Ecofriendly Elimination of Ni (II) Using Fabricated Nanocomposite Based on Chitosan/Silver Nanoparticles/Carbon Nanotubes
Abstract
:1. Introduction
2. Materials
2.1. Synthesis of Chitosan from Chitin
2.2. Synthesis of Silver Nanoparticles (SNPs)
2.3. Nano Formation of Chitosan with SNPs
2.4. Fabrication of the Nanocomposite (Chit-CNTs-SNPs)
2.5. Removal of Ni (II) Ions Using the Nanocomposite (Chit-CNTs-SNPs)
2.5.1. Preparation of Ni (II) Solutions
2.5.2. Effect of Time on Removal of Ni Ions
2.5.3. Effect of pH on Removal of Ni Ions
2.5.4. Nanocomposite Mass Effect on Sorption of Ni Ions
3. Experimental Analyses
3.1. Infrared Spectrometer (FTIR) and Ultraviolet Absorption Measurements (UV)
3.2. Scanning Electron Microscope, Energy-Dispersive X-Ray Spectroscopy, and Transmission Electron Microscope (TEM)
3.3. X-Ray Diffraction (XRD) and X-Ray Photoelectronic Spectroscopy (XPS)
3.4. Inductively Coupled Plasma Emission (ICPE) and Atomic Adsorption
4. Results and Discussion
4.1. Chemical Structure of the Synthesized Chitosan
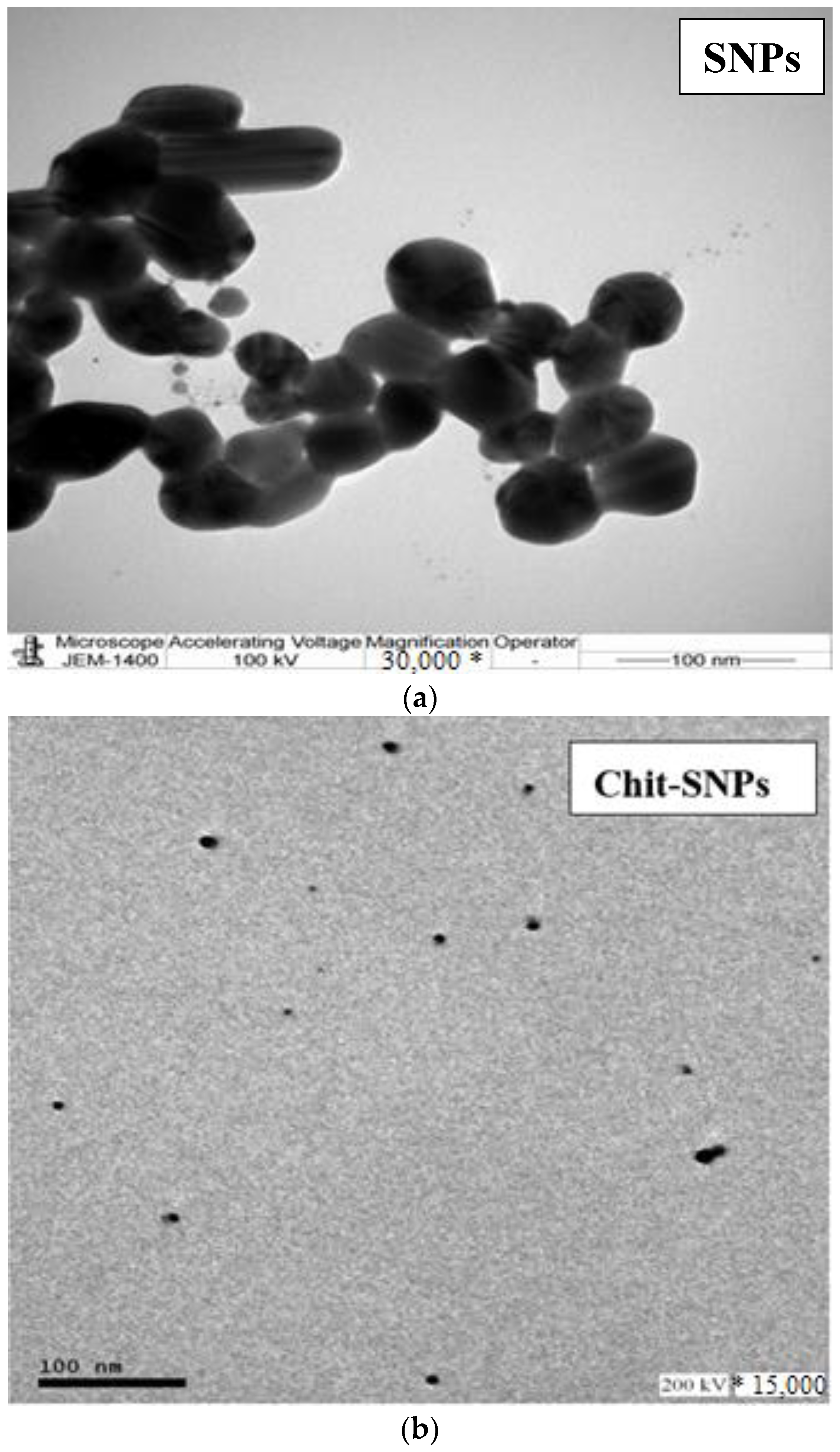
4.2. Chitosan Assembling on Silver Nanoparticles (SNPs)
4.3. Establishment of the Morphology and Composition of the Prepared Nanocomposite (Chit-SNPs-CNTs)
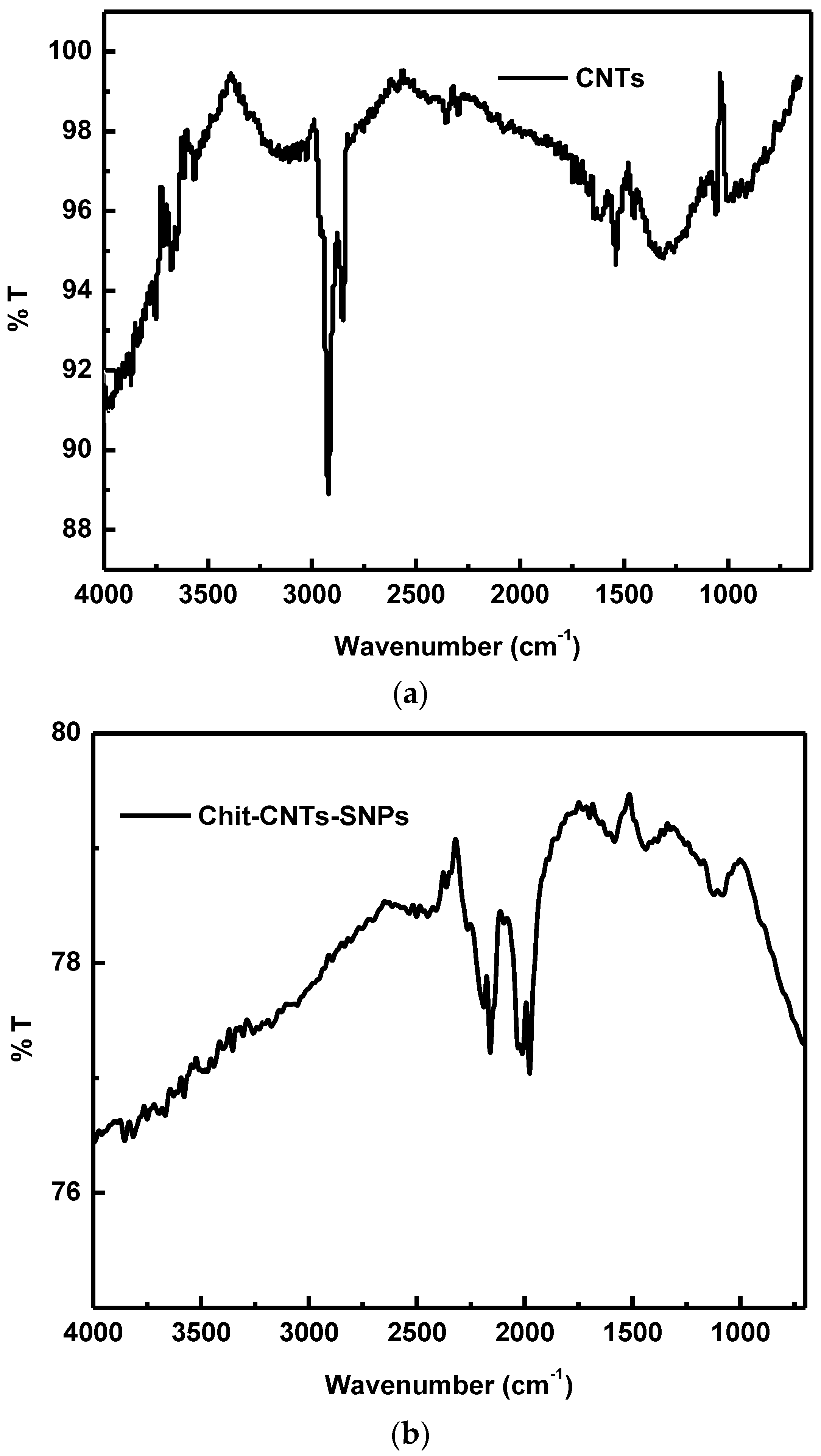
4.3.1. FTIR Spectroscopy of the (Chit-SNPs-CNTs)
4.3.2. Energy-Dispersive X-ray (EDX)
4.3.3. Scanning Electron Microscope (SEM)
4.3.4. X-ray Diffraction (XRD)
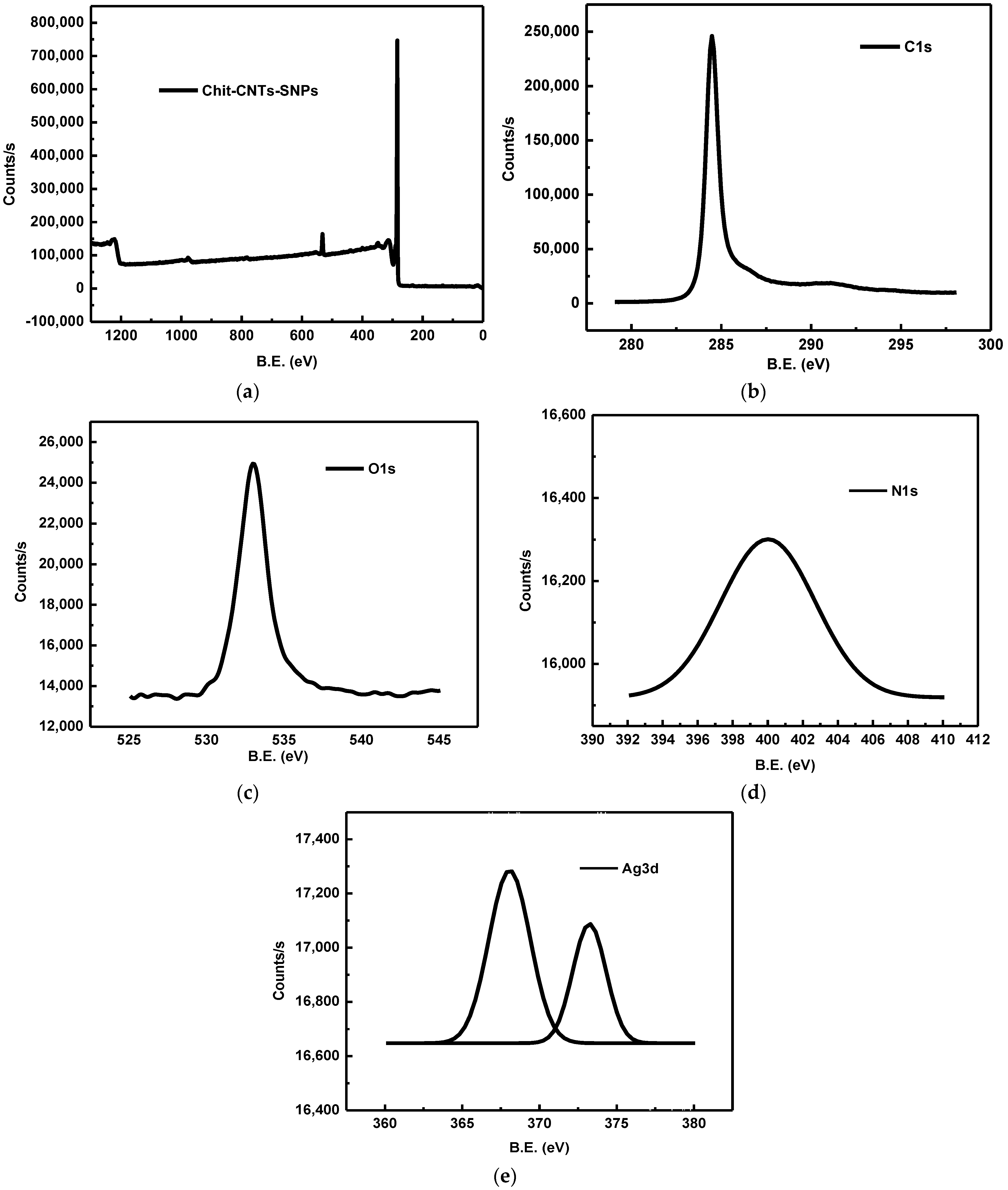
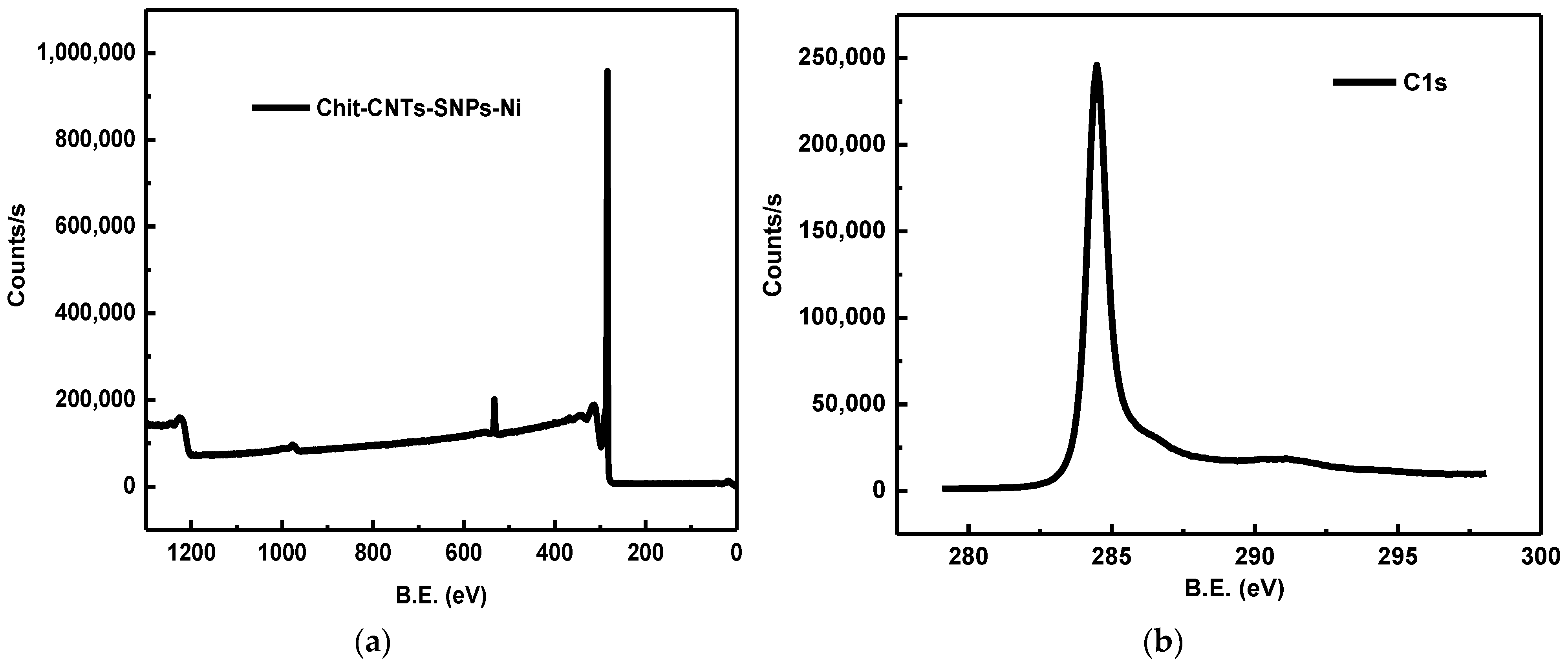
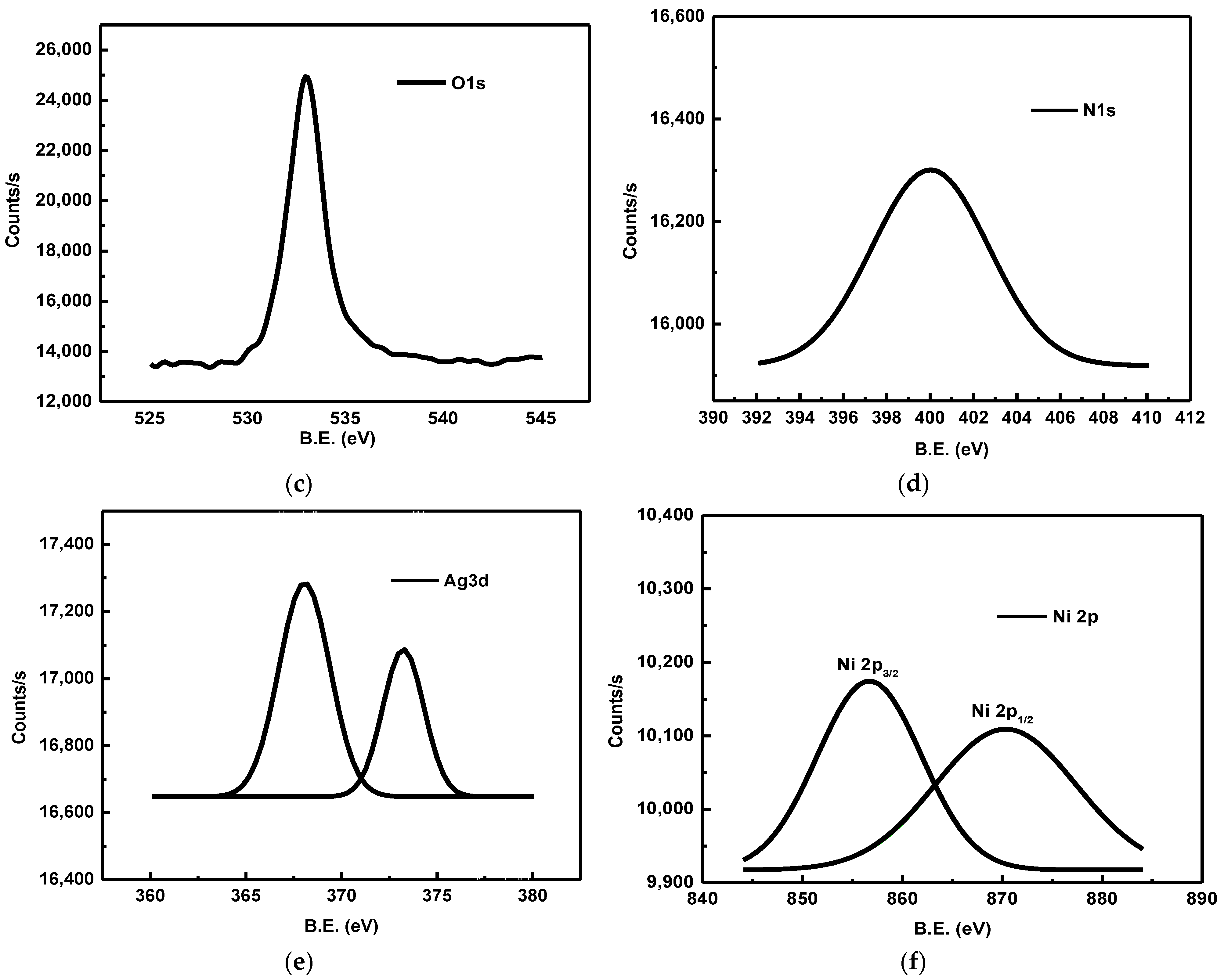
4.3.5. X-ray Photoelectronic Spectroscopy (XPS)
4.4. The Uptake of Ni (II) Ions from Nickel Sulphate Solution
4.5. Variation in Contact Time
4.6. Variation in pH
4.7. Variation in the Nanocomposite Mass
4.8. The Mechanism for the Uptake Process of Ni (II) Ions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Dwivedi, P. Heavy metal water pollution—A case study. Recent Res. Sci. Technol. 2013, 5, 98–99. [Google Scholar]
- Azzam, E.M.; Elsofany, W.I.; Alrashdi, G.K.; Alenezi, K.M.; Alsukaibi, A.K.; Latif, S.; Abdulaziz, F.; Atta, A.M. New route for removal of Cu (II) using fabricated nanocomposite based on cationic surfactant/Ag-nanoparticles/silica gel. Arab. J. Chem. 2022, 15, 103897. [Google Scholar] [CrossRef]
- Jain, N.; Kanu, N.J. The potential application of carbon nanotubes in water treatment: A state-of-the-art-review. Mater. Today Proc. 2021, 43, 2998–3005. [Google Scholar] [CrossRef]
- Wang, X.; Yin, H.; Sheng, G.; Wang, W.; Zhang, X.; Lai, Z. Fabrication of self-entangled 3D carbon nanotube networks from metal–organic frameworks for Li-ion batteries. ACS Appl. Nano Mater. 2018, 7, 7075–7082. [Google Scholar] [CrossRef] [Green Version]
- Kandah, M.I.; Meunier, J.L. Removal of nickel ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, H.; Jing, C.; Wang, X. Soluble polymers with intrinsic porosity for efficient removal of phenolic compounds from water. Micropor. Mesopor. Mater. 2021, 1, 111068. [Google Scholar] [CrossRef]
- Azzam, E.M.; Eshaq, G.H.; Rabie, A.M.; Bakr, A.A.; Abd-Elaal, A.A.; El Metwally, A.E.; Tawfik, S.M. Preparation and characterization of chitosan-clay nanocomposites for the removal of Cu (II) from aqueous solution. Int. J. Biol. Macromol. 2016, 89, 507–517. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Antonino, R.S.; Lia Fook, B.R.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, E.M.; Sami, R.M.; Alenezi, K.M.; Haque, A.; El Moll, H.; Soury, R.A.; Ismail, A.R. Inhibition of Sulfate-Reducing Bacteria by Para-amino-N-((1-Alkylpyridin-1-Ium Bromide)-4-Yl) Benzamide Surfactants and Surfactant-Coated Silver Nanoparticles. J. Surfactants Deterg. 2022, 25, 125–131. [Google Scholar] [CrossRef]
- Azzam, E.M.; Fathy, N.A.; El-Khouly, S.M.; Sami, R.M. Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2/AgNPs/Surfactant nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayalakshmi, K.; Devi, B.M.; Sudha, P.N.; Venkatesan, J.; Anil, S. Synthesis, characterization and applications of nanochitosan/sodium alginate/microcrystalline cellulose film. J. Nanomed. Nanotechnol. 2016, 7, 419. [Google Scholar]
- Azzam, E.M.S.; El-Farargy, A.F.M.; Abd-Elaal, A.A. Enhancement the detection of Ni2+ and Zn2+ ions using nanostructure of synthesized dithiol surfactants with gold nanoparticles. J. Ind. Eng. Chem. 2014, 20, 3905–3912. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral Montanheiro, T.L.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT function-alization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Velmurugan, N.; Kumar, G.G.; Han, S.S.; Nahm, K.S.; Lee, Y.S. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran. Polym. J. 2009, 18, 383–392. [Google Scholar]
- Abdulrazzak, F.H.; Alkiam, A.F.; Hussein, F.H. Behavior of X-Ray Analysis of Carbon Nanotubes. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019. [Google Scholar]
- Azzam, E.M.S.; Abd El-aal, A.A.; Shekhah, O.; Arslan, H.; Wöll, C. Fabrication of spr nanosensor using gold nanoparticles and self-assembled monolayer technique for detection of Cu2+ in an aqueous solution. J. Dispers. Sci. Technol. 2014, 35, 717–724. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Ahmad, S.H.A.; Durrani, S.M.A.; Faiz, M.M.; Ul-Hamid, A. Application of nickel oxide thin films in NiO/Ag multilayer energy-efficient coatings. Mater. Sci. Semiconduct. Process. 2015, 39, 84–89. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Meenakshi, S. Preparation and characterization of silica gel/chitosan composite for the removal of Cu (II) and Pb (II). Int. J. Biol. Macromol. 2012, 50, 650–657. [Google Scholar] [CrossRef] [PubMed]








| Time (min) | 20 min | 40 min | 60 min | 80 min | 100 min | 120 min | 140 min | 160 min |
|---|---|---|---|---|---|---|---|---|
| Removal % | 50% | 55% | 60% | 65% | 75% | 82% | 82% | 82% |
| Ce (g/l) | 0.050 | 0.045 | 0.040 | 0.035 | 0.025 | 0.018 | 0.018 | 0.018 |
| qe (mg/g) | 250 | 275 | 300 | 325 | 375 | 410 | 410 | 410 |
| pH of Ni (II) Solution | pH = 1 | pH = 2 | pH = 10 | pH = 11 |
|---|---|---|---|---|
| Removal % | 35.50% | 25.50% | 40.60% | 45.70% |
| Ce (g/l) | 0.035 | 0.0255 | 0.0406 | 0.0457 |
| qe (mg/g) | 325 | 373 | 297 | 272 |
| Mass of (Chit-CNTs-SNPs) | 0.1 gm | 0.2 gm | 0.3 gm | 0.4 gm |
|---|---|---|---|---|
| Removal % | 95.44% | 97.53% | 99.70% | 99.70% |
| Ce (g/l) | 0.0955 | 0.0080 | 0.0003 | 0.0003 |
| qe (mg/g) | 450 | 460 | 498 | 498 |
| Absorbent | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Chitosan–zeolite composites | 14.75–51.32 | 3 |
| Semi-IPN hydrogel based on chitosan and gelatin | 153.9 | 3 |
| Xanthate-modified magnetic chitosan | 34.5 | 3 |
| Chitosan nanofibrils | 186.6 | 3 |
| Chitosan (Ch)/clay, chitosan (Ch)/AgNPs/clay, and chitosan (Ch)/AuNPs/clay | 181.5 | 3 |
| Chit-CNts-SNPs | 498 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzam, E.M.S.; Elsofany, W.I.; Abdulaziz, F.; AlGhamdi, H.A.; AL alhareth, A.Y. Ecofriendly Elimination of Ni (II) Using Fabricated Nanocomposite Based on Chitosan/Silver Nanoparticles/Carbon Nanotubes. Polymers 2023, 15, 2759. https://doi.org/10.3390/polym15132759
Azzam EMS, Elsofany WI, Abdulaziz F, AlGhamdi HA, AL alhareth AY. Ecofriendly Elimination of Ni (II) Using Fabricated Nanocomposite Based on Chitosan/Silver Nanoparticles/Carbon Nanotubes. Polymers. 2023; 15(13):2759. https://doi.org/10.3390/polym15132759
Chicago/Turabian StyleAzzam, Eid M. S., Walaa I. Elsofany, Fahad Abdulaziz, Hind A. AlGhamdi, and Abdullah Y. AL alhareth. 2023. "Ecofriendly Elimination of Ni (II) Using Fabricated Nanocomposite Based on Chitosan/Silver Nanoparticles/Carbon Nanotubes" Polymers 15, no. 13: 2759. https://doi.org/10.3390/polym15132759





