Research Progress on Up-Conversion Fluorescence Probe for Detection of Perfluorooctanoic Acid in Water Treatment
Abstract
:1. Introduction
2. Method of Detecting PFOA
2.1. Liquid Chromatography-Mass Spectrometry
2.1.1. High Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC–MS/MS)
2.1.2. High Performance Liquid Chromatography–Quadrupole-Time of Flight-Mass Spectrometry (HPLC–Q-TOF-MS)
2.2. Gas Chromatography–Mass Spectrometry
2.2.1. GC-MS
2.2.2. Pre-Column Derivatization–Gas Chromatography
2.3. Fluorescence Detection
3. Fluorescent Materials
3.1. Fluorescent Polymer Materials
3.2. Rare Earth Up-Conversion Nanomaterials
3.2.1. Up-Conversion Fluorescence
3.2.2. Composition of UCNPs
4. Preparation and Optimization of UCNPs
4.1. Method of Preparing UCNPs
4.2. Optimization of Luminescence Efficiency of Up-Conversion Nanomaterials
4.3. Surface Functionalization of Up-Conversion Nanomaterials
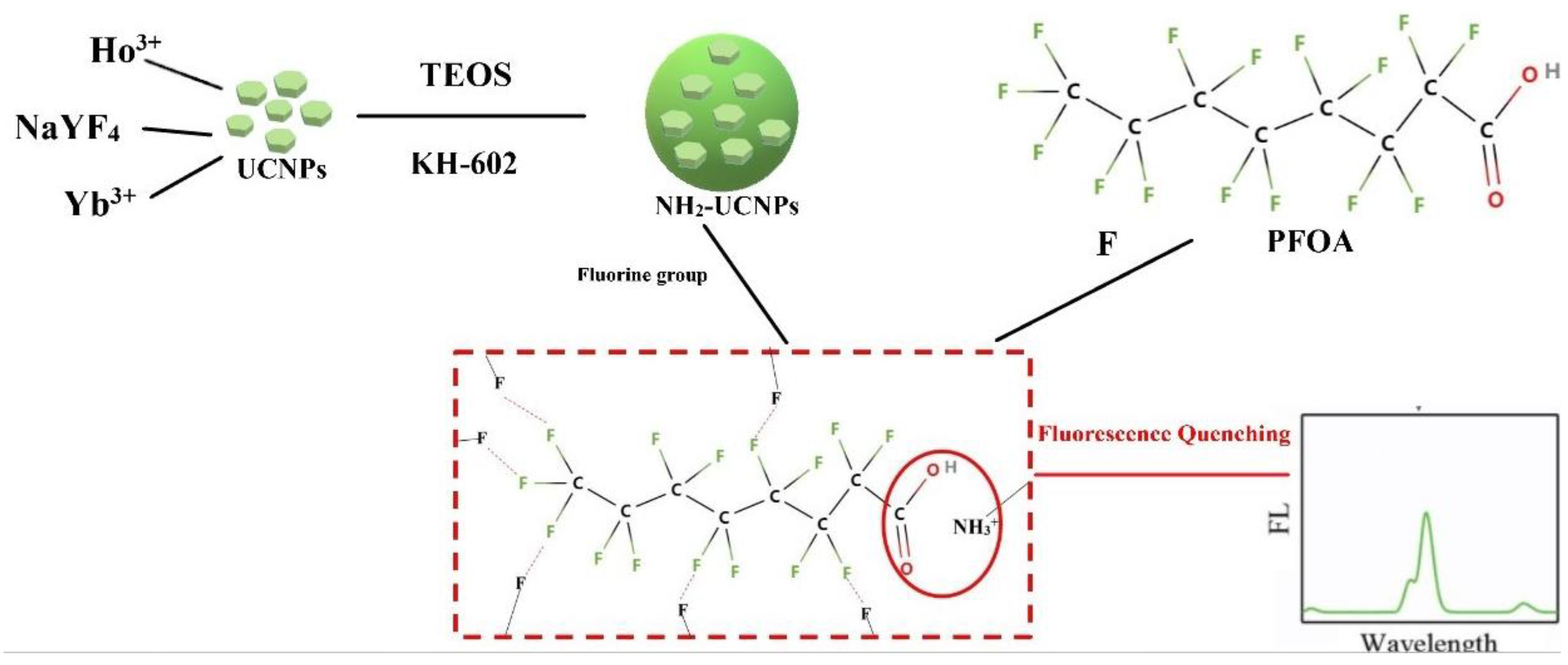
4.4. Principle of Up-Conversion Fluorescent Probe Detecting of PFOA
5. Application Progress and Recovery
5.1. Applications of Up-Conversion Fluorescent Probes in Detection
5.2. Recovery and Challenges of Up-Conversion Fluorescent Probes
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Valsecchi, S.; Rusconi, M.; Mazzoni, M.; Viviano, G.; Pagnotta, R.; Zaghi, C.; Serrini, G.; Polesello, S. Occurrence and Sources of Perfluoroalkyl Acids in Italian River Basins. Chemosphere 2015, 129, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, F.S.; Qin, R.X.; Zhuo, L.; Hu, F.Q.; Shi, Y.G.; Zheng, J. Pollution Characteristics of 16 Kinds of Perfluorinated Compounds in Wastewater from Typical Industries in Chongqing. Acta Ecotoxicol. 2021, 16, 44–58. [Google Scholar]
- Wang, K.M.; Wu, X.Y.; Zhang, R.Z.; Hu, P. Determination of Perfluorooctanoate and Perfluorooctanesulfonate in Drinking Water by Solid Phase Extraction and High-Performance Liquid Chromatography Tandem Mass Spectrometry. Guangdong Chem. Ind. 2022, 49, 102–103. [Google Scholar]
- Rushing, B.R.; Hu, Q.; Franklin, J.N.; McMahen, R.; Dagnino, S.; Higgins, C.P.; Strynar, M.J.; DeWitt, J.C. Evaluation of the Immunomodulatory Effects of 2,3,3,3-Tetrafluoro-2-(heptafluoropropoxy)-Propanoate in C57BL/6 mice. Toxicol. Sci. 2017, 156, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, J.H.; Yao, J.Z.; Sun, S.J.; Sheng, N.; Zhang, X.W.; Guo, X.J.; Guo, Y.; Sun, Y.; Dai, J.Y. Comparative Hepatotoxicity of Novel PFOA Alternatives(perfluoropolyether carboxylic acids)on Male Mice. Environ. Sci. Technol. 2019, 53, 3929–3937. [Google Scholar] [CrossRef]
- Sheng, N.; Pan, Y.T.; Guo, Y.; Sun, Y.; Dai, J.Y. Hepatotoxic Effects of Hexafluoropropylene Oxide Trimer Acid(HFPO-TA),a Novel Perfluorooctanoic Acid (PFOA) Alternative on Mice. Environ. Sci. Technol. 2018, 52, 8005–8015. [Google Scholar] [CrossRef]
- Shi, Y.L.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.X.; Liang, Y.; Cai, Y.Q. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids(Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404. [Google Scholar] [CrossRef]
- Sun, W.Q.; Ma, X.Z.; Zhou, Y.B.; Wang, L.; Liu, H. Effect of Perfluorooctanoic Acid on Lipid Accumulation of Rat Hepatocytes BRL-3A. Med. Health Sci. Technol. 2022, 51, 107–112. [Google Scholar]
- Dong, G.H.; Zhang, Y.H.; Zhen, L.; Liang, Z.F.; He, Q.C. Effects of Perfluorooctane Sulfonic Acid on Proliferation and Cytokine Secretion of Mouse Lymphocytes in Vitro. J. Toxicol. 2011, 25, 125–128. [Google Scholar]
- Zhang, L.M.; Rimal, B.P.; Nichols, R.G.; Tian, Y.; Smith, P.B.; Hatzakis, E.; Chang, S.C.; Butenhoff, J.L.; Peters, J.M.; Patterson, A.D. Perfluorooctane Sulfonate Alters Gut Microbiota-host Metabolic Homeostasis in Mice. Toxicology 2020, 413, 152365. [Google Scholar] [CrossRef]
- Wei, J.P.; Xia, J.; Wang, X.J. Analysis of Detection Standards for PFOA and PFOS of Perfluorinated Compounds. Org. Fluoride Ind. 2014, 162, 38–42. [Google Scholar]
- Cennamo, N.; Zeni, L.; Tortora, P.; Regonesi, M.E.; Giusti, A.; Staiano, M.; D’Auria, S.; Varriale, A. A High Sensitivity Biosensor to detect the presence of perfluorinated compounds in environment. Talanta 2018, 178, 955–961. [Google Scholar] [CrossRef]
- Xu, X.L. Production, Processing and Use status of PFOA Substances in China. Chem. Manag. 2017, 459, 60–61. [Google Scholar]
- Chen, H.; Zhang, Y.L. Current Situation and Safety Analysis of Food Packaging Materials in China. Packag. Food Mach. 2017, 35, 53–57. [Google Scholar]
- Pontius, F. Regulation of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water: A Comprehensive Review. Water 2019, 11, 2003. [Google Scholar] [CrossRef]
- Baduel, C.; Paxman, C.J.; Mueller, J.F. Perfluoroalkyl Substances in a FireFigurehting Training Ground (FTG), Distribution and Potential Future Release. J. Hazard. Mater. 2015, 296, 46–53. [Google Scholar] [CrossRef]
- Tsuda, S. Differential Toxicity between Perfluorooctane Sulfonate(PFOS)and Perfluorooctanoic Acid(PFOA). J. Toxicol. Sci. 2016, 41, 27–36. [Google Scholar] [CrossRef]
- Lorenzo, M.; Campo, J.; Picó, Y. Analytical Challenges to Determine Emerging Persistent Organic Pollutants in Aquatic Ecosystems. TrAC Trends Anal. Chem. 2018, 103, 137–155. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of highly active g-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation. J. Photochem. Photobiol. A Chem. 2021, 418, 113393. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Leng, T.H.; Wang, L.; Zheng, Y. Determination of 12 Perfluorinated Compounds in Rice Powder for Infants and Young Children by High Performance Liquid Chromatography-tandem Mass Spectrometry. Food Saf. Qual. 2019, 10, 8087–8092. [Google Scholar]
- Liu, S.Y.; Jin, Q.; Ren, R. Determination of Perfluorocarboxylic Acids in Fish by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Prev. Med. 2020, 32, 1204–1207. [Google Scholar]
- Guo, J.; Guo, M.M.; Wu, H.Y.; Zhuo, Y.X.; Mou, H.J.; Lu, L.N.; Tan, Z.J. Simultaneous Determination of Perfluorinatedacids and Their Precursors in Bivalve Shellfish by Double SPE Columns Purification and Ultra Fast Liquid Chromatography-tandem Mass Spectrometry. Food Sci. 2017, 38, 248–255. [Google Scholar]
- He, J.L.; Peng, T.; Xie, J.; Dai, H.H.; Chen, D.D.; Yue, Z.F.; Fan, C.L.; Li, C. Development of a Qu ECh ERs Method for Determination of 20 Perfluorinated Compounds in Animal Liver by HPLC–MS/MS. Chin. J. Anal. Chem. 2015, 43, 40–48. [Google Scholar] [CrossRef]
- Feng, S.; Lan, F.; Wu, X.P.; Shen, J.C.; Yue, Z.F.; Xiong, B.B. Determination of 15 Perttuorinated Compounds in Chinese Spirit by Dispersive Solid Phase Extraction and HPLC-MS-MS. Food Sci. 2013, 34, 143–149. [Google Scholar]
- Huang, B.; Liu, W.J.; Fu, J.W.; Su, D.S.; Wu, J.H.; Ai, Y.Z. Simultaneous Detection of 7 Neonicotinoid Insecticides in Camellia Oil by QuEChERS Method Coupled with Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. Qual. Saf. Agric. Prod. 2019, 97, 45–48. [Google Scholar]
- Yang, W.L.; Guo, J.; Du, W. Determination of Perfluorooctanoic Acid and Perfluorooctanesulfonates in Environmental Water and Soil Matrixes Using ultra High Performance Liquid Chromatography Coupled with State-of-art Tandem Quadrupole Mass Spectrometry. Environ. Chem. 2018, 37, 2820–2823. [Google Scholar]
- Yu, L.; Song, W.; Lv, Y.L.; Zhao, M.Y.; Zhao, M.Y.; Zhou, F.F.; Hu, Y.Y.; Zheng, P. Rapid Determination of 204 Pesticide Residues in Tea by Ultra Performance Liquid Chromatography Coupled with Quadrupole-time of Flight Mass Spectrometry. Colour Spectr. 2015, 33, 597–612. [Google Scholar] [CrossRef]
- Zabaleta, I.; Negreira, N.; Bizkarguenaga, E. Screening and Identification of Per- and Polyfluoroalkyl Substances in Microwave Popcorn Bags. Food Chem. 2017, 230, 497. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.W.; Wu, H.; Yu, W.; Wang, Y.; Wang, F.C.; Zhang, C.C.; Zhou, L.S.; Li, Z.G. Rapid Identification of Chemical Constituents of Qiyu Sanlong Decoction by Ultra Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry. Chromatogr. J. 2021, 39, 730–743. [Google Scholar]
- Buszewska-Forajta, M.; Bujak, R.; Yumba-Mpanga, A.; Siluk, D.; Kaliszan, R. GC/MS Technique and AMDIS Software Application in Identification of Hydrophobic Compounds of Grasshoppers’ Abdominal Secretion (Chorthippus spp.). J. Pharm. Biomed. Anal. 2015, 102, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L. Rapid Determination of Perfluorooctanoic Acid in Food Contact Materials by Pre-column Derivatization and Gas Chromatography. Packag. Food Mach. 2019, 37, 69–72. [Google Scholar]
- Luo, S.P.; Shang, G.Q.; Chen, M. Research Progress of Detection Methods of Perfluorinated Compounds in Food Contact Materials. J. Food Saf. Qual. Insp. 2013, 4, 993–998. [Google Scholar]
- Wang, X.Y.; Shen, W.J.; Wang, H.; Yu, K.Y.; Wu, B.; Hu, G.S.; Yang, G.J. Determination of 10 Perfluorocarboxylic Acid Compounds in Water by Gas Chromatography-negative Chemical Source-mass Spectrometry. Chromatography 2019, 37, 32–39. [Google Scholar]
- Zheng, Z.; Yu, H.J.; Geng, W.C.; Hu, X.Y.; Wang, Y.Y.; Li, Z.H.; Wang, Y.F.; Guo, D.S. Guanidinocalix[5]arene for sensitive fluorescence detection and magnetic removal of perfluorinated pollutants. Nat. Commun. 2019, 10, 5762. [Google Scholar] [CrossRef] [PubMed]
- Tian, F. Application of Rhodamine Fluorescent Probes in the Detection of Heavy Metals and Transition Metal atoms. Petrochem. Technol. 2019, 26, 2693–2699. [Google Scholar]
- Chen, Y.Y.; Ding, M.M.; Li, J.Q.; Sheng, W.; Liu, B.; Zhang, Y.; Wang, S. Fluorescence quenching immunoaffinity test column with quantum dots as fluorescence donors for the quick detection of malachite green and crystal violet in aquatic products. Food Anal. Methods 2018, 11, 3362–3370. [Google Scholar] [CrossRef]
- Hua, G.S.; Sheng, W.; Li, J.M.; Zhang, Y.; Wang, J.P.; Wang, S. Fluorescent quenching immune chromatographic strips with quantum dots and up-conversion nanoparticles as fluorescent donors for visual detection of sulfaquinoxaline in foods of animal origin. Anal. Chim. Acta 2017, 982, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhou, H.L.; Wu, F.; Zhang, Y. Temperature Sensing Characteristics of Up-conversion Luminescence in Tm3+/Yb3+Co-dopeed LuYO3Phospho. J. Lumin. 2021, 42, 1872–1881. [Google Scholar]
- Chen, J.Y.; Su, W.; Wang, E.J. Highly selective ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer between 1,8-naphthalimide and rhodamine B. Chem. J. Chin. Univ. 2016, 37, 232–238. [Google Scholar]
- Li, M.; Jiang, X.; Wu, H.; Jiang, X.J.; Wu, H.H.; Xu, H.; Zang, S.Q. A dual functional probe for “turn-on” fluorescence response of Pb2+ and colorimetric detection of Cu2+ based on a rhodamine derivative in aqueous media. Dalton Trans. 2015, 44, 17326–17334. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Kumar, V.; Jayakannan, M. Triple action polymer probe: Carboxylic distilbene fluorescent polymer chemosensor for temperature, metal-ions and biomolecules. Chem. Commun. 2014, 50, 842. [Google Scholar] [CrossRef] [PubMed]
- Duret, D.; Haftek-Terreau, Z.; Carretier, M.; Berki, Z.; Ladavière, C.; Monier, K.; Bouvet, P.; Marvel, J.; Leverrier, Y.; Charreyre, M.T.; et al. Labeling of native proteins with fluorescent RAFT polymer probes: Application to the detection of a cell surface protein using flow cytometry. Polym. Chem. 2020, 9, 1857. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, M.M.; Wu, X.X.; Wang, F.; Liu, L. Methionine-capped gold nanoclusters as a fluorescenceenhanced probe for cadmium(II)sensing. Sensors 2018, 18, 658–668. [Google Scholar] [CrossRef]
- Kelsey, L.R.; Hwang, J.-H.; Esfahani, A.R.; Esfahani, A.R.; Anwar, S.A.H.M.; Lee, W.H. Recent Developments of PFAS-Detecting Sensors and Future Direction: A Review. Micromachines 2020, 11, 667. [Google Scholar]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for trace analysis of perfluorooctanesulfonate in water based on a molecularly imprinted poly (o-phenylenediamine) polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–Organic Framework-Based Microfluidic Impedance Sensor Platform for Ultrasensitive Detection of Perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514. [Google Scholar] [CrossRef]
- Feng, H.; Wang, N.Y.; Tran, T.T.T.; Yuan, L.J.; Li, J.Z.; Cai, Q.Y. Surface molecular imprinting on dye–(NH2)–SiO2 NPs for specific recognition and direct fluorescent quantification of perfluorooctane sulfonate. Sens. Actuators B Chem. 2014, 195, 266–273. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Sequeira, F.; Mattiello, F.; Porto, G.; Biasiolo, A.; Nogueira, R.; Bilro, L.; Zeni, L. A simple and low-cost optical fiber intensity-based configuration for perfluorinated compounds in water solution. Sensors 2018, 18, 3009. [Google Scholar] [CrossRef]
- Takayose, M.; Akamatsu, K.; Nawafune, H.; Murashima, T.; Matsui, J. Colorimetric detection of perfluorooctanoic acid (PFOA) utilizing polystyrene-modified gold nanoparticles. Anal. Lett. 2012, 45, 2856–2864. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, A.M.; Zhang, L.Z.; Gong, J.M. Molecularly imprinted ultrathin graphitic carbon nitridenanosheets–Based electrochemiluminescence sensing probe for sensitive detection of perfluorooctanoic acid. Anal. Chim. Acta 2015, 896, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wan, Y.J.; Wang, X.L.; Li, Y.Y.; Wang, W.J.; Wang, C.X.; Xu, S.Q. Sensitive bioassay for detection of PPARα potentially hazardous ligands with gold nanoparticle probe. Hazard. Mater. 2011, 192, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Monitoring of perfluorinated compounds from the imported fishery products in Korea by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Environ. Epidemiol. 2019, 3, 452–453. [Google Scholar]
- Lertassavakorn, T.; Pholphana, N.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Determination of perfluorooctane sulphonate and perfluorooctanoic acid in seafood and water from Map Ta Phut Industrial Estate area, Thailand. Food Addit. Contam. Part A 2021, 38, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.D.; Gan, Z.W.; Cui, S.F.; Fan, R.J.; Fu, Y.Z.; Peng, M.Y.; Yang, J.Y. Agricultural activities impact on soil and sediment fluorine and perfluorinated compounds in an endemic fluorosis area. Sci. Total Environ. 2021, 771, 144809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, H.Q.; Yang, J.; Fu, Y.Q.; Wang, H.; Yao, J.H.; Gao, T.Q.; Deng, J.G. Simultaneous determination of fluoroalkanes in aquatic products by ultra tandem high performance liquid chromatography mass spectrometry. J. Food Saf. Qual. 2022, 13, 1132–1140. [Google Scholar]
- Wang, Y.K.; Chen, W.Y.; Wen, S.G.; Meng, K.Q.; Xie, S.Z.; Zhen, J.X.; Qiu, C.S. Headspace gas chromatography detection method for perfluorohydrocarbon tracer. Fine Spec. Chem. 2020, 28, 10–13. [Google Scholar]
- Gebbink, W.A.; Letcher, R.I. Linear and branched perfluorooctane sulfonate isomer patterns in herring gull eggs from colonial sites across the Laurentian Great Lakes. Environ. Sci. Technol. 2010, 44, 3739–3745. [Google Scholar] [CrossRef]
- Su, C.Y.; Zheng, N.; Li, S.L.; Qu, X.Y.; Zhou, X.W.; Ma, T.; Yang, H.J.; Wang, J.Q. Research progress on detection methods of perfluorinated compounds in milk. China Dairy Ind. 2018, 46, 29–33. [Google Scholar]
- Wang, J.; Chen, H.Y.; Ru, F.; Zhang, Z.; Mao, X.; Shan, D.L.; Chen, J.; Lu, X.Q. Encapsulation of dual-emitting fluorescent magnetic nanoprobe in metal-organic frameworks for ultrasensitive ratiometric detection of Cu2+. Chem. A Eur. J. 2018, 24, 3499–3505. [Google Scholar] [CrossRef]
- Chen, X.Y.; Sameer Hussain Tang, Y.H.; Chen, X.; Zhang, S.J.; Wang, Y.; Zhang, P.; Gao, R.X.; Wang, S.; Hao, Y. Two-in-one platform based on conjugated polymer for ultrasensitive ratiometric detection and efficient removal of perfluoroalkyl substances from environmental water. Sci. Total Environ. 2022, 860, 160467. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V. Conjugated Polymers-Based Biosensors for Virus Detection: Lessons from COVID-19. Biosensors 2022, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Hussain, S.; Kalita, A.; Iyer, P.K. Conjugated Polymer Nanoparticles for the Amplified Detection of Nitro-explosive Picric Acid on Multiple Platforms. ACS Appl. Mater. Interfaces 2015, 7, 26968–26976. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Hao, Y.; Tian, X.M.; Gao, R.X. Review—Recent Advances of Signal Amplified Smart Conjugated Polymers for Optical Detection on Solid Support. ECS J. Solid State Sci. Technol. 2021, 10, 037006. [Google Scholar] [CrossRef]
- Feng, F.D.; Liu, L.B.; Yang, Q.; Wang, S. Water-soluble conjugated polymers for fluorescent-enzyme assays. Macromol. Rapid Commun. 2010, 31, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.W.; Peng, Y. A selective and ratiometric bifunctional fluorescent probe for Al3+ ion and proton. Org. Lett. 2012, 14, 3420–3423. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.T.; Sonkar, S.K.; Yang, L.J.; Broglie, J.J.; Liu, Y.; Sun, Y.P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10797. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6148. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.M.; Jayakumar, M.K.G.; Bansal, A.; Zhang, Y. Up-conversion nanoparticles as versatile light nanotransducers for photoactivation applicatatoms. Chem. Soc. Rev. 2015, 44, 1449. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, B.Y.; Jin, D.Y.; Liu, X.G. Controlling up-conversion nanocrystals for emerging applicatatoms. Nat. Nanotechnol. 2015, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Zhang, J.; Liu, J.L.; Zhang, Y. Recent progress of rare-earth dopeed up-conversion nanoparticles: Synthesis, optimization, and applicatatoms. Adv. Sci. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Up-conversion and anti-stokes processes with f and d atoms in solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Song, X.W.; Jin, Q.; Li, W.H. Tissue distribution and bio-accumulation of a novel polyfluoroalkyl benzenesulfonate in crucian carp. Environ. Int. 2020, 135, 105418. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, S.; Huttad, L. An NIR-II/MR dual modal nanoprobe for liver cancer imaging. Nanoscale 2021, 12, 11510–11517. [Google Scholar] [CrossRef]
- Chen, H.Q.; Tang, W.; Liu, Y.C.; Wang, L. Quantitative image analysis method for detection of nitrite with cyanine dye-NaYF4:Yb,Tm@NaYF4 up-conversion nanoparticles composite luminescent probe. Food Chem. 2021, 367, 130660. [Google Scholar] [CrossRef]
- Huang, D. Theoretical research and application of rare earth up-conversion luminescent materials. J. Chifeng Univ. (Nat. Sci. Ed.) 2017, 33, 7–8. [Google Scholar]
- Dan, S.; Wu, H.; Ma, X.J.; Tan, M.Q. Preparation and biological application of rare earth up-conversion fluorescent nanomaterials. Prog. Biochem. Biophys. 2013, 40, 925–934. [Google Scholar]
- Zhao, X.; Zhang, L.; Yan, X.; Zhang, L.; Yang, L.; Pan, J.L.; Zhang, M.L.; Wang, C.G.; Suo, H.; Jia, X.T.; et al. A near-infrared light triggered fluormetric biosensor for sensitive detection of acetylcholinesterase activity based on NaErF4: 0.5% Ho3+@NaYF4 up-conversion nano-probe. Talanta 2021, 235, 122784. [Google Scholar] [CrossRef]
- Tian, J.; Luo, H.F. Research Development of Up-Converting Rare-earth Nanophosphors. Front. Chem. 2014, 35, 1–4. [Google Scholar]
- Xu, J.T.; Gulzar, A.; Yang, P.P.; Bi, H.T.; Yang, D.; Gai, S.L.; He, F.; Lin, J.; Xing, B.G.; Jin, D.Y. Recent advances in near-infrared emitting lanthanide-dopeed nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2019, 381, 104. [Google Scholar] [CrossRef]
- Yamini, S.; Gunaseelan, M.; Kumar, G.A.; Singh, S.; Dannangoda, G.C.; Martirosyan, K.S.; Sardar, D.K.; Sivakumar, S.; Girigoswami, A.; Senthilselvan, J. NaGdF4:Yb,Er-Ag nanowire hybrid nanocomposite for multifunctional up-conversion emission, optical imaging, MRI and CT imaging applicatatoms. Microchim. Acta 2020, 187, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Chang, C.A. Optimising FRET-efficiency of Nd3+ sensitised up-conversion nanocomposites by shortening the emitter-photosensitizer distance. Nanoscale 2020, 12, 8742. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, Q.C. Recent advances on functionalized up-conversion nanoparticles for detection of small molecules and atoms in biosystems. Adv. Sci. 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Z.; Loh, K.Y.; Wang, Y.; Chen, Q.S.; Fan, J.Y.; Jung, T.; Nam, S.H.; Suh, Y.D.; Liu, X.G. Recent advances in up-conversion nanocrystals: Expanding the kaleidoscopic toolbox for emerging applicatatoms. Nano Today 2019, 29, 100797. [Google Scholar] [CrossRef]
- Nie, J.H.; Gao, H.; Li, X.; Liu, S.M. Up-conversion luminescence properties of different fluoride substrates materials NaREF4(RE: Gd, Lu, Y) dopeed with Er3+/Yb3+. J. Lumin. 2018, 204, 333–340. [Google Scholar] [CrossRef]
- Fan, X.; Huang, W.; Ying, W.T.; Xu, S.Q. High efficient upconversion luminescence of NaGdF4: Yb3+/Er3+ nanoparticle: First-principles calculation, dual-wavelength stimuli and logic gate application. Mater. Technol. 2021, 37, 1–10. [Google Scholar] [CrossRef]
- Wu, X.F.; Chen, Z.H. Preparation of up-conversion fluorescent probe and its plant cell imaging. Electron. Test. 2015, 7, 43–45. [Google Scholar]
- Li, X.F.; Wu, X.M.; Tao, J. Hydrothermal Synthesis of ZnO:Ho, Yb Luminescent Powder and Its Up-conversion Luminescence Characteristics. J. Synth. Cryst. 2016, 45, 2567–2571. [Google Scholar]
- Mettra, B.; Appaix, F.; Olesiak-Banska, J.; Le Bahers, T.; Leung, A.; Matczyszyn, K.; Samoc, M.; van der Sanden, B.; Monnereau, C.; Andraud, C. A fluorescent polymer probe with high selectivity toward vascular endothelial cells for and beyond noninvasive two-photon intravital imaging of brain vasculature. ACS Appl. Mater. Interfaces 2016, 8, 17047–17059. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, K.X.; Yang, Q.Z.; Yang, Y.X.; Yuan, L. Synthesis and properties of a highly selective fluorescent probe for Al3+. Mod. Chem. Ind. 2022, 43, 254–258. [Google Scholar]
- Yu, Y.C.; Feng, J.K.; Liu, B.; Feng, J.K.; Liu, B.; You, J.; Wu, W.J. A turn-on type Al3+ photoprobe and its in vivo bioassay. China Environ. Sci. 2020, 40, 5422–5427. [Google Scholar]
- Zhang, Y.M.; Chen, Y.C.; Bai, Y.; Bai, Y.; Xue, X.L.; He, W.J.; Guo, Z.J. FRET-based fluorescent ratiometric probes for the rapid detection of endogenous hydrogen sulphide in living cells. Analyst 2020, 145, 4233–4238. [Google Scholar] [CrossRef]
- Hu, W.R.; Song, Q.J.; Dong, M.M. Enhanced fluorescent probe for detection of BSA based on aggregation-induced emission. Chin. J. Anal. Lab. 2022. [Google Scholar] [CrossRef]
- Li, C.J.; Xing, K.Q.; Liu, Y.C.; Xiang, K.Q.; Liu, Y.C.; Zheng, Y.C.; Tian, B.Z.; Zhang, J.L. A novel colorimetric chemosensor for Cu2+ with high selectivity and sensitivity based on Rhodamine B. Res. Chem. Intermed. 2015, 41, 10169–10180. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.Q.; Yu, H.; Tian, L.X.; Yan, L.S.; Liu, X.M.; Lin, L.K.; Wang, J.L. Mesoporous Structure-based Up-conversion Molecularly Imprinted Fluorescent Probes for the Detection of Perfluorooctanoic Sulfonic Acid. Chin. J. Anal. Chem. 2020, 48, 1493–1501. [Google Scholar]
- Zhang, T.; Zhao, H.; Lei, A.; Quan, X. Electrochemical Biosensor for Detection of Perfluorooctane Sulfonate Based on Inhibition Biocatalysis of Enzymatic Fuel Cell. Electrochemistry 2014, 82, 94–99. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.Q.; Qian, H.S.; Hu, Y.; Muhammad, I.N. Seed-mediated synthesis of NaYF4:Yb, Er/NaGdF4 nanocrystals with improved up-conversion fluorescence and MR relaxivity. Nanotechnology 2010, 21, 125602. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Shan, J.; Xu, L.; Zhao, D. Shape, Size, and Phase- Controlled Rare- Earth Fluoride Nanocrystals with Optical Up-Conversion Properties. Chem.-Eur. J. 2009, 15, 11010–11019. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.H.; Wang, J.; Xu, J.; Chen, H.Y.; Zhang, C.; Hong, M.H.; Liu, X.G. Simultaneous phase and size control of up-conversion nanocrystals through lanthanide dopeing. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Radunz, S.; Schavkan, A.; Wahl, S.; Wuerth, C.; Tschiche, H.; Krurnrey, M.; Resch-Genger, U. Evolution of size and optical properties of upconverting nanoparticles during high-temperature synthesis. Phys. Chem. C 2018, 122, 28958–28967. [Google Scholar] [CrossRef]
- Skripka, A.; Marin, R.; Benayas, A.; Canton, P.; Hemmer, E.; Vetrone, F. Double rare-earth nanothermometer in aqueous media: Opening the third optical transparency window to temperature sensing. Phys. Chem. Chem. Phys. 2017, 7, 44531–44536. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, J.; Han, Q.; Zhao, X.; Yan, X.; Liu, J.; Ge, H.; Gao, W. Tuning and enhancing the red upconversion emission of Er3+ in LiYF4 nanoparticles. J. Lumin. 2019, 207, 361–368. [Google Scholar] [CrossRef]
- Wang, H.D.; Wang, L.; Ye, S.Y.; Song, X.L. Construction of Bi2WO6–TiO2/starch nanocomposite films for visible-light catalytic degradation of ethylene. Food Hydrocoll. 2019, 88, 92–100. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Yu, L.X.; Jia, W. One-step solvothermal preparation of Bi-Bi2O3-BiOBr ternary complex and its visible light-driven catalytic degradation of methylene blue. Acta Mater. Compos. Sin. 2022, 1000–3851. [Google Scholar]
- Hu, X.K.; Shang, X.Y.; Huang, P.; Zheng, W.; Chen, X.Y. Polarization up-conversion luminescence and orientation tracing of NaYF4:Yb3+/Er3+single particle nanorods. J. Chem. 2022, 80, 244–248. [Google Scholar]
- Li, Y.T.; Guo, W.; Wu, Z.W.; Jin, L.; Ke, Y.; Guo, Q.; Hu, S. Determination of ultra-trace rare earth elements in high-salt groundwater using aerosol dilution inductively coupled plasma-mass spectrometry (ICP-MS) after iron hydroxide co-precipitation. Microchem. J. 2016, 126, 194–199. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide dopeed up-conversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef]
- Du, H.; Zhang, W.; Sun, J. Structure and up-conversion luminescence properties of BaYF5:Yb3+, Er3+ nanoparticles prepared by different methods. J. Alloys Compd. 2011, 509, 3413–3418. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, H.J.; Qiu, H.L.; Wei, Y.J.; Wang, C.Z.; Chen, G.; Zhang, D. Nano-particle assembled porous core–shell ZnMn2O4 microspheres with superb performance for lithium batteries. Nanotechnology 2017, 28, 105403. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.S.; Shen, Y.X.; Li, F.F.; Zhang, M.X. Preparation of silver vanadate photocatalyst by coprecipitation method and the effect of pH on its photocatalytic activity. Rare Met. 2022, 46, 906–912. [Google Scholar]
- Yi, G.S.; Lu, H.C.; Zhao, S.Y.; Yang, W.J.; Chen, D.P.; Guo, L.H. Synthesis, Characterization, and Biological Application of Size-Controlled Nanocrystalline NaYF4:Yb,Er Infrared-to-Visible Up-Conversion Phosphors. Nano Lett. 2004, 4, 2191–2196. [Google Scholar] [CrossRef]
- Zhou, F.R.; Zhang, M.X. Preparation of Nano-SiO2 by Microemulsion Method and Performance of lts Modified PVA-CS Films. Anhui Chem. Ind. 2022, 48, 52–54. [Google Scholar]
- Qin, Y.K.; Li, S.; Hong, Y.; Wang, Z.G.; Han, D.P.; Peng, Y.; Zhou, H.Y.; Gao, Z.X.; Han, T.; Wang, Z.G.; et al. Research Process of Synthesis Functionalization and Application of Up-conversion Nanoparticles in Food Safety Detection. Anal. Chem. 2021, 49, 1955–1969. [Google Scholar]
- Shao, Q.Q.; Zhang, H.; Dai, J.Y.; Yang, C.; Chen, X.X.; Feng, G.Y.; Zhou, S.H. Preparation, growth mechanism, size manipulation and near-infrared luminescence enhancement of beta-NaYF4:Nd3+microcrystals via Ca2+ dopeing. Crystengcomm 2019, 21, 741. [Google Scholar] [CrossRef]
- Dibaba, S.T.; Xiao, Q.G.; Ren, W.; Sun, L. Recent progress of energy transfer and luminescence intensity boosting mechanism in Nd3+ sensitized up-conversion nanoparticles. J. Rare Earths 2019, 37, 791. [Google Scholar] [CrossRef]
- Tian, G.; Gu, Z.J.; Zhou, L.J.; Yin, W.Y.; Liu, X.X.; Yan, L.; Jin, S.; Ren, W.L.; Xing, G.M.; Li, S.J.; et al. Mn2+ dopeant-controlled Synthesis of NaYF4:Yb/Er Up-conversion Nanoparticles for in Vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226. [Google Scholar] [CrossRef]
- Kang, N.; Zhao, J.; Zhou, Y.; Ai, C.C.; Wang, X.M.; Ren, L. Enhanced Up-conversion Luminescence Intensity of Core-shell NaYF4 Nanocrystals Guided by Morphological Control. Nanotechnology 2019, 30, 105701. [Google Scholar] [CrossRef]
- Würth, C.; Fischer, S.; Grauel, B.; Alivisatos, A.P.; Resch-Genger, U. Quantum yields, surface quenching, and passivation efficiency for ultrasmall core/shell upconverting nanoparticles. J. Am. Chem. Soc. 2018, 140, 4922–4928. [Google Scholar] [CrossRef]
- Sasidharan, S.L.; Niagara, M.I.; Li, Z.Q.; Huang, K.; Soo, K.C.; Zhang, Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 2015, 9, 191–205. [Google Scholar]
- Rong, J.M.; Li, P.C.; Ge, Y.K.; Chen, H.L.; Wu, J.; Zhang, R.W.; Lao, J.; Lou, D.W.; Zhang, Y.X. Histone H2A-peptide-hybrided upconversion mesoporous silica nanoparticles for bortezomib/p53 delivery and apoptosis induction. Colloids Surf. B 2020, 186, 110674. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, B.P.; Karmakar, A.; Ghosh, A. Recent Advancements in Ln-Ion-Based Upconverting Nanomaterials and Their Biological Applications. Part. Part. Syst. Charact. 2019, 36, 1900153. [Google Scholar] [CrossRef]
- Han, S.Y.; Qin, X.; An, Z.F.; Zhu, Y.H.; Liang, L.L.; Han, Y.; Huang, W.; Liu, X.G. Multicolour synthesis in lanthanide-doped nanocrystals through cation exchange in water. Nat. Commun. 2016, 7, 13059. [Google Scholar] [CrossRef]
- Bogdan, N.; Rodriguez, E.M.; Sanz-Rodriguez, F.; De La Cruzm, C.I.; Juarranz, A.; Jaque, D.; Sole, J.G.; Capobianco, J.A. Bio-functionalization of ligand-free upconverting lanthanide doped nanoparticles for bio-imaging and cell targeting. Nanoscale 2012, 4, 3647–3650. [Google Scholar] [CrossRef]
- Xie, X.H.; Song, J.L.; Hu, L.Y.; Zhang, S.Y.; Wang, Y.T.; Zhao, Y.L.; Lu, Q. Tailor-made PL-UC-C3 nanoparticles for fluorescence/computed tomography imaging-guided cascade amplified photothermal therapy. Int. J. Nanomed. 2018, 13, 7633–7646. [Google Scholar] [CrossRef]
- Tian, L.X.; Guo, H.Q.; Li, J.; Yan, L.S.; Zhu, E.Z.; Liu, X.M.; Li, K.X. Fabrication of a Near-infrared Excitation Surface Molecular Imprinting Ratiometric Fluorescent Probe for Sensitive and Rapid detecting Perfluorooctane Sulfonate in Complex Matrix. J. Hazard. Mater. 2021, 413, 125353. [Google Scholar] [CrossRef]
- Su, H.; Luo, J.X.; Zhao, J.B. A Commercial Enzyme Inhibition-Colorimetric Assay Kit for Rapid Detection of Pesticide Residues was Developed for the Determination of Sensitivity to 10 Organophosphorus Pesticides. Food Saf. Food Qual. 2019, 10, 5512–5516. [Google Scholar]
- Zhang, B.H.; Wei, W. Study on the Effect of Enzyme Inhibition Method in the Detection of Pesticide Residues in Vegetables. South China Agric. 2018, 12, 105–106. [Google Scholar]
- Li, Z.M.; Wang, Y.; Leng, H.X.; Li, X.A.; Kang, X. Simultaneous Determination of Clenbuterol and Ractopamine Residues in Food by Protein chip. Instrum. Anal. 2019, 38, 913–919. [Google Scholar]
- Wang, P.Y.; Li, H.H.; Hassan, M.M.; Guo, Z.; Zhang, Z.Z.; Chen, Q. Fabricating an Acetylcholinesterase Modulated UCNPs-Cu2+ Fluorescence Biosensor for Ultrasensitive Detection of Organophosphorus Pesticides-Diazinon in Food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Zhang, L.X.; Jiang, S.; Fu, Z.F. A Facile Luminescence Resonance Energy Transfer Method for Detecting Cyano-Containing Pesticides in Herbal Medicines. Microchem. J. 2020, 152, 104451. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Yang, Q.; Barnych, B.; González-Sapienza, G.; Hammock, B.D.; Wang, M.H.; Hua, X.D. Fluorescent “Turn off-on” Small-Molecule-Monitoring Nanoplatform Based on Dendrimer-like Peptides as Competitors. ACS Appl. Mater. Interfaces 2019, 11, 33380–33389. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.L. Detection Technology of Veterinary Drug Residues in Food of Animal Origin. Sci. Technol. Inf. Anim. Husb. Vet. 2019, 11, 63. [Google Scholar]
- Hlavacek, A.; Farka, Z.; Hubner, M.; Hornakova, V.; Nemecek, D.; Niessner, R.; Skladal, P.; Knopp, D.; Gorris, H.H. Competitive Up-conversion-Linked Immunosorbent Assay for the Sensitive Detection of Diclofenac. Anal. Chem. 2016, 88, 6011–6017. [Google Scholar] [CrossRef]
- Xu, S.; Xu, S.H.; Zhu, Y.S.; Xu, W.; Zhou, P.W.; Zhou, C.Y.; Dong, B.; Song, H.W. A Novel up-conversion, Fluorescence resonance Energy Transfer Biosensor (FRET) for Sensitive Detection of Lead Atoms in Human Serum. Nanoscale 2014, 6, 12573–12579. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.Y.; Xie, Y.N.; Chen, B.; Zhu, S.S. Single Fluorescent Probe Responds to H2O2, NO, and H2O2 with Three Different Sets of Fluorescence Signals. J. Am. Chem. Soc. 2012, 134, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Bhardwaj, V.; Kaur, N.; Singh, N. Fluorescent Primary Sensor for Zinc and Resultant Complex as Secondary Sensor towards Phosphorylated Biomolecules: INHIBIT Logic Gate. Inorg. Chim. Acta 2013, 399, 1–5. [Google Scholar] [CrossRef]
- Tang, L.J.; Cai, M.J.; Zhao, J.; Zhong, K.L.; Hou, S.H.; Bian, Y.J. A Highly Selective and Ratiometric Fluorescent Sensor for Relay Recognition of Zinc(ii) and Sulfide Ions Based on Modulation of Excited-state Intramolecular Proton transfer. RSC Adv. 2013, 3, 16802–16809. [Google Scholar] [CrossRef]
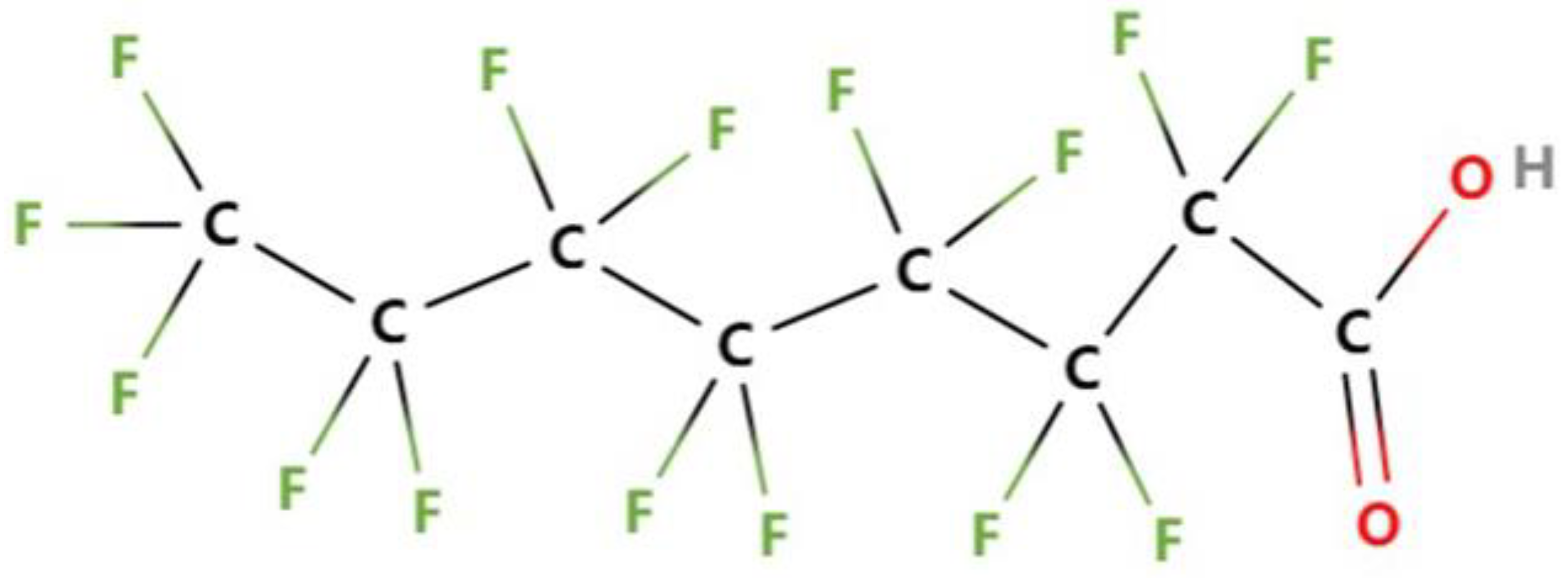
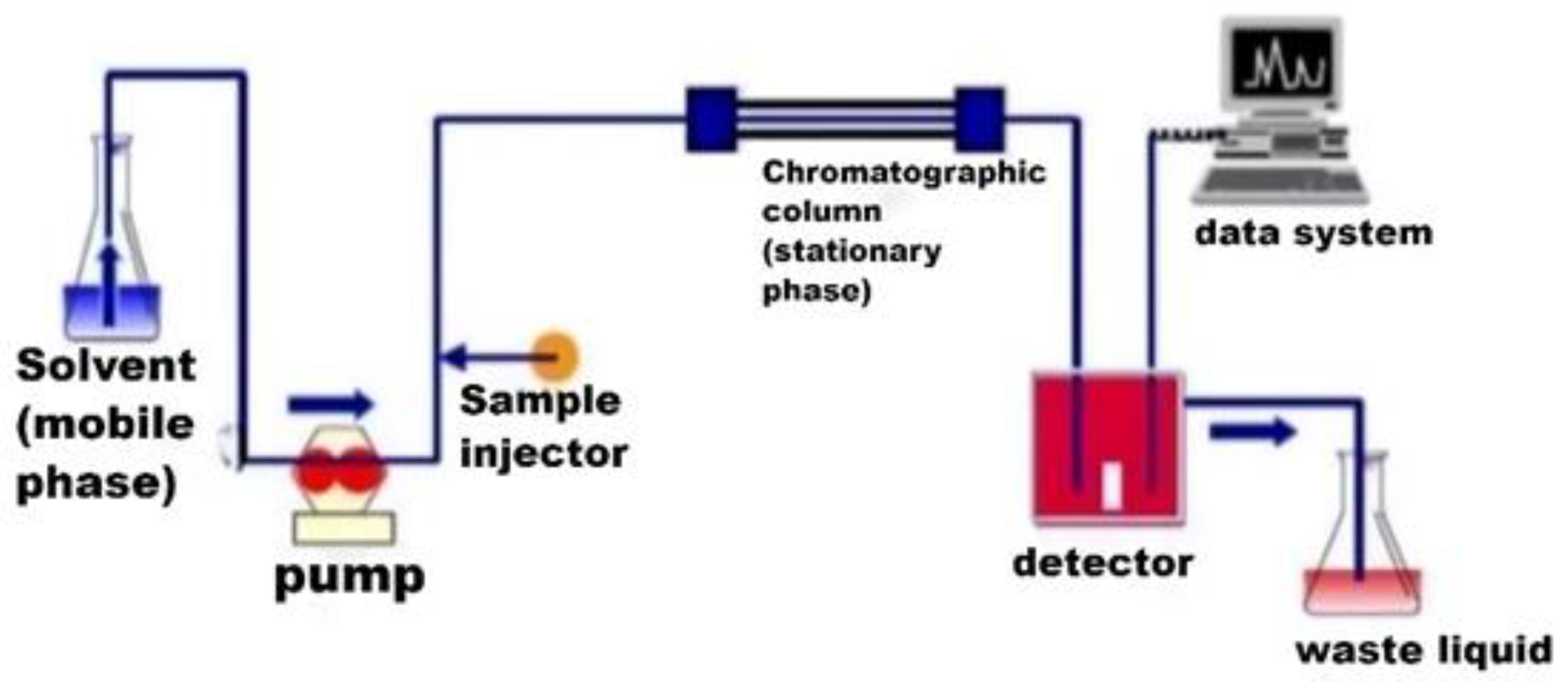
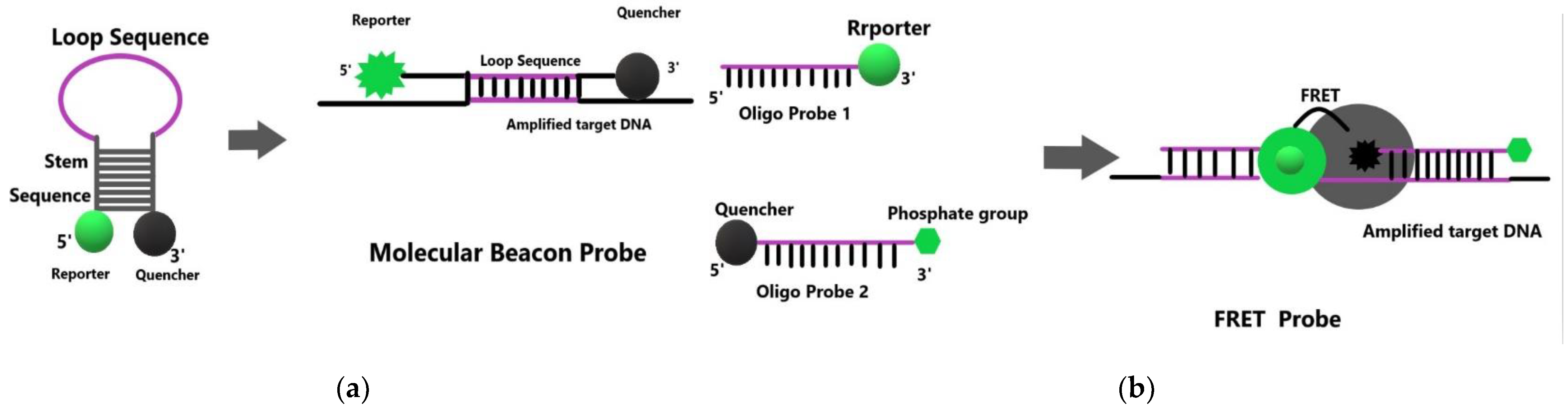
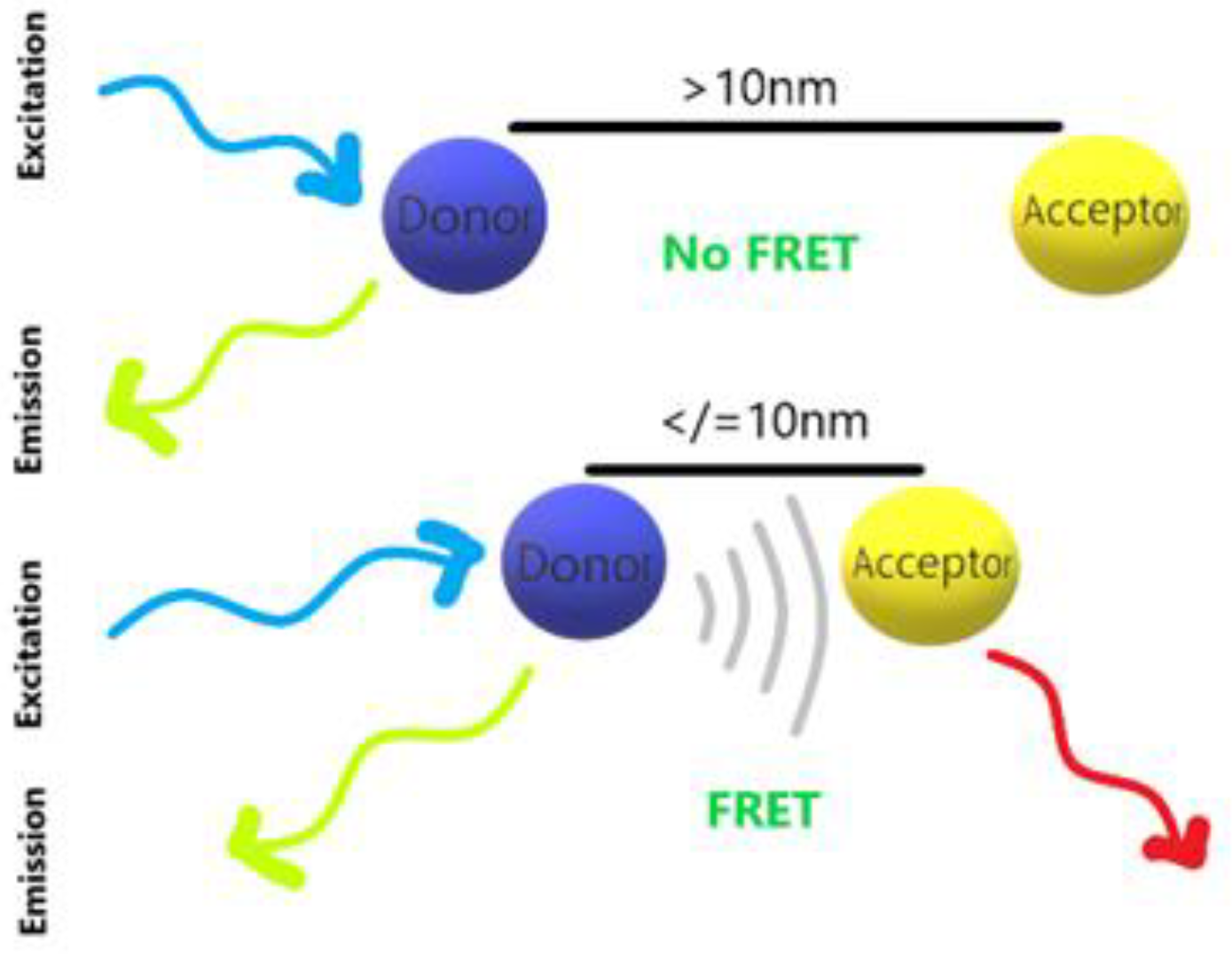
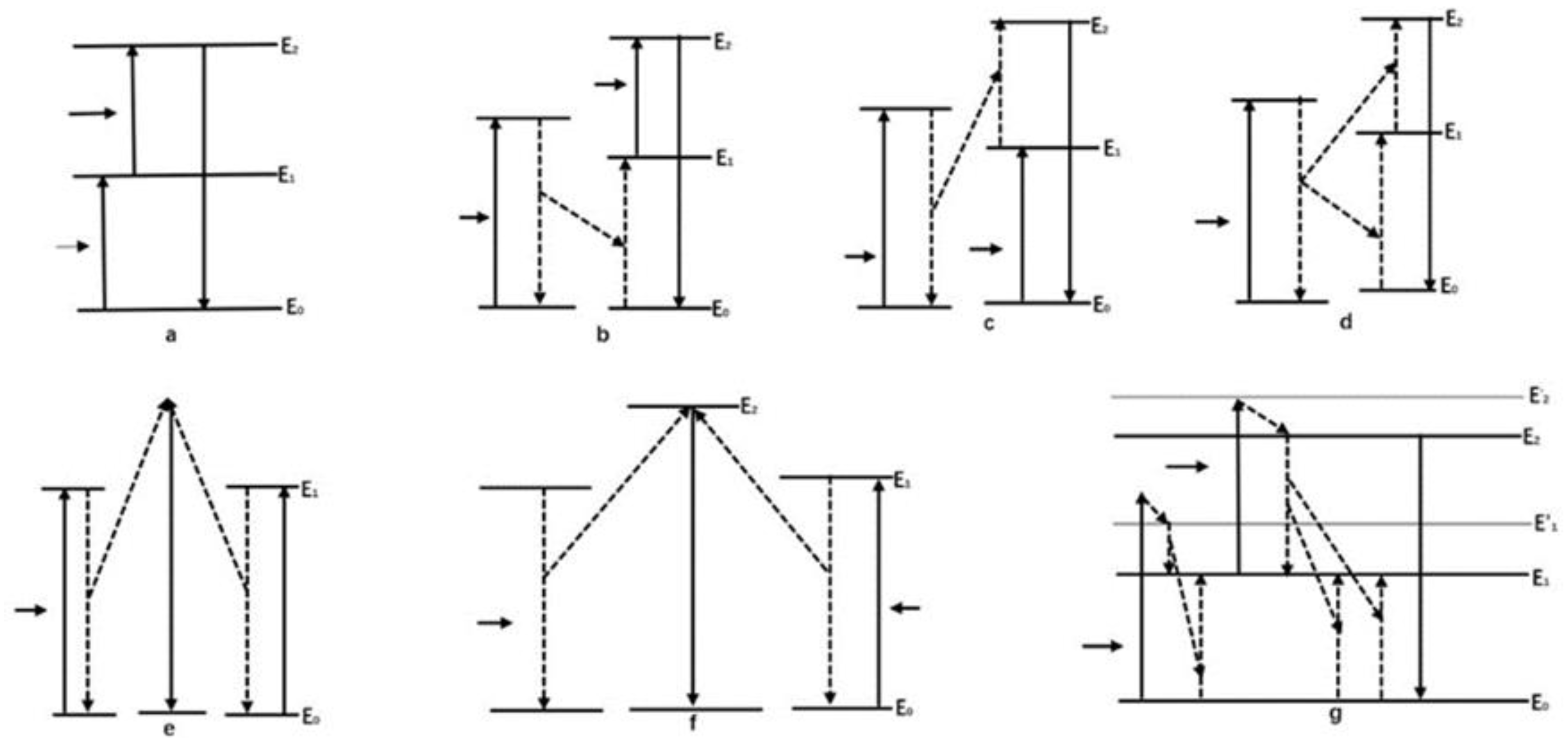
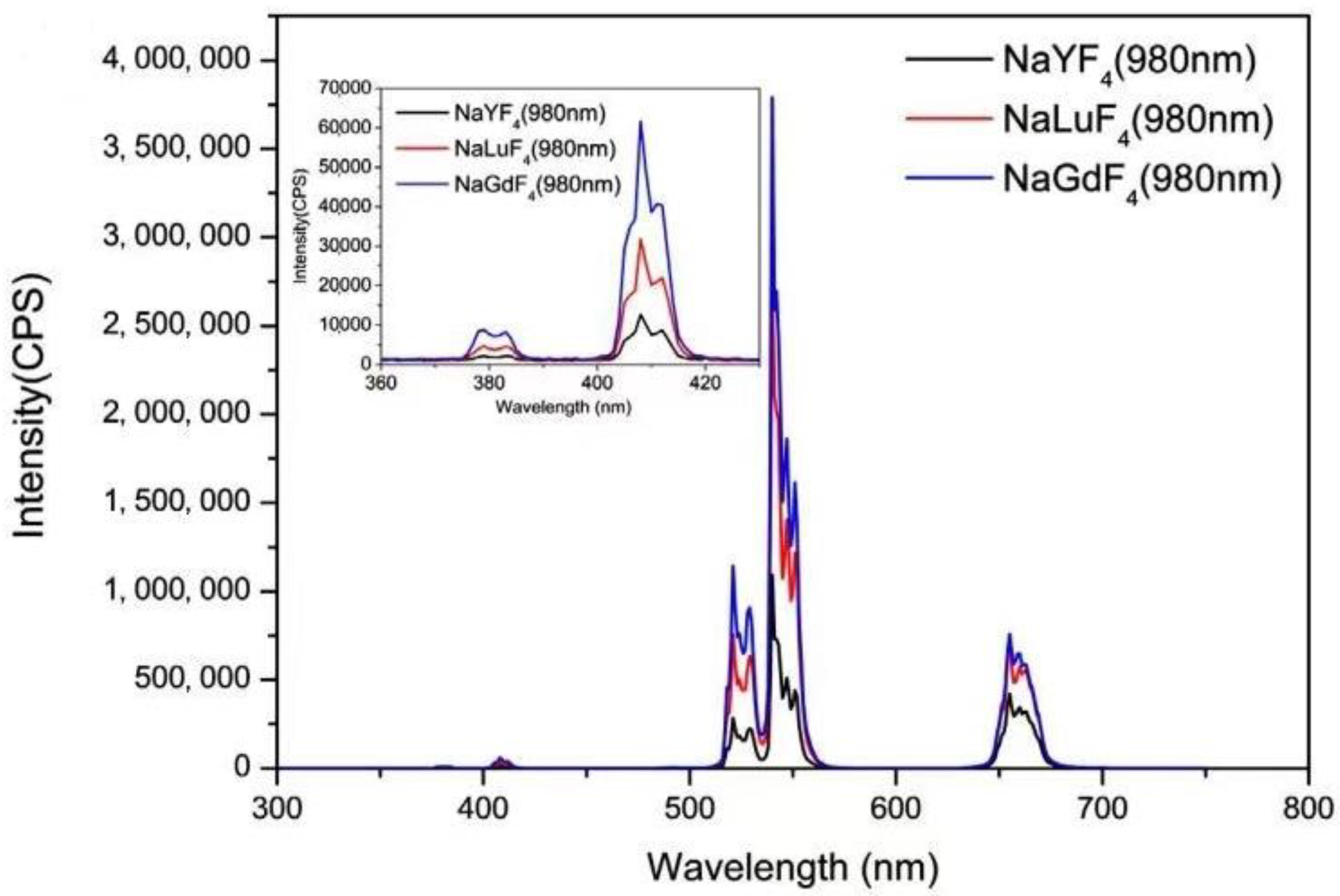

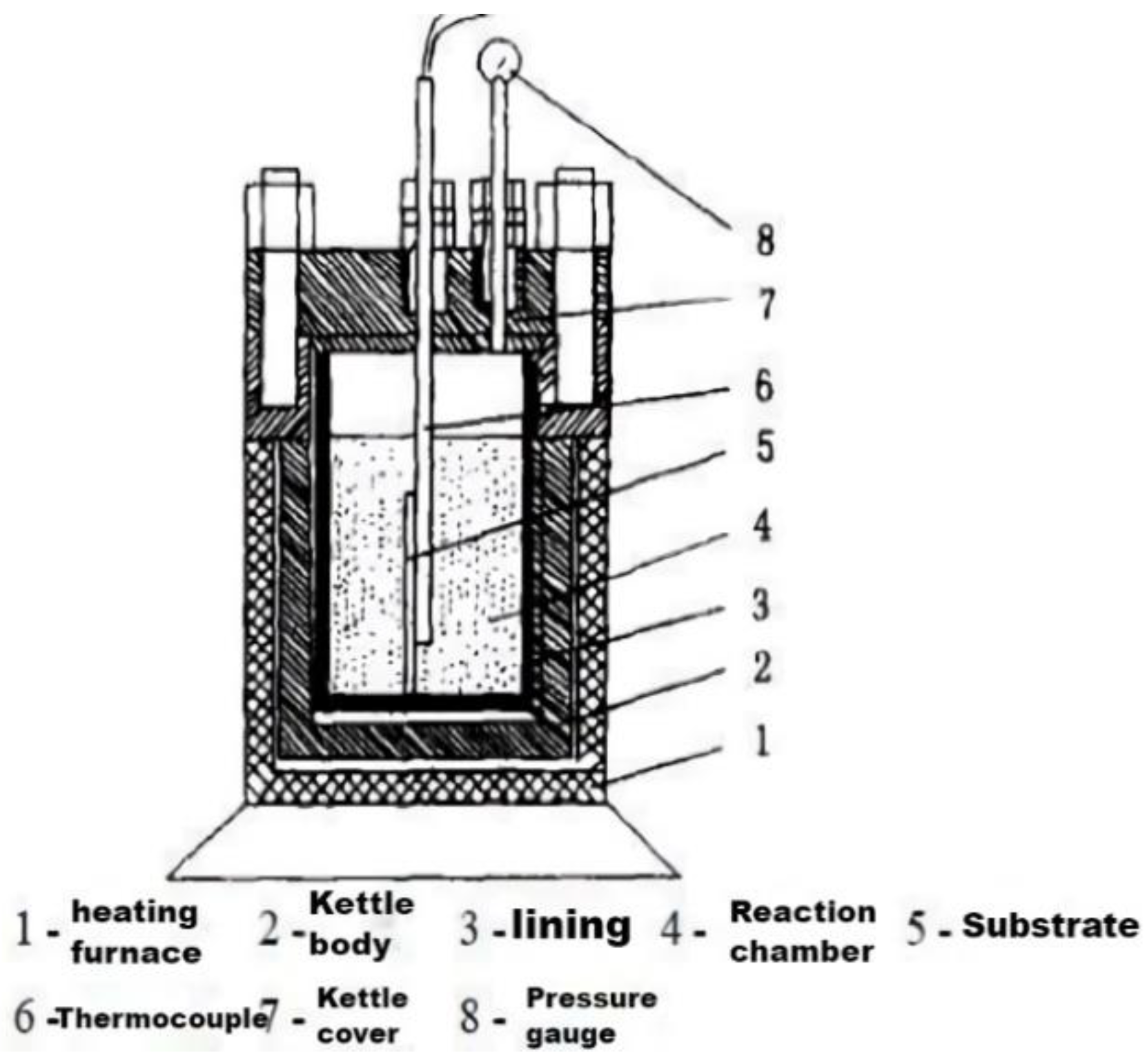

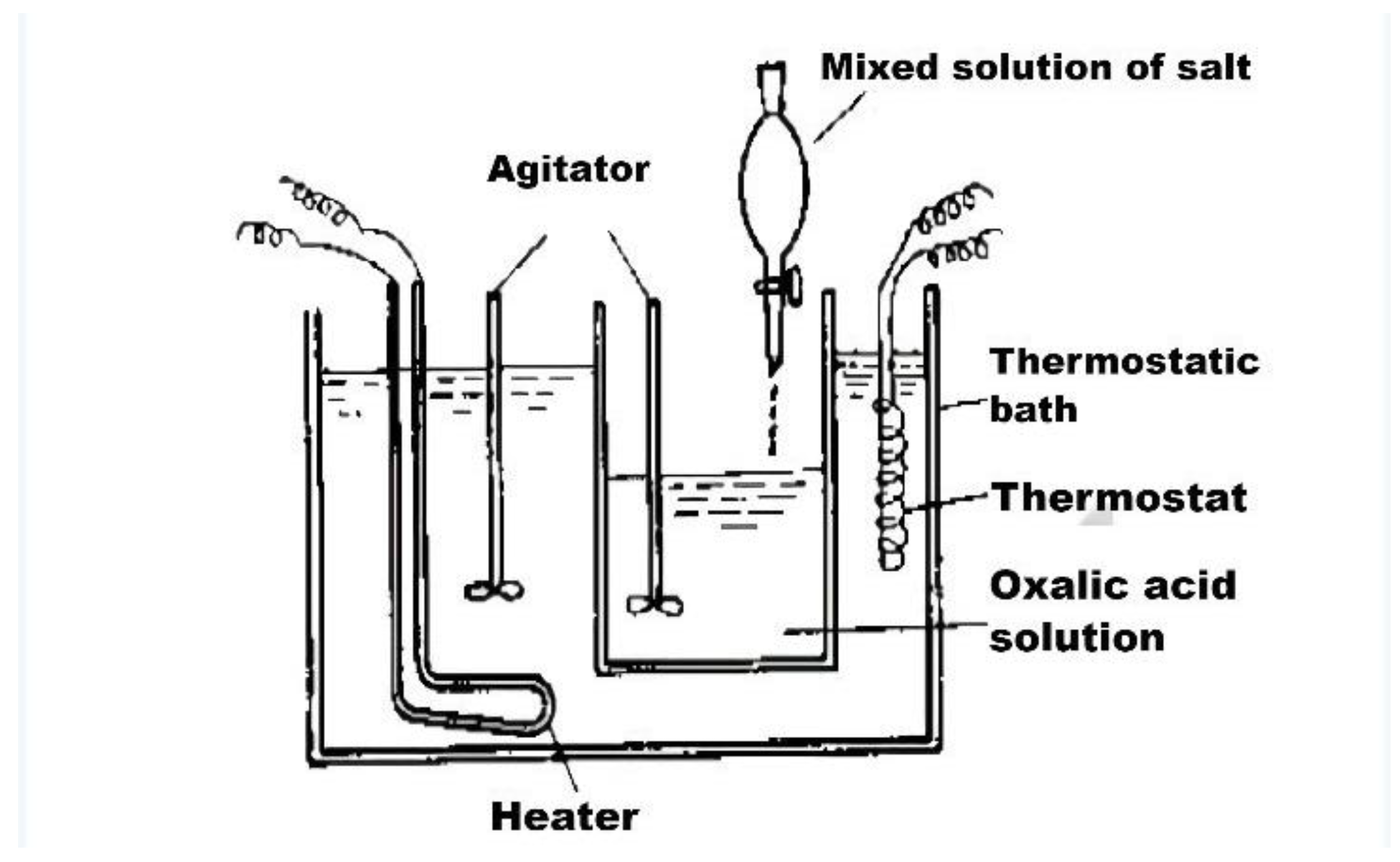

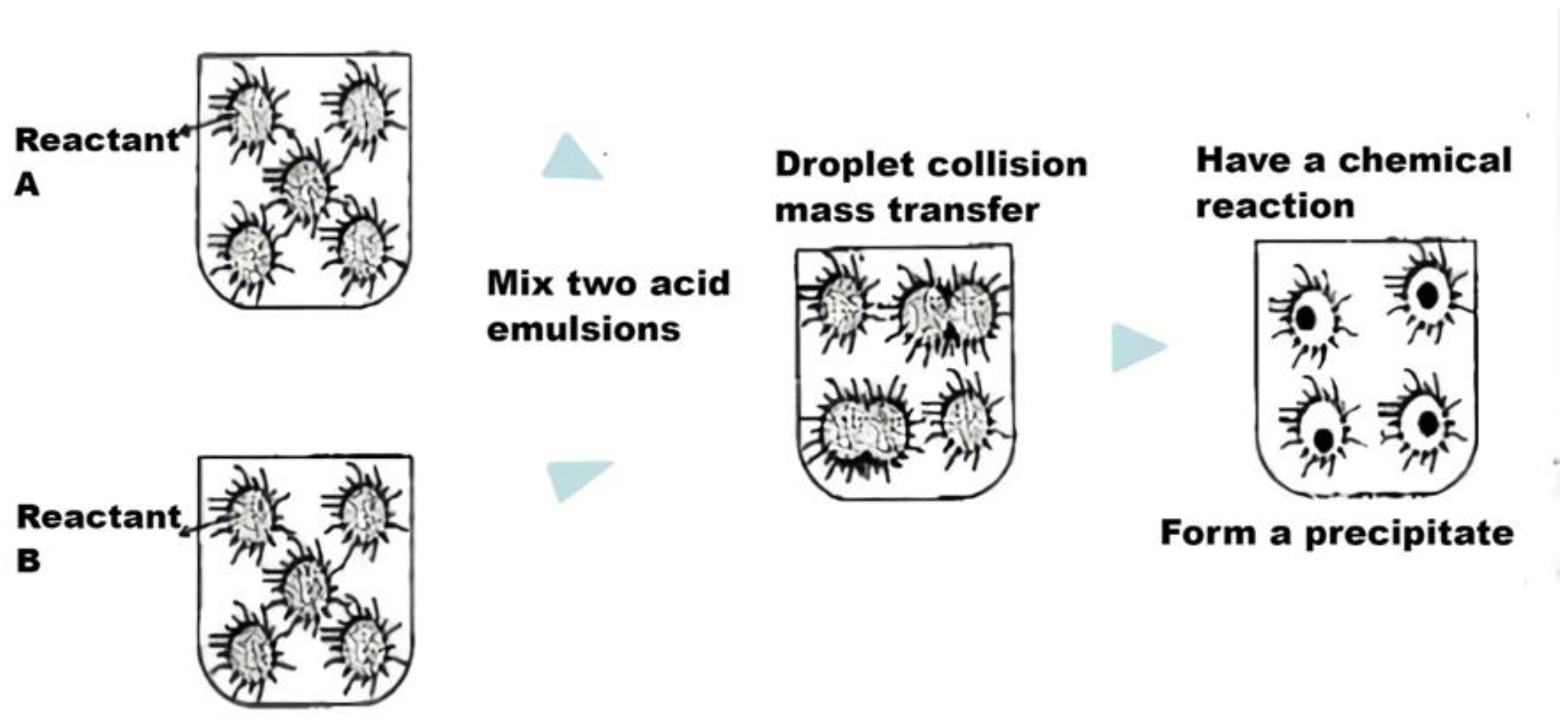

| Category | Advantages | Detection Limit | References |
|---|---|---|---|
| Small molecule fluorescent probes | Small molecule fluorescent probes have significant advantages such as high sensitivity, good membrane permeability, real-time in situ analysis, minimal biological damage, and easy handling. | 1.05 × 10−8~5.8 × 10−7 mol/L | [40,41] |
| Polymeric fluorescent probes | It has a long fluorescence lifetime, good biocompatibility, and high quantum yield. It can also enhance intramolecular electron transport and increase the sensitivity of the probe. | 0.17~2.3 μg/mL | [42,43] |
| Fluorescent probes based on nanomaterials | Compared with traditional fluorescent dyes, fluorescent nanomaterials not only have high fluorescence intensity and good light stability, but also have the characteristics of small size effect, quantum effect, and surface effect that are unique to nanomaterials, which can make up for the shortcomings of traditional fluorescent dyes. | 6.4 × 10−10~1.225 × 10−8 mol/L | [44,45] |
| Category | Advantages | Detection Limit | References |
|---|---|---|---|
| Electrochemical Sensors | Electrochemical sensors can be used to detect chemical and biological compounds. Molecularly imprinted polymers (MIPs) functionalized sensors are effective in detecting PFASs, but may have the disadvantage of long detection times and high costs, as well as the inability to differentiate between similar molecules. | 0.07~1.0 µg/L | [47,48,49] |
| Fluorescence Sensors | Fluorescent sensors occurred fluorescence quenching because of electron transfer between fluorescent dyes and PFASs, which can easily be detected using fluorescence spectrophotometers. However, this method can increase costs and cause environmental hazards | 2.5 ppt~120 ppb | [50,51] |
| Optical Sensors/ Colorimetric sensors | Optical sensors/ Colorimetric sensors usually use organic dyes and can be detected by the naked eye based on visible color changes. Although it is not overly dependent on fluorescence spectrophotometry, co-existing atoms can interfere with the sensor. In addition, they require a good platform to improve sensor availability. | 1 ppb~5 ppt | [52,53] |
| HPLC–MS/MS | HPLC–MS/MS is based on the principle of using liquid chromatography monopole mass spectrometry or multistage mass spectrometry as the separation and detection system. It has the advantages of low detection limits, high resistance to matrix effect interference, convenience, and rapid detection. The detection limit is approximately 0.07~0.15 μg/kg. | The method is expensive, costly, and highly specialized and requires a high level of operator expertise | [54,55,56,57] |
| HPLC–Q-TOF-MS | HPLC–Q-TOF-MS has a certain optimization in the ability to analyze samples when in a complex environment, which increase the correctness of detection. The detection limit is approximately 20~30 ng/L. | The method is too costly to be suitable for universal detection. | [28] |
| GC–MS | GC–MS is a tandem gas chromatograph and mass spectrometer method that offers the advantages of simplicity and convenience. The detection limit is approximately 15~40 ng/L. | PFOA is less volatile and needs to be derivatized before it can be detected by injection. The application of GC–MS is narrow, and the experimental process is accompanied by toxic substances. | [58,59,60] |
| Pre-column Derivatization GC | It is a method that improves on the shortcomings of GC–MS. The derivatization technique reduces the temperature of the target, and improves the signal required to detect the target. The detection limit is approximately 0.01~0.05 mg/kg. | The formation of by-products may cause greater difficulties in chromatographic separation. Impurities or interfering peaks can easily be introduced or samples lost during derivatization. | [34] |
| Rare earth up-conversion fluorescent probe | Fluorescence burst reaction of the probe with the detected substance, offering the advantage of simple, efficient, and highly sensitive detection. The detection limit is approximately 0.01~1.2 nmol/L. | PFOA is a perfluorinated compound. The method is not selectively detectable for PFOA | [61] |
| Fluorescent Probe | Quantum Yields | Reference |
|---|---|---|
| A highly selective Al3+ fluorescent probe | 5.7% (366 nm) | [91,92] |
| Near-infrared H2S fluorescent probes | 1.2% (530 nm) | [93] |
| Enhanced fluorescent probes for BSA | 1.5% (645 nm) | [94] |
| Visualization of iron ion fluorescent probes | 2.1% (610 nm) | [95] |
| Rare-earth up-conversion fluorescent probes | 4.7% (980 nm) | [96] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Thermal decomposition method | The product has high quality, is monodisperse, and has excellent morphology | Reaction requires high temperature, no oxygen, and no water, easy to produce toxic gases |
| Coprecipitation method | Simple process, low cost, relative environmental protection, high yield | The reaction requires high-temperature calcination, and the particle size of the product is not uniform |
| Hydrothermal method | The synthesis method is efficient, simple, and morphologically controllable | The size of the product is commonly large |
| Sol–gel method | The product has good dispersion and uniform morphology | The product agglomerates easily |
| Microemulsion method | Particle size controllable, good morphology | The operation is complicated, the cost is high and the reaction condition is harsh |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Shi, X.; Lin, L.; Cheng, Y.; Luo, X.; Fang, C. Research Progress on Up-Conversion Fluorescence Probe for Detection of Perfluorooctanoic Acid in Water Treatment. Polymers 2023, 15, 605. https://doi.org/10.3390/polym15030605
Mao T, Shi X, Lin L, Cheng Y, Luo X, Fang C. Research Progress on Up-Conversion Fluorescence Probe for Detection of Perfluorooctanoic Acid in Water Treatment. Polymers. 2023; 15(3):605. https://doi.org/10.3390/polym15030605
Chicago/Turabian StyleMao, Tan, Xiaoting Shi, Liyuan Lin, Youliang Cheng, Xueke Luo, and Changqing Fang. 2023. "Research Progress on Up-Conversion Fluorescence Probe for Detection of Perfluorooctanoic Acid in Water Treatment" Polymers 15, no. 3: 605. https://doi.org/10.3390/polym15030605





