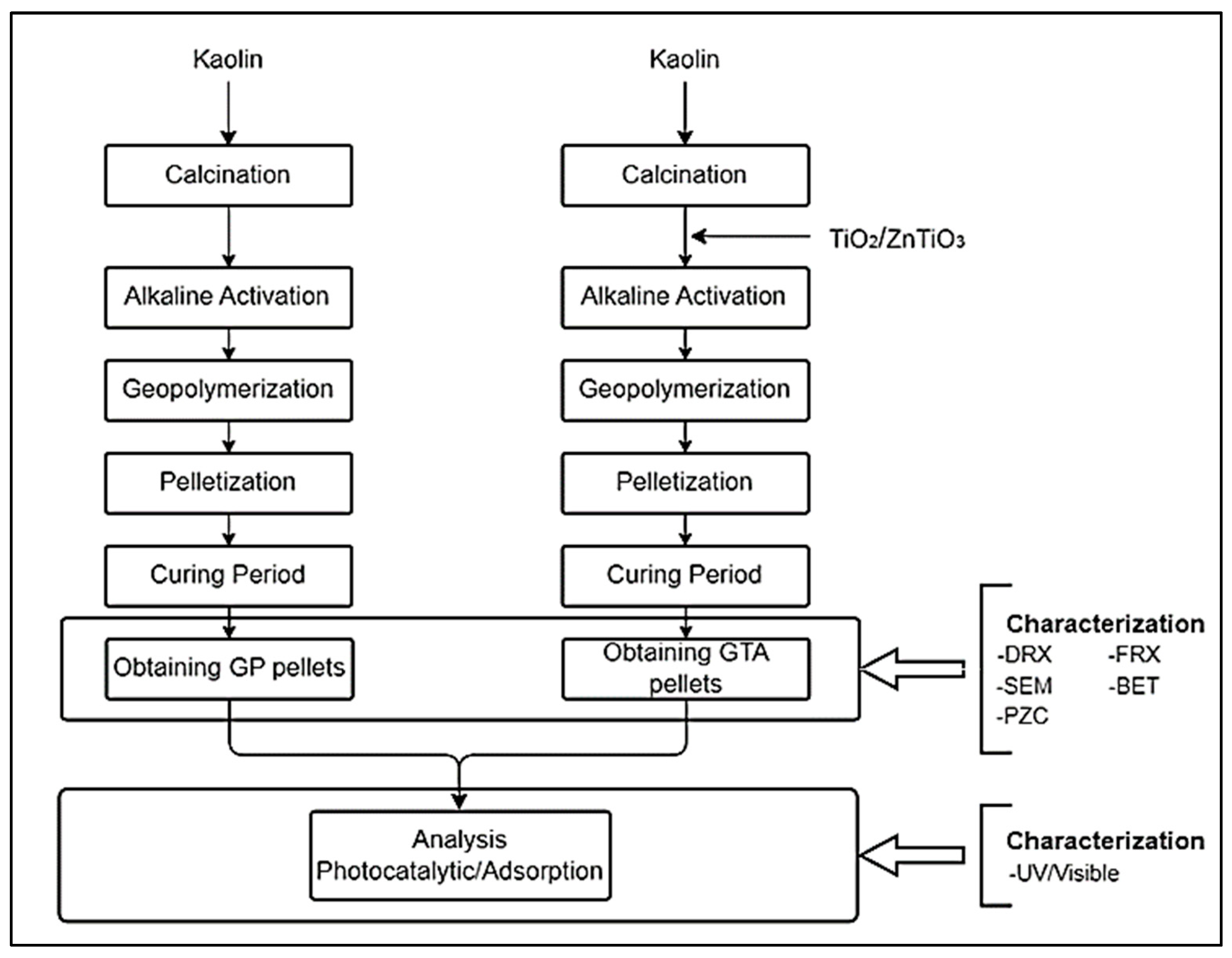
2.2. Synthesis of GP and GTA Compounds
2.3. Characterization
2.4. Adsorption Studies
2.5. Photodegradation Studies
2.6. Total Efficiency and Reuse
3. Results
3.1. Characterization
3.1.1. XRD Analysis
3.1.2. SEM-EDX Analysis
3.1.3. Point of Zero Charge (PZC) and Specific Surface Area (SSA) Analysis
3.2. Adsorption Studies
3.2.1. Effect of Solution pH
3.2.2. Effect of Initial Dye Concentration
3.2.3. Effect of Temperature
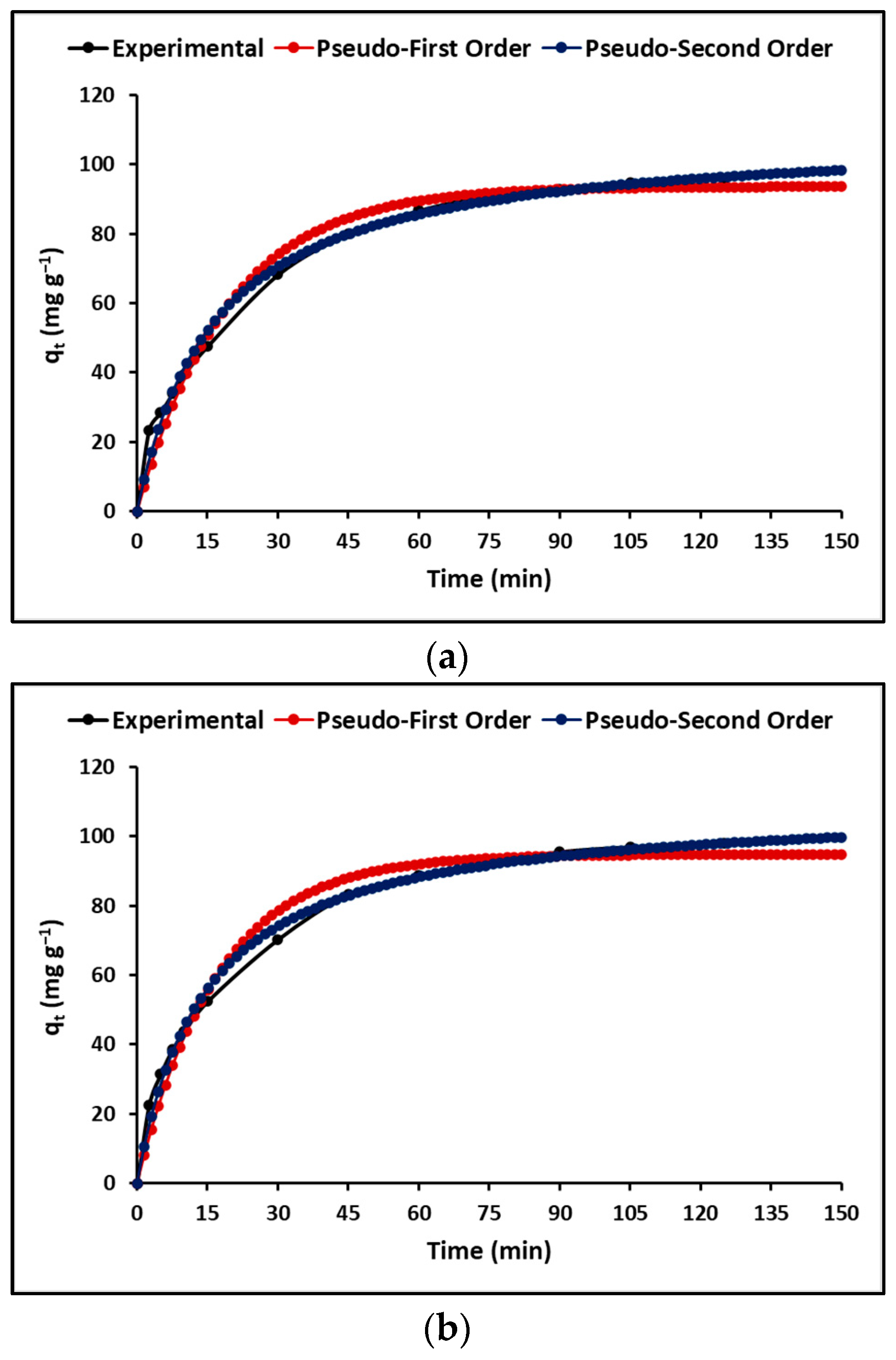
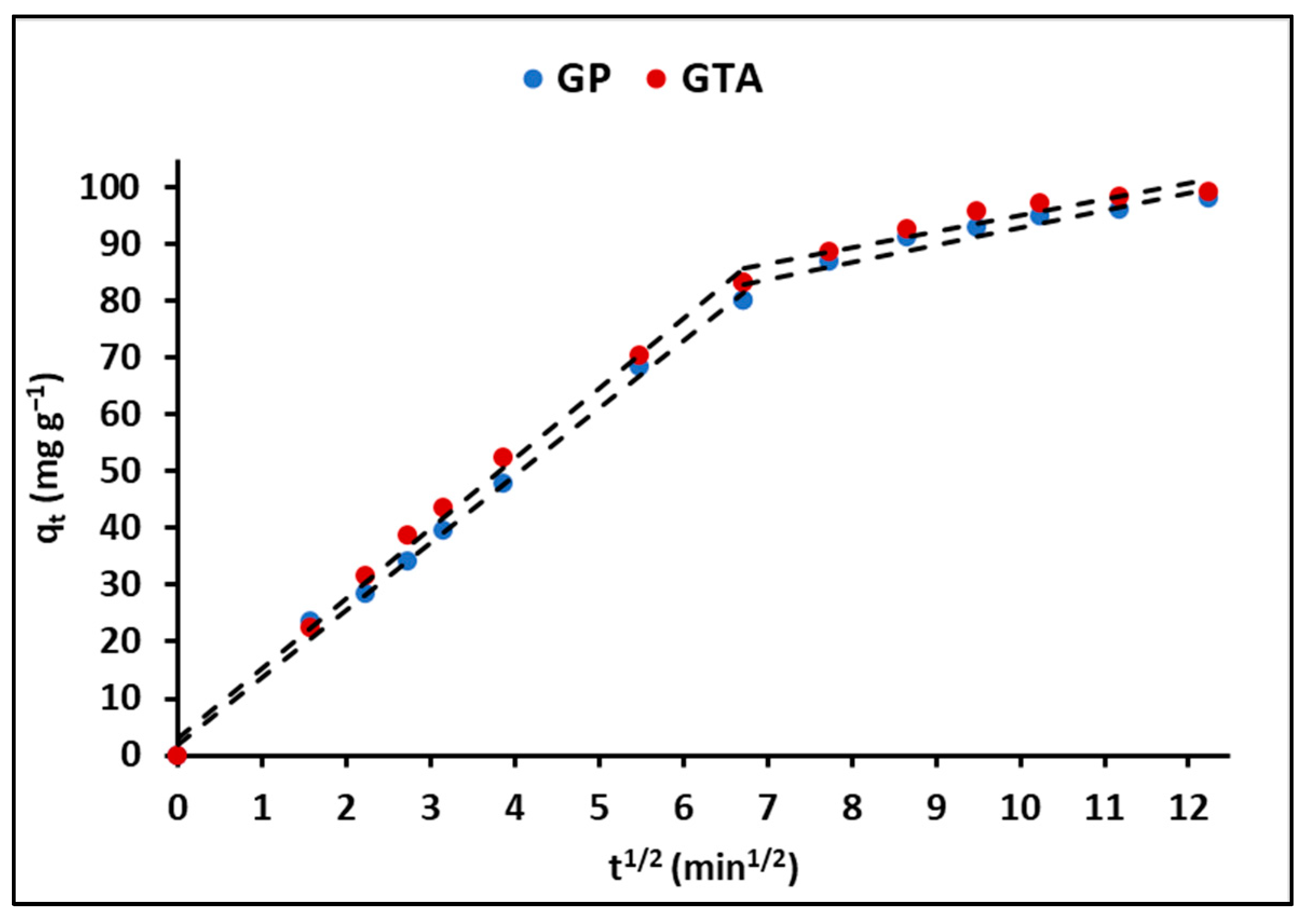
3.2.4. Effect of Contact Time
3.3. Photocatalytic Studies
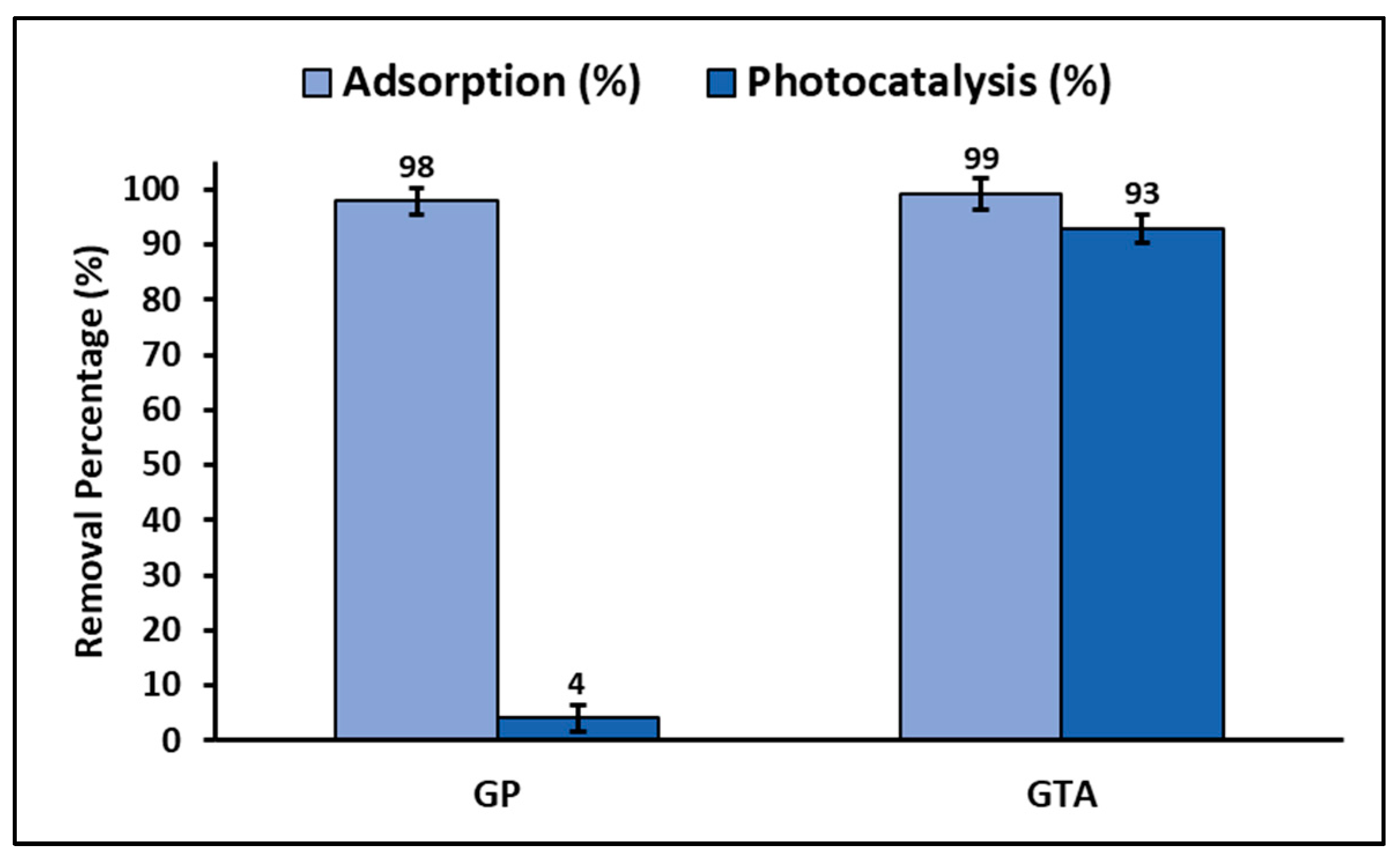
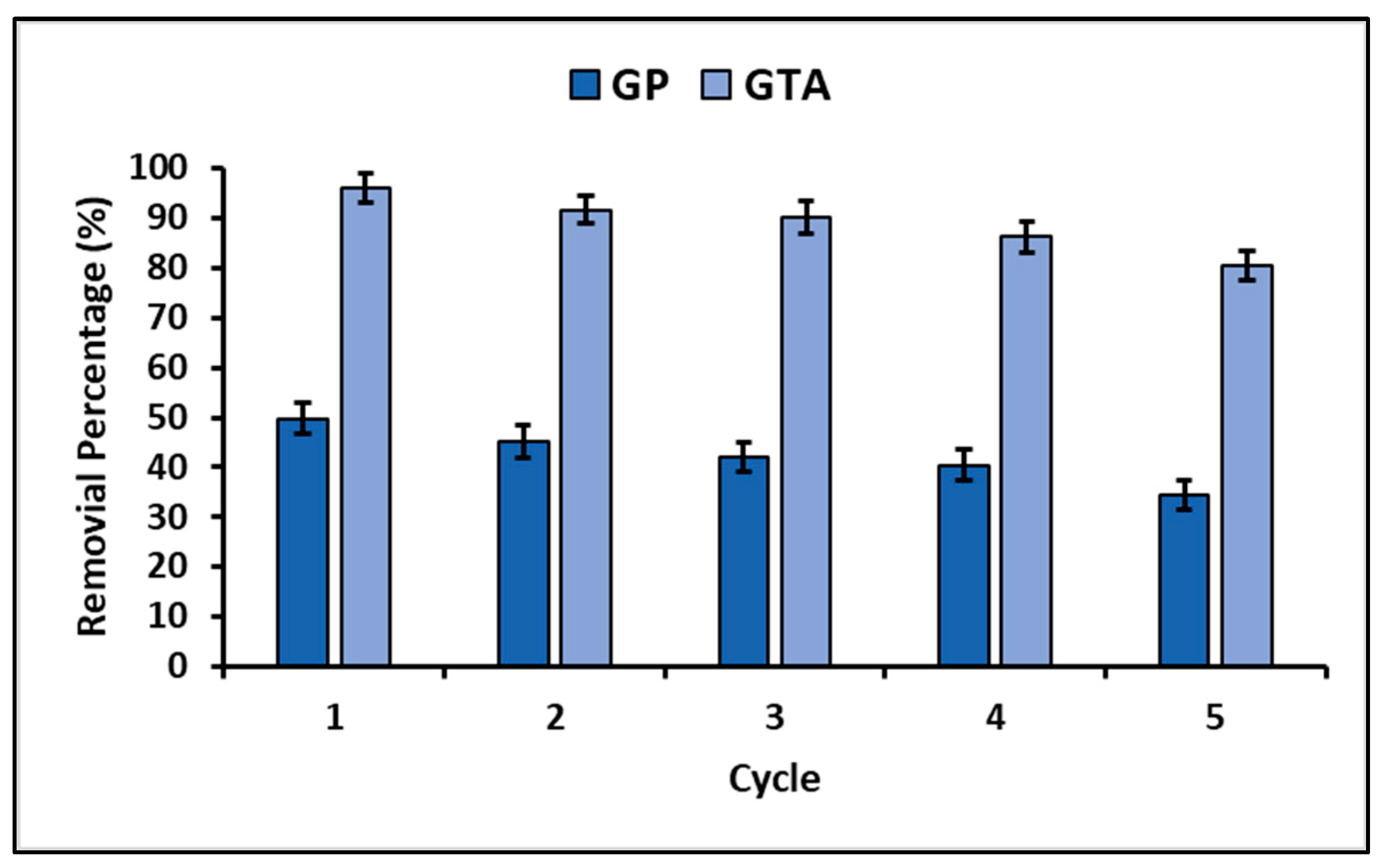
3.4. Total Efficiency and Reuse
4. Discussion
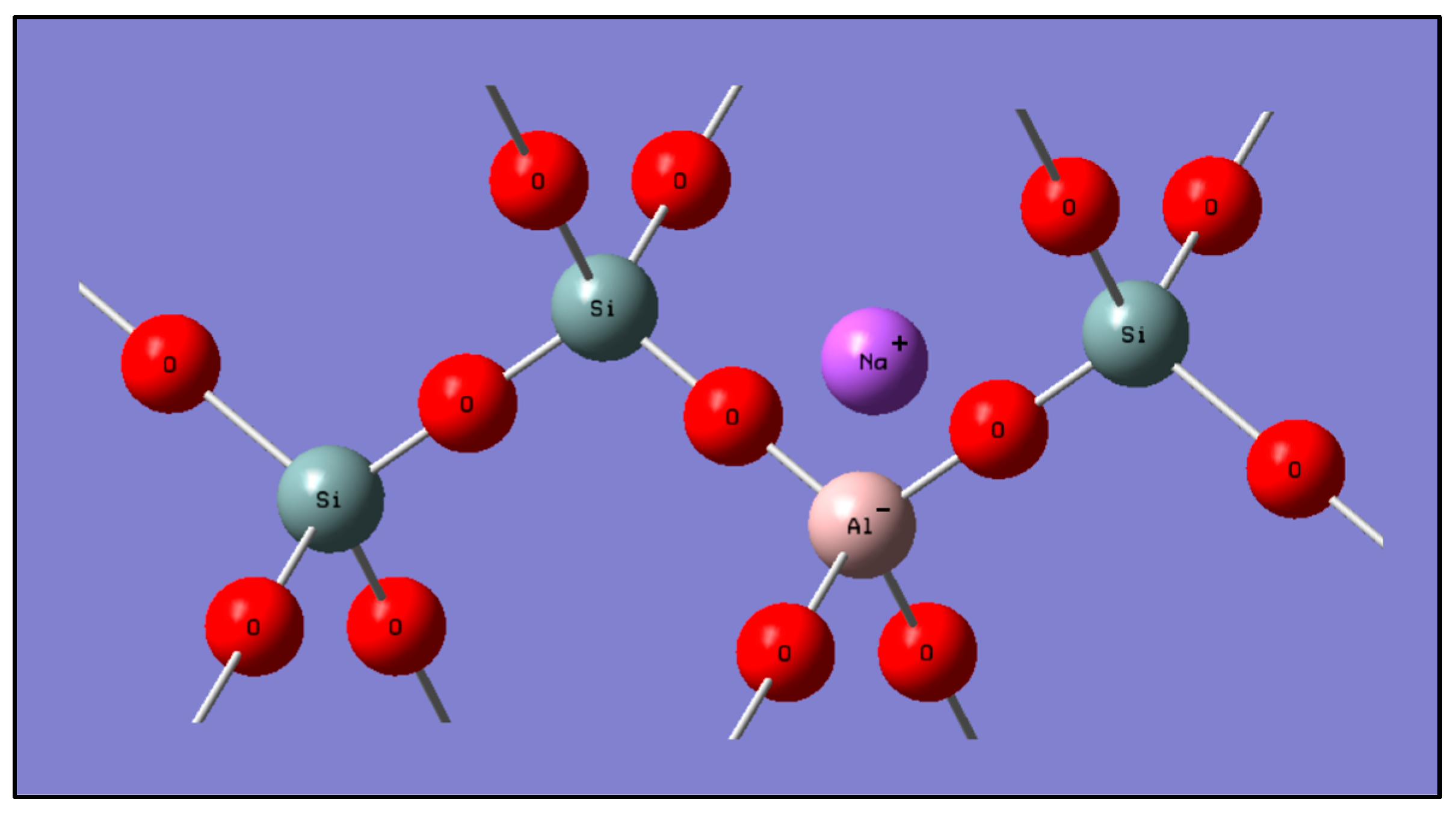
4.1. Characterization
4.1.1. XRD and XRF Analysis
4.1.2. SEM-EDX Analysis
4.1.3. Specific Surface Area (SSA) and Point of Zero Charge (PZC) Analysis
4.2. Adsorption Studies
4.2.1. Effect of Solution pH
4.2.2. Effect of Initial Dye Concentration
4.2.3. Effect of Temperature
4.2.4. Effect of Contact Time
4.3. Photocatalytic Studies
4.4. Total Efficiency and Reuse
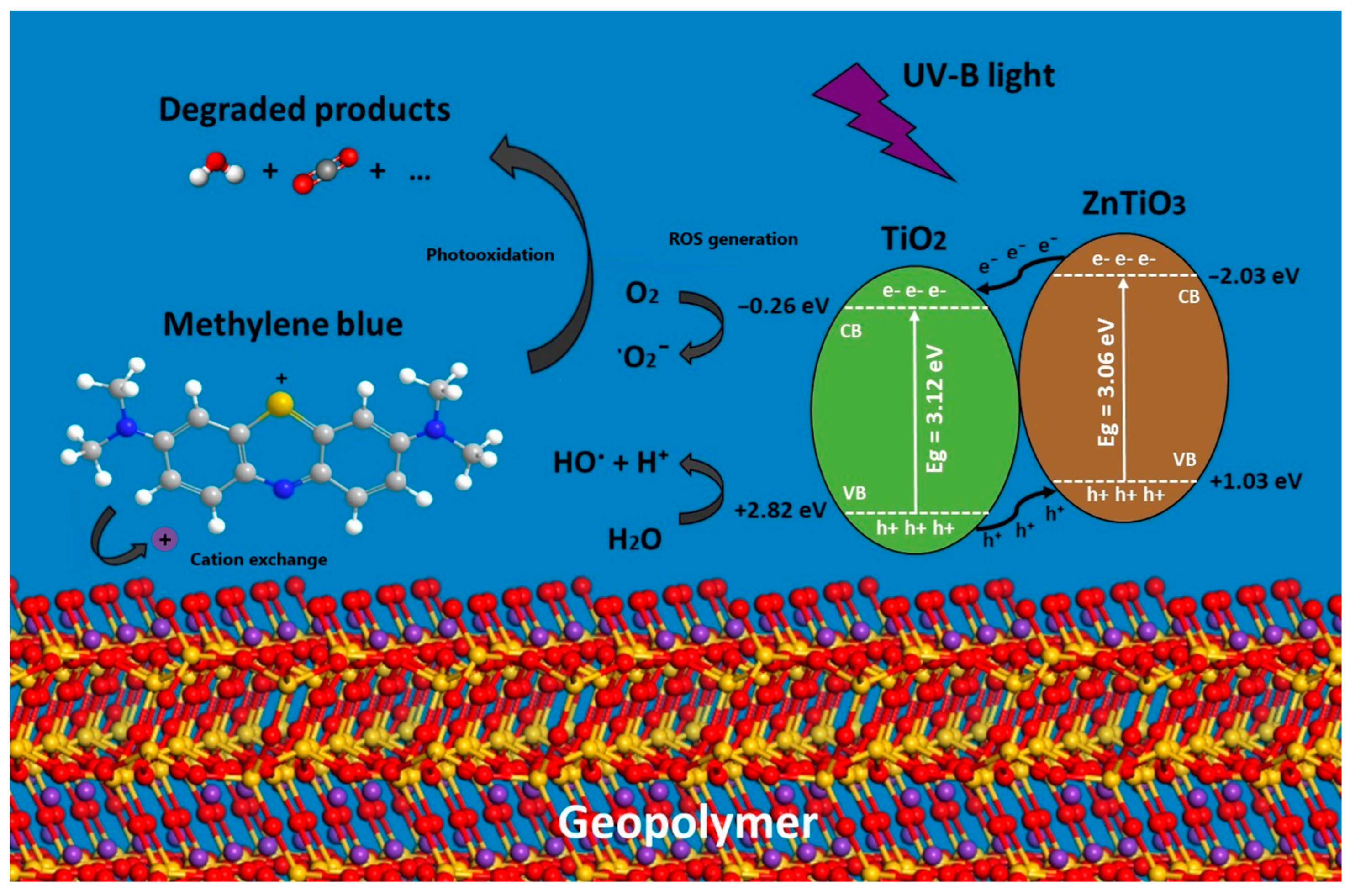
4.5. Proposed Mechanism of Removal of the MB Dye
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Kumar Ponnusamy, V.; Ashokkumar, M. A review on hybrid techniques for the degradation of organic pollutants in aqueous environment. Ultrason. Sonochem. 2020, 67, 105130. [Google Scholar] [CrossRef] [PubMed]
- Salh, D.M.; Aziz, B.K.; Kaufhold, S. High Adsorption Efficiency of Topkhana Natural Clay for Methylene Blue from Medical Laboratory Wastewater: A Linear and Nonlinear Regression. Silicon 2020, 12, 87–99. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Sahu, J.K.; Sahu, U.K.; Patel, R.K. Kendu (Diospyros melanoxylon Roxb) fruit peel activated carbon—An efficient bioadsorbent for methylene blue dye: Equilibrium, kinetic, and thermodynamic study. Environ. Sci. Pollut. Res. 2020, 27, 22579–22592. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X.; Li, F. Methylene blue adsorption properties of mechanochemistry modified coal fly ash. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 2133–2141. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study. Nanomaterials 2022, 12, 3137. [Google Scholar] [CrossRef]
- Qi, J.; Hou, Y.; Hu, J.; Ruan, W.; Xiang, Y.; Wei, X. Decontamination of methylene Blue from simulated wastewater by the mesoporous rGO/Fe/Co nanohybrids: Artificial intelligence modeling and optimization. Mater. Today Commun. 2020, 24, 100709. [Google Scholar] [CrossRef]
- Srivastava, A.; Rani, R.M.; Patle, D.S.; Kumar, S. Emerging bioremediation technologies for the treatment of textile wastewater containing synthetic dyes: A comprehensive review. J. Chem. Technol. Biotechnol. 2022, 97, 26–41. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Kumar, V.; Shah, M.P. Advanced oxidation processes for complex wastewater treatment. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–31. ISBN 9780128210116. [Google Scholar]
- Ji, Z.; Yang, X.; Qi, X.; Zhang, H.; Zhang, Y.; Xia, X.; Pei, Y. Facile synthesis of waste-based CdS-loaded hierarchically porous geopolymer for adsorption-photocatalysis of organic contamination and its environmental risks. Chemosphere 2022, 308, 136144. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Han, Z.C.; He, P.Y.; Chen, H. Geopolymer-based catalysts for cost-effective environmental governance: A review based on source control and end-of-pipe treatment. J. Clean. Prod. 2020, 263, 121556. [Google Scholar] [CrossRef]
- Shah, U.; Jan, F.A.; Ullah, R.; Wajidullah; Salman; Ullah, N. Tin Decorated Zinc Sulphide Nanoparticles for Photocatalytic Degradation of Bromophenol Blue Dye and Their Therapeutic Applications: A Kinetic and Thermodynamic Approach. ECS J. Solid State Sci. Technol. 2022, 11, 033011. [Google Scholar] [CrossRef]
- Boikanyo, D.; Masheane, M.L.; Nthunya, L.N.; Mishra, S.B.; Mhlanga, S.D. Carbon-supported photocatalysts for organic dye photodegradation. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–138. ISBN 9780128110348. [Google Scholar]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin, G.A.N.; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Pawar, M.; Sendoǧdular, S.T.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Farhadyar, N.; Sadr, M.H. Improving the photocatalytic performance of a perovskite ZnTiO3 through ZnTiO3@S nanocomposites for degradation of Crystal violet and Rhodamine B pollutants under sunlight. Inorg. Chem. Commun. 2020, 119, 108091. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N.; Zamanian, A. Synthesis of cubic and hexagonal ZnTiO3 as catalyst support in steam reforming of methanol: Study of physical and chemical properties of copper catalysts on the H2 and CO selectivity and coke formation. Int. J. Hydrog. Energy 2020, 45, 9484–9495. [Google Scholar] [CrossRef]
- Mofokeng, S.J.; Noto, L.L.; Kroon, R.E.; Ntwaeaborwa, O.M.; Dhlamini, M.S. Up-conversion luminescence and energy transfer mechanism in ZnTiO3: Er3+,Yb3+ phosphor. J. Lumin. 2020, 223, 117192. [Google Scholar] [CrossRef]
- Singh, S.; Perween, S.; Ranjan, A. Enhancing the visible light induced photocatalytic activity in sol-electrospun zinc titanate nanopowders by nitrogen doping. J. Phys. Chem. Solids 2021, 158, 110221. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Hernández, K.; González, S. Cu(C3H3N3S3)3 Adsorption onto ZnTiO3/TiO2 for Coordination-Complex Sensitized Photochemical Applications. Materials 2022, 15, 3252. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, L.; Gbozee, M. Thermal stability of geopolymers used as supporting materials for TiO2 film coating through sol-gel process: Feasibility and improvement. Constr. Build. Mater. 2016, 125, 1114–1126. [Google Scholar] [CrossRef]
- Koleżyński, A.; Król, M.; Żychowicz, M. The structure of geopolymers—Theoretical studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- El Alouani, M.; Saufi, H.; Moutaoukil, G.; Alehyen, S.; Nematollahi, B.; Belmaghraoui, W.; Taibi, M. Application of geopolymers for treatment of water contaminated with organic and inorganic pollutants: State-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 105095. [Google Scholar] [CrossRef]
- Mohd Mortar, N.A.; Abdullah, M.M.A.B.; Abdul Razak, R.; Abd Rahim, S.Z.; Aziz, I.H.; Nabiałek, M.; Jaya, R.P.; Semenescu, A.; Mohamed, R.; Ghazali, M.F. Geopolymer Ceramic Application: A Review on Mix Design, Properties and Reinforcement Enhancement. Materials 2022, 15, 7567. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C.G. Calcined kaolinite-bentonite clay blends as supplementary cementitious materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouna, L.; Hanna, J.V.; Benlhachemi, A.; Pilsworth, H.L.; Bouddouch, A.; Bakiz, B. Pyrophyllite clay-derived porous geopolymers for removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2023, 296, 127281. [Google Scholar] [CrossRef]
- Yu, H.; Xue Xu, M.; Chen, C.; He, Y.; Cui, X. A review on the porous geopolymer preparation for structural and functional materials applications. Int. J. Appl. Ceram. Technol. 2022, 19, 1793–1813. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Biomass fly ash geopolymer monoliths for effective methylene blue removal from wastewaters. J. Clean. Prod. 2018, 171, 783–794. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Wang, Q.; Chang, Q.; Li, C. Rapid removal of methylene blue and nickel ions and adsorption/desorption mechanism based on geopolymer adsorbent. Colloids Interface Sci. Commun. 2021, 45, 100551. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Tirado, J.R.; Manzano-Ramírez, A.; Vazquez-Landaverde, P.A.; Herrera-Díaz, E.I.; Rodríguez-Ugarte, M.E.; Rubio-Ávalos, J.C.; Amigó-Borrás, V.; Chávez-Páez, M. Ion-exchanged geopolymer for photocatalytic degradation of a volatile organic compound. Mater. Lett. 2014, 134, 222–224. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Bai, C.; Yang, K.; Zheng, T.; Lu, S.; Li, H.; Qiao, Y.; Colombo, P. Porous alkali-activated material from hypergolic coal gangue by microwave foaming for methylene blue removal. J. Am. Ceram. Soc. 2023, 106, 1473–1489. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.A.; Medina, F. Synthesis of the ZnTio3/Tio2 nanocomposite supported in ecuadorian clays for the adsorption and photocatalytic removal of methylene blue dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 2019, 4212901. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Fabrication of nanocomposite materials. In Mechanical Alloying; William Andrew Publishing: Norwich, UK, 2015; pp. 152–181. ISBN 978-1-4557-7752-5. [Google Scholar]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Benmessaoud, A.; Nibou, D.; Mekatel, E.H.; Amokrane, S. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020, 39, 153–171. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of ZnTio3 /Tio2 adsorbents for the removal of methylene blue, using zeolite precursor clays as natural additives. Nanomaterials 2021, 11, 898. [Google Scholar] [CrossRef]
- Eke-emezie, N.; Etuk, B.R.; Akpan, O.P.; Chinweoke, O.C. Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl. Water Sci. 2022, 12, 157. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Hashemian, S.; Shayesteh, M.R. Kinetics and thermodynamics of cyanide removal by ZnO@NiO nanocrystals. Trans. Nonferrous Met. Soc. China 2017, 27, 1394–1403. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-doped ZnTio3 /Tio2 nanocomposite supported on ecuadorian diatomaceous earth as a highly efficient photocatalyst driven by solar light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.; Falcinelli, S.; Rol, C.; Rosi, M.; Sebastiani, G.V. Gas-Phase TiO2 Photosensitized Mineralization of Some VOCs: Mechanistic Suggestions through a Langmuir-Hinshelwood Kinetic Approach. Catalysts 2020, 11, 20. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2021, 18, 297–316. [Google Scholar] [CrossRef]
- Ke, S.; Cheng, X.; Wang, Q.; Wang, Y.; Pan, Z. Preparation of a photocatalytic TiO2/ZnTiO3 coating on glazed ceramic tiles. Ceram. Int. 2014, 40, 8891–8895. [Google Scholar] [CrossRef]
- Maulana, I.; Takahashi, F. Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J. Water Process Eng. 2018, 22, 80–86. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Biswas, P.; Bhunia, P.; Saha, P.; Sarkar, S.; Chandel, H.; De, S. In situ photodecyanation of steel industry wastewater in a pilot scale. Environ. Sci. Pollut. Res. 2020, 27, 33226–33233. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment—A review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Blanco, I.; D’Angelo, A.; Viola, V.; Vertuccio, L.; Catauro, M. Metakaolin-based geopolymers filled with volcanic fly ashes: FT-IR, thermal characterization, and antibacterial property. Sci. Eng. Compos. Mater. 2023, 30, 20220192. [Google Scholar] [CrossRef]
- Dahal, S. Synthesis of Alkali Activated Geopolymer Cement from Clay. J. Civ. Environ. Eng. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- An, F.; Liu, J.; Xu, Z.; Zheng, S. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution. Water Sci. Technol. 2020, 82, 3023–3031. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef]
- Cigdem, S.O. Adsorption and desorption kinetics behaviour of methylene blue onto activated carbon. Physicochem. Probl. Miner. Process. 2012, 48, 441–454. [Google Scholar] [CrossRef]
- Aldegs, Y.; Elbarghouthi, M.; Elsheikh, A.; Walker, G. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Baraka, A. Adsorptive removal of tartrazine and methylene blue from wastewater using melamine-formaldehyde-tartaric acid resin (and a discussion about pseudo second order model). Desalin. Water Treat. 2012, 44, 128–141. [Google Scholar] [CrossRef]
- Hu, J.; Dai, W.; Yan, X. Comparison study on the adsorption performance of methylene blue and congo red on Cu-BTC. Desalin. Water Treat. 2016, 57, 4081–4089. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Falah, M.; Mackenzie, K.J.D. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts 2020, 10, 1158. [Google Scholar] [CrossRef]
- Van Hung, N.; Nguyet, B.T.M.; Nghi, N.H.; Khieu, D.Q. Photocatalytic Degradation of Methylene Blue by Using ZnO/Longan Seed Activated Carbon Under Visible-Light Region. J. Inorg. Organomet. Polym. Mater. 2021, 31, 446–459. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.F.; Medina, F.; González, S. Dft study of methylene blue adsorption on ZnTio3 and Tio2 surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Muthirulan, P.; Nirmala Devi, C.; Meenakshi Sundaram, M. Synchronous role of coupled adsorption and photocatalytic degradation on CAC–TiO2 composite generating excellent mineralization of alizarin cyanine green dye in aqueous solution. Arab. J. Chem. 2017, 10, S1477–S1483. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Shakeri, M.; Emdadi, Z.; Samsudin, N.A.; Soltani, S.; Mohammad, M.; Amin, N. Development of green photocatalytic geopolymers for dye removal. Mater. Chem. Phys. 2022, 283, 126020. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Tobaldi, D.M.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Synthesis of porous biomass fly ash-based geopolymer spheres for efficient removal of methylene blue from wastewaters. J. Clean. Prod. 2019, 207, 350–362. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Alshaaer, M.; Khalili, F.; Khoury, H. The influence of using Jordanian natural zeolite on the adsorption, physical, and mechanical properties of geopolymers products. J. Hazard. Mater. 2009, 165, 379–387. [Google Scholar] [CrossRef]
- Khan, M.I.; Min, T.K.; Azizli, K.; Sufian, S.; Ullah, H.; Man, Z. Effective removal of methylene blue from water using phosphoric acid based geopolymers: Synthesis, characterizations and adsorption studies. RSC Adv. 2015, 5, 61410–61420. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, Y.J.; Chen, H.; He, P.Y.; Meng, Q. Development of porous and reusable geopolymer adsorbents for dye wastewater treatment. J. Clean. Prod. 2022, 348, 131278. [Google Scholar] [CrossRef]
- Kaewmee, P.; Song, M.; Iwanami, M.; Tsutsumi, H.; Takahashi, F. Porous and reusable potassium-activated geopolymer adsorbent with high compressive strength fabricated from coal fly ash wastes. J. Clean. Prod. 2020, 272, 122617. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mor, M.; Vaccari, A.; Natali Murri, A.; Piotte, L.; Melandri, C.; Landi, E. Mechanical strength and cationic dye adsorption ability of metakaolin-based geopolymer spheres. Appl. Clay Sci. 2020, 193, 105678. [Google Scholar] [CrossRef]
- Falah, M.; MacKenzie, K.J.D. Synthesis and properties of novel photoactive composites of P25 titanium dioxide and copper (I) oxide with inorganic polymers. Ceram. Int. 2015, 41, 13702–13708. [Google Scholar] [CrossRef]
- Padmapriya, M.; Ramesh, S.T.; Biju, V.M. Synthesis of seawater based geopolymer: Characterization and adsorption capacity of methylene blue from wastewater. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 51, pp. 1770–1776. [Google Scholar]
- El-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Hu, X.S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Varmazyar, A.; Sedaghat, S.; Khalaj, M. Highly efficient removal of methylene blue by a synthesized TiO2/montmorillonite-albumin nanocomposite: Kinetic and isothermal analysis in water. RSC Adv. 2017, 7, 37214–37219. [Google Scholar] [CrossRef]
- Marsiezade, N.; Javanbakht, V. Novel hollow beads of carboxymethyl cellulose/ZSM-5/ZIF-8 for dye removal from aqueous solution in batch and continuous fixed bed systems. Int. J. Biol. Macromol. 2020, 162, 1140–1152. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Guo, D.X.; Li, S.F.; Cao, J.P.; Wei, X.Y. Removal of methylene blue by nax zeolites synthesized from coal gasification fly ash using an alkali fusion-hydrothermal method. Desalin. Water Treat. 2020, 185, 355–363. [Google Scholar] [CrossRef]
- Xu, R.; Mao, J.; Peng, N.; Luo, X.; Chang, C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef]
- Bée, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef]
- Marrakchi, F.; Bouaziz, M.; Hameed, B.H. Activated carbon–clay composite as an effective adsorbent from the spent bleaching sorbent of olive pomace oil: Process optimization and adsorption of acid blue 29 and methylene blue. Chem. Eng. Res. Des. 2017, 128, 221–230. [Google Scholar] [CrossRef]
- Woolard, C.D.; Strong, J.; Erasmus, C.R. Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl. Geochem. 2002, 17, 1159–1164. [Google Scholar] [CrossRef]
- Ge, S.; Geng, W.; He, X.; Zhao, J.; Zhou, B.; Duan, L.; Wu, Y.; Zhang, Q. Effect of framework structure, pore size and surface modification on the adsorption performance of methylene blue and Cu2+ in mesoporous silica. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 154–162. [Google Scholar] [CrossRef]
- Sahoo, S.; Uma; Banerjee, S.; Sharma, Y.C. Application of natural clay as a potential adsorbent for the removal of a toxic dye from aqueous solutions. Desalin. Water Treat. 2014, 52, 6703–6711. [Google Scholar] [CrossRef]
- Isabel Bravo, P.; Shimizu, E.; Alvin Malenab, R.; Anne Tigue, A.; Mae Dela Cerna, K.; Isagani Janairo, J.; Angelo Promentilla, M.; Ethelbhert Yu, D. Nanocrystalline Titania Coated Metakaolin and Rice Hull Ash Based Geopolymer Spheres for Photocatalytic Degradation of Dyes in Wastewater. Orient. J. Chem. 2019, 35, 167–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L. Fly ash-based geopolymer as a novel photocatalyst for degradation of dye from wastewater. Particuology 2013, 11, 353–358. [Google Scholar] [CrossRef]
- Pimraksa, K.; Setthaya, N.; Thala, M.; Chindaprasirt, P.; Murayama, M. Geopolymer/Zeolite composite materials with adsorptive and photocatalytic properties for dye removal. PLoS ONE 2020, 15, e0241603. [Google Scholar] [CrossRef]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red mud- and metakaolin-based geopolymers for adsorption and photocatalytic degradation of methylene blue: Towards self-cleaning construction materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Li, P.; Guo, H.; Yan, B.; Chen, D.; Zhao, W.; Cheng, Y.; Wang, K.; Li, P.; et al. A Porous Geopolymer Containing Ti-Bearing Blast Furnace Slag: Synthesis, Characterization, and Adsorption-Photodegradation Studies towards Methylene Blue Removal under Visible Light Condition. Molecules 2023, 28, 3673. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D.; Chen, M.; Li, D.; Zhu, J.; Lü, X.; Yan, C. Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 107–114. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 Photocatalysts toward Visible Photooxidation for Water and Wastewater Treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Takagi, H.; Fujishiro, Y.; Awano, M. Preparation and characterization of the Sb-doped TiO2 photocatalysts. J. Mater. Sci. 2001, 36, 949–955. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 2008, 260, 128–133. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Zhu, J.; Yang, D. A facile method to synthesize nitrogen and fluorine co-doped TiO2 nanoparticles by pyrolysis of (NH4)2TiF6. J. Nanopart. Res. 2008, 11, 303–313. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, F. Characterization, activity and mechanisms of a visible light driven photocatalyst: Manganese and iron co-modified TiO2 nanoparticles. Russ. J. Phys. Chem. A 2011, 85, 1825–1831. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M.S. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Rattanakam, R.; Supothina, S. Visible-light-sensitive N-doped TiO2 photocatalysts prepared by a mechanochemical method: Effect of a nitrogen source. Res. Chem. Intermed. 2009, 35, 263–269. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Y.J.; Kanbara, T.; Zhu, H.Z.; Xiao, C.F. Effects of I and F codoped TiO2 on the photocatalytic degradation of methylene blue. Desalination 2009, 249, 621–625. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Shen, F. Preparation and characterization of TiO2 photocatalysts co-doped with iron (III) and lanthanum for the degradation of organic pollutants. Appl. Surf. Sci. 2011, 257, 7671–7677. [Google Scholar] [CrossRef]
- Abirami, R.; Kalaiselvi, C.R.; Kungumadevi, L.; Senthil, T.S.; Kang, M. Synthesis and characterization of ZnTiO3 and Ag doped ZnTiO3 perovskite nanoparticles and their enhanced photocatalytic and antibacterial activity. J. Solid State Chem. 2020, 281, 121019. [Google Scholar] [CrossRef]









| Oxides | Kaolin | GP | GTA |
|---|---|---|---|
| Al2O3 | 22.20 (±1.12) | 19.62 (±1.07) | 11.77 (±0.92) |
| SiO2 | 37.80 (±2.07) | 35.22 (±2.15) | 21.13 (±1.84) |
| K2O | 1.53 (±0.10) | 1.33 (±0.15) | 0.82 (±0.01) |
| CaO | 1.92 (±0.13) | 1.65 (±0.13) | 1.04 (±0.09) |
| Fe2O3 | 0.43 (±0.02) | 0.36 (±0.03) | 0.22 (±0.01) |
| TiO2 | - | - | 21.15 (±1.42) |
| ZnO | - | - | 6.72 (±1.05) |
| Si/Al | 2.89 (±0.21) | 3.05 (±0.35) | 3.05 (±0.34) |
| Samples | C | O | Na | Al | Si | K | Ca | S | Ti | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaolin | 9.12 | 34.85 | 1.09 | 13.34 | 39.28 | 1.39 | 0.92 | - | - | - |
| GP | 8.44 | 34.75 | 1.73 | 13.19 | 38.78 | 1.72 | 0.73 | 0.65 | - | - |
| GTA | 5.39 | 29.17 | 0.96 | 4.17 | 12.72 | 0.97 | 0.19 | 0.37 | 34.89 | 11.16 |
| Samples | pHPZC | SSA (m2 g−1) |
|---|---|---|
| Kaolin (powder) | 4.6 | 36.8 |
| Geopolymer (powder) | 6.8 | 151.3 |
| Geopolymer + ZnTiO3/TiO2 (powder) | 7.2 | 290.2 |
| Geopolymer (pellet) | 6.8 | 97.8 |
| Geopolymer + ZnTiO3/TiO2 (pellet) | 7.2 | 187.7 |
| Isotherm Parameters | 293.15 K | 298.15 K | 303.15 K | ||||
|---|---|---|---|---|---|---|---|
| GP | GTA | GP | GTA | GP | GTA | ||
| Langmuir | qmax (mg g−1) | 48.97 (±1.33) | 61.96 (±2.52) | 53.87 (±1.46) | 68.16 (±2.17) | 58.76 (±1.60) | 74.35 (±3.02) |
| KL (L mg−1) | 0.33 (±0.03) | 0.37 (±0.05) | 0.40 (±0.04) | 0.46 (±0.06) | 0.48 (±0.03) | 0.54 (±0.04) | |
| RL | 0.13 | 0.12 | 0.11 | 0.10 | 0.09 | 0.08 | |
| χ2 | 2.01 | 1.47 | 2.21 | 1.62 | 2.43 | 1.78 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 0.98 | |
| Freundlich | KF (L mg−1) | 14.94 (±1.79) | 20.30 (±2.15) | 16.43 (±1.97) | 22.33 (±2.37) | 17.93 (±2.15) | 24.36 (±2.58) |
| n | 2.81 (±0.36) | 2.91 (±0.40) | 3.04 (±0.40) | 3.20 (±0.44) | 3.37 (±0.39) | 3.49 (±0.41) | |
| 1/n | 0.42 | 0.41 | 0.32 | 0.31 | 0.30 | 0.29 | |
| χ2 | 3.67 | 2.37 | 3.70 | 2.61 | 2.97 | 2.87 | |
| R2 | 0.94 | 0.95 | 0.96 | 0.95 | 0.95 | 0.94 | |
| Samples | Temperature (K) | ln kC | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1 K−1) |
|---|---|---|---|---|---|
| GP | 293.15 | 13.11 | −37.98 | −27.95 | 0.22 |
| 298.15 | 13.29 | −39.08 | |||
| 303.15 | 13.49 | −40.23 | |||
| GTA | 293.15 | 13.23 | −38.26 | −27.97 | 0.23 |
| 298.15 | 13.43 | −39.43 | |||
| 303.15 | 13.61 | −40.52 |
| Kinetic Parameters | GP | GTA | |
|---|---|---|---|
| Pseudo-first-order | qmax (mg g−1) | 93.63 (±2.24) | 94.83 (±2.17) |
| k1 (L mg−1) | 0.05 (±4.79 × 10−3) | 0.06 (±5.30 × 10−3) | |
| χ2 | 2.57 | 2.26 | |
| R2 | 0.98 | 0.98 | |
| Pseudo-second-order | qmax (mg g−1) | 109.11 (±2.34) | 109.21 (±1.74) |
| k2 (L mg−1) | 5.57 × 10−4 (±5.62 × 10−5) | 6.43 × 10−4 (±4.96 × 10−5) | |
| χ2 | 1.03 | 0.74 | |
| R2 | 0.99 | 0.99 | |
| Intraparticle diffusion | k3 (mg g−1 min−1/2) | 8.80 (±0.11) | 8.84 (±0.12) |
| A | 13.14 (±0.27) | 12.91 (±0.29) | |
| R2 | 0.95 | 0.95 | |
| External-film diffusion | Df (m2 min−1) | 5.84 × 10−12 | 6.77 × 10−12 |
| R2 | 0.96 | 0.94 | |
| Internal-pore diffusion | Dp (m2 min−1) | 5.10 × 10−18 | 6.00 × 10−18 |
| R2 | 0.93 | 0.90 | |
| Material | qe (mg g−1) | References |
|---|---|---|
| Py-GP4 porous geopolymer based pyrophyllite | 64.10 | [31] |
| Biomass fly ash geopolymer monoliths | 20.50 | [33] |
| Metakaolin based geopolymer | 7.64 | [34] |
| Metakaolin based geopolymer | 43.48 | [39] |
| Diatomaceous earth | 77.05 | [49] |
| ZnTiO3/TiO2-Diatomaceous earth | 37.32 | [49] |
| a-TiO2/ZnTiO3 | 16.00 | [52] |
| a-TiO2 | 15.00 | [52] |
| Agricultural and industrial waste by-products based geopolymer | 0.0449 | [78] |
| TiO2/Agricultural and industrial waste by-products based geopolymer | 0.0451 | [78] |
| Biomass fly ash based geopolymer spheres | 30.10 | [79] |
| Kaolin geopolymer (2009) | 25.60 | [80] |
| Metakaolin and rice husk based geopolymer | 4.90 | [81] |
| Hierarchical xNGeo particle | 115 | [82] |
| Coal FA-geopolymer cube | 84.00 | [83] |
| Metakaolin-geopolymer sphere | 4.20 | [84] |
| Cu2O/TiO2 geopolymer nanoparticle | 14.76 | [85] |
| Seawater based geopolymer | 59.52 | [86] |
| Kaolin | 21.41 | [87] |
| Activated lignin-chitosan composite extrudates | 36.25 | [88] |
| TiO2/montmorillonite-albumin nanocomposite | 18.18 | [89] |
| Carboxymethyl cellulose/ZSM-5/ZIF-8 | 10.49 | [90] |
| ZSM-5 zeolite | 105.82 | [91] |
| NaX zeolite | 127.13 | [92] |
| Chitosan/clay microspheres | 152.20 | [93] |
| Magnetic chitosan/clay beads | 82.00 | [94] |
| Activated carbon-clay composite | 178.64 | [95] |
| Hydroxysodalite | 10.82 | [96] |
| Nonporous silica | 91.10 | [97] |
| Natural clay | 15.40 | [98] |
| Geopolymer (GP) | 48.97 | This study |
| Geopolymer/ZnTiO3/TiO2 (GTA) | 61.96 | This study |
| Type of Dopant | MB (mg L−1) | Type of Light | Reaction Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| TiO2-coated geopolymer spheres | - | UV irradiation | 600 | 93 | [99] |
| Fly ash-based geopolymer | 15 | UV irradiation | 240 | 92.7 | [100] |
| Geopolymer/TiO2-doped-Zeolite | 40 | UV irradiation | 180 | 99.1 | [101] |
| Geopolymer/Zeolite | 40 | UV irradiation | 180 | 92.5 | [101] |
| Perlite-based geopolymer | 30 | UV irradiation | 240 | 97.9 | [102] |
| RMGP | 20 | UV irradiation | 720 | 94.6 | [103] |
| TBBFS/geopolymer | 20 | Visible irradiation | 180 | 93.5 | [104] |
| TiO2/C | 28.5 | Visible irradiation | 420 | 70 | [105] |
| TiO2/Ce | 32 | Visible irradiation | 180 | 90 | [106] |
| TiO2/Au | 12 | Visible irradiation | 48 | 92 | [107] |
| TiO2/Sb | 100 | Visible irradiation | 60 | 100 | [108] |
| TiO2/C/N | 10 | Visible irradiation | 180 | 85 | [109] |
| TiO2/N/F | 5.74 | Visible irradiation | 140 | 16 | [110] |
| TiO2/Mn/Fe | 10 | Visible irradiation | 150 | 85 | [111] |
| ZnTiO3/PANI/Ag | 10 | Visible irradiation | 25 | 96 | [112] |
| TiO2/N | 10 | Solar light | 120 | 97 | [113] |
| TiO2/F | 10 | Solar light | 120 | 55 | [114] |
| TiO2/La | 0.1 | UV irradiation | 120 | 85 | [115] |
| TiO2/Fe | 0.1 | UV irradiation | 120 | 75 | [115] |
| ZnTiO3/Ag | 10 | UV irradiation | 150 | 93 | [116] |
| Geopolymer (GP) | 20 | UV irradiation | 150 | 4.0 | This study |
| Geopolymer/ZnTiO3/TiO2 (GTA) | 20 | UV irradiation | 150 | 93 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Gaona, S.; Ramón, J.; Valarezo, E. Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye. Polymers 2023, 15, 2697. https://doi.org/10.3390/polym15122697
Jaramillo-Fierro X, Gaona S, Ramón J, Valarezo E. Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye. Polymers. 2023; 15(12):2697. https://doi.org/10.3390/polym15122697
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Sneyder Gaona, John Ramón, and Eduardo Valarezo. 2023. "Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye" Polymers 15, no. 12: 2697. https://doi.org/10.3390/polym15122697
APA StyleJaramillo-Fierro, X., Gaona, S., Ramón, J., & Valarezo, E. (2023). Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye. Polymers, 15(12), 2697. https://doi.org/10.3390/polym15122697








