Cooperative Effect of Ni-Decorated Monolayer WS2, NiO, and AC on Improving the Flame Retardancy and Mechanical Property of Polypropylene Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
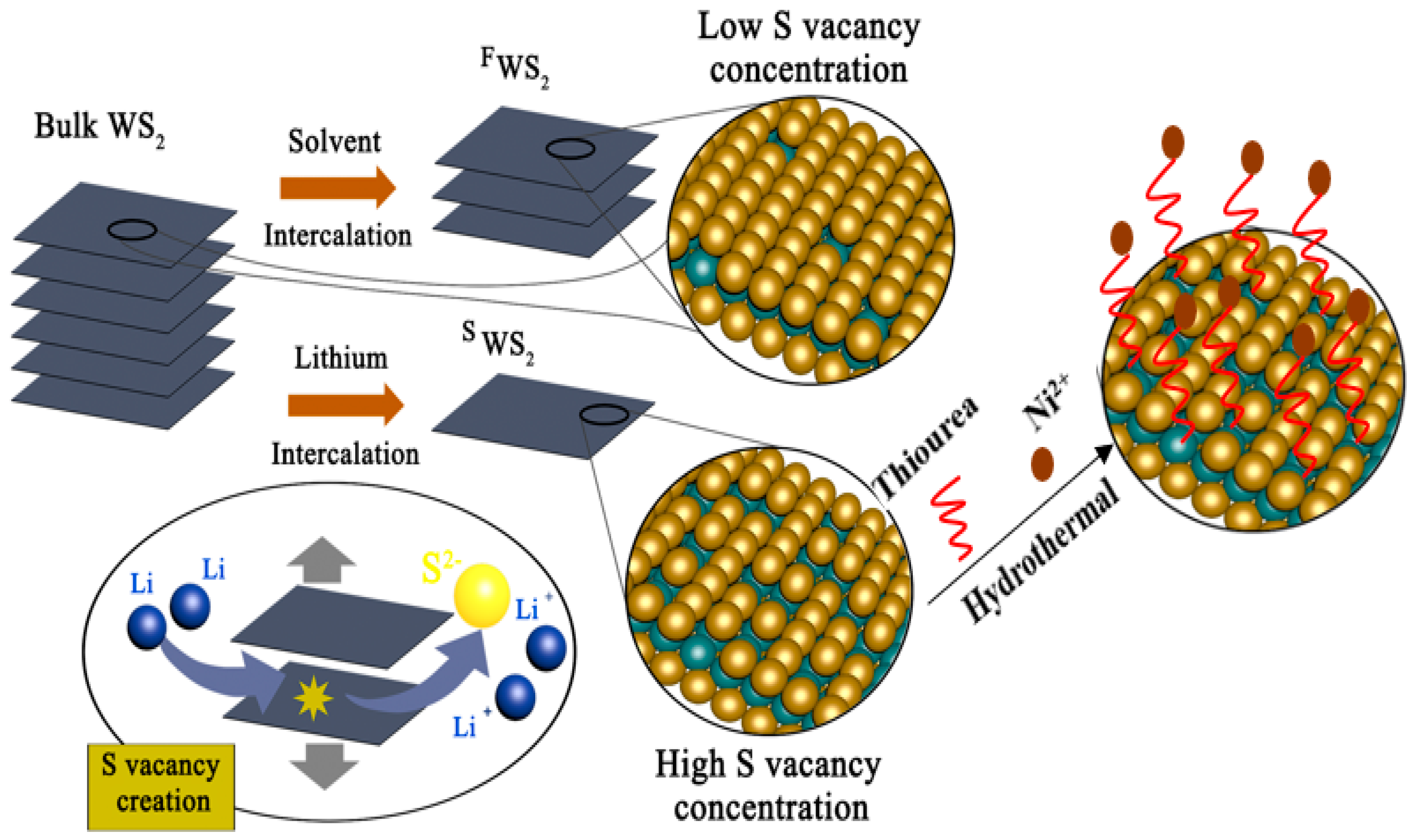
2.2. Preparation of Single Layer WS2
2.3. Preparation of Ni-Decorated WS2
2.4. Preparation of PP Blends
2.5. Characterization
3. Results and Discussion
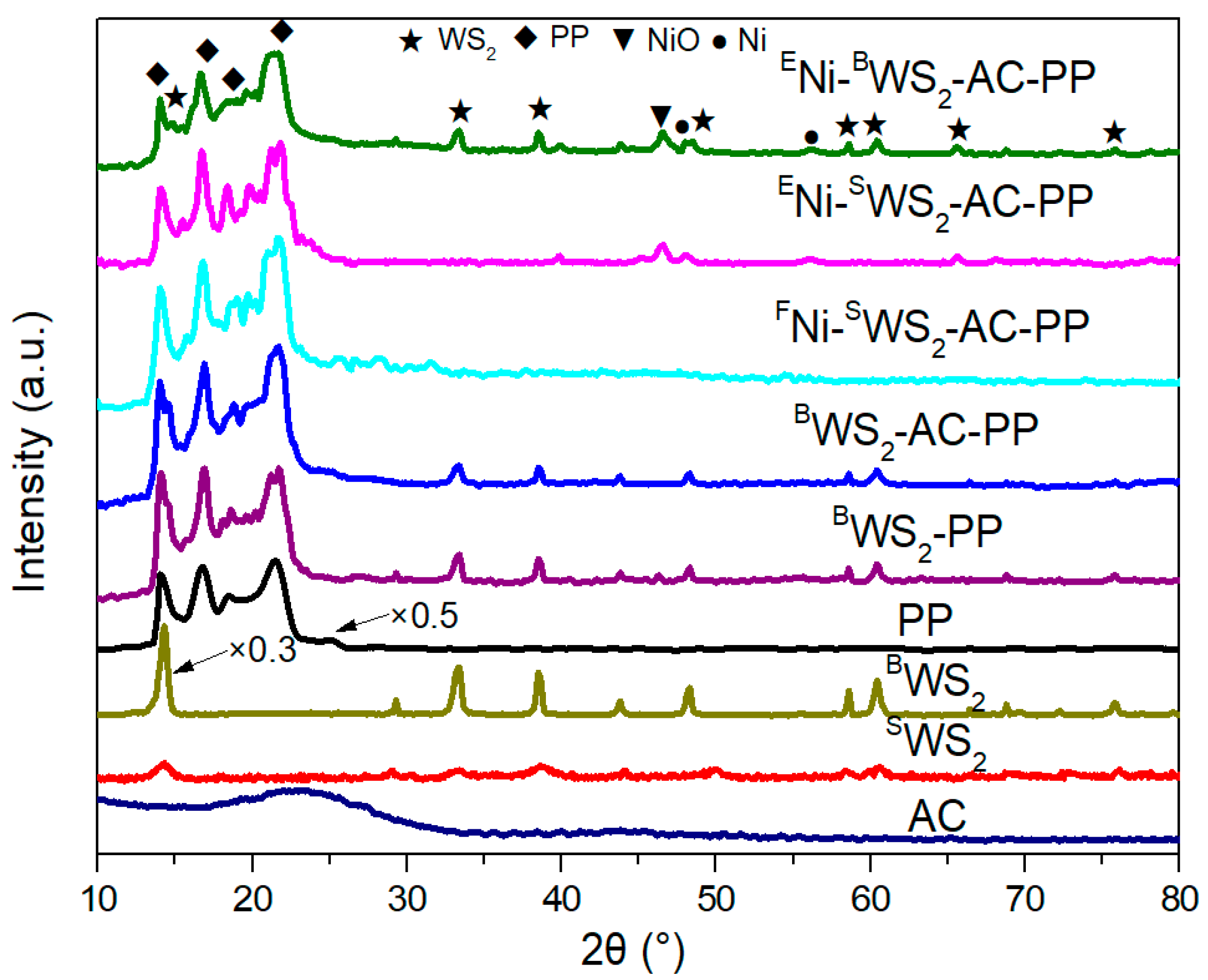
3.1. Characterization of Monolayer WS2
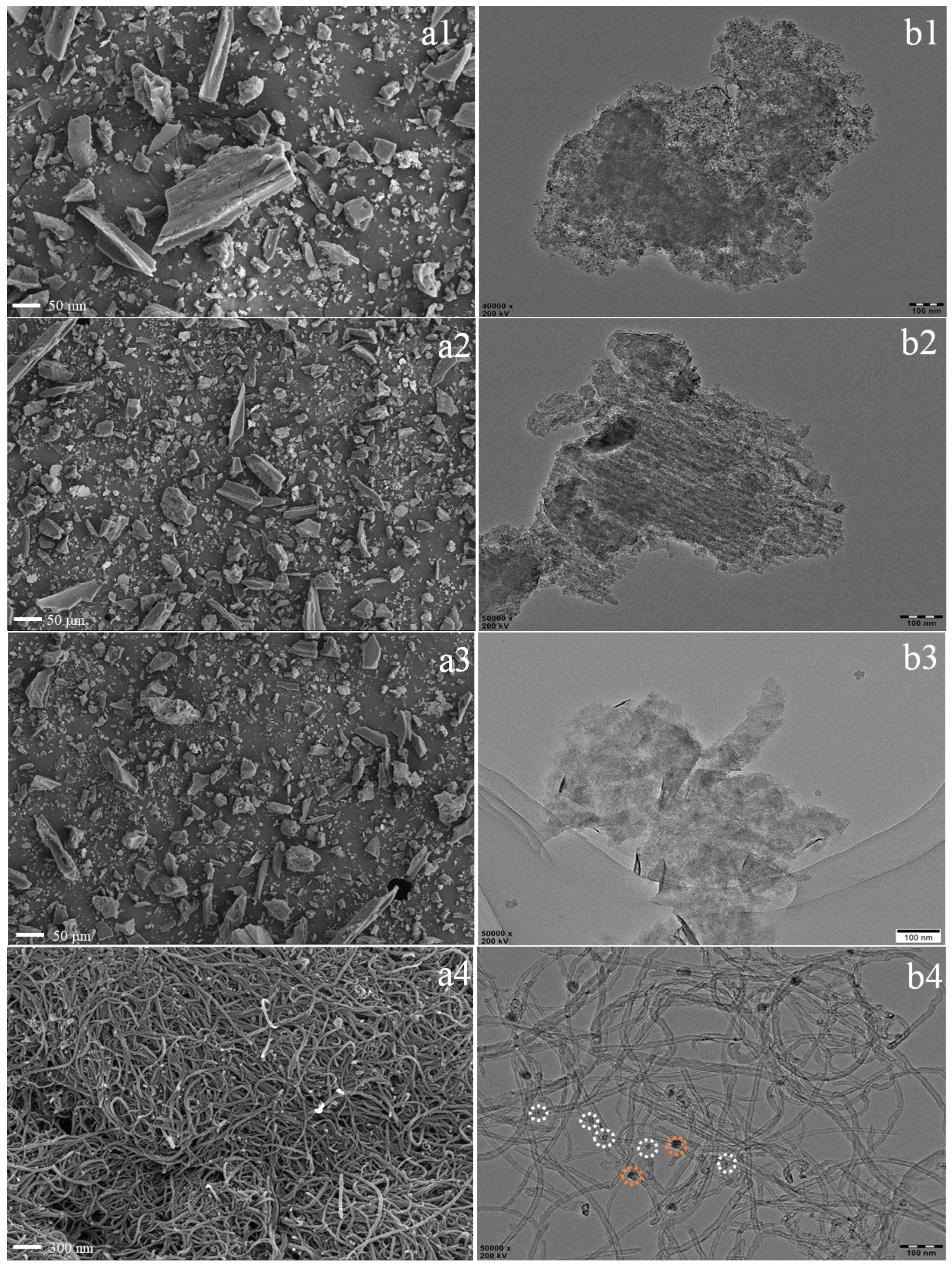
3.2. Dispersion States of Ni and WS2 in PP Matrix
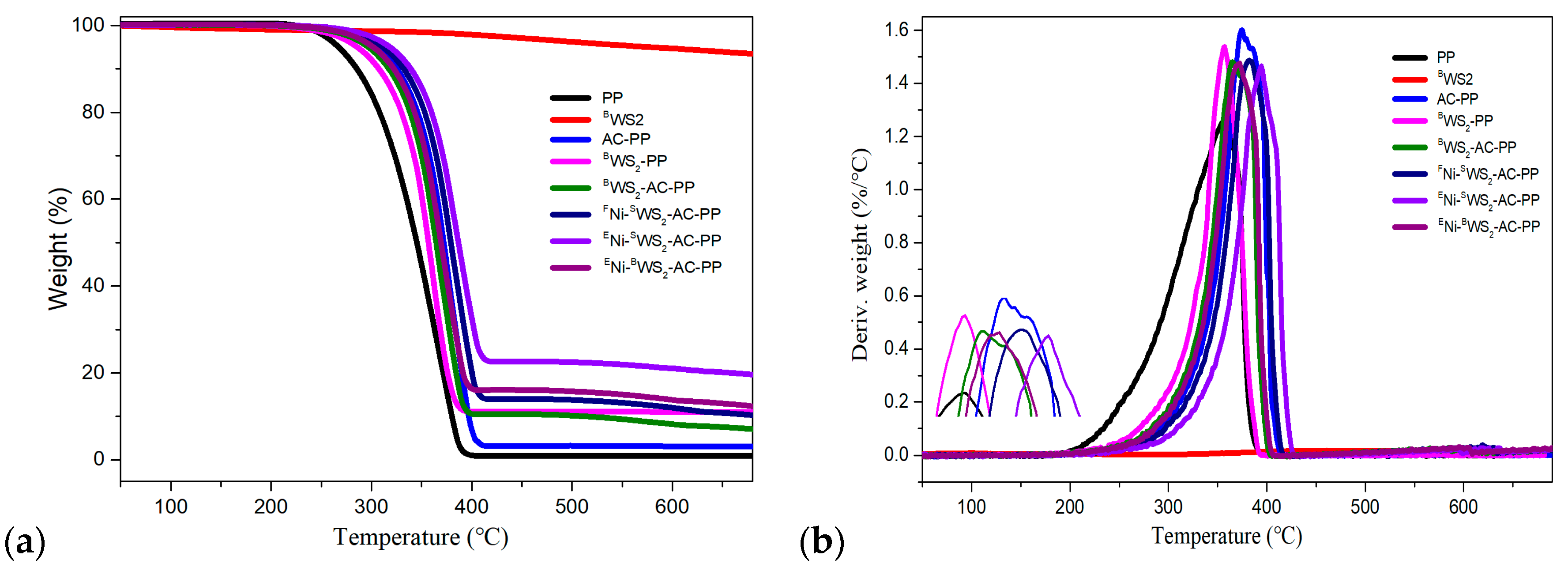
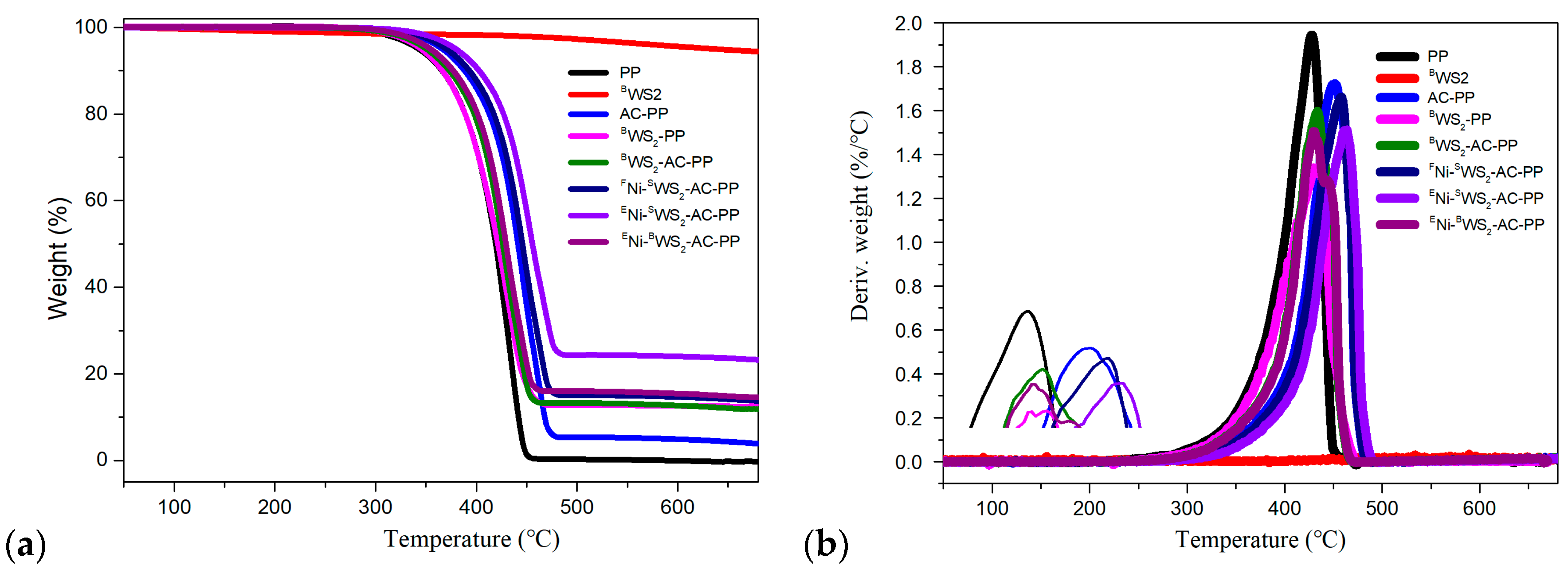
3.3. Thermal Decomposition Behaviors
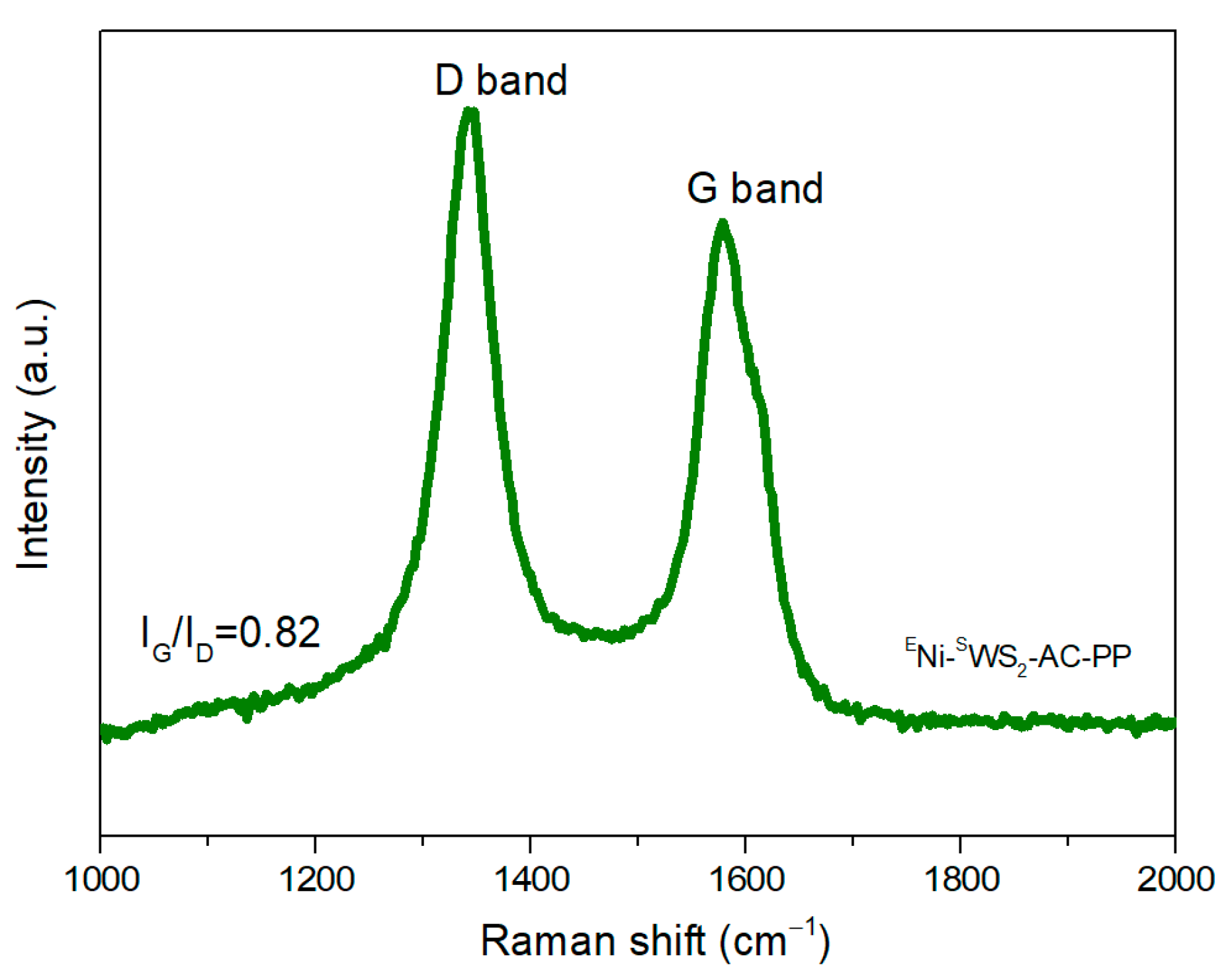
3.4. Characterization of Residual Char
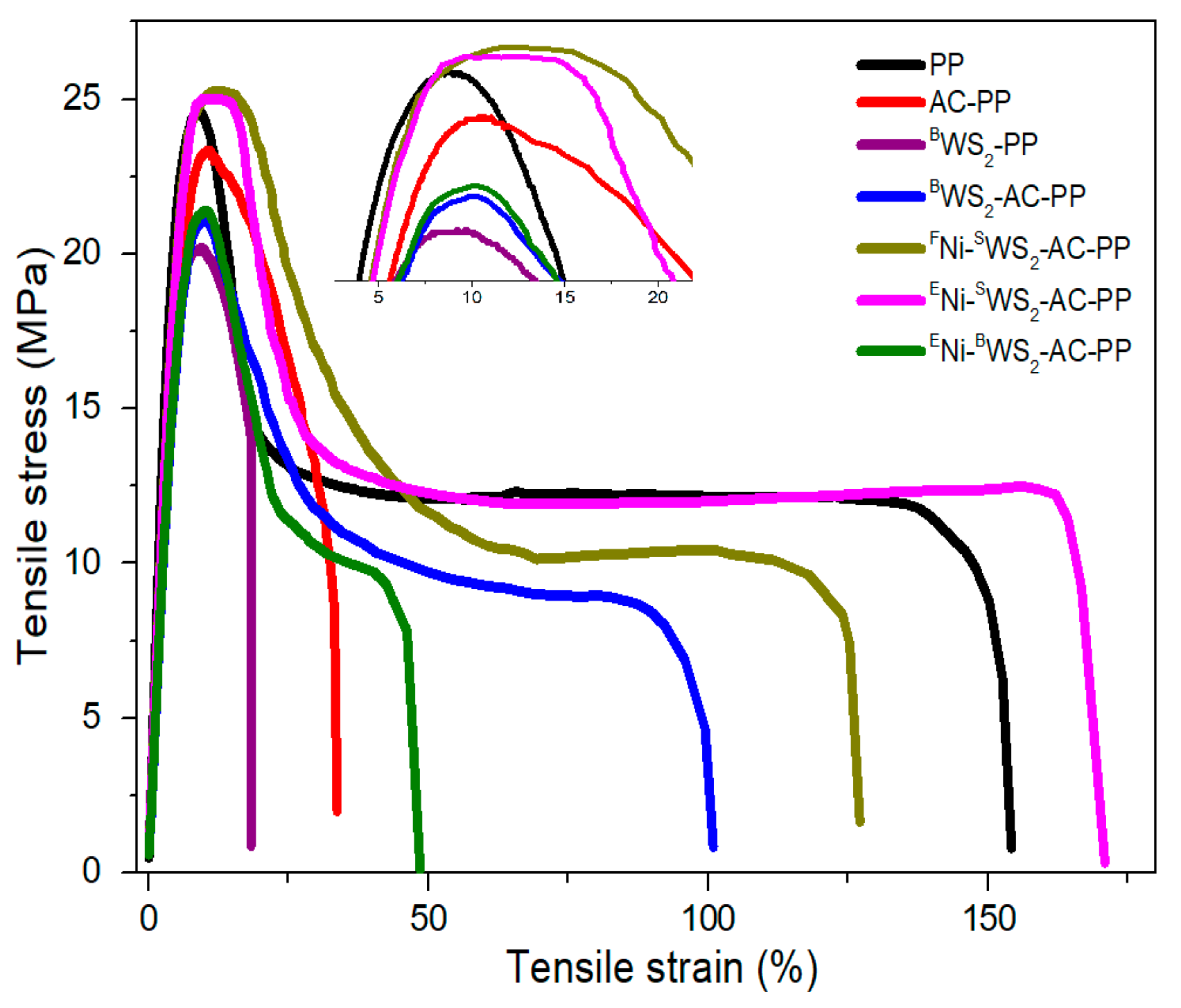
3.5. Mechanical Properties
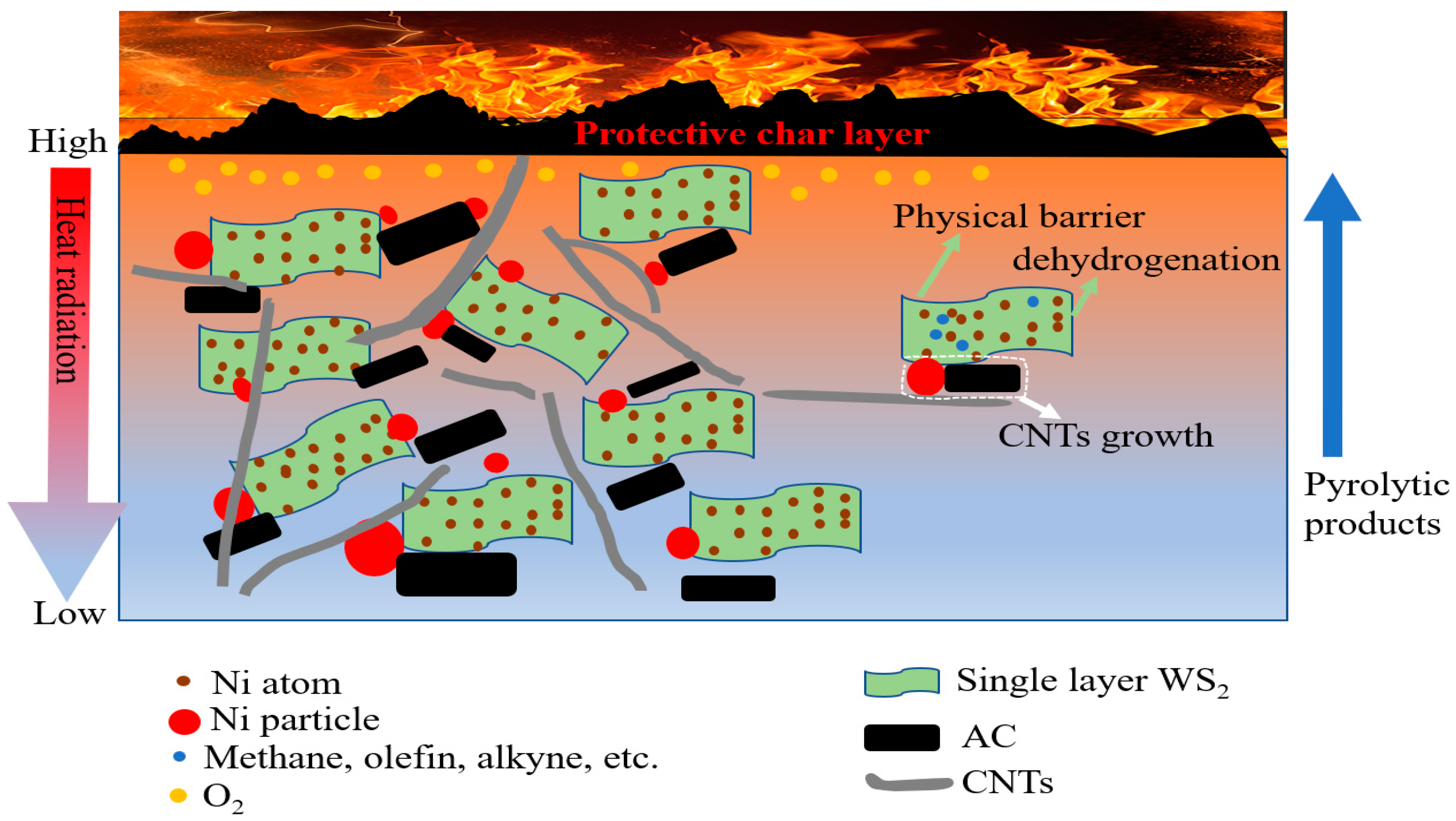
3.6. Flame Retardancy and Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, P.; Wu, M.; Pang, Y.; Shen, B.; Wu, F.; Lan, X.; Luo, H.; Zheng, W. Ultrastrong, flexible and lightweight anisotropic polypropylene foams with superior flame retardancy. Compos. Part A Appl. Sci. Manuf. 2018, 116, 180–186. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Li, H.; Zeng, X. Remarkably improving the fire-safety of polypropylene by synergism of functionalized ZrP nanosheet and N-alkoxy hindered amine. Appl. Clay Sci. 2018, 166, 61–73. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T.; Bilotti, E. Thermal degradation and flammability behavior of polypropylene/clay/carbon nanotube composite systems. Polym. Adv. Technol. 2012, 24, 331–338. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Gong, J.; Liu, J.; Tian, N.; Wang, Y.; Jiang, Z.; Qiu, J.; Tang, T. Thermal and flammability properties of polypropylene/carbon black nanocomposites. Polym. Degrad. Stabil. 2012, 97, 793–801. [Google Scholar] [CrossRef]
- Kang, F.; Deng, J.; Pang, Q.; Yuan, C.; Liu, B.; Yi, X.; Li, H. Flame retardancy and smoke suppression of silicone foams with modified microencapsulated Mg/Zn/Al-layered double hydroxide. J. Therm. Anal. Calorim. 2023. [Google Scholar] [CrossRef]
- Battig, A.; González, K.I.G.; Schartel, B. Valorizing “non-vegan” bio-fillers: Synergists for phosphorus flame retardants in epoxy resins. Polym. Degrad. Stab. 2022, 198. [Google Scholar] [CrossRef]
- Shen, R.; Quan, Y.; Zhang, Z.; Ma, R.; Wang, Q. Metal–Organic Framework as an Efficient Synergist for Intumescent Flame Retardants against Highly Flammable Polypropylene. Ind. Eng. Chem. Res. 2022, 61, 7292–7302. [Google Scholar] [CrossRef]
- He, S.; Deng, C.; Zhao, Z.-Y.; Chen, Z.-X.; Wang, Y.-Z. Hyperbranched polyamide-amine based phosphorous-containing flame retardant for simultaneous flame retardancy and high performance of polypropylene. Compos. Part B Eng. 2023, 250. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Gao, D.; Wen, X.; Guan, Y.; Czerwonko, W.; Li, Y.; Gao, Y.; Mijowska, E.; Tang, T. Flame retardant effect and mechanism of nanosized NiO as synergist in PLA/APP/CSi-MCA composites. Compos. Commun. 2020, 17, 170–176. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2021, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Synthesis and characterization of flame-retardant-wrapped carbon nanotubes and its flame retardancy in epoxy nanocomposites. Polym. Polym. Compos. 2021, 29, S835–S843. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, J.; Tian, W.; Liew, K.M.; Zhang, Y.; Wang, B. Engineering carbon nanotubes wrapped ammonium polyphosphate for enhancing mechanical and flame retardant properties of poly(butylene succinate). Compos. Part A Appl. Sci. Manuf. 2018, 115, 215–227. [Google Scholar] [CrossRef]
- Gong, J.; Tian, N.; Liu, J.; Yao, K.; Jiang, Z.; Chen, X.; Wen, X.; Mijowska, E.; Tang, T. Synergistic effect of activated carbon and Ni2O3 in promoting the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2013, 99, 18–26. [Google Scholar] [CrossRef]
- Gong, J.; Tian, N.; Wen, X.; Chen, X.; Liu, J.; Jiang, Z.; Mijowska, E.; Tang, T. Synergistic effect of fumed silica with Ni2O3 on improving flame retardancy of poly(lactic acid). Polym. Degrad. Stabil. 2014, 104, 18–27. [Google Scholar] [CrossRef]
- Huang, S.; Cai, X.; Liu, J. Growth of Millimeter-Long and Horizontally Aligned Single-Walled Carbon Nanotubes on Flat Substrates. J. Am. Chem. Soc. 2003, 125, 5636–5637. [Google Scholar] [CrossRef]
- Tang, T.; Chen, X.; Meng, X.; Chen, H.; Ding, Y. Synthesis of multiwalled carbon nanotubes by catalytic combustion of polypropylene. Angew. Chem. Int. Ed. Engl. 2005, 44, 1517–1520. [Google Scholar] [CrossRef]
- Wang, D.; Wen, X.; Chen, X.; Li, Y.; Mijowska, E.; Tang, T. A novel stiffener skeleton strategy in catalytic carbonization system with enhanced carbon layer structure and improved fire retardancy. Compos. Sci. Technol. 2018, 164, 82–91. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Dai, Y.; Wang, C.-H. Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem. Eng. J. 2020, 408, 127268. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, W.; Zheng, B.; Gao, L. Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B 2016, 181, 769–778. [Google Scholar] [CrossRef]
- Liu, G.; Robertson, A.W.; Li, M.M.-J.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.-C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017, 9, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Nikulshin, P.; Minaev, P.; Mozhaev, A.; Maslakov, K.I.; Kulikova, M.; Pimerzin, A.A. Investigation of co-effect of 12-tungstophosphoric heteropolyacid, nickel citrate and carbon-coated alumina in preparation of NiW catalysts for HDS, HYD and HDN reactions. Appl. Catal. B Environ. 2015, 176–177, 374–384. [Google Scholar] [CrossRef]
- Shao, M.; Cui, H.; Guo, S.; Zhao, L.; Tan, Y. Preparation and characterization of NiW supported on Al-modified MCM-48 catalyst and its high hydrodenitrogenation activity and stability. RSC Adv. 2016, 6, 61747–61757. [Google Scholar] [CrossRef]
- Yağci, Ö.; Eker Gümüş, B.; Taşdemir, M. Thermal, structural and dynamical mechanical properties of hollow glass sphere-reinforced polypropylene composites. Polym. Bull. 2020, 78, 3089–3101. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Yu, Y.; Fu, S.n.; Song, P. Engineering Interfaces toward High-Performance Polypropylene/Coir Fiber Biocomposites with Enhanced Friction and Wear Behavior. ACS Sustain. Chem. Eng. 2019, 7, 18453–18462. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Intraramongkol, N.; Samoechip, W.; Potiyaraj, P.; Mongkholrattanasit, R.; Jamnongkan, P.; Wongwachirakorn, P.; Sugimoto, M.; Ito, H.; Huang, C.-F. Towards a Circular Economy: Study of the Mechanical, Thermal, and Electrical Properties of Recycled Polypropylene and Their Composite Materials. Polymers 2022, 14, 5482. [Google Scholar] [CrossRef]
- Ebadi-Dehaghani, H.; Reiszadeh, M.; Chavoshi, A.; Nazempour, M.; Vakili, M.H. The Effect of Zinc Oxide and Calcium Carbonate Nanoparticles on the Thermal Conductivity of Polypropylene. J. Macromol. Sci. Part B 2013, 53, 93–107. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M. Polypropylene/Glass Fiber Hierarchical Composites Incorporating Inorganic Fullerene-like Nanoparticles for Advanced Technological Applications. ACS Appl. Mater. Interfaces 2013, 5, 9691–9700. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C. Polymer blend nanocomposites based on poly(L-lactic acid), polypropylene and WS2 inorganic nanotubes. RSC Adv. 2016, 6, 40033. [Google Scholar] [CrossRef] [Green Version]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Dong, J.; Li, X.; Zhao, X.; Tan, W.; Ji, C.; Zhang, Q. Tough polyimide composites synergistically reinforced by carbon nanofiber-grafted carbon fiber and rGO for improved heat dissipation and electromagnetic interference shielding. J. Mater. Res. Technol. 2023, 149, 225–236. [Google Scholar] [CrossRef]
- Shi, Z.; Sheng, J.; Liu, Z.; Yang, Z.; Xing, C.; Li, J.; Chen, W.; Wang, M.; Wang, L.; Fei, W. Graphene-like carbon nanosheet/copper composite with combined performance designed by pyrolyzing trimesic acid@copper formate. J. Mater. Res. Technol. 2021, 13, 111–120. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Xing, W.; Zhou, K.; Song, L.; Hu, Y. Liquid-exfoliated MoS2 by chitosan and enhanced mechanical and thermal properties of chitosan/MoS2 composites. Compos. Sci. Technol. 2014, 93, 76–82. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M. Mechanical and thermal behaviour of isotactic polypropylene reinforced with inorganic fullerene-like WS2 nanoparticles: Effect of filler loading and temperature. Mater. Chem. Phys. 2013, 141, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.-L.; Gu, S.; Xiao, Y.-F.; Wang, Y.-Z.; Chen, L. Novel organophosphonate-decorated WS2 nanosheets towards flame retardancy and mechanical enhancement of epoxy resin. Polym. Degrad. Stabil. 2022, 206, 110170. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Fu, S. Tannic acid-assisted green exfoliation and functionalization of MoS2 nanosheets: Significantly improve the mechanical and flame-retardant properties of polyacrylonitrile composite fibers. Chem. Eng. J. 2019, 384, 123288. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Zhang, L.; Li, M.; Liu, M.; Wang, C.; Fu, S. Amorphous cobalt borate nanosheets grown on MoS2 nanosheet for simultaneously improving the flame retardancy and mechanical properties of polyacrylonitrile composite fiber. Compos. Part B Eng. 2020, 201, 108298. [Google Scholar] [CrossRef]
- Chen, D.; Qin, X.; Cao, X.; Wang, N.; Zhu, Y. Selective laser sintering of PEG treated inorganic fullerene-like tungsten disulfide nanoparticles/polyamide 12 nanocomposites and fire safety behavior. Chem. Eng. J. 2022, 450. [Google Scholar] [CrossRef]
- He, Q.; Yuan, T.; Wei, S.; Guo, Z. Catalytic and synergistic effects on thermal stability and combustion behavior of polypropylene: Influence of maleic anhydride grafted polypropylene stabilized cobalt nanoparticles. J. Mater. Chem. A 2013, 1, 13064–13075. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912. [Google Scholar] [CrossRef]
- Zuo, C.; Liu, Y.; Guo, Y.; Tan, W.; Ren, Y.; Liu, X. Preparation of a copper porphyrin derivative and its surface modification for simultaneously endowing PET fibers with dyeing, flame retardant and anti-dripping performance. Polym. Degrad. Stab. 2023, 209, 110273. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, W.; Jiang, M.; Liu, P.; Xu, J. Flame retardant study of formalized polyvinyl alcohol fiber coated with melamine formaldehyde resins and the synergistic effect of copper ions. Polym. Degrad. Stab. 2017, 144, 331–343. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Wan, D.; Chen, X.; Wen, X.; Mijowska, E.; Jiang, Z.; Wang, Y.; Tang, T. Catalytic carbonization of polypropylene by the combined catalysis of activated carbon with Ni2O3 into carbon nanotubes and its mechanism. Appl. Catal. A Gen. 2012, 449, 112–120. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, R.; Bi, W.; Lu, J.; Tang, T. Polypropylene as a carbon source for the synthesis of multi-walled carbon nanotubes via catalytic combustion. Carbon 2007, 45, 449–458. [Google Scholar] [CrossRef]



| Sample | PP (g) | AC (g) | WS2 (g) | Ni (Coordinated with Thiourea) (mg) | NiO (g) | Sum (g) | Residue (wt%) |
|---|---|---|---|---|---|---|---|
| PP | 100 | - | - | - | - | 100 | 0 |
| BWS2 | - | - | 100 | - | - | 100 | - |
| AC-PP | 92 | 8 | - | - | - | 100 | 3.1 |
| BWS2-PP | 92 | - | 8 | - | - | 100 | 8.6 |
| BWS2-AC-PP | 92 | 4 | 4 | - | - | 100 | 6.4 |
| FNi-SWS2-AC-PP | 92 | 4 | 4 | 8.4 × 10−2 | - | ≈100 | 8.3 |
| ENi-SWS2-AC-PP | 92 | 2 | 2 | 8.4 × 10−2 | 4 | ≈100 | 41.6 |
| ENi-BWS2-AC-PP | 92 | 2 | 2 | 0 | 4 | 100 | 14.9 |
| Sample | T5%wt a | T10%wt b | T50%wt c | Tmax d | Residue (wt%) |
|---|---|---|---|---|---|
| PP | 265.9 | 285.8 | 344.1 | 356.5 | 0.33 |
| BWS2 | 577.9 | - | - | - | 93.36 |
| AC-PP | 306.5 | 327.1 | 373.8 | 374.5 | 3.05 |
| BWS2-PP | 268.3 | 293.9 | 345.4 | 356.9 | 9.94 |
| BWS2-AC-PP | 298.3 | 318.8 | 366.5 | 367.8 | 8.57 |
| FNi -SWS2-AC-PP | 312.4 | 332.5 | 381.7 | 383.1 | 9.93 |
| ENi-SWS2-AC-PP | 314.1 | 336.9 | 394.1 | 395.7 | 21.29 |
| ENi-BWS2-AC-PP | 302.4 | 322.9 | 369.7 | 373.1 | 12.73 |
| Sample | T5%wt | T10%wt | T50%wt | Tmax | Residue (wt%) |
|---|---|---|---|---|---|
| PP | 342.9 | 365.9 | 418.8 | 427.6 | 0.21 |
| BWS2 | 638.3 | - | - | 94.26 | |
| AC-PP | 367.6 | 389.5 | 443.1 | 451.3 | 3.91 |
| BWS2-PP | 345.6 | 366.3 | 421.1 | 429.3 | 12.45 |
| FNi-SWS2-AC-PP | 370.3 | 393.6 | 445.9 | 458.2 | 10.76 |
| ENi-SWS2-AC-PP | 374.6 | 397.9 | 455.6 | 464.7 | 23.15 |
| ENi-BWS2-AC-PP | 353.8 | 375.9 | 429.3 | 439.8 | 14.69 |
| Sample | Tensile Strength | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| PP | 24.6 ± 0.6 | 146 ± 15 | 601 ± 82 |
| AC-PP | 23.4 ± 0.4 | 32 ± 4 | 568 ± 53 |
| BWS2-PP | 20.1 ± 0.5 | 18 ± 2 | 487 ± 61 |
| BWS2-AC-PP | 21.1 ± 0.7 | 91 ± 11 | 533 ± 49 |
| FNi-SWS2-AC-PP | 25.4 ± 0.3 | 120 ± 14 | 624 ± 84 |
| ENi-SWS2-AC-PP | 25.1 ± 0.5 | 162 ± 5 | 609 ± 75 |
| ENi-BWS2-AC-PP | 21.4 ± 0.6 | 43 ± 4 | 546 ± 68 |
| Sample | pHRR (W/g) | pHRR Reduction (%) | TpHRR (°C) | HRC (J/g K) | THR (kJ/g) |
|---|---|---|---|---|---|
| PP | 1317 | - | 483.5 | 1241 | 43.4 |
| BWS2-PP | 1237 | 6.07 | 485.6 | 1150 | 40.2 |
| BWS2-AC-PP | 1131 | 14.12 | 487.1 | 1086 | 39.5 |
| FNi-SWS2-AC-PP | 1084 | 17.69 | 493.2 | 1002 | 38.9 |
| ENi-SWS2-AC-PP | 707 | 46.32 | 495.6 | 643 | 32.1 |
| ENi-BWS2-AC-PP | 1012 | 23.16 | 488.3 | 996 | 37.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Shi, Y.; Liu, J.; Xue, B.; Niu, M. Cooperative Effect of Ni-Decorated Monolayer WS2, NiO, and AC on Improving the Flame Retardancy and Mechanical Property of Polypropylene Blends. Polymers 2023, 15, 2791. https://doi.org/10.3390/polym15132791
Shao M, Shi Y, Liu J, Xue B, Niu M. Cooperative Effect of Ni-Decorated Monolayer WS2, NiO, and AC on Improving the Flame Retardancy and Mechanical Property of Polypropylene Blends. Polymers. 2023; 15(13):2791. https://doi.org/10.3390/polym15132791
Chicago/Turabian StyleShao, Mingqiang, Yiran Shi, Jiangtao Liu, Baoxia Xue, and Mei Niu. 2023. "Cooperative Effect of Ni-Decorated Monolayer WS2, NiO, and AC on Improving the Flame Retardancy and Mechanical Property of Polypropylene Blends" Polymers 15, no. 13: 2791. https://doi.org/10.3390/polym15132791
APA StyleShao, M., Shi, Y., Liu, J., Xue, B., & Niu, M. (2023). Cooperative Effect of Ni-Decorated Monolayer WS2, NiO, and AC on Improving the Flame Retardancy and Mechanical Property of Polypropylene Blends. Polymers, 15(13), 2791. https://doi.org/10.3390/polym15132791






