Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives
Abstract
:1. Introduction
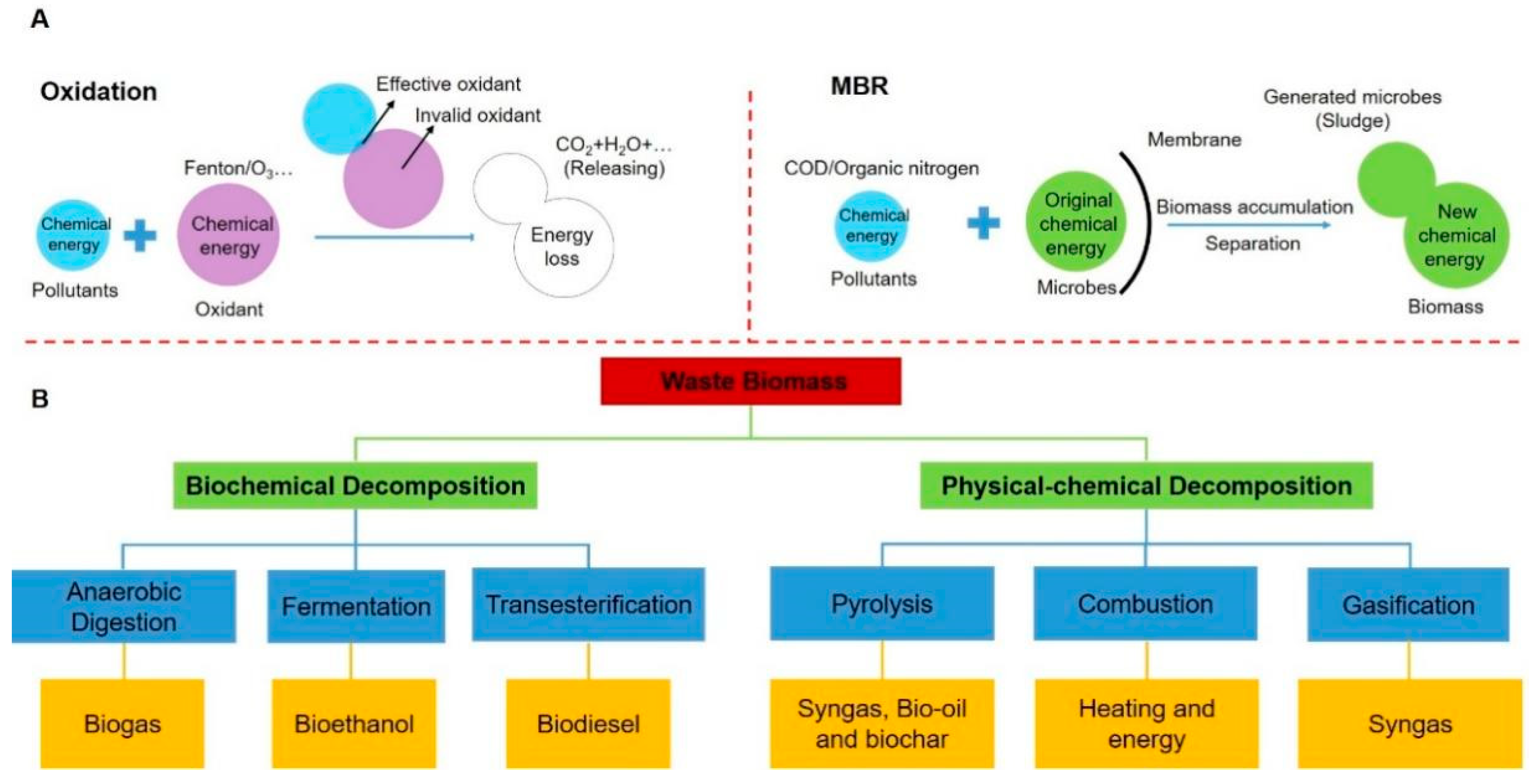
2. Biochar Production from Polymers in Waste Biomass
2.1. Technologies of Biomass Polymers Derived Biochar Preparation
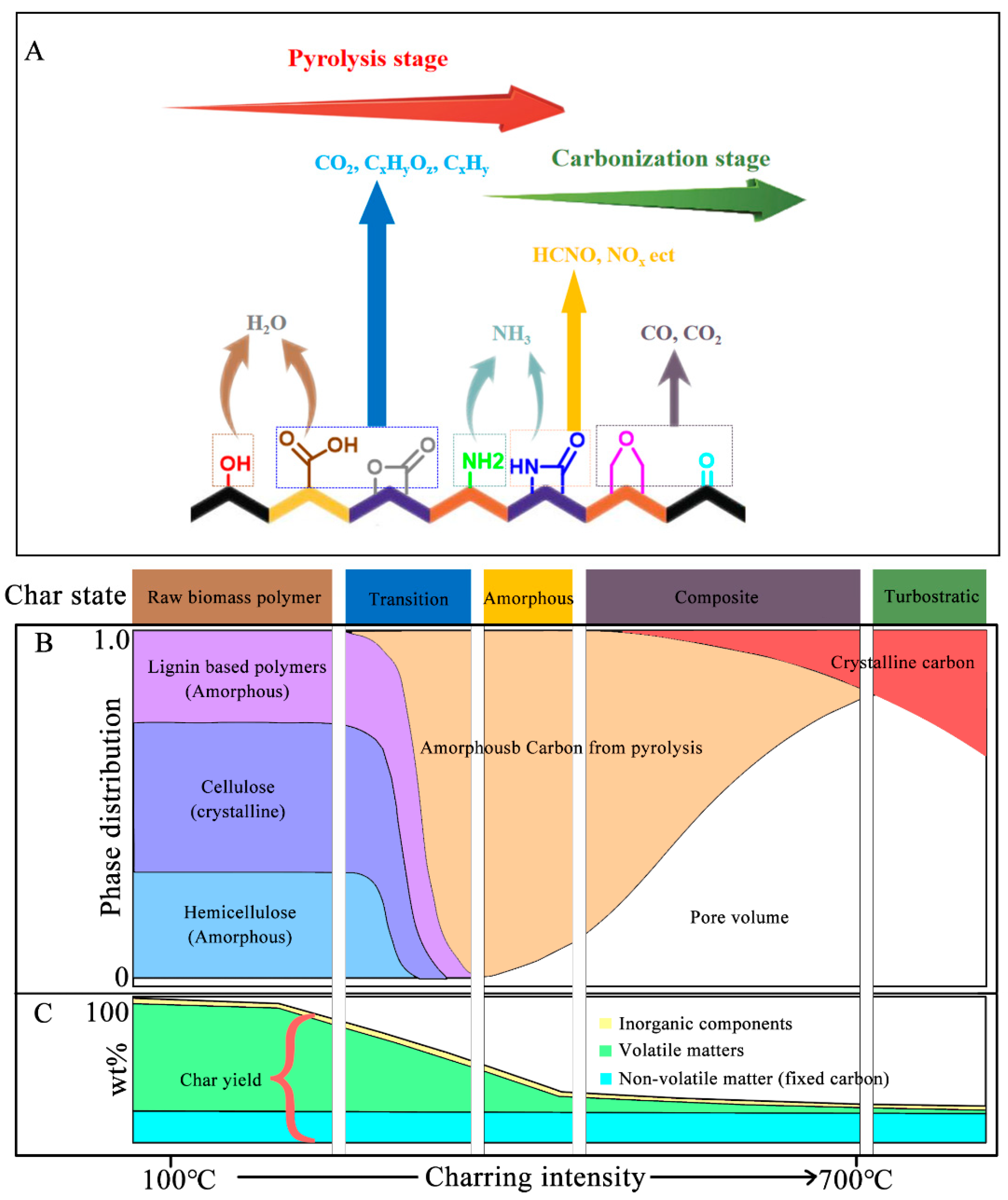
2.2. The Formation Mechanism of Biomass Polymer Biochar
2.3. Effect of Pyrolysis Conditions on Biochar Formation
2.4. Element Composition of Biomass Polymeric Component-Derived Biochar
3. Postprocessing Modification of Biomass Polymer-Derived Biochar for Energy Storage
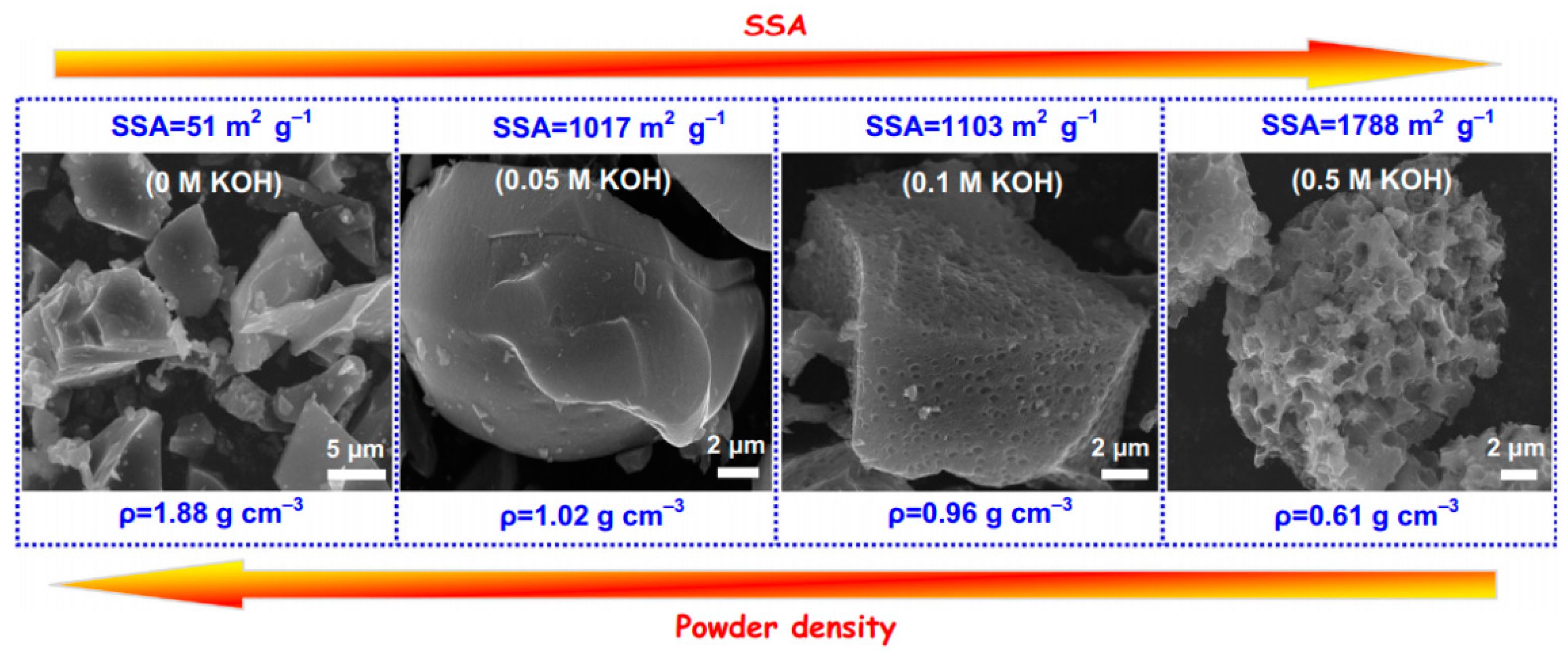
3.1. Biochar Activation
3.2. Biochar Modification
3.2.1. Surface Doping
3.2.2. Surface Recombination
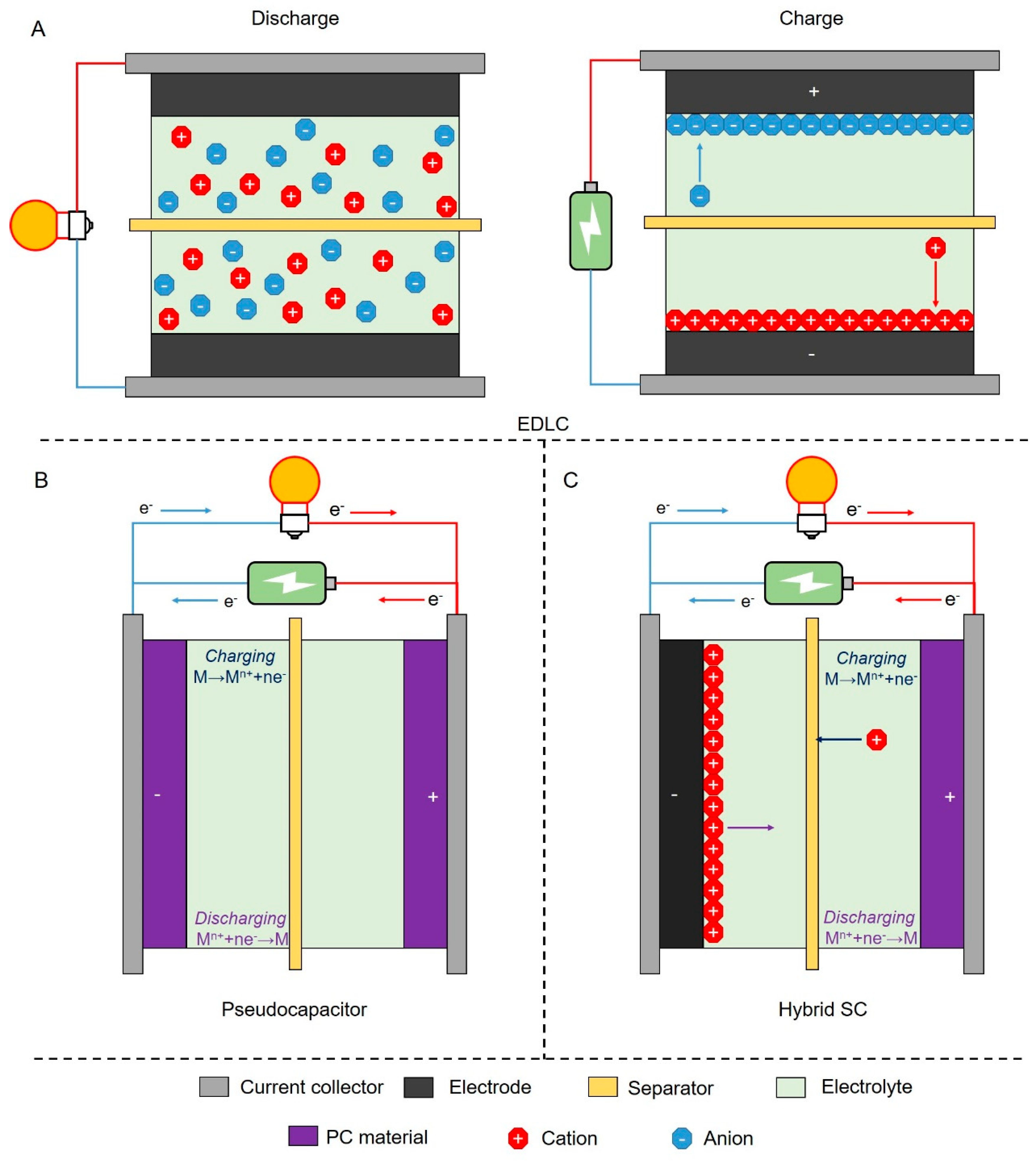
4. Recent Advances in Biochar-Based SCs
4.1. Biochar-Derived EDLC
4.2. Biochar-Derived Hybrid SCs
4.3. Biochar-Derived Flexible and Self-Healing SCs
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Xuan, Z.; Wenyan, G.; Xilin, Z.; Yaran, S.; Qingrui, Z. Research progress on removal mechanism and purification technology of heavy metal complexes in wastewater. J. Yanshan Univ. 2022, 46, 297–308. [Google Scholar]
- Cai, M.; Zhang, H.; Zhang, Y.; Wu, H. Bioelectrochemical assisted landfill technology for the stabilization and valorization of food waste anaerobic digestate. Bioresour. Technol. 2022, 351, 126935. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.-H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Ravishankar, E.; Booth, R.E.; Hollingsworth, J.A.; Ade, H.; Sederoff, H.; DeCarolis, J.F.; O’Connor, B.T. Organic solar powered greenhouse performance optimization and global economic opportunity. Energy Environ. Sci. 2022, 15, 1659–1671. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Y.; Guo, C.; Li, Y.; Jiang, N.; Yin, C. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Bioresour. Technol. 2018, 260, 130–134. [Google Scholar] [CrossRef]
- Deling, Y.; Jiachen, T.; Zhongwen, N.; Shoufeng, T. Study on humic acid removal in water by ultraviolet activated sodium percarbonate. J. Yanshan Univ. 2021, 45, 220–226. [Google Scholar]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Hao, N.; Wang, Y.Y.; Dou, C.; Lin, F.; Shen, R.; Bura, R.; Hodge, D.B.; Dale, B.E.; Ragauskas, A.J.; et al. Transforming biorefinery designs with ‘Plug-In Processes of Lignin’ to enable economic waste valorization. Nat. Commun. 2021, 12, 3912. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrog. Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, H.; Wu, J.; Hu, Y.; Wen, H.; Yang, Z.; Wu, H. Hydrogen peroxide treatment mitigates antibiotic resistance gene and mobile genetic element propagation in mariculture sediment. Environ. Pollut. 2023, 328, 121652. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Ipiales, R.P.; de la Rubia, M.A.; Diaz, E.; Mohedano, A.F.; Rodriguez, J.J. Integration of Hydrothermal Carbonization and Anaerobic Digestion for Energy Recovery of Biomass Waste: An Overview. Energy Fuels 2021, 35, 17032–17050. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Bhaumik, S.; Dong, L.; Vuthaluru, H. Enhanced tar removal in syngas cleaning through integrated steam catalytic tar reforming and adsorption using biochar. Fuel 2023, 331, 125912. [Google Scholar] [CrossRef]
- Amin, M.; Munir, S.; Iqbal, N.; Wabaidur, S.; Iqbal, A. The Conversion of Waste Biomass into Carbon-Supported Iron Catalyst for Syngas to Clean Liquid Fuel Production. Catalysts 2022, 12, 1234. [Google Scholar] [CrossRef]
- Rajendran, N.; Kang, D.; Han, J.; Gurunathan, B. Process optimization, economic and environmental analysis of biodiesel production from food waste using a citrus fruit peel biochar catalyst. J. Clean. Prod. 2022, 3, 339–365. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adeyemo, A.J.; Awodun, M.A. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 2019, 336, 1–11. [Google Scholar] [CrossRef]
- Praneeth, S.; Guo, R.; Wang, T.; Dubey, B.K.; Sarmah, A.K. Accelerated carbonation of biochar reinforced cement-fly ash composites: Enhancing and sequestering CO2 in building materials. Constr. Build. Mater. 2020, 244, 118363. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; You, S.; Zhang, L.; Shang, J.; Lehmann, J.; Wang, X.; Zhu, Y.-G.; Tsang, D.C.W.; Park, Y.-K.; Hou, D.; et al. Progress, Barriers, and Prospects for Achieving a “Hydrogen Society” and Opportunities for Biochar Technology. ACS EST Eng. 2022, 2, 1987–2001. [Google Scholar] [CrossRef]
- Ali, A.; Raza, R.; Shakir, M.I.; Iftikhar, A.; Alvi, F.; Ullah, M.K.; Hamid, A.; Kim, J.-S. Promising electrochemical study of titanate based anodes in direct carbon fuel cell using walnut and almond shells biochar fuel. J. Power Sources 2019, 434, 126679. [Google Scholar] [CrossRef]
- Ngoc-Dan Cao, T.; Mukhtar, H.; Yu, C.-P.; Bui, X.-T.; Pan, S.-Y. Agricultural waste-derived biochar in microbial fuel cells towards a carbon-negative circular economy. Renew. Sustain. Energy Rev. 2022, 170, 112965. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Hasan Johir, M.A.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.D.; Richardson, C.; Moni, M.A.; Bryant, M.R. Activated carbon preparation from biomass feedstock: Clean production and carbon dioxide adsorption. J. Clean. Prod. 2019, 225, 405–413. [Google Scholar] [CrossRef]
- Cheng, B.H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Loganathan, N.N.; Perumal, V.; Pandian, B.R.; Atchudan, R.; Edison, T.N.J.I.; Ovinis, M. Recent studies on polymeric materials for supercapacitor development. J. Energy Storage 2022, 49, 104149. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kim, W.; Kumar, S.; Yoon, T.-H.; Lee, J.-S. Nanocomposite Supercapacitor Electrode from Sulfonated Graphene Oxide and Poly(pyrrole-(biphenyldisulfonic acid)-pyrrole). ACS Appl. Energy Mater. 2020, 3, 6743–6751. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Sahoo, S.; Shim, J.-J.; Tan, W.K.; Matsuda, A.; Singh, R.K. An overview of recent progress in nanostructured carbon-based supercapacitor electrodes: From zero to bi-dimensional materials. Carbon 2022, 193, 298–338. [Google Scholar] [CrossRef]
- Yang, M.; Long, X.; Li, H.; Chen, H.; Liu, P. Porous Organic-Polymer-Derived Nitrogen-Doped Porous Carbon Nanoparticles for Efficient Oxygen Reduction Electrocatalysis and Supercapacitors. ACS Sustain. Chem. Eng. 2018, 7, 2236–2244. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Oladepo, S.; Ali Ehsan, M.; Iali, W.; Alenaizan, A.; Nahid Siddiqui, M.; Oyama, M.; Al-Betar, A.R.; Aziz, M.A. Recent Progress in Polyaniline and its Composites for Supercapacitors. Chem. Rec. 2023, 42, 1–25. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, C.; Zeng, G.; Huang, D.; Tan, X.; Duan, A. Lignocellulosic biomass carbonization for biochar production and characterization of biochar reactivity. Renew. Sustain. Energy Rev. 2022, 157, 112056. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Wiedemeier, D.B.; Abiven, S.; Hockaday, W.C.; Keiluweit, M.; Kleber, M.; Masiello, C.A.; McBeath, A.V.; Nico, P.S.; Pyle, L.A.; Schneider, M.P.W.; et al. Aromaticity and degree of aromatic condensation of char. Org. Geochem. 2015, 78, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.A.; Kercher, A.K.; Nguyen, T.H.; Nagle, D.C.; Ball, W.P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org. Geochem. 2006, 37, 321–333. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 2010, 34, 67–74. [Google Scholar] [CrossRef]
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soi. Int. Biochar Initiat. 2015. Available online: https://biochar-international.org/wp-content/uploads/2023/01/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 15 June 2023).
- Young, C.; Park, T.; Yi, J.W.; Kim, J.; Hossain, M.S.A.; Kaneti, Y.V.; Yamauchi, Y. Advanced Functional Carbons and Their Hybrid Nanoarchitectures towards Supercapacitor Applications. ChemSusChem 2018, 11, 3546–3558. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Seman, R.N.A.R.; Azam, M.A.; Mohamad, A.A. Systematic gap analysis of carbon nanotube-based lithium-ion batteries and electrochemical capacitors. Renew. Sustain. Energy Rev. 2017, 75, 644–659. [Google Scholar] [CrossRef]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.W.; Sajdak, M. Various biomass pyrolysis conditions influence the porosity and pore size distribution of biochar. Energy 2023, 263, 126128. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Horie, Y.; Ozaki, S.; Matsuzawa, Y.; Suezaki, H.; Kim, C.; Miyashita, N.; Endo, M. Correlation between the pore and solvated ion size on capacitance uptake of PVDC-based carbons. Carbon 2004, 42, 1491–1500. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [Green Version]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Frackowiak, E.; Jurewicz, K.; Friebe, M.; Parmentier, J.; Béguin, F. Electrochemical energy storage in ordered porous carbon materials. Carbon 2005, 43, 1293–1302. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef] [Green Version]
- Braghiroli, F.L.; Bouafif, H.; Hamza, N.; Bouslimi, B.; Neculita, C.M.; Koubaa, A. The influence of pilot-scale pyro-gasification and activation conditions on porosity development in activated biochars. Biomass Bioenergy 2018, 118, 105–114. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lu, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef]
- Qu, W.H.; Xu, Y.Y.; Lu, A.H.; Zhang, X.Q.; Li, W.C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- Vu, M.T.; Chao, H.-P.; Van Trinh, T.; Le, T.T.; Lin, C.-C.; Tran, H.N. Removal of ammonium from groundwater using NaOH-treated activated carbon derived from corncob wastes: Batch and column experiments. J. Clean. Prod. 2018, 180, 560–570. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Role of hydrochar properties on the porosity of hydrochar-based porous carbon for their sustainable application. ACS Sustain. Chem. Eng. 2015, 3, 833–840. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Luo, Y.; Street, J.; Steele, P.; Entsminger, E.; Guda, V. Activated carbon derived from pyrolyzed pinewood char using elevated temperature, KOH, H3PO4, and H2O2. BioResources 2016, 11, 10433–10447. [Google Scholar] [CrossRef] [Green Version]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Anfruns, A.; García-Suárez, E.J.; Montes-Morán, M.A.; Gonzalez-Olmos, R.; Martin, M.J. New insights into the influence of activated carbon surface oxygen groups on H2O2 decomposition and oxidation of pre-adsorbed volatile organic compounds. Carbon 2014, 77, 89–98. [Google Scholar] [CrossRef]
- He, H.; Qian, T.-T.; Liu, W.-J.; Jiang, H.; Yu, H.-Q. Biological and chemical phosphorus solubilization from pyrolytical biochar in aqueous solution. Chemosphere 2014, 113, 175–181. [Google Scholar] [CrossRef]
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable preparation of green biochar based high-performance supercapacitors. Ionics 2022, 28, 2525–2561. [Google Scholar] [CrossRef]
- Arvas, M.B.; Gürsu, H.; Gencten, M.; Sahin, Y. Preparation of different heteroatom doped graphene oxide based electrodes by electrochemical method and their supercapacitor applications. J. Energy Storage 2021, 35, 1–17. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a Novel Interconnected 3D Pore Network Algal Biochar Constituting Iron Nanoparticles Derived from a Harmful Marine Biomass as High-Performance Asymmetric Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kim, W.-B.; Kumar, S.; Yoon, T.-H.; Shim, J.-J.; Lee, J.-S. Redox-active supercapacitor electrode from two-monomer-connected precursor (Pyrrole: Anthraquinonedisulfonic acid: Pyrrole) and sulfonated multi-walled carbon nanotube. Electrochim. Acta 2022, 415, 140243. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Li, A.; Zhang, L. Facile synthesis of high-surface area mesoporous biochar for energy storage via in-situ template strategy. Mater. Lett. 2018, 230, 183–186. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Gao, Y.; An, W.; Cao, Z.; Liu, J. Biomass-swelling assisted synthesis of hierarchical porous carbon fibers for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28283–28290. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Z.; Luo, J.; Zhang, H.; Qu, Y.; Yang, Z. Self-templated synthesis of hollow hierarchical porous olive-like carbon toward universal high-performance alkali (Li, Na, K)-ion storage. Carbon 2021, 174, 317–324. [Google Scholar] [CrossRef]
- Bai, Q.; Xiong, Q.; Li, C.; Shen, Y.; Uyama, H. Hierarchical porous carbons from a sodium alginate/bacterial cellulose composite for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2018, 455, 795–807. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
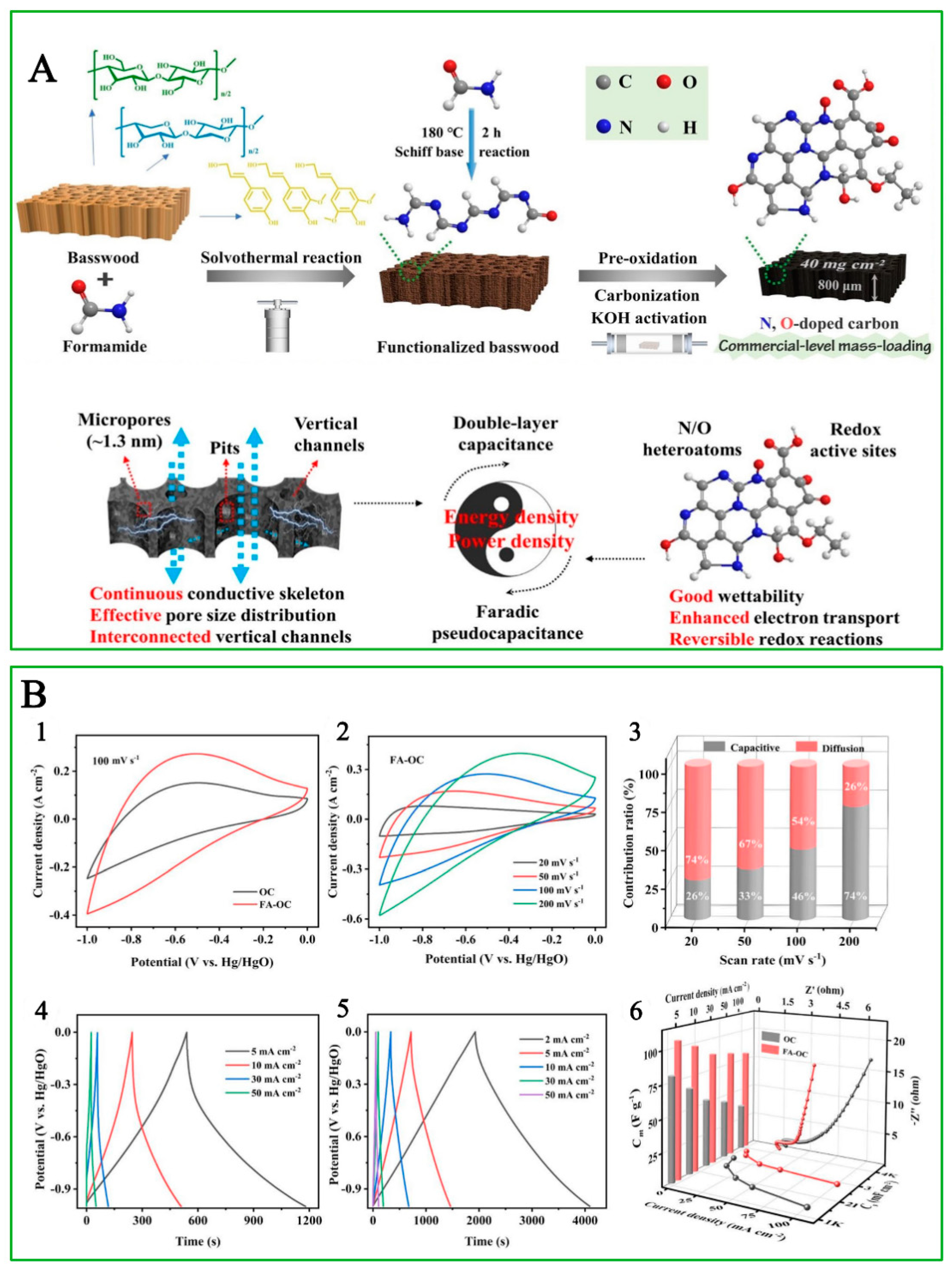
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Lin, Y.; Li, F.; Li, X.; Zhao, H.; Liu, G. Multifunctional Template Prepares N-, O-, and S-Codoped Mesoporous 3D Hollow Nanocage Biochar with a Multilayer Wall Structure for Aqueous High-Performance Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 2265–2275. [Google Scholar] [CrossRef]
- Nirmaladevi, S.; Boopathiraja, R.; Kandasamy, S.K.; Sathishkumar, S.; Parthibavarman, M. Wood based biochar supported MnO2 nanorods for high energy asymmetric supercapacitor applications. Surf. Interfaces 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, M.; Lee, E.; Baeck, S.H.; Shim, S.E. Nylon 6, 6/Polyaniline based sheath nanofibers for high-performance supercapacitors. Electrochim. Acta 2016, 213, 124–131. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Chen, X.; Zhang, H.; Song, C.; Liu, Z.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J. Polyaniline-modified porous carbon tube bundles composite for high-performance asymmetric supercapacitors. Electrochim. Acta 2018, 292, 458–467. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, Y.; Li, J.; Wang, L. Polypyrrole-anchored cattail biomass-derived carbon aerogels for high performance binder-free supercapacitors. Carbohydr. Polym. 2018, 8, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Quan, H.; Wang, W.; Gao, W.; Dong, Y.; Chen, D. Porous carbon with interpenetrating framework from Osmanthus flower as electrode materials for high-performance supercapacitor. J. Environ. Chem. Eng. 2018, 6, 258–265. [Google Scholar] [CrossRef]
- Rawat, S.; Boobalan, T.; Sathish, M.; Hotha, S.; Thallada, B. Utilization of CO2 activated litchi seed biochar for the fabrication of supercapacitor electrodes. Biomass Bioenergy 2023, 171, 106747. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, X.; Hou, X.; Lin, H.; Jia, L. One step synthesis of N, P co-doped hierarchical porous carbon nanosheets derived from pomelo peel for high performance supercapacitors. J. Colloid Interface Sci. 2022, 605, 71–81. [Google Scholar] [CrossRef]
- Pradiprao Khedulkar, A.; Dien Dang, V.; Pandit, B.; Ai Ngoc Bui, T.; Linh Tran, H.; Doong, R.A. Flower-like nickel hydroxide@tea leaf-derived biochar composite for high-performance supercapacitor application. J. Colloid Interface Sci. 2022, 623, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Charoensook, K.; Huang, C.-L.; Tai, H.-C.; Lanjapalli, V.V.K.; Chiang, L.-M.; Hosseini, S.; Lin, Y.-T.; Li, Y.-Y. Preparation of porous nitrogen-doped activated carbon derived from rice straw for high-performance supercapacitor application. J. Taiwan Inst. Chem. Eng. 2021, 120, 246–256. [Google Scholar] [CrossRef]
- Du, X.; Lin, Z.; Zhang, Y.; Li, P. Microstructural tailoring of porous few-layer graphene-like biochar from kitchen waste hydrolysis residue in molten carbonate medium: Structural evolution and conductive additive-free supercapacitor application. Sci. Total Environ. 2023, 871, 162045. [Google Scholar] [CrossRef]
- Dubey, P.; Maheshwari, P.H.; Sundriyal, S. Human Hair-Derived Porous Activated Carbon as an Efficient Matrix for Conductive Polypyrrole for Hybrid Supercapacitors. Energy Fuels 2022, 36, 13218–13228. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Sun, X.; Zhan, J.; Zhang, H.; Zhao, X. Nitrogen-doped hierarchical porous carbon using biomass-derived activated carbon/carbonized polyaniline composites for supercapacitor electrodes. J. Electroanal. Chem. 2018, 827, 213–220. [Google Scholar] [CrossRef]
- Xue, C.-F.; Wei, Y.-Y.; Huang, R.-C.; Zhao, W.; Wu, T.; Li, X.-H.; Liu, G.; Hao, X.-G. Magnesium oxide scaffolded preparation of N, O self-doped biochar with super-hydrophilic surface for aqueous supercapacitor with desired energy density. J. Energy Storage 2022, 53, 105193. [Google Scholar] [CrossRef]
- Bui, T.A.N.; Huynh, T.V.; Tran, H.L.; Doong, R.A. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials 2022, 12, 1939. [Google Scholar] [CrossRef]
- Reddygunta, K.K.R.; Callander, A.; Šiller, L.; Faulds, K.; Berlouis, L.; Ivaturi, A. Sono-exfoliated graphene-like activated carbon from hazelnut shells for flexible supercapacitors. Int. J. Energy Res. 2022, 46, 16512–16537. [Google Scholar] [CrossRef]
- Li, X.; Lou, D.; Wang, H.; Sun, X.; Li, J.; Liu, Y.N. Flexible Supercapacitor Based on Organohydrogel Electrolyte with Long-Term Anti-Freezing and Anti-Drying Property. Adv. Funct. Mater. 2020, 30, 2007291. [Google Scholar] [CrossRef]
- Peng, H.; Lv, Y.; Wei, G.; Zhou, J.; Gao, X.; Sun, K.; Ma, G.; Lei, Z. A flexible and self-healing hydrogel electrolyte for smart supercapacitor. J. Power Sources 2019, 431, 210–219. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, X.; Pan, K.; Pang, H. Development and application of self-healing materials in smart batteries and supercapacitors. Chem. Eng. J. 2020, 380, 122565. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, X.; Ok, Y.S.; Elliott, J.A.; Chang, S.X.; Chung, H.-J. Flexible and self-healing aqueous supercapacitors for low temperature applications: Polyampholyte gel electrolytes with biochar electrodes. Sci. Rep. 2017, 7, 1685. [Google Scholar] [CrossRef] [Green Version]
| Application | Purpose | Reference |
|---|---|---|
| Catalyst | ||
| Syngas purification | Removing syngas tars | [20] |
| Liquid biofuel production | Converting syngas to liquid hydrocarbons | [21] |
| Biodiesel production | Preparing solid acid catalyst for biodiesel production | [22] |
| Soil amendment | ||
| Mitigating GHG emissions | Sequestering solid carbon to mitigate GHG emissions | [19] |
| Increasing soil quality | Increasing soil fertility, pH of acidic soil, and soil cation exchange capacity, and improving soil microbial activity and nutrient retention | [23] |
| Adsorbent | ||
| Pollutant removal | Absorbing contaminates in soil, water, and gases | [8] |
| Gas adsorbents | ||
| Storage material | CO2 sequestration | [24] |
| H2 storage | [25] | |
| Fuel cell system | ||
| DCFC * | Used instead of fossil fuel for power generation | [26] |
| Microbial fuel cell | Enabling a carbon-negative circular economy and lowering the electrode cost | [27] |
| Raw material | ||
| Activated carbon | Producing AC with low cost | [28] |
| Energy storage | ||
| Supercapacitor | For long-life, quickly charging/discharging power supply | [12] |
| Characteristic | Capacitor | SC | Battery |
|---|---|---|---|
| Specific energy (W h/kg) | <0.1 | 1–1091 | 10–1606 |
| Specific power (W/kg) | >>10,000 | 500–19,600 | <1000 |
| Charging time | 10−3–10−6 s | s to min | 0.3–3 h |
| Discharging time | 10−3–10−6 s | s to min | 1–5 h |
| Coulombic efficiency (%) | Around 100 | 85–99 | 70–85 |
| Cycle-life (cycles) | Infinite | >500,000 | Around 1000 |
| Vmax determinants | Dielectric thickness and strength | Electrode and electrolyte stability window | Thermodynamics of phase reactions |
| Charge stored determinants | Electrode area and dielectric | Electrode microstructure and electrolyte | Active mass and thermodynamics |
| Process | Temperature (°C) | Residence Time | Biochar Yield (wt.%) |
|---|---|---|---|
| Slow pyrolysis | 300–800 | min to days | 20–40 |
| Fast pyrolysis | 400–600 | Around 1 s | 10–20 |
| Gasification | 800–1000 | 5–20 s | <10 |
| HTC * | 180–250 | 1–12 h | 30–60 |
| Flash carbonization | 300–600 | <30 min | ~40 |
| Torrefaction | Around 290 | 10–60 min | 61–84 |
| Components | Temperature (°C) | Pathway |
|---|---|---|
| Cellulose | 200–260 | Cellulose → oligosaccharides → d-glucopyranose → levoglucosan → levoglucosenone → biochar |
| Hemicellulose | 240–350 | Hemicellulose → oligosaccharides → 1,4-anhydro-d-xylopyranose → biochar |
| Lignin | 280–500 | Lignin → vanillin/2-methoxy-4-methylphenol → biochar (via β-O-4 linin linkage-based radical reaction) * |
| K and Cl | Low | Vaporization |
| Ca and Mg | High | Ionically or covalently bounding with organic compound, or vaporization |
| P, S, and N | Low | Decomposed |
| Electrode Material | Modification | Capacitance * (Scan Rate) | Electrolyte (Conc.) | Energy Density Power Density * at (Current Density) | Stability after (Cycle Num.) | Ref. |
|---|---|---|---|---|---|---|
| Osmanthus flower | KOH | 255 F/g (5 mV/s) | KOH (6 M) | 7.95 Wh/kg 10 kW/kg (20 A/g) | 92.9% (10,000) | [88] |
| Litchi seed | CO2 activation | 493 F/g (10 mV/s) | H2SO4 (1 M) | 24.6 Wh/kg 0.6 kW/kg (1 A/g) | 92% (10,000) | [89] |
| BC–SA * composite | KOH and O doping | 302 F/g (5 mV/s) | KOH (6 M) | 15.6 Wh/kg 20 kW/kg (20 A/g) | 93.8% (10,000) | [79] |
| Pomelo peel | N/P co-doping by NH4H2PO4 | 314 F/g (5 mV/s) | Li2SO4 (2 M) | 36 Wh/kg 1000 W/kg (1 A/g) | 99% (10,000) | [90] |
| Tea leaves | NaOH activation Ni(OH)2 decoration | 945 F/g (10 mV/s) | Na2SO4 (1 M) | 58 Wh/kg 6.32 kW/kg (1 A/g) | >94% (10,000) | [91] |
| Rice straw | KOH activation N/O doping | 324 F/g (2 mV/s) | EMI-TFSI (N.A.) | 48.9 Wh/kg 750 W/kg (0.5 A/g) | 95% (10,000) | [92] |
| Cladophora glomerata | KOH/H2SO4/HNO3/FeCl3 | 368 F/g (5 mV/s) | KCl (3 M) | 41.5 Wh/kg 900 W/kg (1 A/g) | 91.3% (10,000) | [72] |
| Kitchen waste | Molten K2CO3 method | 237.4 F/g (10 mV/s) | Na2SO4 (1 M) | 4.2 Wh/kg 8 kW/kg (0.5 A/g) | 95% (10,000) | [93] |
| Human hair | KOH activation PPy | 358 F/g (5 mV/s) | H2SO4 (1 M) | 53.3 Wh/kg 408.5 W/kg (0.5 A/g) | 92.7% (10,000) | [94] |
| Celery | KOH/N doping/PANI decoration | 402 F/g (5 mV/s) | H2SO4 (1 M) | 178.2 Wh/kg 473.3 W/kg (1 A/g) | 97% (10,000) | [95] |
| Flammulina velutipes | MgO decoration N and O doping | 470.5 F/g (200 mV/s) | Na2SO4 (1 M) | 26.1 Wh/kg 1.0 kW/kg (0.5 A/g) | 100% (10,000) | [96] |
| Coffee grounds | KOH activation Erbium-doped graphene quantum dot decoration | 699 F/g (5 mV/s) | KOH (2 M) | 94.5 Wh/kg 1.3 kW/kg (1 A/g) | 81% (5000) | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. https://doi.org/10.3390/polym15122741
Yang C, Wu H, Cai M, Zhou Y, Guo C, Han Y, Zhang L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers. 2023; 15(12):2741. https://doi.org/10.3390/polym15122741
Chicago/Turabian StyleYang, Caiyun, Hao Wu, Mengyu Cai, Yuting Zhou, Chunyu Guo, Ying Han, and Lu Zhang. 2023. "Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives" Polymers 15, no. 12: 2741. https://doi.org/10.3390/polym15122741
APA StyleYang, C., Wu, H., Cai, M., Zhou, Y., Guo, C., Han, Y., & Zhang, L. (2023). Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers, 15(12), 2741. https://doi.org/10.3390/polym15122741









