Development of Stereocomplex Polylactide Nanocomposites as an Advanced Class of Biomaterials—A Review
Abstract
:1. Introduction
2. Stereocomplex Polylactide (s-PLA) and Polylactide (PLA) Nanocomposites
2.1. Stereocomplex PLA (s-PLA) Synthesis and Properties
2.2. PLA Nanocomposites Synthesis and Properties
3. Stereocomplex Nanocomposite PLA (Stereo-Nano PLA)
- (i)
- PLLA + PDLA + nanoparticlesThe first and most simple combination to form stereo-nano PLA comprises PDLA, PLLA, and nanoparticles. The chemical structure of the nanoparticle is critical to ensure its miscibility in the PLLA/PDLA matrix. The –OH and C=O groups in lignin enhance the mixing capability with PLLA/PDLA blends through hydrogen bonding [93]. Despite MWCNT in PLLA/PDLA blends being able to yield stereocomplex crystallites, the poor dispersion of MWCNT in the polymer matrix leads to agglomerates and limits stereocomplex crystallization [95]. The presence of organic modifiers in nanoplatelets helps their delamination and improves their dispersion in the PLLA/PDLA matrix [86,101,102]. The presence of 1–10% PDLA in the PLLA matrix prevents the aggregation of carbon black (CB) and helps the formation of a CB nanoparticle network [110,117]. Multifunctional CB increases its compatibility with the PLLA/PDLA matrix [118]. The presence of –OH groups of CNF results in well-dispersed CNF in the PLLA/PDLA matrix [115]. An increasing nanosilica content results in the formation of aggregates, although it also aids in the formation of the stereocomplex crystallites in PLLA/PDLA blends [126]. The functionalization of nanoparticles affects their compatibility and dispersion in the PLLA/PDLA matrix and also affects the formation of stereocomplex crystallites.
- (ii)
- PLLA + PDLA-grafted nanoparticlesA high content of non-functionalized nanoparticles leads to aggregation in the polymer matrix. The functionalization of nanoparticles enhances the miscibility and interfacial interaction between the nanoparticle surface and the PLA chains. A combination of PLLA/nanoparticles-g-PDLA shows better crystallization compared to PLLA/PDLA blends. This is explained by the reduction in the crystallization activation energy by nanomaterials, such as CNT and graphene oxide [88,89]. The PDLA chains grafted on the CNT particles increase the stability of the nanoparticles in the PLLA matrix by forming stable stereocomplex crystallites through interfacial adhesion [90,107,111,116,128].
- (iii)
- PLLA-grafted nanoparticles + PDLA-grafted nanoparticles
4. Properties and Applications of Stereo-Nano PLA
5. Prospective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, C.K.; Hillmyer, M.A. Polymer from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 45, 1–10. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.-J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Infrared spectroscopic study of CH3···O=C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation. Macromolecules 2005, 38, 1822–1828. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide). J. Macromol. Sci. B Phys. 1991, B30, 119–140. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Biopolymer stereocomplexes. Adv. Drug Deliv. Rev. 2003, 55, 549–583. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high performance bio-based polymers: Their formation, properties, and applications. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Kakuta, M.; Hirata, M.; Kimura, Y. Stereoblock polylactides as high-performance biobased polymers. Polym. Rev. 2009, 49, 107–140. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplexation between enantiomeric poly(lactide)s. In Biodegradable Polymer Blends and Composites from Renewable Resources; Yu, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Chapter 7; pp. 163–190. [Google Scholar]
- Saravanan, M.; Domb, A.J. A contemporary review on—Polymer stereocomplexes and its biomedical application. Eur. J. Nanomed. 2013, 5, 81–86. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactic Acid). In Biobased Plastics: Materials and Applications; Kabasci, S., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; Chapter 8; pp. 171–239. [Google Scholar]
- Jing, Y.; Quan, C.; Liu, B.; Jiang, Q.; Zhang, C. A mini-review on the study of functional biomaterials based on poly(lactic acid) stereocomplex. Polym. Rev. 2016, 56, 262–286. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 2. Stereocomplex formation in concentrated solutions. Macromolecules 1991, 24, 2719–2724. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 6. Binary blends from copolymers. Macromolecules 1992, 25, 5719–5723. [Google Scholar] [CrossRef]
- Brochu, S.; Prud’homme, R.E.; Barakat, I.; Jerome, R. Stereocomplexation and morphology of polylactides. Macromolecules 1995, 28, 5230–5239. [Google Scholar] [CrossRef]
- Schmidt, S.C.; Hillmyer, M.A. Polylactide stereocomplex crystallites as nucleating agents for isotactic polylactide. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 300–313. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Yamane, H.; Sasai, K. Effect of the addition of poly(D-lactic acid) on the thermal property of poly(L-lactic acid). Polymer 2003, 44, 2569–2575. [Google Scholar] [CrossRef]
- Tsuji, H.; Tezuka, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 12. Spherulite growth of low-molecular-weight poly(lactic acid)s from the melt. Biomacromolecules 2004, 5, 1181–1186. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of high-molecular-weight polylactide using supercritical fluid. Macromolecules 2010, 43, 1137–1142. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. Melt stability of 8-arms star-shaped stereocomplex polylactide with three-dimensional core structures. Polym. Degrad. Stab. 2013, 96, 1097–1101. [Google Scholar] [CrossRef]
- Bibi, G.; Jung, Y.; Lim, J.C.; Kim, S.H. Novel strategy of lactide polymerization leading to stereocomplex nanoparticles using supercritical fluid technology. ACS Sustain. Chem. Eng. 2016, 4, 4521–4528. [Google Scholar] [CrossRef]
- Im, S.H.; Lee, C.W.; Bibi, G.; Jung, Y.; Kim, S.H. Supercritical fluid technology parameters affecting size and behavior of stereocomplex polylactide particles and their composites. Polym. Eng. Sci. 2017, 58, 1193–1200. [Google Scholar] [CrossRef]
- Anderson, K.S.; Hillmyer, M.A. Melt preparation and nucleation efficiency of polylactide stereocomplex crystallites. Polymer 2006, 47, 2030–2035. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acids) with high molecular weight. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Tsuji, H.; Shimizu, K.; Sakamoto, Y.; Okumura, A. Hetero-stereocomplex formation of stereoblock copolymer of substituted and non-substituted poly(lactide)s. Polymer 2011, 52, 1318–1325. [Google Scholar] [CrossRef]
- Portinha, D.; Belleney, J.; Bouteiller, L.; Pensec, S.; Spassky, N. Formation of nanoparticles of polylactide-containing diblock copolymers: Is stereocomplexation the driving force? Macromolecules 2002, 35, 1484–1486. [Google Scholar] [CrossRef]
- Nurqadar, R.I.; Purnama, P.; Kim, S.H. Preparation and characterization of a stereocomplex of poly(lactide-co-caprolactone)/tricalcium phosphate biocomposite using supercritical fluid technology. Express Polym. Lett. 2013, 7, 974–983. [Google Scholar] [CrossRef]
- Luo, F.; Fortenberry, F.; Ren, J.; Qiang, Z. Recent progress in enhancing poly(lactic acid) stereocomplex formation for material property improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef]
- Purnama, P.; Samsuri, M.; Iswaldi, I. A review on fully bio-based material development from polylactide and cellulose nanowhiskers. Polymers 2022, 14, 4009. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Brzezinski, M.; Biela, T. Polylactide nanocomposites with functionalized carbon nanotubes and their stereocomplexes: A focused review. Mater. Lett. 2014, 121, 244–250. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. Stereocomplexation of poly(L-lactide) and Random Copolymer Poly(D-lactide-co-ε-caprolactone) to enhance melt stability. Macromolecules 2012, 45, 4012–4014. [Google Scholar] [CrossRef]
- Biela, T.; Duda, A.; Penczek, S. Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 2006, 39, 3710–3713. [Google Scholar] [CrossRef]
- Kim, S.H.; Nederberg, F.; Zhang, L.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L. Hierarchical assembly of nanostrtuctured organosilicate networks via stereocomplexation of block copolymers. Nano Lett. 2008, 8, 294–301. [Google Scholar] [CrossRef]
- Purnama, P.; Samsuri, M.; Iswaldi, I. Properties enhancement of high molecular weight polylactide using stereocomplex polylactide as a nucleating agent. Polymers 2021, 13, 1725. [Google Scholar] [CrossRef]
- Sinha Ray, S. Polylactide-based bionanocomposites: A promising class of hybrid materials. Acc. Chem. Res. 2012, 45, 1710–1720. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent advances in clay/polymer nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef] [Green Version]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ueda, K. Polylactide-layered silicate nanocomposite: A novel biodegradable materials. Nano Lett. 2002, 2, 1093–1096. [Google Scholar] [CrossRef]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: Role of organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide/layered silica nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 245, 815–840. [Google Scholar]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silica nanocomposites. 2. Concurrent improvements of materials properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, A.K.; Maiti, S.N.; Raha, S.; Gupta, R.K.; Bhattacharya, S. Morphology and rheological behavior of polylactic acid/clay nanocomposites. Polym. Eng. Sci. 2012, 52, 225–232. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Morphology, electromagnetic properties and electromagnetic interference shielding performance of polylactide/graphene nanoplatelet nanocomposites. Mater. Des. 2016, 95, 119–126. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Dielectric properties and electromagnetic interference shielding effectiveness of graphene-based biodegradable nanocomposites. Mater. Des. 2016, 109, 68–78. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Xu, L.; Sun, Z.; Ren, P.; Yan, D.; Li, Z. Large-scale preparation of segregated PLA/carbon nanotube composite with high efficient electromagnetic interference shielding and favourable mechanical properties. Compos. Part B 2018, 155, 405–413. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Mark, L.H.; Shaayegan, V.; Wang, G.; Li, H.; Zhao, G.; Park, C.B. Ultralow-Threshold and lightweight biodegradable porous PLA/MWCNT with segregated conductive networks for high-performance thermal insulation and electromagnetic interference shielding applications. ACS Appl. Mater. Interfaces 2018, 10, 1195–1203. [Google Scholar] [CrossRef]
- Mina, M.F.; Beg, M.D.H.; Islam, M.R.; Nizam, A.; Alam, A.K.M.M.; Yunus, R.M. Structure and properties of injection-molded biodegradable polyl(lactic acid) nanocomposites prepared with untreated and treated multiwalled carbon nanotubes. Polym. Eng. Sci. 2014, 54, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ding, S.; Zhou, C. Preparation and degradation of PLA/chitosan composite materials. J. Appl. Polym. Sci. 2004, 91, 274–277. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Saad, G.R.; Sayed, A.M. Mechanical, thermal, and dielectric properties of poly(lactic acid)/chitosan nanocomposites. Polym. Eng. Sci. 2016, 56, 987. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Fekry, M.; Sayed, A.M.; Maziad, N.A.; Saad, G.R. Physico-chemical charateristics of biodegradable poly(lactic acid) and poly(lactic acid)/chitosan nano-composites under the influence of gamma irradiation. J. Polym. Environ. 2023, 31, 2705–2714. [Google Scholar] [CrossRef]
- Kramschuster, A.; Turng, L.S.; Li, W.J.; Peng, Y.; Peng, J. The Effect of Nano Hydroxyapatite Particles on Morphology and Mechanical Properties of Microcellular Injection Molded Polylactide/Hydroxyapatite Tissue Scaffold. In Proceedings of the ASME 1st Global Congress on NanoEngineering for Medicine and Biology 2010, NEMB2010, Houston, TX, USA, 7–10 February 2010; American Society of Mechanical Engineers Digital Collection. pp. 175–178. [Google Scholar]
- Haaparanta, A.; Haimi, S.; Ella, V.; Hopper, N.; Miettinen, S.; Suuronen, R.; Kellomaki, M. Porous polylactide/β-tricalcium phosphate composite scaffolds for tissue engineering applications. J. Tissue Eng. Regen. Med. 2010, 4, 366–373. [Google Scholar] [CrossRef]
- Morelli, S.; Salerno, S.; Holopainen, J.; Ritala, M.; De Bartolo, L. Osteogenic and osteoclastogenic differentiation of co-cultured cells in polylactic acid-nanohydroxyapatite fibe scaffolds. J. Biotechnol. 2015, 204, 53–62. [Google Scholar] [CrossRef]
- Yanoso-Scholl, L.; Jacobson, J.A.; Bradica, G.; Lerner, A.L.; O’Keefe, R.J.; Schwarz, E.M.; Zuscik, M.J.; Awad, H.A. Evaluation of dense polylactide acid/beta-tricalcium phosphate scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2010, 95, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Kang, M.-H.; Kang, I.-G.; Kim, H.-E.; Jeong, S.-H. In vitro and in vivo evaluation of polylactic acid-based composite with tricalcium phosphate microsphere for enhanced biodegradability and osseointegration. J. Biomater. Appl. 2018, 32, 1360–1370. [Google Scholar] [CrossRef]
- Pietrzykowska, E.; Mukhovskyi, R.; Chodara, A.; Wojnarowicz, J.; Koltsov, I.; Chudoba, T.; Lojkowski, W. Composites of polylactide and nanohydroxyapatite created by cryomilling and warm isostatic pressing for bone implants applications. Mater. Lett. 2019, 236, 625–628. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(L-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Wan Yunus, W.D.Z.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly(lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA-ZNO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132, 42480. [Google Scholar] [CrossRef]
- Campardelli, R.; Della Porta, G.; Gomez, V.; Irusta, S.; Reverchon, E.; Santamaria, J. Encapsulation of titanium dioxide nanoparticles in PLA microsphere using supercritical extraction to produce bactericidal nanocomposites. J. Nanopart. Res. 2013, 15, 1987. [Google Scholar] [CrossRef]
- Urbanczyk, L.; Ngoundjo, F.; Alexandre, M.; Jerome, C.; Detrembleur, C.; Calberg, C. Synthesis of polylactide/clay nanocomposites by in situ intercalative polymerization in supercritical carbon dioxide. Eur. Polym. J. 2009, 45, 643–648. [Google Scholar] [CrossRef]
- Ming, Y.; Zhou, Z.; Hao, T.; Nie, Y. Polymer nanocomposites: Role of modifier filler content and interfacial interaction on crystallization. Eur. Polym. J. 2022, 162, 110894. [Google Scholar] [CrossRef]
- Wang, L.-N.; Wang, P.-Y.G.; Wei, J.-C. Graphene oxide-graft-poly(l-lactide)/poly(L-lactide) nanocomposites: Mechanical and thermal properties. Polymers 2017, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, J.; Sinha Ray, S. The Quantitative Analysis of Nano-clay Dispersion in Polymer Nanocomposites by Small-angle X-ray Scattering Combined with Electron Microscopy. Polymer 2010, 51, 1434–1449. [Google Scholar] [CrossRef]
- Sinha Ray, S. A New Possibility for Microstructural Investigation of Clay-Based Polymer Nanocomposite by Focused-Ion-Beam Tomography. Polymer 2010, 51, 3966–3970. [Google Scholar]
- Bandyopadhyay, J.; Sinha Ray, S. Determination of Structural Changes of Dispersed Clay Platelets in a Polymer Blend during Solid-state Rheological Property Measurement by Small-Angle X-ray Scattering. Polymer 2011, 52, 2628–2642. [Google Scholar] [CrossRef]
- Choi, H.J.; Sinha Ray, S. A Review on Melt-State Viscoelastic Properties of Polymer Nanocomposites. J. Nanosci. Nanotechnol. 2011, 11, 8421–8449. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Zaman, I.; Kuan, H.-C.; Dai, J.; Kawashima, N.; Michelmore, A.; Sovi, A.; Dong, S.; Luong, L.; Ma, J. From carbon nanotubes and silicate layers to graphene platelets for polymer nanocomposites. Nanoscale 2012, 4, 4578. [Google Scholar] [CrossRef] [Green Version]
- Sinha Ray, S.; Bousmina, M. Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Materials World. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R.; Hollingsworth, L.O. Supra-molecular ecobionanocomposites based on polylactide and cellulosic nanowhiskers: Synthesis and properties. Biomacromolecules 2012, 13, 2013–2019. [Google Scholar] [CrossRef]
- Tingaut, P.; Zimmermann, T.; Lopez-Suevos, F. Syntheis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 2010, 11, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M. Green esterification: A new approach to improve thermal and mechanical properties of poly(lactic acid) composites reinforced by cellulose nanocrystals. J. Appl. Polym. Sci. 2018, 135, 46468. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Hartman, K. Esterified cellulose nanocrystals as reinforcement in poly(lactic acid) nanocomposites. Cellulose 2019, 26, 2349–2362. [Google Scholar] [CrossRef]
- Yoon, J.T.; Jeong, Y.G.; Lee, S.C.; Min, B.G. Influences of poly(lactic acid)-grafted carbon nanotube on thermal, mechanical, and electrical properties of poly(lactic acid). Polym. Adv. Technol. 2009, 20, 631–638. [Google Scholar] [CrossRef]
- Chen, G.-X.; Kim, H.-S.; Park, B.H.; Yoon, J.-S. Synthesis of poly(L-lactide)-functionalized multiwalled carbon nanotubes by ring-opening polymerization. Macromol. Chem. Phys. 2007, 208, 389–398. [Google Scholar] [CrossRef]
- Zhou, S.; Zhen, X.; Yu, X.; Wang, J.; Weng, J.; Li, X.; Feng, B.; Yin, M. Hydrogen bonding interaction of poly(D,L-lactide)/hydroxyapatite nanocomposites. Chem. Mater. 2007, 19, 247–253. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Hazeli, K.; Niu, J.; Kontsos, A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials 2012, 33, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Purnama, P.; Lim, S.H.; Jung, Y.; Kim, S.H. Stereocomplex-nanocomposite formation of polylactide/fluorinated-clay with superior thermal property using supercritical fluid. Macromol. Res. 2012, 20, 545–548. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. An advanced class of bio-hybrid materials: Bionanocomposites of inorganic clays and organic stereocomplex polylactides. Macromol. Mater. Eng. 2013, 298, 263–269. [Google Scholar] [CrossRef]
- Tan, B.H.; Hussain, H.; Lin, T.T.; Chua, Y.C.; Leong, Y.W.; Tjiu, W.W.; Wong, P.K.; He, C.B. Stable dispersions of hybrid nanoparticles induced by stereocomplexation between enantiomeric poly(lactide) star polymers. Langmuir 2011, 27, 10538–10547. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Synthesis, stereocomplex crystallization, morphology and mechanical property of poly(lactide)-carbon nanotube composites. RSC Adv. 2013, 3, 2219. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Synthesis and stereocomplex crystallization poly(lactide)-graphene oxide nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Wu, G. Forming CNT-guided stereocomplex networks in polylactide-based nanocomposites. Compos. Sci. Technol. 2016, 128, 8–16. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Y.; Wu, G. Polylactide-based nanocomposites with stereocomplex networks enhanced by GO-g-PDLA. Compos. Sci. Technol. 2017, 138, 57–67. [Google Scholar] [CrossRef]
- Souza, D.H.; Santoro, P.V.; Dias, M.L. Isothermal crystallization kinetics of poly(lactic acid) stereocomplex/graphene nanocomposites. Mater. Res. 2018, 21, e20170352. [Google Scholar] [CrossRef] [Green Version]
- Brzezinski, M.; Boguslawska, M.; Ilcikova, M.; Mosnacek, J.; Biela, T. Unusual thermal properties of polylactides and polylactide stereocomplexes containing polylactide-functionalized multi-walled carbon nanotubes. Macromolecules 2012, 45, 8714–8721. [Google Scholar] [CrossRef]
- Habibi, Y.; Aouadi, S.; Raquez, J.-M.; Dubois, P. Effect of interfacial stereocomplexation in cellulose nanocrystal-filled polylactide nanocomposites. Cellulose 2013, 20, 2877–2885. [Google Scholar] [CrossRef]
- De Arenaza, I.M.; Sarasua, J.R.; Amestoy, H.; Lopez-Rodriguez, N.; Zuza, E.; Meaurio, E.; Meyer, F.; Santos, J.I.; Raquez, J.-M.; Dubois, P. Polylactide stereocomplex crystallization promted by multiwall carbon nanotubes. J. Appl. Polym. Sci. 2013, 130, 4327–4337. [Google Scholar]
- Purnama, P.; Kim, S.H. Biodegradable blends of stereocomplex polylactide and lignin by supercritical carbon dioxide-solvent system. Macromol. Res. 2014, 22, 74–78. [Google Scholar] [CrossRef]
- Tan, B.H.; Hussain, H.; Leong, Y.W.; Lin, T.T.; Tjiu, W.W.; He, C. Tuning self-assembly of hybrid PLA-P(MA-POSS) block copolymers in solution via stereocomplexation. Polym. Chem. 2013, 4, 1250. [Google Scholar] [CrossRef]
- Kum, C.H.; Cho, Y.; Seo, S.H.; Joung, Y.K.; Ahn, D.J.; Han, D.K. A poly(lactide) stereocomplex structure with modified magnesium oxide and its effects in enhancing the mechanical properties and surpressing inflammation. Small 2014, 10, 3783–3794. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Synergism of cellulosic nanowhiskers and graft structure in stereocomplex-based materials: Formation in solution and a stereocomplex memory study. Cellulose 2014, 21, 2539–2548. [Google Scholar] [CrossRef]
- Re, G.L.; Benali, S.; Habibi, Y.; Raquez, J.-M.; Dubois, P. Stereocomplexed PLA nanocomposites: From in situ polymerization to material properties. Eur. Polym. J. 2014, 54, 138–150. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chen, E.-C.; Wu, T.-M. Organically modified layered zinc phenilphosphonate reinforced stereocomplex-type poly(lactic acid) nanocomposites with highly enhanced mechanical properties and degradability. J. Mater. Sci. 2015, 50, 7770–7778. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chen, E.-C.; Wu, T.-M. Lamellae evolution of stereocomplex-type poly(lactic acid)/organically-modified layered zinc phenylphosphonate nanocomposites induced by isothermal crystallization. Materials 2016, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Jiang, L.; Xu, P.; Dong, W.; Chen, M.; Lemstra, P.J. Rapid stereocomplexation between enantiomeric comb-shaped cellulose-g-poly(L-lactide) nanohybrids and poly(D-lactide) from the melt. Biomacromolecules 2015, 16, 3723–3729. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G.; Qin, J. Synthesis, stereocomplex crystallization and properties of poly(L-lactide)/four-armed star poly(D-lactide) functionalized carbon nanotubes nanocomposites. Polym. Adv. Technol. 2015, 26, 223–233. [Google Scholar] [CrossRef]
- Liu, H.; Bai, H.; Bai, D.; Liu, Z.; Zhang, Q.; Fu, Q. Design of high-performance poly(L-lactide) /elastomer blends through anchoring carbon nanotubes at the interface with the aid of stereocomplex crystallization. Polymer 2017, 108, 38–49. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Zhou, L.; Duan, Y.; Zhang, J. Synthesis and characterization of cellulose nanocrystal-graft-poly(D-lactide) and its nanocomposite with poly(L-lactide). Polymer 2016, 103, 365–375. [Google Scholar] [CrossRef]
- Gupta, A.; Katiyar, V. Cellulose functionalized high molecular weight stereocomplex polylactic acid biocomposite films with improved barrier, thermomechanical properties. ACS Sustain. Chem. Eng. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Fan, X.; Cao, M.; Zhang, X.; Li, Z. Synthesis of star-like hybrid POSS-(PDMAEMA-b-PDLA)8 copolymer and its stereocomplex properties with PLLA. Mater. Sci. Eng. C 2017, 76, 211–216. [Google Scholar] [CrossRef]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable silica rubber core-shell nanoparticles and their stereocomplex for efficient PLA toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Liu, H.; Bai, D.; Bai, H.; Zhang, Q.; Fu, Q. Manipulating the filler network structure and properties of polylactide/carbon black nanocomposites with the aid of stereocomplex crystallites. J. Phys. Chem. C 2018, 122, 4232–4240. [Google Scholar] [CrossRef]
- Huang, G.; Du, Z.; Yuan, Z.; Gu, L.; Cai, Q.; Yang, X. Poly(L-lactide) nanocomposites containing poly(D-lactide) grafted nanohydroxyapatite with improved interfacial adhesion via stereocomplexation. J. Mech. Behav. Biomed. Mater. 2018, 78, 10–19. [Google Scholar] [CrossRef]
- Xu, P.; Lv, P.; Wu, B.; Ma, P.; Dong, W.; Chen, M.; Du, M.; Ming, W. Smart design of rapid crystallizing and non-leaching antibacterial poly(lactide) nanocomposites by sustainable aminolysis grafting and in situ interfacial stereocomplexation. ACS Sustain. Chem. Eng. 2018, 6, 13367–13377. [Google Scholar] [CrossRef]
- Benali, S.; Khelifa, F.; Lerari, D.; Mincheva, R.; Habibi, Y.; Lahem, D.; Debliquy, M.; Dubois, P. Supramolecular Approaches for efficient processing of polylactide/starch nanocomposites. ACS Omega 2018, 3, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Jin, L.; He, X.; Huang, X.; Xie, M.; Wang, C.; Zhang, C.; Yang, W.; Meng, F.; Lu, J. Glowing stereocomplex biopolymers are generating power: Polylactide/carbon quantum dot hybrid nanofibers with high piezoresponse and multicolor luminescence. J. Mater. Chem. A 2019, 7, 1810–1823. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Wang, C.; Peng, Z.; Xu, Y.; He, X.; Zhang, C.; Lu, J. Nanocellulose-assisted construction of hydrophilic 3D hierarchical stereocomplex meshworks in enantiomeric polylactides: Towards thermotolerant biocomposites with enhanced environmental degradation. Cryst. Eng. Comm. 2019, 21, 6405–6413. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, P.; Lv, P.; Lemstra, P.J.; Cai, X.; Yang, W.; Dong, W.; Chen, M.; Liu, T.; Du, M.; et al. Excellent UV Resistance of polylactide by interfacial stereocomplexation with double-shell-structured TiO2 nanohybrids. ACS Appl. Mater. Interfaces 2020, 12, 49090–49100. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Miyamoto, K.; Akasaka, S.; Asai, S. Formation of polylactide stereocomplex crystallites and the electrical properties of carbon black-filled PLLA/PDLA composites. Polym. J. 2020, 52, 1093–1102. [Google Scholar] [CrossRef]
- Liu, Z.; Ling, F.; Diao, X.; Fu, M.; Bai, H.; Zhang, Q.; Fu, Q. Stereocomplex-type polylactide with remarkably enhanced melt-processability and electrical performance via incorporating multifunctional carbon black. Polymer 2020, 188, 122136. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, Z.-M.; Pu, J.-H.; Feng, C.-P.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Highly thermally conductive electrospun stereocomplex polylactide fibrous film dip-coating with silver nanowires. Polymer 2020, 194, 122390. [Google Scholar] [CrossRef]
- Niu, H.; Li, J.; Cai, Q.; Wang, X.; Luo, F.; Gong, J.; Qiang, Z.; Ren, J. Molecular stereocomplexation for enhancing the stability of nanoparticles encapsulated in polymeric micelles for magnetic resonance imaging. Langmuir 2020, 36, 13881–13889. [Google Scholar] [CrossRef]
- Chuan, D.; Ran, R.; Wang, Y.; Ren, Y.; Wang, C.; Du, Y.; Zhou, L.; Yu, J.; Gu, Y.; Chen, H.; et al. Stereocoomplex poly(lactic acid)-based composite nanofiber membranes with highly dispersed hydroxyapatite for potential bone tissue engineering. Compos. Sci. Technol. 2020, 192, 108107. [Google Scholar] [CrossRef]
- Chai, H.; Chang, Y.; Zhang, Y.; Chen, Z.; Zhong, Y.; Zhang, L.; Sui, X.; Mao, Z. The fabrication of polylactide/cellulose nanocomposites with enhanced crystallization and mechanical properties. Int. J. Biol. Macromol. 2020, 155, 1578–1588. [Google Scholar] [CrossRef]
- Fan, X.; Luo, Z.; Ye, E.; You, M.; Liu, M.; Yun, Y.; Loh, X.J.; Wu, Y.-L.; Li, Z. AuNPs decorated PLA stereocomplex micelles for synergetic photothermal and chemotherapy. Macromol. Biosci. 2021, 21, 2100062. [Google Scholar] [CrossRef]
- Gu, T.; Sun, D.-X.; Qi, X.-D.; Yang, J.-H.; Lei, Y.-Z.; Wang, Y. Heat resistant and thermally conductive polylactide composites achieved by stereocomplex crystallite tailored carbon nanofiber network. Chem. Eng. J. 2021, 418, 129287. [Google Scholar] [CrossRef]
- Gu, T.; Sun, D.-X.; Qi, X.-D.; Yang, J.-H.; Zhao, C.-S.; Lei, Y.-Z.; Wang, Y. Sinchronously enhanced thermal conductivity and heat resistance poly(L-lactide)/graphene nanoplatelets composites via constructing stereocomplex crystallites at interface. Compos. Part B 2021, 224, 109163. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Han, C.; Xiao, L. Thermal and mechanical properties of stereocomplex polylactide enhanced by nanosilica. Colloid Polym. Sci. 2021, 299, 1161–1172. [Google Scholar] [CrossRef]
- Lyu, Y.; Wen, X.; Wang, G.; Zhang, Q.; Lin, L.; Schlarb, A.K.; Shi, X. 3D printing nanocomposites with controllable “strength-toughness” transition: Modification of SIO2 and construction of stereocomplex crystallites. Compos. Sci. Technol. 2022, 218, 109167. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Peng, S.; Pan, H.; Bai, X. Construction of a stereocomplex between poly(D-lactide) grafted hydroxyapatite and poly(L-lactide): Toward a bioactive composite scaffold with enhanced interfacial bonding. J. Mater. Chem. B 2022, 10, 214. [Google Scholar] [CrossRef]
- Baek, S.-W.; Kim, J.H.; Song, D.H.; Kim, D.-S.; Park, C.G.; Han, D.K. Enhanced mechanical properties and anti-inflammation of poly(L-lactic acid) by stereocomplexes of PLLA/PDLA and surface-modified magnesium hydroxide nanoparticles. Polymers 2022, 14, 3790. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, T.; Ning, Z.; Jiang, N.; Gan, Z. Melt and nucleation reinforcement for stereocomplex crystallites in poly(L-lactide)/lignin-grafted-poly(D-lactide) blend. Eur. Polym. J. 2022, 167, 111072. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, M.; Weng, Z.; Zhu, X.; Li, W.; Huang, P.; Wang, L.; Zheng, W.; Ohshima, M. Promoted formation of stereocomplex in enantiomeric poly(lactic acid)s induced by cellulose nanofibers. Carbohydr. Polym. 2022, 276, 118800. [Google Scholar] [CrossRef]
- Rong, C.; Chen, Y.; Chen, C.; Hu, L.; Wang, H.; Li, Y. Toward simultaneous compatibilization and nucleation of fully biodegradable nanocmposites: Effect of nanorod-assisted interfacial stereocomplex crystals in immiscible polymer blends. Compos. Part B 2022, 234, 109708. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2000; pp. 168–195. [Google Scholar]
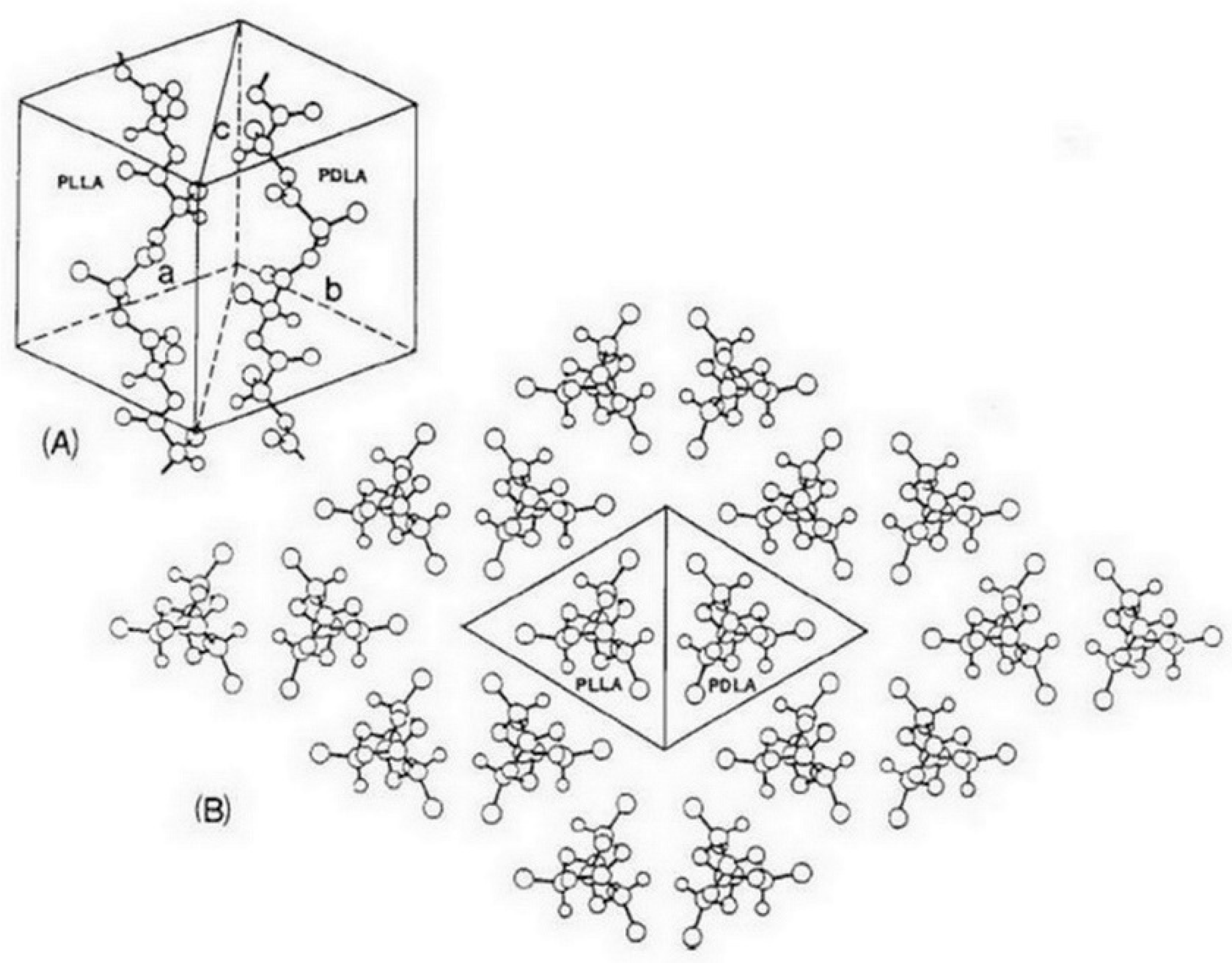



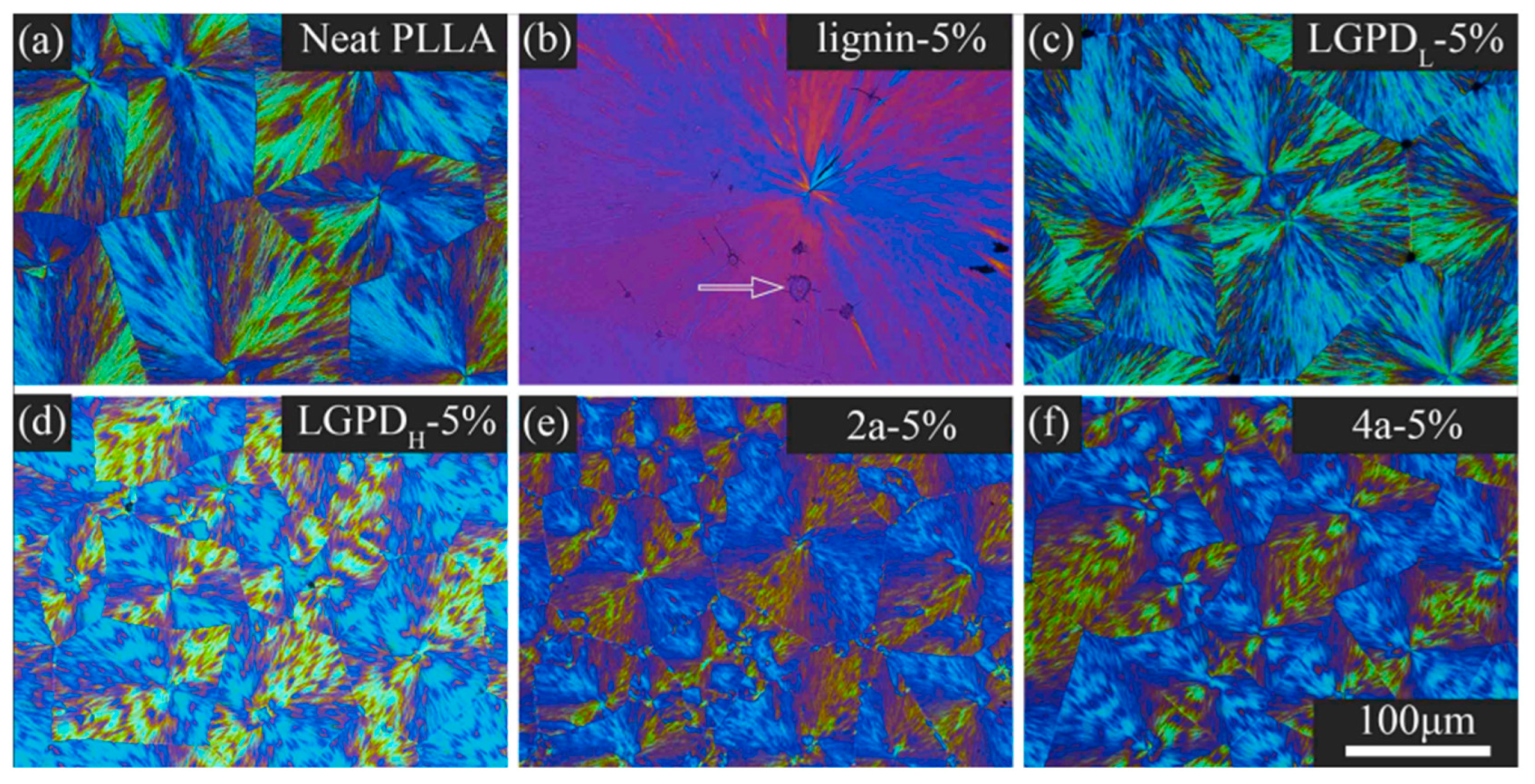


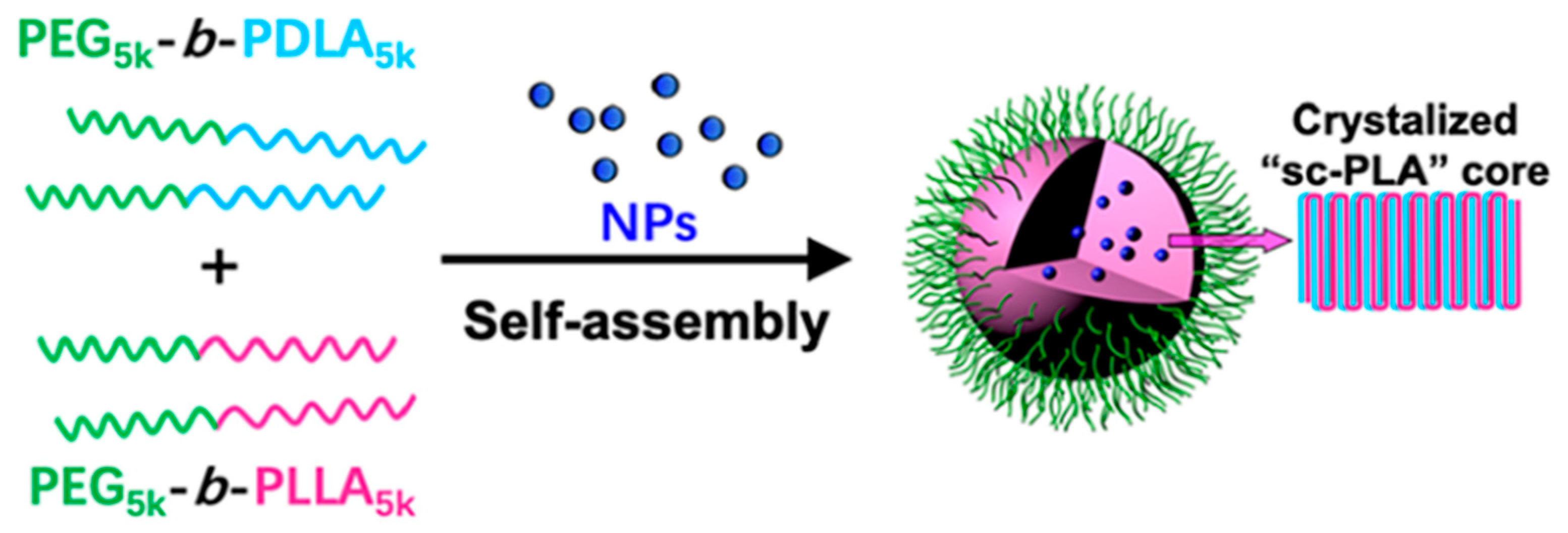
| Polymers | Processing | Condition | s-PLA Shape | Findings | Ref. |
|---|---|---|---|---|---|
| PLLA and PDLA (MV = 7.0 × 104 g/mol) | Solution-precipitation | Solvent: dichloromethane-methanol Variable: PLLA:PDLA ratio Concentration: 1.0 g/dL | Fibrous materials | Formation of s-PLA crystallites was observed via a differential scanning calorimeter (DSC) and X-ray diffraction (XRD) analysis | [2] |
| PLLA (MW = 150,000 g/mol) and PDLA (MW = 100,000 g/mol) | Solution casting | Solvent: chloroform Fixed PLLA:PDLA ratio (1:1) Concentration: 1.0 g/dL | Films | Formation of s-PLA was caused by CH3–O=C interaction based on FT-IR | [4] |
| PLLA and PDLA | Solution casting | Solvent: dichloromethane Variable: molecular weight Fixed PLLA:PDLA ratio (1:1) Concentration: 1.0 g/dL | Films | In the range MW 1 × 105–1 × 106, PLLA/PDLA blends show higher mechanical properties caused by s-PLA crystallites | [14] |
| PLLA and PDLA (MV = ~4.0 × 104 g/mol) | Sol-Gel process | Solvent: chloroform Variable: PLLA:PDLA ratio Concentration: 7.5–17.5 g/dL | Gels, microgels | Rapid complex formation at 1:1 ratio at higher concentrations | [15] |
| PLLA and PDLA (MV = ~1.0–9.9 × 104 g/mol) | Solution casting | Solvent: dichloromethane Variable: optical purity Fixed PLLA:PDLA ratio (1:1) Concentration: 1.0 g/dL | Films | High optical purity affects the formation of s-PLA crystallites more than their homocrystallites | [16] |
| PLLA (MV = 1.32 × 104 g/mol) PDLA (MV = 4.42 and 1.45 × 104 g/mol) | Melt crystallization | Variable: optical-purity PDLA, 80% and 100% Concentration:2.0 g/dL | Films | High optical purity supports the fast rate of the s-PLA formation | [17] |
| PLLA and PDLA | Melt crystallization | Variable: Molecular weight, PLLA/PDLA ratio Nucleation comparison | Films | The s-PLA crystallites were form at low concentration of PDLA. The s-PLA nucleation effect was superior to that of talc and PLLA homopolymers. | [18] |
| PLLA and PDLA | Melt crystallization | Variable: molecular weight, PLLA/PDLA ratio, crystallization temperature and time | Films | The addition of PDLA to PLLA formed s-PLA crystallites which acted as nucleation site and improved its properties | [19,20,21] |
| PLLA and PDLA | Supercritical CO2-cosolvent | High-molecular-weight, fixed 1:1 ratio Variable: solvent type, pressure, temperature, and time | Dry powder | Rapid formation of 100% s-PLA from high-molecular-weight PLA in dry powder shapes | [22,23] |
| PLLA and PDLA | Supercritical fluid | Variable: molecular weight, Fixed 1:1 ratio, pressure, temperature, and time | Dry powder | Supercritical fluid parameter affects the degree of s-PLA formation and final s-PLA shapes | [24,25] |
| PLA Matrix | Nanoparticles/Modification | Preparation Methods | Property Improvement | Ref. |
|---|---|---|---|---|
| PLLA and PDLA (Mn = 25,000–75,000 g/mol) | Cloisite 30 B Fluorinated clay | Supercritical fluid | Increased melting temperature (up to 64 °C) and thermal degradation temperature (up to 30 °C) | [85,86] |
| Star-shaped PLLA and PDLA (MW = 12,000–35,000 g/mol) | Octafunctional polyoctahedral silsesquioxanes (POSS) as initiator (core of star polymer) | Solution method by tetrahydrofuran (THF) | Self-formation of s-PLA, increased melting temperature, doubled improvement of hardness (GPa) | [87] |
| Commercial PLLA (Mn = 130,000 g/mol) | CNT-grafted PDLA Graphene oxide-grafted PDLA | Solution casting in chloroform | Crystallinity and mechanical properties | [88,89,90,91,92] |
| MWCNT-PLLA MWCNT-PDLA | Multiwall CNT (MWCNT) as initiator (core of grafted PLA) | Solution casting in chloroform | Reversible s-PLA crystallization after melting (stereocomplex memory) | [93] |
| Commercial PLLA (Mn = 130,000 g/mol) | Cellulose nanocrystal -grafted PDLA | Melt blending in mini-extruder | Thermal and mechanical properties | [94] |
| PDLA; PLLA; pyrene-end-functionalized PLLA | Pristine MWCNT | Solution casting in chloroform | Pyrene end-group prevention of the formation of crystalline s-PLA, and enhancement of w-PLA crystallization by MWCNT but with poor dispersion | [95] |
| PDLA (Mn = 126,537 g/mol) PLLA (Mn = 194,597 g/mol) | Lignin | Supercritical carbon dioxide—solvent | Thermal degradation properties | [96] |
| PLLA and PDLA block copolymer | Methacrylisobutyl POSS (MA-POSS) | Solution casting in THF | Self-assembly stereocomplex, thermal properties | [97] |
| PLLA | Magnesium oxide–oligo D-lactide (MgO-ODLA) | Solution casting in chloroform | Enhanced mechanical properties and suppressed severe acid-induced inflammation. | [98] |
| PLA-graft-cellulose nanowhiskers (PLLA-g-CNW; PDLA-g-CNW) | Acetylated-CNW | Solution casting in chloroform | Crystallization, thermal and mechanical properties, stereocomplex memory | [99] |
| PLLA (Mn = 100,000 g/mol) and Cloisite 30B-g-PDLA | Cloisite 30 B | Melt process | Thermal and mechanical properties | [100] |
| PLLA (Mn = 132,000 g/mol) PDLA (Mn = 64,800 g/mol) | Oleylamine zinc phenylphosphonate | Solution casting in dichloromethane | Enhanced thermal and mechanical properties, higher disintegration rate compares neat s-PLA | [101,102] |
| PLLA (Mn = 51,000 g/mol) PDLA (Mn = 86,000 g/mol) | Cellulose nanocrystalline (CNC) grafted PLLA | Solution casting in chloroform, evaluated after melting | CNC-g-PLLA/PDLA with a higher s-PLA degree after cooling, and higher crystallinity | [103] |
| PLLA (MW = 200,000 g/mol) | Functionalized CNT (Branched PDLA-g-CNT) | Melt blending in mini extruder | Excellent thermal stability, and higher tensile strength (62.5 MPa) | [104] |
| PLLA (MW = 170,000 g/mol) PDLA grafted ethylene-acrylic ester (MW = 96,000 g/mol) | MWCNT | Melt mixing at 190 °C | Improve interfacial strength and impact toughness) | [105] |
| PLLA (MW = 100,000 g/mol) | Cellulose nanocrystalline (CNC) grafted PDLA | Solution casting in chloroform | Enhanced crystallization, storage modulus, and heat distortion temperature | [106] |
| PLLA (MW = 239,000 g/mol) PDLA (MW = 182,000 g/mol) | PDLA-grafted-cellulose microcrystals (CMC) | Melt process using twin-screw extruder | Enhanced gas barrier and thermomechanical properties | [107] |
| Commercial PLLA | 8-arms POSS-(PDMAEMA-b-PDLA)8 | Solution casting in chloroform | Enhanced thermal and mechanical properties up to certain content nanoparticle, higher disintegration rate compares neat s-PLA | [108] |
| PLLA | SiO2-r-PDLA nanoparticles | Solution blending, injection molding | Improved interface control, thermal and mechanical properties | [109] |
| PLLA (MW = 170,000 g/mol) PDLA (MW = 167,000 g/mol) | Carbon black | Melt mixing | Enhanced matrix crystallization and mechanical properties | [110] |
| PLLA (MW = 100,000 g/mol) | PDLA-grafted-nanohydroxyapatite (nanoHA) | Solution casting in chloroform | Enhanced interfacial adhesion, crystallization, mechanical and thermal properties | [111] |
| PLLA (Mn = 180,000 g/mol) PDLA (Mn = 70,000 g/mol) | PLLA-grafted-ZnO | Solution casting in chloroform | Improved crystallization rate, non-leaching properties | [112] |
| PLLA (Mn = 143,000 g/mol) | PDLA-b-PDMA-Starch nanoparticles | Solution casting in chloroform | Synergetic effect on thermal and mechanical properties | [113] |
| PLLA and PDLA (MW = 100,000 g/mol) | Carbon quantum dots (CQD) | Solution electrospinning | High shear piezoelectricity, photoluminescence and improved heat resistant | [114] |
| PLLA (MW = 253,000 g/mol) PDLA (MW = 100,000 g/mol) | CNCs | Solution casting in dichloromethane | Improve hydrolytic degradation rate and heat resistant | [115] |
| PLLA (Mn = 180,000 g/mol) | TiO2@SiO2-g-PDLA | Solution casting in chloroform | Enhanced mechanical properties (by 49%), UV shielding and UV resistance | [116] |
| PLLA (MW > 160,000 g/mol) PDLA (MW > 185,000 g/mol) | Carbon black, multifunctional carbon black | Melt mixing | Crystallinity, electrical properties | [117,118] |
| PLLA (MW = 210,000 g/mol) PDLA (MW = 200,000 g/mol) | Silver nanowires (AgNWs) | Electrospinning–dip coating | Thermal conductivity | [119] |
| PEG5k-b-PDLA5k PEG5k-b-PLLA5k (PEG: polyethyleneglycol) | MnFe2O4 MnFe2O4@Fe3O4 | Solution dispersion | Biocompatibility and superparamagnetic properties Excellent negative contrast enhancement of MR signals | [120] |
| PLLA (MW = 210,000 g/mol) | HA-g-PDLA | Electrospinning | Mechanical properties, BMSC proliferation, osteogenic differentiation. | [121] |
| PLLA (Mn = 150,000 g/mol) | CNCs-PLLA CNCs-PDLA | Solution casting in dichloromethane | Crystallinity (up to 86.7%), mechanical properties (up to 36%) | [122] |
| Poly(2-(dimethylamino) ethyl methacrylate)-block-poly(D-lactide) (PDMAEMA-PDLA) PEG-PLLA | Gold nanoparticles | Solution mixing | The light-responsive drug release and therapeutic efficacy | [123] |
| PLLA, PDLA | Cellulose nanofibers (CNF) PLLA-g-graphene PDLA-g-graphene | Solution precipitation followed by compression molding | Heat resistance and thermal conductivity | [124,125] |
| PLLA (MW = 207,000 g/mol) PDLA (MW = 110,000 g/mol) | Nanosilica (AEROSIL 200) | Melt blending | Thermal stability (~33 °C higher), storage modulus, tensile strength and modulus | [126] |
| PLLA (MW = 74,000 g/mol) | SiO2-PDLA PLLA-g-GMA (compatibilizer) | Melt mixing–3D printing | Heat resistance and tensile strength | [127] |
| PLLA (MW = 88,400 g/mol) | PDLA-g-nanoHA | Selective laser sintering (SLS) | Interfacial bonding, cytocompatibility | [128] |
| PLLA | Mg(OH)2-g-Oligo(D-lactide-co-caprolactone) (MH-ODLCL) S-PLA microparticles | Solution casting–compression molding | Mechanical properties, anti-inflammatory | [129] |
| PLLA | Lignin-g-PDLA Lignin-g-2-armed PDLA Lignin-g-4-armed PDLA | Solution casting in Chloroform | Thermal and mechanical properties | [130] |
| PLLA (MW = 170,000 g/mol) PDLA (MW = 130,000 g/mol) | Cellulose nanofibers | Melt compounding | Interchain molecular interactions, crystallization rate | [131] |
| PLLA (Mn = 68,000 g/mol) Poly(1,4-butylene succinate (PBSU) (Mn = 68,000 g/mol) | PDLA-PBSU-g-nanorod (AlOOH-g-(D&B) PLLA-PBSU-g-nanorod (AlOOH-g-(L&B) | Melt blending | Interfacial stabilization, phase compatibility, thermal resistance, high modulus, fast crystallization rate | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsuri, M.; Purnama, P. Development of Stereocomplex Polylactide Nanocomposites as an Advanced Class of Biomaterials—A Review. Polymers 2023, 15, 2730. https://doi.org/10.3390/polym15122730
Samsuri M, Purnama P. Development of Stereocomplex Polylactide Nanocomposites as an Advanced Class of Biomaterials—A Review. Polymers. 2023; 15(12):2730. https://doi.org/10.3390/polym15122730
Chicago/Turabian StyleSamsuri, Muhammad, and Purba Purnama. 2023. "Development of Stereocomplex Polylactide Nanocomposites as an Advanced Class of Biomaterials—A Review" Polymers 15, no. 12: 2730. https://doi.org/10.3390/polym15122730




